Abstract
The pursuit of alternate therapies for end-stage heart failure post-myocardial infarction has led to the development of a variety of in situ gelling materials to be used as cellular or acellular scaffolds for cardiac repair. Previously, a protocol was established to decellularize human and porcine pericardia and process the extracellular matrix into an injectable form. The resulting gels were found to retain components of the native extracellular matrix; cell infiltration was facilitated in vivo and neovascularization was observed by two weeks. However, the assertion that an injectable form of human pericardial tissue could be a potentially autologous scaffold for myocardial tissue engineering requires assessment of the patient-to-patient variability. With this work, seven human pericardia from a relevant patient demographic are processed into injectable matrix materials that gel when brought to physiologic conditions. The resulting materials are compared with respect to their protein composition, glycosaminoglycan content, in vitro degradation, in vivo gelation, and microstructure. It is observed that a diminished collagen content in a subset of samples prevents in vitro gelation, but not in vivo gelation at lower ECM concentrations. The structure is similarly fibrous and porous across all samples, implying the cell infiltration may be similarly facilitated. The biochemical composition as characterized by tandem mass spectrometry is comparable; basic ECM components are conserved across all samples and the presence of a wide variety of ECM proteins and glycoproteins demonstrate the retention of biochemical complexity postprocessing. It is concluded that the variability within human pericardial tissue specimens does not prevent them from being processed into injectable scaffolds for cardiac repair; therefore, pericardial tissue offers a promising source for an autologous biomaterial therapy.
Keywords: autologous, injectable, pericardium, biomaterial, MI, scaffold
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the Western world, resulting in a coronary event every 25 seconds in the United States alone [1]. Heart failure (HF) subsequent to myocardial infarction (MI) is responsible for the majority of CVD-related deaths. Given the scarcity of appropriate donor hearts, the difficulties associated with chronic use of a left ventricular assist device (LVAD), and the inherent risk in the surgery for either scenario, there is a pressing clinical need for alternate therapies. Thus, developing injectable therapies that can be delivered via minimally invasive methods is attractive. Recently, biomaterials that can be injected into the myocardium and gel in situ have been investigated as scaffolds for cardiac repair post-MI. The success of these materials has been attributed to a variety of characteristics, although exact mechanisms are unknown. These include that a fibrous, porous microstructure may support cell infiltration [2–4], mechanical support may decrease stress on the ventricle [5], appropriate degradation time may allow for cell in-growth and tissue remodeling [6, 7], and the degradation products of naturally-derived materials may offer biochemical cues for cellular migration and angiogenesis [8, 9]. Additionally, these biomaterials may provide delivery vehicles for cellular cardiomyoplasty, increasing viability and retention [7, 10].
Investigated materials vary from synthetic hydrogels such as polyethylene glycol (PEG) [11] and poly(N-isopropyl acrylamide) [12] to natural materials such as alginate [13], collagen [14], and fibrin [15]. Most recently, materials derived from decellularized tissues have emerged as injectable scaffolds [7]. These materials offer the unique advantage of mimicking the complexity of the extracellular matrix of human tissues, a characteristic unmatched in the aforementioned materials. Injectable forms of the ECM of small intestinal submucosa (SIS) [16] and myocardial tissue [17] have been produced. Work with SIS ECM gels in a small animal infarct model has shown that one type (SIS-B) preserved end-systolic left ventricular geometry and improved cardiac contractility at 6 weeks compared saline-injected control animals. Myocardial matrix has also been shown to preserve ejection fraction 6 weeks post-MI in a small animal model (data unpublished).
These, and most other complex ECM materials are necessarily derived from xenogeneic or allogeneic sources. Using materials derived from animal tissue raises concerns about pathogen transmission [18], ethical issues [19], and most importantly, immunogenicity [20–23]. This becomes especially important if multiple treatments are determined to be of therapeutic benefit. In this case, an antibody response may be mounted against the injected biomaterial [24]. While some preliminary immune response studies have been carried out with ECM materials, the long-term effects these scaffolds will have on the host are still largely undetermined [20]. If an autologous source for a biomaterial therapy could be established, such concerns would be obviated.
Previously, the pericardium was established as a source of autologous ECM that could be used in the design of injectable scaffolds for cardiac tissue engineering applications. Injectable forms of both porcine and human pericardial ECM were developed and characterized. The matrix gels were found to have a porous structure that facilitated cellular infiltration and neovascularization at two weeks [25]. With an autologous option, the bioactivity of a naturally derived scaffold could be harnessed while avoiding the potential complications associated with xenogeneic tissue sources. Recently, Lu, et.al. produced a three-dimensional ECM scaffold using the matrix secreted by human bone marrow mesenchymal stem cells (MSCs), normal human articular chondrocytes (NHAC), and normal human dermal fibroblasts (NHDF) cultured in vitro. After removing the original polymer template, the ECM mesh was used as an implantable scaffold in a small animal model [26]. Assuming the secreted matrix is unaffected by in vitro culture conditions, this could be considered a potentially autologous approach for a patch or scaffold, though surgical implantation would be required.
Thus, pericardial tissue has a promising foundation for use as an autologous source for an injectable biomaterial therapy. For the subset of patients where tissue can be collected at no additional risk, it is prudent to identify if this tissue can be processed consistently into a suitable scaffold. While previous studies were performed on a combined pool of material, it is necessary to determine if the tissue collected from any given patient could be processed and used in the same manner. It is understood that tissue composition changes with age and disease; some of the more well-documented age-related changes include the elastinization and loss of collagen in both human and porcine mitral and aortic valves [27]. While pericardial ECM composition has not been studied for age-related changes, it is not unreasonable to expect differences between patients of varied ages and pathologies. In this study, seven human pericardium specimens were collected and processed. The resulting matrix gels were compared with respect to material composition, gelation, degradation, and microstructure to determine if patient-to-patient variability would prevent the application of this technology across a wide patient population.
Materials and Methods
All experiments in this study were performed in accordance with the guidelines published by the Institutional Animal Care and Use Committee at the University of California, San Diego, and the American Association for Accreditation of Laboratory Animal Care.
Tissue Collection
Human pericardium samples were collected from consenting patients scheduled for cardiothoracic surgery; the surgeon removed a small piece (approximately 4–5 cm2) of tissue during surgery, in compliance with the University of California, San Diego, Institutional Review Board.
Decellularization
After dissecting away any adherent adipose tissue, fresh human pericardia were decellularized using hypotonic and hypertonic rinses in deionized (DI) water and sodium dodecyl sulfate (SDS), an ionic detergent previously shown to decellularize pericardium [28]. The samples were first washed in DI water for 30 min, and then stirred continuously in 1% SDS in PBS for 60–65 h, followed by an overnight DI rinse. Specimens were then removed from solution and again rinsed under running DI water. This protocol has previously demonstrated consistent decellularization of both human and porcine pericardial tissue samples [25].
Preparation of injectable ECM
Injectable ECM was prepared via methods modified from previously published protocols for bladder [29] and myocardial matrix [17]. Decellularized human pericardia were lyophilized, ground into a coarse powder with a Wiley mini mill (Thomas Scientific, Swedesboro, NJ), and then frozen and stored at −80 °C until further use. Milled samples were pepsin-digested in 0.1 M HCl at a concentration of 10 mg ECM per 1 mL HCl. Pepsin (Sigma, St. Louis, MO) was used at a concentration of 1 mg/mL. The ECM powder was digested for 60–65 h and then brought to physiologic pH and osmolarity by adding 1 M NaOH (1/10 of original digest volume) and 10× PBS (1/10 of final neutralized volume). Bringing the solution to ph 7.4 stops pepsin activity; the enzyme is deactivated above pH 6 [30]. All processing steps, including the pepsin digestion, were performed at room temperature. The resulting viscous solution was diluted with 1× PBS to the appropriate concentration.
Characterization of injectable ECM composition
One-dimensional gel electrophoresis was performed on the injectable ECM (10 mg/mL) compared to rat tail collagen type I (2.5 mg/mL; BD Biosciences, San Jose, CA). The three solutions were run on a Tris-HCl, 12% polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA) inTris/glycine/SDS buffer (Fisher Scientific, Hanover Park, IL), and 80 mM reducing agent dithiothreitol (Invitrogen, Carlsbad, CA). NuPAGE Plus2 Prestained Standard (1Â) (Invitrogen) was used as the protein standard; all other samples were stained with Imperial Protein Stain (Pierce, Rockford, IL). Samples were prepared and run in an XCell Surelock MiniCell (Invitrogen). Glycosaminoglycan (GAG) content of the injectable ECM was quantified using a colorimetric Blyscan GAG assay (Biocolor, Carrickfergus, United Kingdom). Samples were run in triplicate.
To analyze the protein content of the injectable ECM, tandem mass spectroscopy (MS/MS) was performed. Injectable ECM samples were briefly pepsin-digested before analysis by liquid chromatography (LC)-MS/MS with electrospray ionization, using a QSTAR-Elite hybrid mass spectrometer (Applied Biosciences, Foster City, CA) interfaced to a Tempo nanoscale reversed-phase high-pressure liquid chromatograph (Applied Biosciences) using a 10 cm 180 ID glass capillary packed with 5 mm C-18 Zorbax beads (Agilent Technologies, Santa Clara, CA) as previously described [25]. Peptides were identified using paragon algorithm executed in Protein Pilot 2.0 (Life Technologies, Carlsbad, CA). Detected peptide sequences were run against the Swiss Prot databank for protein identification. Proteins were identified based on at least one peptide detected with a confidence of above 95% for that peptide identification. MASCOT was used to supplement the proteins identified by ProteinPilot. Proteins were identified based on a MASCOT score above 27. Additionally, a brief trypsin digest was performed on the injectable ECM samples and the LC-MS/MS and analysis was repeated as described. All mass spectroscopy work was performed at the Biomolecular Mass Spectroscopy Facility at the University of California, San Diego (La Jolla, CA).
Peptide mass distributions were compiled from the full spectra list for each sample. All MS2 precursor masses found by the QSTAR mass spectrometer are represented, positive identification notwithstanding.
Rheometry
A TA instruments AR-G2 rheometer was used to test pericardial ECM gel rheological properties. Pericardial matrix gels were tested at 37°C using a 20 mm parallel plate geometry, with a 1.0 mm gap between plates. Three frequency sweeps from 0.1 to 50 rad/s were conducted with a constant strain of 2.5%, a value within the linear viscoelastic strain region (determined experimentally). Each gel was tested in triplicate, and the resulting values were averaged. Additionally, each sample was run in triplicate and a representative curve was obtained. The storage modulus (G′) is reported at 1 rad/s.
Scanning electron microscopy
Environmental scanning electron microscopy (ESEM) was used to visualize the structure of the pericardial matrix gels. ESEM is preferred for hydrogels because a humid environment can be maintained and uncoated samples can be examined [31]. Gels were prepared for ESEM by fixation with 2.5% glutaraldehyde for two hours at 37 °C, followed by a serial dehydration in ethanol rinses (30 – 100%), as has been done previously with both decellularized tissue and gels made from natural materials [17, 29, 32, 33]. Images were taken with a Phillips XL30 Environmental SEM Field Emission microscope. Fiber diameter was evaluated by taking 20–40 measurements of fiber diameter per image. An average and standard deviations are reported for each sample.
In vitro degradation
To assess degradation properties of the different pericardial matrix gels, ninhydrin reactivity was evaluated after incubation with collagenase [34, 35]. Sets of 100 uL gels at 6 mg/mL (HP-A – E) or 8 mg/mL (HP-F and G) were formed overnight in microcentrifuge tubes. Collagen gels at a concentration of 2.5 mg/mL were used for comparison. Bacterial collagenase (Worthington Biomedical Corporation, Lakewood, NJ) at 100 U/mL in a 0.1 M Tris-base, 0.25 M CaCl2 solution, at pH 7.4, was added to the gels. Gels were incubated with collagenase at 37 °C for 2, 4, 12, or 24 hours. Visual observations made throughout the incubation; at the end of the experiment, samples were centrifuged at 15,000 rpm for 10 min and the supernatant was reacted with 2% ninhydrin (Sigma-Aldrich) at 100 °C for 10 min. Samples were read on a BioTek Synergy 4 (BioTek Instruments, Winooski, TX) spectrophotometer at 570 nm.
In vivo gelation
To determine whether each pericardium sample would form a fibrous, porous scaffold in vivo, the matrix material was injected into the left ventricular (LV) free wall of healthy female Harlan Sprague-Dawley rats (225–250 g), as previously described [14, 15, 36, 37]. Briefly, rats were anesthetized with 5% isoflurane, intubated, and maintained at 2.5% isoflurane during surgery. The apex of the heart was visualized via access through the diaphragm and 75 μL of pericardial ECM material was injected intramurally. A total of seven rats were injected with ECM, all were euthanized at 45 min. Immediately after euthanasia, the hearts were resected and fresh-frozen in OCT. 10 μm short-axis cross-sections were taken every 100 μm from the apex to the base. Sections were stained with hematoxylin and eosin for visualization of the tissue and injection region.
Results
Preparation of injectable ECM
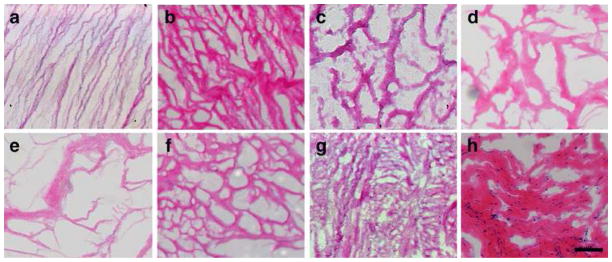
Samples were collected from patients across a wide range of age and condition (Table 1). Each human pericardium sample presented differently upon gross examination. Samples varied in the amount of adipose tissue present, thickness, and size. After preparation for decellularization, all visible adherent adipose tissue was removed, as extant lipid content prevents material processing [38]. Previous results with multiple human pericardium samples demonstrated the efficacy of the decellularization method [25] and verification via H&E staining indicated a removal of nuclear material for all samples (Fig. 1). After decellularization, all samples could be lyophilized and milled in a similar manner. Pepsin digestion was less consistently homogeneous than when working with porcine tissue, but with no discernable patterns between the gels. When brought to physiologic conditions, all samples except HP-F and HP-G form weak gels at a concentration of 6 mg/mL. HP-F and HP-G required a concentration of 8 mg/mL to gel in vitro at 37 °C. After digestion, it was observed that HP-F and HP-G were also less viscous than the other matrix materials.
Table 1.
Specimen Information. Information regarding the tissue sources for each sample. Mean donor age was 56 years. M, Male; F, Female; CABG, coronary artery bypass graft; PTE, pulmonary thrombo endarterectomy, AVR, aortic valve replacement.
| Sample | Gender | Age | Surgery |
|---|---|---|---|
| HP-A | F | 35 | PTE |
| HP-B | M | 86 | CABG, AVR |
| HP-C | M | 46 | CABG |
| HP-D | F | 59 | PTE |
| HP-E | M | 23 | PTE |
| HP-F | M | 66 | CABG |
| HP-G | F | 77 | AVR |
Fig. 1.
Decellularization verification. Hematoxylin and eosin staining on 10 μm sections of decellularized samples reveals a lack of nucleic material (A–G) in all seven human pericardium samples (HP-A through HP-G) compared to the section of a fresh sample of HP-A (H), scale bar is 1 mm.
Biochemical characterization
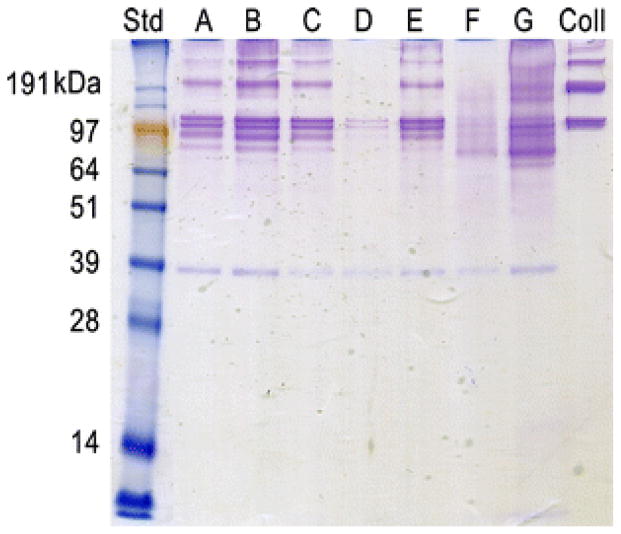
In order to elucidate potential differences in biochemical composition, the pericardial matrix materials were characterized and compared. According to a Blyscan Colorimetric GAG Assay, the human pericardial matrix samples range in sulfated GAG content from 10 ug/mg to 30 ug/mg dry ECM weight (Table 2). This is lower than previous results [25], perhaps due to variability in the extent of digestion seen with individual human samples. The SDS-PAGE gel reveals the samples are not equal in protein content (Fig. 2). HP-F and HP-G do not have as defined bands and HP-D and HP-F appear to have less overall protein content, specifically less collagen I. Variable protein content is supported with the results of the ninhydrin assay described later.
Table 2.
Glycosaminoglycan Content. Results of a colorimetric Blyscan assay for sulfated GAGs in the human pericardial matrix materials. All materials retain these carbohydrates, though the relative amount varies.
| Sample | GAG (ug/mg) |
|---|---|
| HP-A | 25 ± 2.7 |
| HP-B | 23 ± 3.3 |
| HP-C | 27 ± 2.3 |
| HP-D | 20 ± 4.1 |
| HP-E | 30 ± 3.2 |
| HP-F | 20 ± 7.4 |
| HP-G | 18 ± 5.5 |
Fig. 2.
SDS-PAGE. An Imperial Protein stain of samples run on a poly-acrylamide gel indicates variable protein content, specifically lower collagen content in HP-D and HP-F.
Tandem mass spectrometry results indicate that all human pericardia samples retain components of structural ECM proteins and glycoproteins after processing (Table 3). These results correlate well with previously published results for human and porcine pericardia [25]. The characterization performed here indicates that the biochemical composition by mass spectrometry is comparable and while relative percentages cannot be quantified, basic components such as collagen I, collagen V, collagen VIII, elastin, fibrillin are retained across all samples. Other proteins present in most, but not all samples include collagen III, collagen IV, microfibrillar associated protein 4 (MFAP4), and procollagen I.
Table 3.
MS/MS Results. A summary of the results of tandem mass spectrometry data is presented here. Presence of peptides identified from a wide range of ECM proteins and glycoproteins indicates the retention of these components after material processing.
| Identified Protein | HP-A | HP-B | HP-C | HP-D | HP-E | HP-F | HP-G |
|---|---|---|---|---|---|---|---|
| Angiomotin | x | ||||||
| Collagen I | x | x | x | x | x | x | x |
| Collagen II | x | ||||||
| Collagen III | x | x | x | x | x | x | |
| Collagen IV | x | x | x | x | x | x | |
| Collagen V | x | x | x | x | x | x | x |
| Collagen VI | x | ||||||
| Collagen VII | x | x | x | x | |||
| Collagen VIII | x | x | x | x | x | x | x |
| Collagen XI | x | x | x | ||||
| Collagen XII | x | ||||||
| Collagen XIX | x | ||||||
| Collagen XXII | x | ||||||
| Decorin | x | x | x | ||||
| Dermatopontin | x | x | x | ||||
| Elastin | x | x | x | x | x | x | x |
| Emilin | x | ||||||
| Fibrillin 1 | x | x | x | x | x | x | x |
| Fibulin 2 | x | ||||||
| Follistatin | x | ||||||
| Hemicentin | x | x | |||||
| MFAP4 | x | x | x | x | x | x | |
| Procollagen I | x | x | x | x | x | x | |
| Prolargin | x | x | |||||
| EGFR kinase substrate | x |
Some components appeared in only a few samples – collagen II, VI, XI, XII, XIX, decorin, dermatopontin, emilin, fibulin, follistatin, hemicentin, and prolargin. The extracellular matrix proteins identified include hemicentin, which forms track-like structures to facilitate directed cell movement [39]. Prolargin binds perlecan and collagens I and II [40]. Microfibrillar associated protein 4 (MFAP4) has a role in cell adhesion and intercellular interactions [41]. Fibulin-2 binds various extracellular ligands and calcium and has been shown to interact with laminin and perlecan [42–44]. Dermatopontin has a role in cell-matrix interactions and matrix assembly. Additionally, it may modify the behavior of TGF beta through interaction with decorin [45].
ECM glycoproteins and proteoglycans were also identified. Emilin (elastin microfibril interface located protein) is an ECM glycoprotein found in elastin-rich tissues; recombinant EMILIN-1 has been shown to be a very efficient ligand for cell adhesion of several cell types [46]. Follistatin, or activin binding protein, is an autocrine glycoprotein [47, 48]. Another glycoprotein, fibrillin is essential for the formation of elastic fibers – it is secreted into the ECM and becomes incorporated into the insoluble microfibrils that provide a scaffold for elastin deposition [49–51]. Decorin is a proteoglycan that consists of a protein core containing leucine repeats with a glycosaminoglycan (GAG) chain consisting of either chondroitin sulfate (CS) or dermatan sulfate (DS); it binds to type I collagen fibrils and plays a role in matrix assembly [52]. Decorin has also been shown to influence fibrillogenesis, interact with fibronectin, epidermal growth factor receptor (EGFR) and transforming growth factor-beta (TGF-beta) [53, 54].
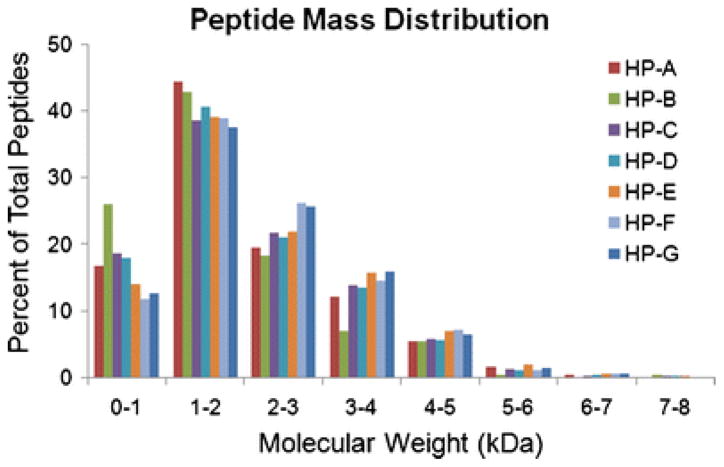
Other components identified include EGFR kinase substrate, which binds to EGFR and enhances EGF-dependent mitogenic signals and angiomotin, which increases migration of endothelial cells toward growth factors [55]. Peptide mass distributions were analyzed and all samples except HP-B have similar curves. HP-B is shifted to the left – there were more small peptides (< 2 kDa) resolved by the mass spectrometer than in the other samples (Fig. 3).
Fig. 3.
Peptide mass distribution. Precursor mass information for all peptides was derived from the MS/MS data, here binned in 1 kDa increments to identify differences in the mass distribution, such as the left-ward shift of peptides in HP-B.
Structural and mechanical characterization
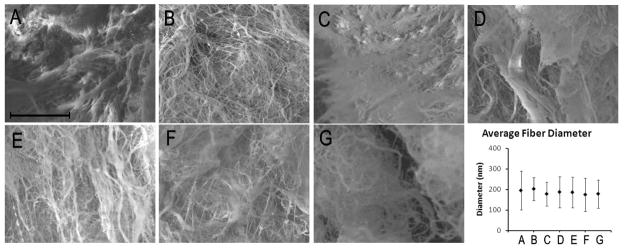
After determining that there were slight variations in the biochemical composition, it was interesting to investigate if that would have any effect on the structure and mechanical properties of the materials after gelation. Electron microscopy allowed for the visualization of the fibrous microstructure of the matrix gels. Samples HP-F and HP-G did not gel in vitro at 6 mg/mL, so SEM was performed on gels from 8 mg/mL samples. Figure 4 shows representative images from the different gels, all illustrating a fibrous mesh. Fibers were on the order of 200 nm. While fiber size varied throughout each sample, the different specimens were similar when compared to each other (p = 0.19) implying the differences in biochemical composition did not prevent similar self-assembly.
Fig. 4.
Scanning electron microscopy. ESEM images reveal a fibrous network in each sample (A–G). Quantification of fiber diameter (H) reveals no statistical difference across samples – all with approximately 200 nm fibers (p = 0.19). HP-A through HP-E (A–E) were gelled at 6 mg/mL and HP-F and HP-G (F, G) were gelled at 8 mg/mL. Fiber diameter data shown as mean ± standard deviation.
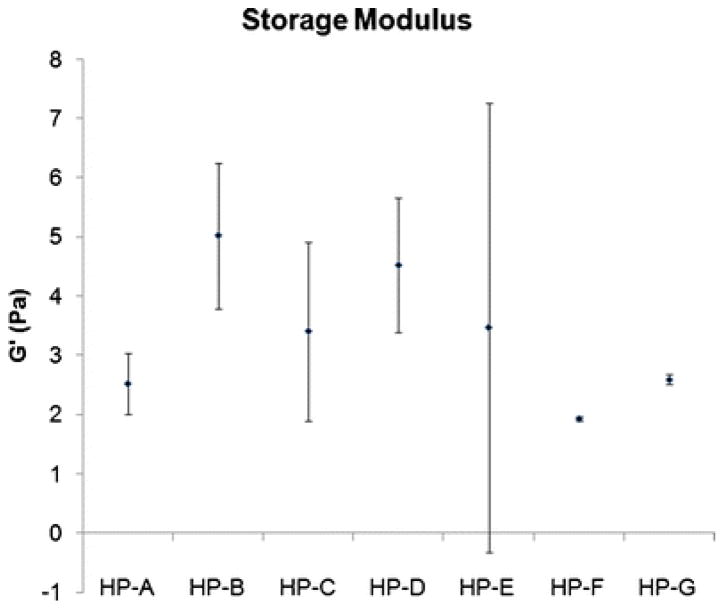
Parallel plate rheometry revealed that all seven samples form relatively weak gels, with average storage moduli ranging from 2.1 to 6.9 Pa (Fig. 5) and average loss moduli from 0.5 to 1.1 Pa. Again, since samples HP-F and HP-G did not gel in vitro at 6 mg/mL, rheometry was performed on 8 mg/mL samples. These values were comparable to the other samples that formed gels at 6 mg/mL. The average storage modulus for each sample was compared, due to high variability within each sample, no significant differences were observed (p = 0.06).
Fig. 5.
Storage modulus. Due to high variability within each matrix gel sample, all samples are statistically similar (p = 0.06). HP-F and HP-G were gelled at 8 mg/mL, all other samples were gelled at 6 mg/mL. Data is presented as mean ± standard deviation.
Scaffold degradation
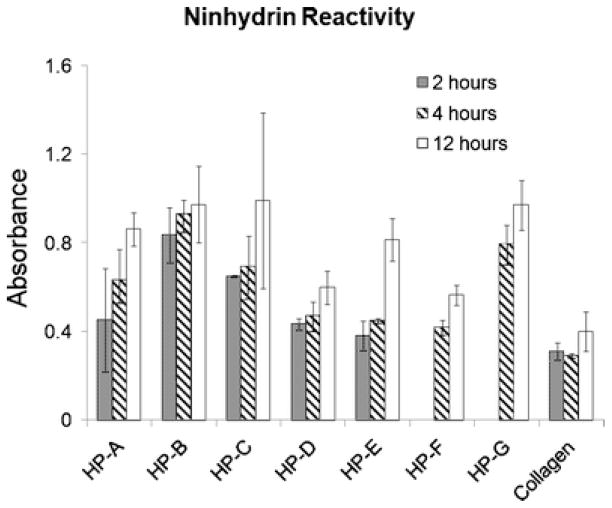
Using a ninhydrin reactivity assay to determine the extent of degradation after collagenase treatment in vitro allowed for the analysis of degradation patterns in the gels formed from the different tissue samples. Collagen gels (2.5 mg/mL) were used as a control for comparison. In the presence of collagenase, ninhydrin reactivity peaked for all samples after 12 hours at 37 °C (Fig. 6); no further reactivity was seen at 24 hours (data not shown). Even after complete collagen degradation, remnants of the gels remained, indicating the presence of other structural ECM components. No visible material remained in the collagen gels after 12 hours. Total ninhydrin reactivity varied across the samples, implying the protein content varied. Specifically, ninhydrin reactivity was lower for HP-D and HP-F, the same samples with an observed decrease in protein content compared to the other samples on the SDS-PAGE gel. Data for the 2-hour time point for samples HP-F and HP-G were not gathered due to insufficient material.
Fig. 6.
Degradation. Ninhydrin reactivity after 2, 4, and 12 hours of incubation with collagenase. Absorbance values indicates a variable protein content across the gels, though the samples degrade in a similar fashion.
In vivo scaffold formation
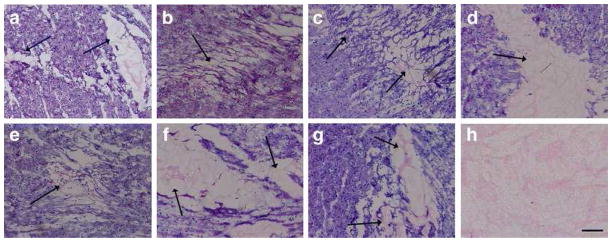
While all of the matrix samples formed gels in vitro at 37 °C, albeit two at a different concentration, we explored whether gelation would occur with the 6 mg/mL concentration in vivo. After injection in the LV free wall of a rat, H&E stained sections of excised tissue were examined for presence of the pericardial scaffold. All seven pericardial matrix samples formed porous scaffolds in vivo similar to what we previously reported with porcine pericardium matrix and pooled human pericardial matrix [25] (Fig. 7). Average pore size ranged from 25 to 35 μm, consistent with previously published work with other decellularized tissue matrices [17]. The interstitial spread of gels varied, a consistent result based on previous work with similar materials. Due to the small sample size, no conclusions could be drawn with respect to whether or not the varied collagen content or donor age contributed to the differences observed in the in vivo gelation.
Fig. 7.
In vivo gelation. Hematoxylin and eosin stain of the injection region identified on a short-axis cross-section. Within 45 minutes of injection, all materials form a porous gel visible with H&E. Arrows indicate injection region. Scale bar is 1 mm (A–G); scale bar is 500 μm (H).
Data analysis
Single factor ANOVAs were performed on all relevant data sets to determine if there was significance within the groups. If significance were determined, student’s t-test with a Tukey-Kramer correction was performed to determine which groups were significantly different.
Discussion
Previously, we demonstrated proof-of-concept for the use of decellularized porcine and human pericardial matrix gels as injectable scaffolds for cardiac repair. In vitro, porcine pericardial matrix gels promoted cellular migration while human pericardial matrix gels did not promote migration when compared to known chemoattractants [25]. Upon injection into the LV free wall of healthy rats, however, the human pericardial matrix gels facilitated a similar degree of vascular cell infiltration and arteriole formation at 2 weeks, implying the in vivo effect may be driven more by the fibrous, porous structure. With that work, the pericardium was identified as a potential source of autologous ECM for use as a biomaterial scaffold [25].
The assertion that an injectable form of human pericardial tissue could be a potentially autologous scaffold for myocardial tissue engineering requires assessment of the patient-to-patient variability. With this work, seven human pericardia from a relevant patient demographic – those undergoing a thoracic surgery where the pericardium was accessed – were processed into injectable matrix materials that gel when brought to physiologic conditions. The resulting matrix gels were compared with respect to their protein composition, glycosaminoglycan content, in vitro degradation, in vivo gelation, and fibrous microstructure. In order to serve as an injectable scaffold, each matrix gel needed to be able to be processed into an injectable form, self-assemble when brought to physiologic conditions, and form a fibrous, porous network in vivo. By fulfilling these criteria, the matrix gels can serve as structural and biochemical scaffolds to support the infiltration of cells and revascularization of the damaged region in order to mitigate negative remodeling post-MI.
It was expected that there would be variability between the tissue samples, as the donors ranged in age and condition. Tissue changes with age, both in composition and material properties. For example, investigation of age-related changes in the mitral and aortic valve revealed that with age the collagen content of the valve decreases, the valves thicken and GAG and elastin content increase dramatically [27]. Significant age-related differences exist in the material properties of these valves as well [56]. Additionally, while the effect of disease on the composition of pericardial ECM has not been examined, changes in the composition of pericardial fluid have been characterized. It has been shown that growth factors such as basic fibroblast growth factor (bFGF), acidic fibroblast growth factor (aFGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) accumulate in the pericardial fluid in patients with ischemic heart disease [57]. Pericardial fluid from these patients has also been shown to have significant effects in vitro – accelerating growth of vascular smooth muscle cells and inducing myocardial cell and vascular endothelial cell apoptosis [57]. Several other disease states can cause inflammation in the pericardium – infections, malignancies, rheumatologic disorders, and uremia. In these cases, the pericardial fluid volume increases and composition may become purulent, exudative, or frank blood [58]. All of these changes could also affect the pericardium and unfortunately, little is understood about the composition of healthy human pericardial tissue; it is therefore difficult to ascertain the effects these disease states and the changes in pericardial fluid have on the composition of the pericardial ECM.
The characterization performed here allowed for a broad comparison of conserved proteins and glycoproteins. While a powerful tool for identification of peptides in a complex sample, mass spectrometry is limited by how well the peptides ionize, crosslinking that causes branched regions and any significant methylation. It is important to acknowledge that mass spectrometry is not an exclusive determination of composition, but it is one of the only ways to start fully characterizing complex samples. Here, we found retention of basic structural ECM components across all samples and the presence of a variety of other protein and glycoprotein components in some of the samples, indicating retention through the material processing steps. Variation was also found in the peptide mass distribution – HP-B was shifted to the lower masses, indicating it was comprised of smaller peptides (Fig. 3). This may be due to an over-digestion of the sample. Since all samples were processed identically, this could be reflecting a property of the composition of HP-B, that it was prone to degradation or digestion more so than the other samples. Peptide mass distribution for HP-F and HP-G were slightly shifted toward the higher masses, indicating they were comprised of larger peptides. These slight differences may contribute to the differences in variability observed in the rheological characterization (Fig. 5). The reduced variability in samples HP-F and HP-G could be due in part to the decreased collagen content, the increased concentration, or the potentially shifted peptide mass distribution. If the mass spectrometry data reflects true differences between the samples, it supports the prediction that there will be patient-to-patient variability.
Mass spectrometry provides no quantification of the different proteins. Varied protein content is, however, apparent with the results of SDS-PAGE and the ninhydrin assay. Decreased collagen content in HP-F and HP-G most likely caused the need to increase the concentration from 6 mg/mL to 8 mg/mL to achieve in vitro gelation. However, the histological sections taken after in vivo injections into a healthy rat heart indicate that each pericardial matrix material can form a gel in vivo even at the lower concentration. This is consistent with previous work with this material and other injectable matrix materials [17, 25].
Characterization of the microstructure of gels formed in vitro reveals a similarly fibrous structure across all samples, implying the self-assembly process for the different matrix samples may be similar, despite differences in composition. The gels collapsed upon preparation for SEM and so porosity could not be quantified, but the average fiber diameter is not statistically different between samples. Fiber diameter has been implicated in a variety of effects in vitro, including cell attachment, migration, and spreading [60–62]; the similar fiber diameter in the matrix gels may imply that cell infiltration will be similarly facilitated across all samples. While it could not be quantified via SEM, the H&E stained sections illustrate the porosity of the gels formed in vivo; the average pore size is approximately 25–35 μm, within a range previously identified as ideal for cellular and vascular infiltration [63–66]. The importance of porosity has been demonstrated repeatedly [2, 3, 67–70], suggesting the structure of these gels is an important factor in determining their success as injectable scaffolds.
Additionally, since degradation products have been implicated as contributing to the chemotactic nature of decellularized tissue scaffolds [8] and the degradation rate of tissue engineered scaffolds is known to affect cell infiltration [71, 72], it is important to note that the degradation patterns for the different materials are also similar. After 12 hours, no additional ninhydrin reactivity was recorded – the collagenase had fully degraded the parts of the gel it could degrade. Gels with lower total ninhydrin reactivity at 24 hours contain a relatively lower collagen concentration compared to the other gels; this was true for HP-D and HP-F, which correlates with the decreased protein content observed with SDS-PAGE. The increased ninhydrin activity compared to a 2.5 mg/mL collagen gel indicates the presence of additional peptides in the HP samples. With respect to the pattern of degradation over time, however, all HP gels behaved similarly – partially degraded at 2 hours and 4 hours and considered fully degraded after 12 hours. The similarities observed may be due to the similar structural properties of the gels moderating enzymatic activity.
As injectable materials derived from decellularized tissue continue to be developed for use as therapies, it will be of utmost importance to determine the short and long-term effects of the materials on the host immune system. Using xenogeneic tissue raises concerns about immunogenicity, but by establishing an autologous source for a complex, tissue-derived scaffold, these concerns can be circumvented. It is important to note here that in order to be a fully autologous therapy, the pepsin used in the processing methods would need to come from the patient. This is feasible, as human pepsin has been isolated in an active form from gastric juice [73]. Relevant quantities can be acquired via minimally invasive measures during the same procedure in which pericardium is harvested.
There is no doubt that working with human tissue is less consistent than working with porcine tissue; however, we have successfully applied a protocol to decellularize, process, and gel seven different human pericardium samples with similar results. The seven donors varied broadly with respect to age and condition, allowing for the evaluation of a wide range of representative pericardia. If future results reveal that the concentration of collagen or other components is important for cellular recruitment, each sample may require optimization of ECM concentration. If it is determined that over- or under-digestion is an important factor, this may also need to be optimized for each sample. Based on previous studies and current cardiomyoplasty clinical trials, even a conservative estimate of the quantity of pericardial tissue that can be collected (4–5 cm2) will provide significantly more material than would be injected therapeutically, allowing material to be used for optimization.
Previously, we demonstrated proof-of-concept for the use of decellularized pericardial matrix gels as injectable scaffolds for cardiac repair [25]. With this work, we examined the effects of inter-patient variability on the processing, composition, and material properties of the matrix gels. Tissue from seven patients spanning a wide range of ages and conditions was examined in order to investigate the applicability of this approach across a broad patient population. The patients were selected from a relevant demographic and the tissue was processed in an identical fashion. Comparison of the samples revealed similarities with respect to composition, rheological properties, fibrous microstructure, gelation and degradation. Collagen content varied from sample to sample; in samples with less collagen in vitro gelation was only achieved after raising the concentration to 8 mg/mL but all samples gelled in vivo at 6 mg/mL. It is concluded that, while not identical, human pericardium samples across a wide patient population can all be processed into injectable scaffolds for cardiac repair.
Acknowledgments
The authors would like to thank Dr. Majid Ghassemian for assistance with mass spectroscopy data, Todd Johnson, Samantha Evans, and Anthony Monteforte for their help in tissue preparation, and Ryan Anderson for assistance with the ESEM. This research was supported in part by the National Institutes of Health Director’s New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004309-01. S.B.S.-N. would like to thank the National Science Foundation for a Graduate Research Fellowship.
Footnotes
Disclosure Statement
The authors have no competing financial interests to disclose.
References
- 1.Roger VL, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 123(4):e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Martina M, et al. Developing macroporous bicontinuous materials as scaffolds for tissue engineering. Biomaterials. 2005;26(28):5609–16. doi: 10.1016/j.biomaterials.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Ifkovits JL, et al. The influence of fibrous elastomer structure and porosity on matrix organization. PLoS One. 5(12):e15717. doi: 10.1371/journal.pone.0015717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall ST, et al. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114(24):2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 6.Davis ME, et al. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97(1):8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res. 3(5):478–86. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reing JE, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15(3):605–14. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 9.Li F, et al. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11(3–4):199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Adv Drug Deliv Rev. 62(7–8):784–97. doi: 10.1016/j.addr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Dobner S, et al. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009;15(7):629–36. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto KL, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30(26):4357–68. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landa N, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 14.Huang NF, et al. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11(11–12):1860–6. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 15.Christman KL, et al. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44(3):654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, et al. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 31(30):7678–83. doi: 10.1016/j.biomaterials.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 17.Singelyn JM, et al. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77(11):1742–54. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins ED, et al. Informed consent: cultural and religious issues associated with the use of allogeneic and xenogeneic mesh products. J Am Coll Surg. 210(4):402–10. doi: 10.1016/j.jamcollsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikos AG, et al. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33(1–2):111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 23.Sefton MV, Babensee JE, Woodhouse KA. Innate and adaptive immune responses in tissue engineering. Semin Immunol. 2008;20(2):83–5. doi: 10.1016/j.smim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Frank DH, et al. Human antibody response following multiple injections of bovine collagen. Plast Reconstr Surg. 1991;87(6):1080–8. doi: 10.1097/00006534-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Seif-Naraghi SB, et al. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 16(6):2017–27. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H, et al. Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials. 32(10):2489–99. doi: 10.1016/j.biomaterials.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 27.McDonald PC, et al. The challenge of defining normality for human mitral and aortic valves: geometrical and compositional analysis. Cardiovasc Pathol. 2002;11(4):193–209. doi: 10.1016/s1054-8807(01)00102-8. [DOI] [PubMed] [Google Scholar]
- 28.Mirsadraee S, et al. Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng. 2006;12(4):763–73. doi: 10.1089/ten.2006.12.763. [DOI] [PubMed] [Google Scholar]
- 29.Freytes DO, et al. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29(11):1630–7. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Johnston N, et al. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope. 2007;117(6):1036–9. doi: 10.1097/MLG.0b013e31804154c3. [DOI] [PubMed] [Google Scholar]
- 31.Danilatos GD, Postle R. The environmental scanning electron microscope and its applications. Scan Electron Microsc. 1982;(Pt 1):1–16. [PubMed] [Google Scholar]
- 32.Ott HC, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 33.Stuart K, Panitch A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers. 2008;89(10):841–51. doi: 10.1002/bip.21024. [DOI] [PubMed] [Google Scholar]
- 34.Cornwell KG, et al. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell-matrix interactions. J Biomed Mater Res A. 2007;80(2):362–71. doi: 10.1002/jbm.a.30893. [DOI] [PubMed] [Google Scholar]
- 35.Partis MD, et al. Cross-linking of protein by ω-maleimido alkanoyl <i>N</i>-hydroxysuccinimido esters. Journal of Protein Chemistry. 1983;2(3):263–277. [Google Scholar]
- 36.Christman KL, et al. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10(3–4):403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 37.Huang NF, et al. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat Protoc. 2006;1(3):1596–609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 38.Young DA, et al. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 7(3):1040–9. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128(6):883–94. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- 40.Bengtsson E, et al. The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissues. J Biol Chem. 1995;270(43):25639–44. doi: 10.1074/jbc.270.43.25639. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Z, et al. The gene for a human microfibril-associated glycoprotein is commonly deleted in Smith-Magenis syndrome patients. Hum Mol Genet. 1995;4(4):589–97. doi: 10.1093/hmg/4.4.589. [DOI] [PubMed] [Google Scholar]
- 42.Pan TC, et al. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J Cell Biol. 1993;123(5):1269–77. doi: 10.1083/jcb.123.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang RZ, et al. Fibulin-2 (FBLN2): human cDNA sequence, mRNA expression, and mapping of the gene on human and mouse chromosomes. Genomics. 1994;22(2):425–30. doi: 10.1006/geno.1994.1404. [DOI] [PubMed] [Google Scholar]
- 44.Utani A, Nomizu M, Yamada Y. Fibulin-2 binds to the short arms of laminin-5 and laminin-1 via conserved amino acid sequences. J Biol Chem. 1997;272(5):2814–20. doi: 10.1074/jbc.272.5.2814. [DOI] [PubMed] [Google Scholar]
- 45.Superti-Furga A, et al. Complementary DNA sequence and chromosomal mapping of a human proteoglycan-binding cell-adhesion protein (dermatopontin) Genomics. 1993;17(2):463–7. doi: 10.1006/geno.1993.1348. [DOI] [PubMed] [Google Scholar]
- 46.Colombatti A, et al. The EMILIN protein family. Matrix Biol. 2000;19(4):289–301. doi: 10.1016/s0945-053x(00)00074-3. [DOI] [PubMed] [Google Scholar]
- 47.Esch FS, et al. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol Endocrinol. 1987;1(11):849–55. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- 48.Ueno N, et al. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1987;84(23):8282–6. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kielty CM, et al. Fibrillin: from microfibril assembly to biomechanical function. Philos Trans R Soc Lond B Biol Sci. 2002;357(1418):207–17. doi: 10.1098/rstb.2001.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kielty CM, et al. Fibrillin-rich microfibrils: elastic biopolymers of the extracellular matrix. J Muscle Res Cell Motil. 2002;23(5–6):581–96. [PubMed] [Google Scholar]
- 51.Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103(6 Pt 1):2499–509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laremore TN, et al. Domain structure elucidation of human decorin glycosaminoglycans. Biochem J. 431(2):199–205. doi: 10.1042/BJ20100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem. 2002;277(38):35671–81. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]
- 54.Iozzo RV, et al. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274(8):4489–92. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 55.Bratt A, et al. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005;280(41):34859–69. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 56.Stephens EH, et al. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng Part A. 16(3):867–78. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujita M, et al. Pericardial fluid as a new material for clinical heart research. Int J Cardiol. 2001;77(2–3):113–8. doi: 10.1016/s0167-5273(00)00462-9. [DOI] [PubMed] [Google Scholar]
- 58.Zipes DP, Braunwald E. Braunwald’s heart disease: a textbook of cardiovascular medicine. 7. Philadelphia, Pa: W.B. Saunders; 2005. p. xxi.p. 2183.p. 74. [Google Scholar]
- 59.Capaldi MJ, Chapman JA. The C-terminal extrahelical peptide of type I collagen and its role in fibrillogenesis in vitro. Biopolymers. 1982;21(11):2291–313. doi: 10.1002/bip.360211115. [DOI] [PubMed] [Google Scholar]
- 60.Sisson K, et al. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J Biomed Mater Res A. 94(4):1312–20. doi: 10.1002/jbm.a.32756. [DOI] [PubMed] [Google Scholar]
- 61.Christopherson GT, Song H, Mao HQ. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30(4):556–64. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Yang F, et al. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–10. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 63.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 64.Jawad H, et al. Myocardial tissue engineering: a review. J Tissue Eng Regen Med. 2007;1(5):327–42. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 65.Ratner BD. A paradigm shift: biomaterials that heal. Polymer International. 2007;56(10):1183–1185. [Google Scholar]
- 66.Marshall AJ, Ratner BD. Quantitative characterization of sphere-templated porous biomaterials. AIChE Journal. 2005;51(4):1221–1232. [Google Scholar]
- 67.Crane GM, Ishaug SL, Mikos AG. Bone tissue engineering. Nat Med. 1995;1(12):1322–4. doi: 10.1038/nm1295-1322. [DOI] [PubMed] [Google Scholar]
- 68.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 69.Ishaug SL, et al. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36(1):17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 70.Stosich MS, et al. Vascularized adipose tissue grafts from human mesenchymal stem cells with bioactive cues and microchannel conduits. Tissue Eng. 2007;13(12):2881–90. doi: 10.1089/ten.2007.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6(8):623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 72.Alsberg E, et al. Regulating bone formation via controlled scaffold degradation. J Dent Res. 2003;82(11):903–8. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 73.Peek K, Roberts NB, Taylor WH. Improved separation of human pepsins from gastric juice by high-performance ion-exchange chromatography. J Chromatogr. 1989;476:291–7. doi: 10.1016/s0021-9673(01)93876-2. [DOI] [PubMed] [Google Scholar]