Abstract
Cachexia is characterized by severe weight loss, including adipose and muscle wasting, and occurs in a large percentage of cancer patients. Insulin resistance contributes to dysregulated metabolism in cachexia and occurs prior to weight loss in mice with colon-26 tumor-induced cachexia. Therefore, we hypothesized that the insulin sensitizer, rosiglitazone, would attenuate the loss of adipose and muscle to result in improved outcomes for mice with late-stage cachexia. Male CD2F1 mice were inoculated with colon-26 adenocarcinoma cells or vehicle. Treatments included vehicle, rosiglitazone (10 mg/kg body weight/day) or rosiglitazone plus pair-feeding to food intake of vehicle-treated mice with tumors. Rosiglitazone delayed weight loss onset by 2 d over the 16 d duration of this aggressive tumor model. This finding was associated, in part, with increased food intake. In addition, adipose mass, adipocyte cross-sectional area and inflammation were improved with rosiglitazone. However, at the time of necropsy 16 d after tumor inoculation rosiglitazone had no effect on retention of muscle mass, strength or proteolysis in late-stage cachexia. We did not measure stamina or endurance in this study. In early-stage cachexia, rosiglitazone normalized PDK4 and PPAR-delta mRNA in quadriceps muscle and rescued the decrease in insulin-stimulated glucose disappearance in mice with tumors. Rosiglitazone may delay weight loss onset by decreasing tumor-induced markers of metabolic change in early-stage cachexia. These changes predict for modest improvement in adipose, but no improvement in muscle strength in late-stage cachexia.
Keywords: rosiglitazone, cancer cachexia, colon-26 adenocarcinoma, insulin resistance
Introduction
Cachexia has recently been defined as a “multifactorial syndrome characterized by severe loss of body weight, adipose mass, and muscle and accompanied by increased protein catabolism due to underlying disease(s).”1 An estimated 30–90% of cancer patients develop cachexia, depending on the type of cancer.2,3 Diminished skeletal muscle mass leads to severe weakness and fatigue, and decreased tolerability and effectiveness of anticancer therapies.2,3 Besides decreased quality of life, cancer cachexia is estimated to cause between 10–20% of cancer-related deaths.4,5
Both anorexia and dysregulated metabolism are key contributors to cachexia-induced weight loss.6 Strategies for improving energy intake are necessary to counteract decreased appetite, and in some cases, tumor-induced increases in metabolic rate.7 However, higher energy consumption is not adequate to stop or reverse cachexia, particularly muscle wasting. Normalization of metabolism is paramount for the effective utilization of nutrients.6
Glucose intolerance was noted in cancer patients nearly a century ago.8 Numerous pre-clinical and clinical studies have utilized exogenous insulin therapy to promote weight gain in cachexia. Generally, insulin increases fat mass with little or no improvement in lean mass in rodents and humans.9-14 Additionally, insulin is an anabolic hormone that promotes growth of many tumors,9-12 making insulin therapy potentially contraindicative for patients with cancer. Another problem with insulin therapy for cachexia is the presence of insulin resistance, rendering insulin less effective in eliciting an anabolic response. Insulin resistance is present in patients with cancer cachexia.15,16 We previously reported that the onset of insulin resistance occurs prior to weight loss in mice with colon-26 tumors, suggesting a role for insulin resistance in cachexia pathogenesis.17
Rosiglitazone is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonist and insulin sensitizing agent primarily used for treating type 2 diabetes mellitus. PPAR-γ is a transcription factor expressed most highly in adipose tissue, where it drives adipocyte differentiation and lipogenesis. Adipogenesis results in lipid partitioning away from non-adipose tissues and into adipose, preventing ectopic lipid accumulation, an important contributor to insulin resistance.18,19 In cachexia, ectopic lipid accumulation and subsequent lipotoxicity would likely occur not as a result of excess lipid as occurs in obesity and type 2 diabetes, but rather as a result of rapid delipidation and wasting of adipose tissue. Rosiglitazone could preserve insulin sensitivity in mice with cachexia by promoting adipogenesis and attenuating delipidation of adipose tissue, potentially preserving insulin sensitivity. In addition, rosiglitazone promotes an improved adipocytokine profile, decreasing pro-inflammatory cytokine production and increasing adiponectin.18,19 Anti-inflammatory properties of rosiglitazone may be important in the study of cachexia as tumor-induced inflammation is thought to be an important component to dysregulated metabolism and weight loss in this syndrome.20 In agreement with the known mechanisms of action of rosiglitazone, treatment with rosiglitazone increased insulin signaling and subsequently decreased proteolysis in muscle of db/db mice,21 a model of insulin resistance and diabetes. We found that rosiglitazone at 10 mg/kg body weight daily preserved insulin sensitivity, decreased proteolytic gene expression, and maintained adipose mass in early-stage cachexia in mice with colon-26 tumors.17
The objective of the current study was to determine if the effects of rosiglitazone to preserve insulin sensitivity in early-stage cachexia17 lead to improved outcomes in mice with late-stage cachexia. We, therefore, tested two hypotheses: 1) rosiglitazone attenuates loss of body weight, adipose and muscle mass, and muscle strength, and decreases proteolysis and inflammation in late-stage cachexia in mice with colon-26 adenocarcinoma tumors; and 2) differences in outcomes are associated with attenuated tumor-induced changes in markers of metabolism.
Results
Late-stage cachexia study
Body and tissue weights
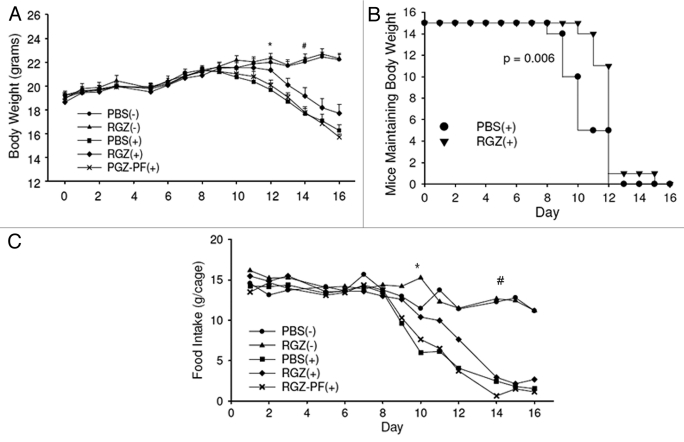
Sixteen days after tumor inoculation, the PBS(+), RGZ(+) and RGZ-PF(+) groups lost 15.1, 5.3 and 17.5% body weight compared with baseline, respectively. This corresponded to 26.8, 20.3 and 29.3% reductions when compared with the PBS(-) group at the end of the study (Fig. 1A). The PBS(+) and RGZ-PF(+) groups had decreased body weight compared with the PBS(-) group beginning on day 12, and the RGZ(+) group had decreased body weight compared with the PBS(-) group beginning on day 14 (Fig. 1A). Total weight loss at the end of the study was significantly attenuated in the RGZ(+) group compared with the PBS(+) group (Table 1). To examine the delayed onset of weight loss in the RGZ(+) group, we analyzed time to weight loss incidence, defined as ≥ 1g loss from the maximum body weight for each mouse. One gram was chosen because it represented approximately 5% of body weight, which is definitive of cachexia in humans.22 Mice maintained weight significantly longer in the RGZ(+) group (median 13 d) compared with the PBS(+) group (median 11 d) (Fig. 1B).
Figure 1.
Body weight and food intake. (A) Mice were inoculated with 1x106 colon-26 adenocarcinoma cells (+) or vehicle (-) and treated daily with intraperitoneal injections of either RGZ or PBS. Some RGZ-treated mice with tumors were pair-fed (PF) to PBS-treated mice with tumors. Body weights were measured daily. Groups with tumors were compared with the PBS(-) group for statistical analyses. *First day that the PBS(+) and RGZ-PF(+) groups had decreased body weight compared with the PBS(-) group. #First day that the RGZ(+) group had decreased body weight compared with the PBS(-) group. Significance continued through the remainder of the study. n = 13–15 mice/group. (B) Kaplan-Meier survival plot illustrating the difference in weight loss incidence between PBS(+) and RGZ(+) groups. p value represents a comparison of the two groups using a Wilcoxon Signed Rank test. (C) Food intake was measured daily throughout the study. Values represent the average intake per cage, n = three cages per treatment group. *First day that the PBS(+) group had decreased food intake compared with the PBS(-) group. #First day that the RGZ(+) group had reduced food intake compared with the PBS(-) group.
Table 1. Body weight change (g), tissue mass (mg) and plasma metabolites for late-stage cachexia study.
| |
No tumor |
Tumor |
Effect p value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBS | RGZ | PBS | RGZ | RGZ-PF | Treatment | Tumor | Treatment x Tumor | |||
| Body weight change |
3.0 ± 0.3 |
3.4 ± 0.3 |
|
−2.9 ± 0.4 |
−1.0 ± 0.6* |
−3.3 ± 0.3 |
|
0.005 |
< 0.001 |
0.064 |
| Quadriceps muscle |
372 ± 19 |
347 ± 22 |
|
281 ± 19 |
271 ± 15 |
221 ± 14* |
|
0.342 |
< 0.001 |
0.693 |
| Tibialis Anterior muscle |
96 ± 4 |
103 ± 4 |
|
68 ± 4 |
72 ± 5 |
65 ± 3 |
|
0.184 |
< 0.001 |
0.778 |
| Gastrocnemius muscle |
223 ± 9 |
227 ± 7 |
|
160 ± 8 |
177 ± 10 |
156 ± 6 |
|
0.226 |
< 0.001 |
0.421 |
| Epidydimal adipose |
664 ± 40 |
862 ± 31 |
|
91 ± 23 |
249 ± 55* |
133 ± 21 |
|
< 0.001 |
< 0.001 |
0.636 |
| Inguinal adipose |
513 ± 28 |
580 ± 53 |
|
35 ± 16 |
179 ± 51* |
64 ± 17 |
|
0.014 |
< 0.001 |
0.355 |
| Brown adipose |
132 ± 6 |
176 ± 9 |
|
40 ± 7 |
87 ± 11** |
78 ± 5*** |
|
< 0.001 |
< 0.001 |
0.878 |
| Tumor |
|
|
|
1648 ± 98 |
1754 ± 87 |
1846 ± 102 |
|
|
|
|
| Fasting glucose (mmol/L) |
8.0 ± 0.3 |
7.5 ± 0.3 |
|
4.4 ± 0.8 |
3.6 ± 0.5 |
3.3 ± 0.4 |
|
0.174 |
< 0.001 |
0.804 |
| Insulin-stimulated glucose change (mmol/L) |
−1.6 ± 0.3 |
−1.9 ± 0.4 |
|
0.2 ± 0.2 |
0.5 ± 0.2 |
0.3 ± 0.2 |
|
0.920 |
< 0.001 |
0.398 |
| Adiponectin (ng/ml) |
9202 ± 592 |
19342 ± 490 |
|
3384 ± 274 |
6431 ± 632** |
6803 ± 574** |
|
< 0.001 |
< 0.001 |
< 0.001 |
| IL-6 (pg/ml) |
2 ± 1 |
5 ± 3 |
|
274 ± 101 |
134 ± 29 |
127 ± 31 |
|
0.087 |
< 0.001 |
0.075 |
| NEFA (mEq/L) | 0.33 ± 0.02 | 0.26 ± 0.02 | 0.25 ± 0.06 | 0.36 ± 0.10 | 0.35 ± 0.10 | 0.698 | 0.898 | 0.128 | ||
Mice were inoculated with 1x106 colon-26 adenocarcinoma cells (+) or vehicle (-) on day 0, and treated daily with IP injections of PBS or RGZ. Some RGZ(+) mice were pair-fed to the PBS(+) group. Mice were sacrificed when there was ~30% difference in body weight between the PBS(-) and PBS(+) groups. Two-way ANOVA was done to analyze tumor, treatment and tumor x treatment effects between the PBS(-), RGZ(-), PBS(+) and RGZ(+) groups. Two-sample t-tests with the Bonferroni correction factor were done to determine differences between the RGZ(+) and RGZ-PF(+) groups compared with the PBS(+) group. n = 13–15 mice/group, *p < 0.05, **p < 0.01, ***p < 0.001.
The presence of tumor had a significant effect on the mass of all tissue collected. Gastrocnemius and tibialis anterior muscle masses were not different among the groups with tumors. Quadriceps muscle mass was not different between the PBS(+) and RGZ(+) groups, but the RGZ-PF(+) group was decreased by 21% compared with the PBS(+) group (Table 1). RGZ increased epididymal, inguinal and brown adipose mass in the RGZ(+) group by 2.75-, 5.10- and 2.15-fold respectively, compared with the PBS(+) group. This significant increase was maintained in the RGZ-PF(+) group for brown adipose (Table 1). No differences were detected in tumor mass between groups (Table 1).
Food intake
Food intake declined with weight loss in all three groups of mice with tumors. Similar to the pattern of delayed weight loss in the RGZ(+) group, this group also had a delayed onset of anorexia (Fig. 1C). Food intake was decreased in the PBS(+) group compared with the PBS(-) groups beginning on day 10 (p < 0.01), whereas the RGZ(+) group did not have significantly reduced food intake until day 14 (p < 0.001).
Proteolysis
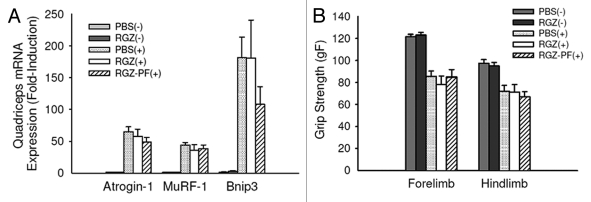
Atrogin-1 and Murf-1 are E3 ligases in skeletal muscle essential for proteasomal proteolysis.23,24 Bnip3 is involved in the regulation of autophagy, or lysosomal proteolysis.25 There was a significant effect of tumor to increase all three transcripts (p < 0.001). Unlike our previous results in mice with early-stage cachexia,17 RGZ was not effective in reducing proteolytic gene expression in tumor-bearing mice with late-stage cachexia (Fig. 2A).
Figure 2.
Markers of muscle proteolysis and strength. (A) Quadriceps muscle mRNA expression of proteolytic genes in mice with (+) and without (-) tumors treated daily with RGZ or PBS. Some tumor-bearing mice treated with RGZ were pair-fed [RGZ-PF(+)] to the PBS(+) group. n = 5–8 mice per group. No differences between tumor groups were detected. (B) Forelimb and hindlimb grip strength (gF, grams of force) 15 d after inoculation with tumor or vehicle. Each test was done in triplicate and averaged for each mouse. n = 13–15 mice per group. No differences between tumor groups were detected.
Grip strength
There was no difference between groups in grip strength at baseline (data not shown). For forelimb and hindlimb tests, there was a significant effect of tumor to decrease grip strength 15 d after tumor inoculation (p < 0.001). No difference was detected when comparing the RGZ(+) and RGZ-PF(+) groups to the PBS(+) group (Fig. 2B).
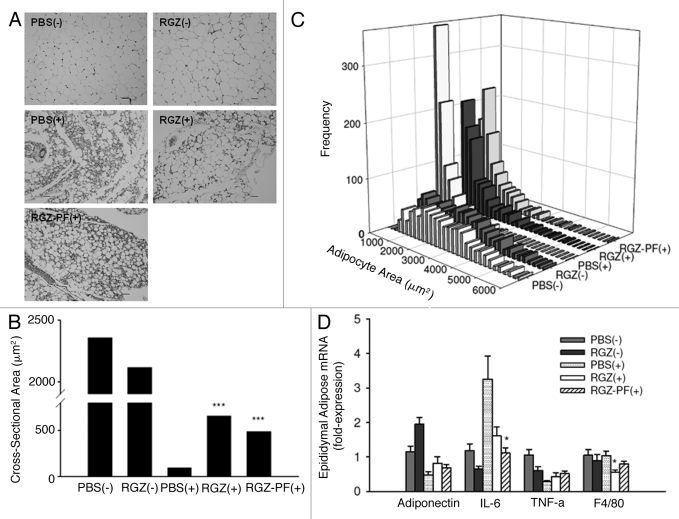
Epididymal adipose cross-sectional area
Epididymal adipocyte cross-sectional area was measured to estimate adipocyte size. There was a significant effect of tumor to decrease adipocyte cross-sectional area regardless of treatment (p < 0.001; Fig. 3A), but tumor-bearing mice treated with RGZ had increased adipocyte area compared with tumor-bearing mice treated with PBS (Fig. 3B and C).
Figure 3.
Adipocyte size and inflammation. (A) Representative images of hematoxylin and eosin-stained epididymal adipose sections. Magnification = 200x; bar = 50 μm. (B) Cross-sectional area of adipocytes was analyzed using Image J software. Graph represents median of 900 cells per group (100 cells/mouse x 9 mice). ***p < 0.001 compared with the PBS(+) group. (C) Histograms illustrating differences in the distribution of adipocyte cross-sectional areas between groups. (D) Epididymal adipose relative mRNA expression of adipocytokines (adiponectin, IL-6, TNF-α), and a marker of macrophage infiltration (F4/80), n = 5–7 mice per group for adiponectin, TNF-α, and F4/80, n = 11–14 mice per group for IL-6, *p < 0.05 compared with the PBS(+) group.
Fasting glucose and insulin resistance
All groups with tumors had decreased fasting glucose values compared with mice without tumors, and the blood glucose response to insulin was blunted, regardless of RGZ treatment (Table 1). On the contrary, plasma levels of adiponectin, an insulin-sensitizing adipocytokine, were improved in both RGZ-treated groups with tumors (Table 1), although this was not reflected by increased transcript levels of adiponectin in adipose tissue (Fig. 3D).
Inflammation
Inflammatory markers were modestly improved with RGZ treatment in mice with tumors. IL-6 is the major inflammatory cytokine driving the wasting process in this model.26 Plasma levels of IL-6 were elevated in mice with tumors, and IL-6 was non-significantly decreased in both the RGZ(+) and RGZ-PF(+) groups compared with the PBS(+) group (Table 1). Levels of IL-6 mRNA in epididymal adipose tissue were decreased in the RGZ(+) and RGZ-PF(+) groups compared with the PBS(+) group (Fig. 3D). Transcript levels of TNF-α mRNA were not changed, and F4/80, a marker of macrophage infiltration, was reduced in RGZ(+) mice compared with PBS(+) mice (Fig. 3D).
Early cachexia studies
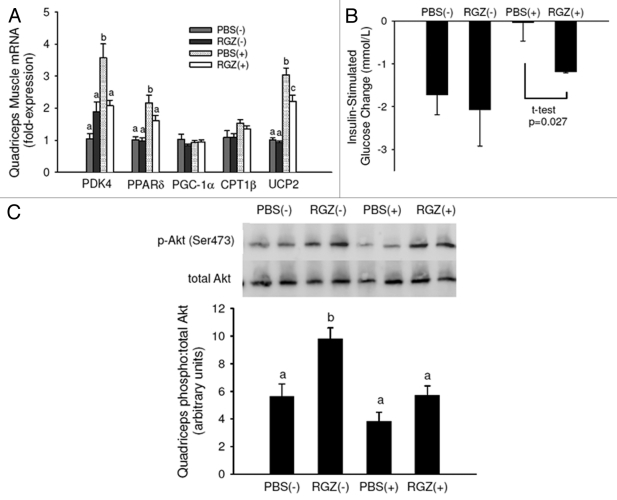
Because of the delayed weight loss in the RGZ(+) group compared with the PBS(+) group in mice with late-stage cachexia, we examined metabolic markers in mice with early-stage cachexia to determine how RGZ induces this weight-maintaining effect. In the quadriceps muscle, tumor increased and RGZ normalized transcript levels of pyruvate dehydrogenase kinase-4 (PDK4), peroxisome proliferator activated receptor-delta (PPAR-δ), and uncoupling protein-2 (UCP2) (Fig. 4A), genes involved in the substrate utilization switch from glucose to fatty acids.27,28 There was a significant effect of tumor to diminish insulin-stimulated removal of blood glucose (p = 0.027), and when comparing the two tumor-bearing groups, RGZ significantly increased glucose disappearance from the blood (Fig. 4B). There was a significant effect of tumor to lower Akt activation (p = 0.006) and RGZ to increase Akt activation (p = 0.005), but the difference between PBS and RGZ-treated mice with tumors was not significant (Fig. 4C). Overall, these data indicate that RGZ may normalize the tumor-induced increase in lipid utilization and decrease in glucose utilization in muscle of mice with early-stage cachexia.
Figure 4.
Early-stage cachexia quadriceps muscle gene expression and insulin sensitivity. (A) Quadriceps muscle mRNA expression in mice with (+) and without (-) tumors and treated daily with RGZ or PBS. n = 4 for PBS(-) and RGZ(-), n = 12 for PBS(+) and RGZ(+). Different letters represent significant differences between groups for each gene (p < 0.05). (B) After an overnight fast, blood glucose was tested before and 15 min after insulin administration (IP injection, 0.75 U/kg body weight). The graph represents glucose change in 15 min. PBS(+) and RGZ(+) are significantly different using a Student’s t-test. n = 4–5 mice per group. (C) Mice were sacrificed after 15 min glucose test and quadriceps Akt activation measured with protein gel blot. Pictured is relative density of the ratio of phosporylated (Ser473) to total Akt with representative blots. n = 3–5 mice per group.
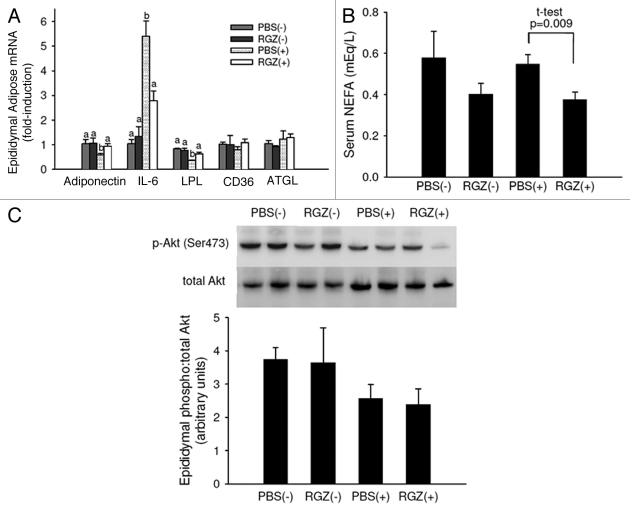
The mechanisms by which RGZ normalizes metabolism within the muscle are likely multifactorial, and may occur as a result of RGZ-induced metabolic changes in adipose tissue.19 In epididymal adipose, RGZ normalized the tumor-induced decrease in adiponectin and lipoprotein lipase gene expression and the tumor-induced increase in IL-6 gene expression (Fig. 5A). There was a significant effect of RGZ to lower serum NEFA (Fig. 5B; p = 0.008), and comparing the two tumor groups, RGZ(+) had decreased NEFA compared with PBS(+). No differences were detected in Akt activation between groups (Fig. 5C).
Figure 5.
Early-stage cachexia epididymal adipose gene expression and Akt activation, and serum NEFA. (A) Epididymal adipose mRNA expression in mice with (+) and without (-) tumors and treated daily with RGZ or PBS. n = 4 for PBS(-) and RGZ(-), n = 12 for PBS(+) and RGZ(+). Different letters represent significant differences between groups for each gene (p < 0.05). (B) Fasting serum NEFA. n = 4 for PBS(-) and RGZ(-) and n = 9–11 for PBS(+) and RGZ(+). Tumor-bearing groups are significantly different using a Student’s t-test. (C) Mice were sacrificed 15 min after insulin administration (IP injection, 0.75 U/kg body weight) and epididymal Akt activation measured with protein gel blot. Pictured is relative density of the ratio of phosporylated (Ser473) to total Akt with representative blots. n = 3–5 mice per group.
Discussion
We previously demonstrated that RGZ improves insulin sensitivity, decreases proteolytic gene expression, and maintains body weight and adipose mass in tumor-bearing mice early in cachexia pathogenesis, before significant muscle wasting occurs.17 The goal of the present work was to determine if these early positive effects translated to improved outcomes in late-stage cachexia, with the clinical application being to determine potential strategies for improving quality of life in patients with severe cachexia. We found RGZ attenuated loss of body weight, adipose mass and anorexia while decreasing markers of inflammation in mice with late-stage cachexia. There was no effect of RGZ on muscle mass, proteolytic gene expression, or grip strength. We did not measure stamina or endurance in this study.
Delayed onset of weight loss was responsible for improved body weight at the end of the study in RGZ-treated mice compared with PBS-treated mice with tumors. Due to the short time-course of this model, 2 d of weight maintenance may be clinically relevant because preventing weight loss even for a short time may improve responsiveness to and tolerability of anticancer therapies.3 The 2 d delay in weight loss in our 16 d study represents a 12.5% increase in the time RGZ-treated mice maintained body weight compared with PBS-treated mice. However, once mice began to lose weight, RGZ did not appear to slow the rate of weight loss, indicating RGZ may be more effective in the prevention rather than the slowing of weight loss.
Some of the modest improvements in the RGZ-treated mice were dependent on the delayed onset of anorexia as shown by the difference in outcomes between RGZ-treated mice fed ad libitum and those pair-fed to PBS-treated tumor-bearing mice. Previously, we showed that pair-feeding mice without tumors to mice with tumors resulted in a similar weight loss in both groups, despite an absence of wasting in mice without tumors.29 Together these data provide compelling evidence to support the necessity of treating both decreased energy intake and dysregulated metabolism in cachexia in order to see optimal outcomes.
Although rosiglitazone did not improve muscle mass in late-stage cachexia, it did attenuate adipose wasting, an effect that was diminished but not completely lost with pair-feeding. Loss of muscle mass and strength is a primary contributor to the morbidity of cachexia, but severe wasting of the adipose tissue may contribute indirectly to muscle wasting through its contribution to whole body metabolism.30
Cachexia and RGZ had opposing effects on adiponectin and IL-6. Cachexia severely depleted plasma adiponectin concentrations, but RGZ, independent of food intake, increased adiponectin compared with cachectic mice without RGZ. Adiponectin, an insulin-sensitizing adipocytokine with receptors in skeletal muscle, is an important contributor to the cross-talk between adipose and muscle. Binding to the adiponectin receptor in skeletal muscle, adiponectin activates AMPK, subsequently activating pathways to increase cellular energy status including glucose uptake and fatty acid β-oxidation.31 AMPK phosphorylation was not measured in the present study due to the confounding effects of the insulin injection prior to necropsy, but this mechanism should be explored in a future study. Due to the very late stage of cachexia in our mice, it is possible that despite an improvement in circulating adiponectin with RGZ, substrate availability was so depleted that improvement in cellular energy status was no longer possible without upregulation of proteolysis. Likewise, RGZ decreased adipose transcript levels of IL-6 and non-significantly decreased plasma IL-6, potentially relieving inhibition of insulin signaling in muscle.18 However, the hypoglycemia characteristic of this model in late-stage cachexia may render the RGZ-induced decrease in IL-6 less effective because of a lack of substrate availability. Alternatively, although RGZ decreased IL-6 in mice with tumors, plasma concentrations were still far higher than non-tumor controls, suggesting that a possible threshold in IL-6 reduction must be attained in order to relieve inhibition of insulin signaling.
RGZ exerted more potent effects in early stages of cachexia in our mouse model, evidenced by the delayed onset of weight loss. As a PPAR-gamma agonist, RGZ has adipogenic effects through its regulation of adipocyte differentiation and lipid uptake in adipose tissue.19 We have previously shown this adipogenic effect of RGZ in early stage cachexia.17 Because adipose delipidation begins earlier than muscle wasting in the colon-26 model,32 adipose mass preservation by RGZ may delay the catabolic milieu characteristic of cachexia. Normalization of IL-6, adiponectin, and LPL gene expression in adipose likely contributed to delayed delipidation seen with RGZ. Adipose preservation may in turn decrease circulating NEFA and IL-6 and increase circulating adiponectin, as shown in the present study. Decreased exposure of muscle to fatty acids, coupled with improved availability of glucose as an energy source, most likely contributed to normalization of fatty acid metabolism-related gene expression in muscle.
Although the adipogenic effect of RGZ contributes to the normalization of muscle metabolism in early-stage cachexia,17 these early improvements do not translate to improved muscle mass or strength in late-stage cachexia. These findings support recent recommendations for clinicians to distinguish between patients with pre-cachexia and cachexia.1 The present study is evidence for significant metabolic differences and treatment responses in early and late stages of cachexia. Treatment plans for patients should be developed on the basis of cachexia severity.
Materials and Methods
Late-stage cachexia study
Study procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University. Five-week-old male CD2F1 mice (BALB/c x DBA/2; Charles River Laboratories) were housed five per cage at 22 ± 0.5°C, with a 12h light/dark cycle, and free access to water and pelleted AIN-93G purified diet (Research Diets). Mice acclimated to the environment for approximately one week before the study started.
Treatment groups
Mice were randomized into one of five groups with 15 mice per group to equate average body weights. Groups were: (1) no tumor with phosphate-buffered saline (PBS) treatment [PBS(-)]; (2) no tumor with rosiglitazone treatment [RGZ(-)]; (3) tumor with PBS treatment [PBS(+)]; (4) tumor with rosiglitazone treatment [RGZ(+)]; (5) tumor with rosiglitazone treatment, pair-fed to the PBS(+) group [RGZ-PF(+)]. The fifth group was pair-fed to the PBS(+) group to control for any differences in food intake occurring as a result of RGZ treatment. RGZ (Cayman Chemical) was solubilized in dimethyl sulfoxide (DMSO) as a stock solution and diluted in PBS prior to use. Beginning on study day 0, mice were treated daily with intraperitoneal (IP) injections of RGZ (10 mg/kg body weight) or an equal volume of PBS containing DMSO. Body weights and food intake were also measured daily.
Colon-26 adenocarcinoma cell culture and tumor cell inoculation
Colon-26 adenocarcinoma cells were cultured with RPMI 1640 + L-glutamine medium (Sigma-Aldrich) supplemented with 5% fetal bovine serum and 1% Penicillin-Streptomycin at 37°C and 5% CO2. On study day 0, 1.0 × 106 cells suspended in 100 µl PBS were injected subcutaneously into the right flanks of mice in the tumor groups. An equal volume of PBS was injected into mice in groups without tumors as a control.
Necropsy
Mice were sacrificed when there was approximately a 30% difference in body weight between the PBS(-) and PBS(+) groups. After a 4 h fast, blood glucose was tested using the One Touch Ultra® glucose meter and tail vein blood. An IP injection of insulin (0.75 U/kg body weight; Humulin® R, Eli Lilly and Co.) was administered and 10 min later blood glucose was tested again. Immediately thereafter, mice were over-anesthetized with isofluorane and blood was collected via cardiac puncture. Blood was collected in EDTA-containing tubes, centrifuged at 1500 x g for 20 min to separate plasma, and frozen at -80°C until analysis. Muscles, adipose depots and other organs were harvested, weighed, frozen in liquid nitrogen, and stored at -80°C until analysis. A section of epididymal adipose tissue was fixed in 4% paraformaldehyde for histological analysis.
Early-stage cachexia study
Samples for quadriceps muscle and epididymal adipose mRNA analysis and serum non-esterified fatty acids (NEFA) were obtained from a study described elsewhere.17 Briefly, male CD2F1 mice with and without colon-26 tumors were treated daily with either PBS or RGZ (10 mg/kg body weight). Mice were sacrificed after an overnight fast when there was a 9% body weight difference between PBS-treated mice with and without tumors. Insulin-stimulated glucose change and Akt activation were measured from a study in which mice with and without tumors were treated daily with either PBS or RGZ (10 mg/kg body weight). There were five mice in each group. Mice were sacrificed 10 d after tumor inoculation, when there was a 2.4% body weight difference between the PBS(-) and PBS(+) groups, with the PBS(+) group weighing more. After a 12 h overnight fast, an IP injection of insulin (0.75 U/kg body weight) was given. Blood glucose was tested before and 15 min after insulin administration. Immediately after the second glucose test, mice were sacrificed and quadriceps muscle and epididymal adipose tissue were harvested for western blotting.
RT-PCR
Muscle RNA was extracted with TRIzol (Invitrogen), and epididymal adipose RNA with the RNeasy Lipid Tissue Mini Kit (QIAGEN, Inc.). RNA concentration was determined spectrophotometrically, and quality visualized on a 1% agarose gel. RNA was reversed transcribed to cDNA using the High Capacity cDNA Archive Kit (ABI). cDNA was amplified by real-time PCR with TaqMan Gene Expression Assays (ABI) using pre-designed and validated primers (FAM probes, ABI) under universal cycling conditions defined by ABI. Target gene expression was normalized to the endogenous control (VIC probe, mouse GAPD for muscle and 18 s for adipose). Samples were run in triplicate and data expressed as 2-ΔΔct relative to the control group.33
Western blotting
Quadriceps muscle and epididymal adipose tissue were homogenized in 10x and 3x lysis buffer respectively (20 mM trizma base, 1% triton-X100, 50 mM NaCl, 250 mM sucrose, 50 mM NaF, 5 mM Na4P2O7 · 10H2O) with Complete Mini Protease Inhibitor Cocktail Tablets (Roche Diagnostics). Supernatant protein concentration was determined with the BCA Protein Assay (Pierce). Protein (60 µg/sample) was separated on 10% polyacrylamide gels and transferred to 0.45 µm nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk and incubated with primary antibody against phosphorylated Akt (Ser 473; Cell Signaling). Bands were visualized with chemiluminescense using Kodak Image Station 2000RT (Eastman Kodak). Membranes were then stripped and re-probed for total-Akt (Cell Signaling). The relative ratio of phosphorylated to total Akt was calculated from band densities using Kodak 1D 3.6 software.
Plasma metabolites
Plasma IL-6 and adiponectin were measured by ELISA (Invitrogen and Millipore, respectively) and NEFA by colorimetric assay (NEFA, WAKO) according to the manufacturers’ protocols.
Grip strength
Forelimb and hindlimb grip strength were measured one day prior to and 15 d after tumor inoculation with the Columbus Instruments Grip Strength Meter. Forelimbs and hind limbs of each mouse were tested three times consecutively with ~5–10 s between replicates. The average of the replicates was used for analysis.
Histology
Histology sections were prepared (OSU Pathology Core Facility) from paraffin blocks with a thickness of 5 µm and stained with hematoxylin and eosin (H&E) for analysis by light microscopy. The cross-sectional area of 100 adipocytes from nine mice in each group was measured using Image J software (NIH) to quantify adipocyte size.
Statistics
Data were analyzed with two-sample t-tests to compare differences between two groups, with the Bonferroni correction factor applied for multiple comparisons. ANOVA was used to determine differences among multiple groups, with Tukey’s test being applied for multiple comparisons. Differences in the medians for weight loss incidence and adipocyte cross-sectional area were analyzed with the Wilcoxon Rank Sum test for non-parametric data. Systat 13 was used for all analyses. p < 0.05 was considered statistically significant.
Conclusions
RGZ delayed weight loss onset and improved adipose mass, adipocytokine profile and adipocyte cross-sectional area without improving muscle mass, strength or proteolytic gene expression in mice with late-stage cancer cachexia. The delayed onset of overt cachexia seen with RGZ appears to be due to the normalization of metabolic markers in both adipose and muscle, as well as delayed anorexia. Because of the differential responses of mice to RGZ in early- and late-stages of cachexia, future work should focus on determining optimal treatment options for various stages of cachexia.
Acknowledgments
The authors thank Donna McCarthy and Michael Stout for helpful discussion and contributions to necropsy. This research was supported by the Carol S. Kennedy Endowment (M.A.B.), the Ohio Agricultural Research and Development Center (M.A.B.), and the P.E.O. Scholar Award (M.L.A).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/18134
References
- 1.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Argilés JM, Almendro V, Busquets S, Lopez-Soriano FJ. The pharmacological treatment of cachexia. Curr Drug Targets. 2004;5:265–77. doi: 10.2174/1389450043490505. [DOI] [PubMed] [Google Scholar]
- 3.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med. 1980;69:491–7. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 4.Warren S. The immediate causes of death in cancer. Am J Med Sci. 1932;184:610–5. doi: 10.1097/00000441-193211000-00002. [DOI] [Google Scholar]
- 5.Inagaki J, Rodriguez V, Bodey GP. Causes of death in cancer patients. Cancer. 1974;33:568–73. doi: 10.1002/1097-0142(197402)33:2<568::AID-CNCR2820330236>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Argilés JM. López-Soriano, Busquets S. Novel approaches to the treatment of cachexia. Drug Discov Today. 2008;13:73–8. doi: 10.1016/j.drudis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Bozzetti F, Ammatuna M, Migliavacca S, Bonalumi MG, Facchetti G, Pupa A, et al. Total parenteral nutrition prevents further nutritional deterioration in patients with cancer cachexia. Ann Surg. 1987;205:138–43. doi: 10.1097/00000658-198702000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohdenburg GL, Bernhard A, Krehbiel O. Sugar tolerance in cancer. J Am Med Assoc. 1919;72:528–30. [Google Scholar]
- 9.Moley JF, Morrison SD, Norton JA. Preoperative insulin reverses cachexia and decreases mortality in tumor-bearing rats. J Surg Res. 1987;43:21–8. doi: 10.1016/0022-4804(87)90042-4. [DOI] [PubMed] [Google Scholar]
- 10.Moley JF, Morrison SD, Gorschboth CM, Norton JA. Body composition changes in rats with experimental cancer cachexia: improvement with exogenous insulin. Cancer Res. 1988;48:2784–7. [PubMed] [Google Scholar]
- 11.Peacock JL, Norton JA. Impact of insulin on survival of cachectic tumor bearing rats. JPEN J Parenter Enteral Nutr. 1988;12:260–4. doi: 10.1177/0148607188012003260. [DOI] [PubMed] [Google Scholar]
- 12.Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59:677–81. doi: 10.1038/bjc.1989.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearlstone DB, Wolf RF, Berman RS, Burt M, Brennan MF. Effect of systemic insulin on protein kinetics in postoperative cancer patients. Ann Surg Oncol. 1994;1:321–32. doi: 10.1007/BF02303571. [DOI] [PubMed] [Google Scholar]
- 14.Lundholm K, Körner U, Gunnebo L, Sixt-Ammilon P, Fouladiun M, Daneryd P, et al. Insulin treatment in cancer cachexia: effects on survival, metabolism and physical functioning. Clin Cancer Res. 2007;13:2699–706. doi: 10.1158/1078-0432.CCR-06-2720. [DOI] [PubMed] [Google Scholar]
- 15.Cersosimo E, Pisters PW, Pesola G, Rogatko A, Vydelingum NA, Bajorunas D, et al. The effect of graded doses of insulin on peripheral glucose uptake and lactate release in cancer cachexia. Surgery. 1991;109:459–67. [PubMed] [Google Scholar]
- 16.Dodesini AR, Benedini S, Terruzzi I, Sereni LP, Luzi L. Protein, glucose and lipid metabolism in the cancer cachexia: A preliminary report. Acta Oncol. 2007;46:118–20. doi: 10.1080/02841860600791491. [DOI] [PubMed] [Google Scholar]
- 17.Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer. 2010;126:756–63. doi: 10.1002/ijc.24784. [DOI] [PubMed] [Google Scholar]
- 18.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2002;18:S10–5. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 20.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–8. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 22.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–5. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodine SC, Latres E, Baumhueter S, Lai VKM, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 25.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–71. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–4. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lange P, Moreno M, Silvestri E, Lombardi A, Goglia F, Lanni A. Fuel economy in food-deprived skeletal muscle: signaling pathways and regulatory mechanisms. FASEB J. 2007;21:3431–41. doi: 10.1096/fj.07-8527rev. [DOI] [PubMed] [Google Scholar]
- 28.Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, et al. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J. 2008;22:9–18. doi: 10.1096/fj.07-8945com. [DOI] [PubMed] [Google Scholar]
- 29.Tian M, Kliewer KL, Asp ML, Sout MB, Belury MA. c9t11-Conjugated linoleic acid-rich oil fails to attenuate wasting in colon-26 tumor-induced late-stage cancer cachexia in male CD2F1 mice. Mol Nutr Food Res. 2011;55:268–77. doi: 10.1002/mnfr.201000176. [DOI] [PubMed] [Google Scholar]
- 30.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34:12–8. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–8. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]