Abstract
The extended-release formulation of zolpidem (Ambien CR®) is approved for the treatment of insomnia without a treatment duration limit. Acutely zolpidem impairs performance, and no research to date has examined whether tolerance develops to these performance impairments during nighttime awakening. The present double-blind, placebo-controlled study examined whether tolerance develops to zolpidem-induced acute performance impairment after repeated (22–30 days) nightly use. Effects of bedtime administration of zolpidem extended-release (ZOL; 12.5 mg) were tested on a battery of performance measures assessed during a forced nighttime awakening in 15 healthy male volunteers who completed overnight polysomnographic recording sessions in our laboratory at baseline and after approximately a month of at-home ZOL. As expected, bedtime ZOL administration was associated with changes in sleep architecture and impairments across all performance domains during nighttime testing (psychomotor function, attention, working memory, episodic memory, metacognition) with no residual next morning impairment. Tolerance did not develop to the observed ZOL-related impairments on any outcome. Possible evidence of acute abstinence effects following discontinuation of ZOL was observed on some performance and sleep outcomes. Overall, these findings suggest that performance is significantly impaired during nighttime awakening even after a month of nightly ZOL administration and these impairments could significantly impact safety should nighttime awakening require unimpaired functioning (e.g., driving; combat-related activities in the military).
Keywords: zolpidem, Ambien, psychomotor, cognition, tolerance, memory
Introduction
Zolpidem (Ambien®) is a sedative hypnotic that binds selectively at the benzodiazepine site of α1 subunit containing GABAA receptors, and is indicated for the short-term treatment of insomnia (Weinling et al., 2006). In 2005, an extended-release formulation of zolpidem (Ambien CR®) was approved by the United States Food and Drug Administration (FDA) without a specific treatment duration limit (Rosenberg, 2006). Neither zolpidem formulation produces residual next-day effects on psychomotor and cogntive function after nighttime dosing; however, both acutely impair performance during the period of peak drug effects (Berlin et al., 1993; Danjou et al., 1999; DeClerk & Bisserbe; 1998; Hindmarch et al., 2001; Mintzer, Frey, Yingling, & Griffiths, 1997; Mintzer & Griffiths, 1999 a, b; Otmani et al., 2008; Stoops & Rush, 2003; Verster et al., 2002). Acute performance impairment could be problematic when circumstances necessitate proper functioning during nighttime awakenening (e.g., walking up or down stairs; responding to an emergency; driving a car). Despite the fact that Ambien CR® is prescribed without a treatment duration limit, we are aware of no study that has systematically tested whether tolerance develops to the acute performance impairing effects of zolpidem after chronic exposure. Independent of established treatment duration limits for specific drugs, the extent to which tolerance develops to benzodiazepine receptor agonist sedative/hypnotics is an important question as these medications are often used for periods of time longer than medically indicated (e.g., Barker, Greenwood, Jackson, & Crowe, 2004; Curran et al. 2003). As such, the present study was designed to determine if tolerance develops to zolpidem-induced acute performance impairment after repeated nightly use. The acute effects of zolpidem extended-release administration were examined on a battery of performance measures assessed during a forced nighttime awakening in 15 healthy male volunteers who completed overnight polysomnographic recording sessions in our laboratory at baseline and after repeated (22–30 nights) at-home zolpidem extended-release use.
Although no studies have examined tolerance to zolpidem’s acute performance-impairing effects after nightly use for a clinically relevant period, one study examined zolpidem’s performance effects (orignal formuation; 15 mg) across four laboratory sessions that took place in close succession (Stoops & Rush, 2003). Outcomes revealed that performance on three separate tasks was significantly impaired on the first day of testing in the zolpidem condition relative to placebo and performance in the zolpidem condition did not differ significantly on the fourth day relative to the first day. These results suggest that tolerance did not develop to the performance impairing effects of zolpidem. In addition, some studies suggest that complete tolerance may not develop to the amnestic effects of benzodiazepines, even after years of chronic use (Curran et al., 1994; Ghoneim, Mewaldt, Berie, & Hinrichs, 1981; Tata, Rollings, Collins, Pickering, & Jacobsen, 1994). Therefore, we predicted that tolerance would not develop to the acute performance impairing effects of zolpidem in the present study after repeated nightly use.
Outcomes included in the present study were selected based on previous reports of the effects of zolpidem and other benzodiazepine receptor agonists on performance outcomes. Episodic memory was the primary outcome of interest given that impairment of this aspect of performance is consistently observed and particularly pronounced in daytime laboratory studies involving zolpidem and other benzodiazepine receptor agonists (e.g., Danjou et al., 1999; Mintzer et al., 1997; 1999a; Otmani et al., 2008; Verster et al., 2002). In these studies, zolpidem and other drugs impair the initial encoding of new information into memory but do not impair (and may in fact enhance) retrieval of previously encoded information. The enhancement of recall for information studied prior to drug administration is referred to as retrograde facilitation (Fillmore, Kelly, Rush, & Hays, 2001; Hinrichs et al., 1984; Mintzer & Griffiths, 1999a). Assessment of specific episodic memory processes (e.g., encoding, retrieval, retrograde facilitation) was made possible in the present study by administering multiple episodic word memory tasks throughout testing sessions. An added, clinically relevant rationale for focusing the present study on episodic memory outcomes is the accumulation of multiple, recent case reports of individuals completing complex behaviors such as cooking, driving, or email correspondence during nighttime awakening following bedtime hypotic use (many with zolpidem) without recollection of such behaviors the following morning (Gustavsen et al., 2008; Siddigui et al., 2008). Additional outcome measures of importance in the present study included functions shown to be impaired by daytime administration of zolpidem in previous studies (psychomotor function, attention, working memory; Mintzer et al., 1997; 1999a; Stoops & Rush, 2003; Verster et al., 2002). Lastly, secondary outcomes in the present study included estimates of performance (i.e., metacognition, or awareness of changes in performance), polysomnographic sleep recordings, and subjective ratings of drug effects.
Method
Participants
Study completers included 15 healthy male participants (females were excluded from participation based on evidence that sleep parameters may vary across the menstrual cycle; Baker & Driver, 2004). All participants had normal sleep patterns as assessed by: 1) the Pittsburgh Sleep Quality Index (PSQI; score < 5) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), 2) a review of actigraphy and sleep questionnaire data collected during a pre-study screening week, and 3) a review of polysomnographic recording during an initial sleep adaptation night (see “General Study Design” below). The participants ranged in age from 21 to 42 years (M = 30.0, SD = 6.5), in weight from 63.2 to 98.0 kg (M = 78.5, SD = 10.4), and reported having completed 12 to 24 years of education (M = 17.9, SD = 3.2). Participants were nonsmokers who reported consuming 0 to 15 alcoholic beverages per week (M = 2.3, SD = 3.7) and 9.4 to 400 mg of caffeine per day (M = 168.9, SD = 135.6). None reported use of prescription hypnotics or contraindications to sedative drugs. All were in good health (as determined by medical history and vital signs), did not have a history of psychiatric conditions, and reported no history of drug dependence.
Participants were asked to refrain from using alcohol and psychoactive drugs (with the exception of caffeine) during study participation. Abstinence was confirmed at screening, during medication pick-up visits, and at the start of each session by alcohol Breathalyzer and urine drug tests (opiates, methadone, cocaine, and benzodiazepines) using an EMIT system (Syva Co., Palo Alto, CA). The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine; all participants gave their written informed consent before beginning the study and were paid for their participation.
General Study Design
During an initial, face-to-face screening visit participants were familiarized with the experimental procedures and told that the purpose of the study was to learn more about the effects of drugs that are used to treat insomnia. They were informed that during study participation they might receive the following drugs: sedatives, muscle relaxants, anti-anxiety medications (e.g., diazepam, buspirone, temazepam, zolpidem), or placebo. Participants were otherwise blind to the type of drug administered. After screening, participants completed an adaptation night that was designed to acclimate participants to the residential unit and the sleep monitoring procedures; no drugs or performance assessments were administered. Subsequently, participants completed four overnight experimental sessions that involved performance testing (see Outcomes) and double-blind capsule administration of placebo (PL) or 12.5 mg extended-release zolpidem (ZOL) in the following fixed order: session 1 (PL), session 2 (ZOL), session 3 (ZOL), and session 4 (PL). Twenty-two (22) to 30 days of nightly at-home double-blind ZOL ingestion took place between sessions 2 and 3 (see At-Home Dosing Compliance section), allowing for an assessment of tolerance to the acute effects of ZOL (relative to PL) after chronic dosing. As such, sessions 1 and 2 were designated as the “Baseline Phase” (prior to chronic nightly dosing) and sessions 3 and 4 were designated as the “Chronic Phase” (after chronic nightly dosing). An additional 5–8 nights of at-home double-blind ZOL ingestion took place between sessions 3 and 4 (see At-Home Dosing Compliance section). Thus, comparison of performance in session 1 (PL/Baseline Phase) relative to session 4 (PL/Chronic Phase) allowed for an assessment of acute abstinence effects after discontinuation of chronic ZOL ingestion. Primary outcomes included episodic memory, psychomotor function, attention, and working memory. Secondary outcomes included performance estimation (metacognition), polysomnographic (PSG) sleep recordings, and subjective ratings of drug effects. Withdrawal symptoms were monitored in the week following session 4 using a daily, at-home self-report questionnaire and a follow-up phone call (a review of these data provided no evidence for withdrawal in any participant).
Experimental Sessions
The wiring for PSG recordings took place upon the participant’s arrival on the residential unit, according to standard procedures recommended by the American Academy of Sleep Medicine (AASM; Kushida et al., 2005), followed by the “pre-drug performance battery” (see Table 1 for a session overview). Capsules were administered 30 minutes before lights out, which took place at the participant’s usual bedtime (always prior to midnight). Awakening time for the “peak effects battery” took place approximately 80 minutes after capsule administration, and was designed to coincide with the estimated period of peak psychoactive ZOL effects. To ensure consistency in participant sleep state, the exact awakening time was determined during each session by monitoring the PSG recordings, with the goal of awakening participants while in slow-wave sleep (or at minimum during Stage 2, before the participant entered REM) during the first sleep cycle. This goal was met across all sessions and participants with the exception of one session each for two participants. During these two cases, the participant was already awake when the staff member entered the room to awaken him; however, in both cases, the participant had been in Stage 2 prior to spontaneous awakening. Overall, from first lights out to next morning lights on, participants had the opportunity to sleep a total of 8 hours (taking into account the length of the peak battery). In the morning, participants were offered an optional breakfast and shower before completing the “next-morning battery” and subsequent discharge from the unit.
Table 1.
Experimental Session Overview
| Time relative to drug administration |
pre-drug | 0 | 30 | 80 | 90 | 180 | 390 | 450 |
|---|---|---|---|---|---|---|---|---|
| Approximate Time* |
10pm | 7:30am | 8:30am | |||||
| Event |
Pre-drug battery: Word study 1 Sternberg N-back Div attention DSST Subj. ratings Psychomotor Word test 1 |
capsule p.o. |
Lights out | Lights on (forced awakening) |
Peak battery: Word study 2 Sternberg N-back Div attention DSST Subj. ratings Psychomotor Word test 2 Word study 3 |
Lights out | Lights on |
Next morning battery: Word test 3 Word study 4 Sternberg N-back Div attention DSST Subj. ratings Psychomotor Word test 4 Word test 5 |
| Sleep measurement periods | Period 1 | Period 2 | ||||||
timing based on a hypothetical 10pm bedtime
Drug administration
Single oral doses of 12.5 mg ZOL were prepared by encapsulating commercially available Ambien CR® 12.5 mg tablets (G.D. Searle and Co., Chicago, IL, USA) in a size 000 capsule; lactose was used to fill the remainder of the capsule. PL capsules contained only lactose. Drug administration during the four experimental sessions took place on the residential unit. During the two periods of at-home ZOL ingestion (see General Study Design), participants took their nightly capsules at home prior to bedtime. To ensure safety, participants were given standard instructions and precautions for nightly hypnotic use (Physician’s Desk Reference 2003). Take home capsules were supplied on a weekly basis in pillboxes labeled with each day of the week. Although participants were not observed taking their capsules, precautions were taken to maximize compliance, including asking participants to: 1) use an automated telephone voice mail system to "date and time stamp" the time of nightly capsule self-administration (as in Mintzer, Allen, & Griffiths, 2001; Mintzer & Griffiths, 2005) and 2) report to the unit once a week to pick up capsules, drop off any capsules that were not taken, and provide urine and breathalyzer samples.
Outcome Measures
Episodic memory
Episodic memory was measured with free recall and recognition tasks. Stimuli for these tasks consisted of unique sets (i.e., no stimulus was repeated across sessions) of 36 common concrete nouns selected from the Thorndike and Lorge (1944) corpus; further details on word lists are presented elsewhere (Carter, Griffiths, & Mintzer, 2009). During the study phase, participants read each word aloud and clicked the mouse to proceed to the next word. Four word lists were studied each session, and memory was tested with free recall and recognition tests for each of these lists. The administration of study lists and tests was as follows for each session (see Table 1): Test 1- studied at the start of the predrug battery (List 1) and memory tested at the end of the predrug battery 60 min later (study/test predrug), Test 2- studied at the start of the peak effects battery (List 2) and memory tested at the end of the peak battery 60 min later (study/test peak), Test 3 - studied at the end of the peak battery (List 3) and memory tested at the beginning of the next morning battery (study peak/test next morning), Test 4 - studied at the beginning of the next morning battery (immediately after Test 3; List 4) and memory tested at the end of the next-morning battery 60 min later (study/test next morning). In addition, Test 5 was a cumulative free recall test given at the end of the next-morning battery to test for memory across all four study lists administered during that session; a cumulative test of recognition memory was not completed. ZOL’s effects on memory encoding were tested by examining memory for lists administered during the period of peak drug effects (List 2 and 3, as assessed by Tests 2, 3, and 5). Residual effects of ZOL on episodic memory (encoding and retrieval) were tested by examining memory for List 4 (as assessed by Tests 4 and 5; encoding and retrieval next morning). In addition, ZOL’s retrograde facilitation effects were examined by testing later recall for words studied immediately prior to drug administration (List 1, as assessed by Test 5).
The dependent measure for the four separate free recall tests was the number of correct words written down out of the 36 possible “old” (i.e., studied) words. The dependent variable for the Test 5/cumulative free recall test was the number of correct words out of the 144 total possible words (i.e., 36 “old” words from each of the four lists). Dependent measures associated with the recognition memory test included proportion of old responses made to old words (hit rate), proportion of old responses to new (i.e., non-studied) words (false alarm rate), and signal detection measures of sensitivity in distinguishing between old and new words (d’) and response bias (C; Snodgrass & Corwin 1988). Lastly, metamemory was measured within the recognition memory test by calculating the Goodman–Kruskal gamma correlation (between confidence ratings made during the recognition memory test and recognition memory accuracy computed for each participant and collapsed across old and new words; Goodman & Kruskal 1954).
Psychomotor
Psychomotor function was measured using standing balance and circular lights tasks (Mintzer et al., 1997).
Attention
Attention was measured using the digit symbol substitution task (DSST; McLeod, Griffiths, Bigelow, & Yingling, 1982; based on Wechsler, 1981) and a divided attention task [simultaneous visual tracking (moving a mouse curser to track a crosshair moving on a horizontal plane in the center of the screen) and monitoring (clicking a mouse whenever a number stimulus was presented that matched a target stimulus in the periphery of the screen)] (Kleykamp, Griffiths, & Mintzer, 2010).
Working memory
Working memory was measured using the N-back task (Jonides et al., 1997; Mintzer and Stitzer, 2002) and a modified Sternberg task (Kleykamp et al., 2010).
Performance estimation
In addition to the above objective measures of performance, participants were asked to rate their performance on the DSST, N-back task, Sternberg working memory, and recognition memory before (pre-performance estimates) and after (post-performance estimates) the task (See Table 2 for specific rating measures). The participant estimates of performance were compared with the actual task scores by calculating difference scores for each post-drug time point as the percentage of pre-drug estimate minus the percentage of pre-drug actual performance. For all performance estimate outcomes, a positive score represents an under-estimation of performance impairment (over confident), while a negative score represents an over-estimation (under confident). Additional details on the performance estimation outcomes are described elsewhere (Kleykamp et al., 2010).
Table 2.
Data from the peak effects battery as function of phase and drug condition
| Placebo | Zolpidem | Placebo | Zolpidem | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline phase | Baseline phase | Chronic phase | Chronic phase | Significance | |||||
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | ||
| Episodic Memory | |||||||||
| Test 2 | |||||||||
| Recognition task | |||||||||
| Hit rate* | 0.76 | (0.03) | 0.65 | (0.04) | 0.76 | (0.04) | 0.63 | (0.06) | Z < P |
| d'* | 1.24 | (0.14) | 0.37 | (0.11) | 1.37 | (0.16) | 0.51 | (0.17) | Z < P |
| False alarm rate* | 0.33 | (0.05) | 0.52 | (0.05) | 0.30 | (0.04) | 0.47 | (0.07) | Z > P |
| C/Response bias | −0.13 | (0.11) | −0.25 | (0.13) | −0.10 | (0.11) | −0.07 | (0.24) | - |
| Gamma* | 0.49 | (0.07) | 0.16 | (0.10) | 0.44 | (0.09) | 0.23 | (0.11) | Z < P |
| Free Recall | |||||||||
| Number recalled* | 2.07 | (0.55) | 0.27 | (0.12) | 3.53 | (0.86) | 0.93 | (0.46) | Z < P |
| Test 3 | |||||||||
| Recognition task | |||||||||
| Hit rate* | 0.74 | (0.03) | 0.56 | (0.05) | 0.71 | (0.06) | 0.57 | (0.04) | Z < P |
| d'* | 1.14 | (0.17) | 0.42 | (0.07) | 1.17 | (0.20) | 0.58 | (0.09) | Z < P |
| False alarm rate | 0.35 | (0.04) | 0.42 | (0.05) | 0.33 | (0.05) | 0.36 | (0.04) | - |
| C/Response bias* | −0.13 | 0.09 | 0.03 | 0.14 | −0.06 | 0.14 | 0.09 | 0.11 | Z > P |
| Gamma* | 0.46 | (0.08) | 0.22 | (0.10) | 0.47 | (0.08) | 0.26 | (0.10) | Z < P |
| Free Recall | |||||||||
| Number recalled* | 2.73 | (0.84) | 0.93 | (0.66) | 2.40 | (1.09) | 0.20 | (0.20) | Z < P |
| Psychomotor | |||||||||
| Balance* | 92.85 | (12.81) | 30.85 | (5.60) | 99.38 | (6.75) | 35.96 | (6.65) | Z < P |
| Circular Lights+ | 100.54 | (3.93) | 77.31 | (2.79) | 90.19 | (3.19) | 81.91 | (2.77) | Z1 < P1 |
| Attention | |||||||||
| Digit Symbol Substitition Task | |||||||||
| Attempted+ | 102.92 | (3.07) | 71.76 | (3.15) | 94.73 | (1.79) | 79.34 | (3.11) | Z1<P1, Z2<P2 |
| Proportion Correct* | 99.62 | (1.24) | 90.77 | (2.56) | 98.51 | (1.32) | 92.90 | (2.22) | Z < P |
| Divided Attention | |||||||||
| Accuracy (cross hair/cursor overlap; tracking)* | 98.24 | (1.51) | 64.93 | (3.64) | 95.31 | (3.67) | 68.72 | (3.28) | Z < P |
| Mean RT (monitoring)* | 115.09 | (5.37) | 148.53 | (10.90) | 109.67 | (4.78) | 124.68 | (7.05) | Z > P |
| Working Memory | |||||||||
| Nback task # | |||||||||
| 1-back Task | |||||||||
| d' | 98.69 | (3.55) | 76.65 | (6.94) | 94.05 | (5.02) | 61.69 | (11.01) | - |
| Median response time | 93.19 | (2.99) | 144.77 | (9.22) | 109.36 | (4.86) | 127.16 | (6.17) | Z > P |
| 2-back Task | |||||||||
| d' | 105.31 | (7.02) | 56.20 | (8.80) | 78.57 | (8.79) | 63.73 | (10.49) | - |
| Median response time | 101.86 | (3.86) | 138.31 | (8.45) | 98.12 | (3.87) | 123.82 | (3.83) | Z > P |
| 3-back Task | |||||||||
| d' | 114.19 | (11.72) | 61.50 | (10.15) | 62.69 | (14.34) | 73.12 | (11.02) | Z1, P2 < P1 |
| Median response time | 100.83 | (2.65) | 124.20 | (6.87) | 104.88 | (4.45) | 115.67 | (5.83) | - |
| Sternberg task | |||||||||
| Proportion correct | 99.54 | (2.16) | 90.57 | (3.33) | 93.82 | (3.27) | 95.38 | (2.90) | - |
| Median Response Time* | 103.73 | (3.23) | 136.78 | (6.34) | 107.24 | (5.33) | 132.07 | (4.28) | Z > P |
| Performance estimation | |||||||||
| DSST | |||||||||
| Speed | −11.43 | (2.92) | 12.01 | (4.71) | −0.94 | (2.09) | 11.29 | (3.97) | Z > P |
| Accuracy | −9.62 | (3.94) | 0.62 | (16.06) | −0.94 | (2.62) | 14.25 | (4.29) | - |
| Recognition task | |||||||||
| Test 2 d' | 5.70 | (7.11) | 54.70 | (6.24) | 6.79 | (7.36) | 45.43 | (9.12) | Z > P |
Note. SE = standard error. Significance column: P = Placebo, Z = Zolpidem, 1 = baseline phase, 2 = chronic phase.
Symbols:
dash (-) = not significant,
asterisk (*) = main effect of drug condition,
plus sign (+) = drug by phase interaction;
number sign (#) = drug by task interaction observed for all N-back outcomes except false alarm rate; additionally, a significant interaction of drug by task condition by phase was observed for N-back hit rate, d', and C.
Sternberg: data are collapsed across delay condition. Performance estimation: data are collapsed across time of testing (pre/post) and peak % of predrug values; difference scores between ratings and actual performance were calculated as follows: Speed = speed rating minus actual trials attempted, Accuracy = accuracy rating minus actual proportion correct, Test 2 d' = accuracy rating minus actual d'.
Polysomnographic sleep recordings
Polysomnographic recordings were acquired following standard procedures (American Academy of Sleep Medicine (AASM); Kushida et al., 2005), using an Embla N7000™ system with EEG (F4-M1, C4-M1, 02-M1, F3-M2, C3-M2, O1-M2), EOG (E1-M2, E2-M1), chin EMG, and ECG. EEG signals were digitized on-line at a rate of 500 Hz and saved to disk for post-analysis. During the adaptation night only, EMG was recorded from the left and right anterior tibialis muscles, respiratory activity was recorded using oximetry, nasal pressure transducer and oral-nasal airflow thermocouple, and respiratory effort measured by abdominal and thoracic strain gauges. Polysomnograms were visually scored by certified polysomnographers from the Johns Hopkins Center for Interdisciplinary Sleep Research and Education (CISRE) who were blinded to participant condition using the AASM standards (Kushida et al., 2005). Records were subsequently reviewed and finalized by a physician, board certified in sleep medicine that was blind to study hypotheses. Sleep architecture variables (as defined by the AASM) were: initial sleep latency, wake after sleep onset time, total sleep time, sleep efficiency, % Stage 1 sleep, % Stage 2 sleep, % Slow wave sleep (SWS), % REM sleep, and REM latency. PSG data were analyzed separately for the period from initial lights out to lights on prior to the peak battery (sleep period 1) and the period from lights out after the peak battery to lights on the next morning (sleep period 2; see Table 1).
Subjective ratings of drug effects
Participants completed a total of 37 questions pertaining to drug effects using questionnaires described in detail previously (Mintzer et al., 1997).
Data Analysis
All outcomes included complete data sets (N=15) with the exception of one missing data point for the circular lights task due to experimenter error. All data were analyzed using PROC MIXED in SAS (SAS Institute Inc., Cary, NC). Analyzed data were percentage of predrug values (psychomotor, attention, working memory, performance estimation), difference from predrug values (subjective ratings), or raw values [episodic memory; Test 3 data were not analyzed relative to predrug scores due to the longer retention interval (i.e., interval between study and test) for Test 3 (overnight retention) relative to Tests 1 or 2 (60 min retention). Thus, all episodic memory data were analyzed as raw scores for consistency].
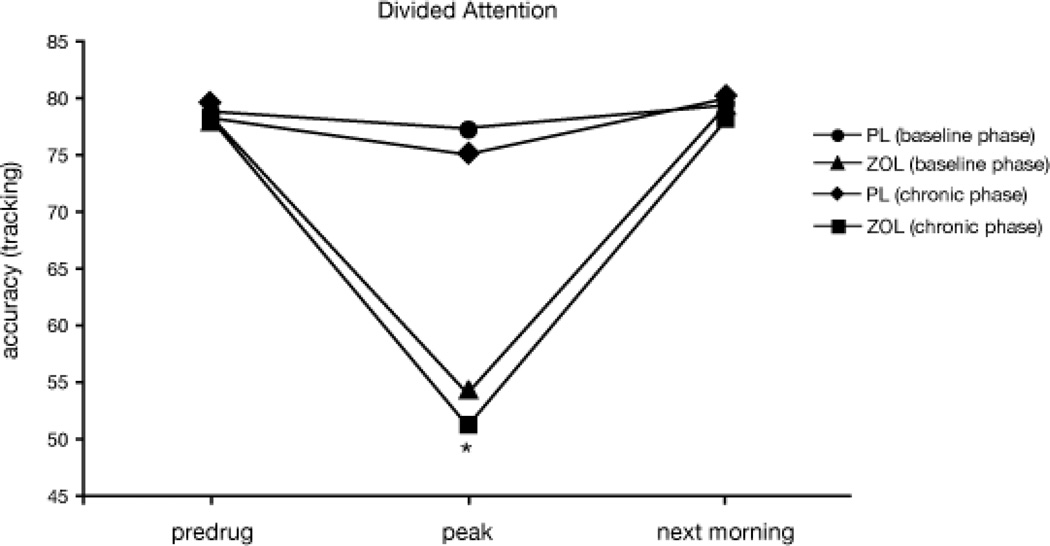
Visual inspection of the data revealed that performance across all tasks returned to predrug levels the next morning in all conditions, thus indicating no residual drug effects (see Figure 1 for representative data from the divided attention task). Therefore, for all performance-related outcomes only data from the peak effects battery will be presented. For episodic memory, this included Test 2 (study/test peak), Test 3 (study peak/test next-morning), and Test 5 (cumulative free recall). Performance battery and sleep data (polysomnographic recordings for sleep period 1 and sleep period 2) were entered into a within-subject ANOVA with factors of phase (Baseline, Chronic) and drug condition (PL, ZOL) for all outcomes. The following outcomes included additional factors: N-back task (memory load: 1-, 2- and 3-back; 0-back control condition entered as a covariate), Sternberg task (delay between stimulus presentation and testing: 0 and 12 s; nonmemory control condition entered as a covariate), Performance estimation (time of rating: pre- and post-task), and Free recall Test 5 (cumulative free recall; lists: 1, 2, 3, and 4). Significant interactions (p ≤ .05) were followed up with simple effects tests with modified Bonferroni corrections (Keppel, 1991). Simple effects tests were limited to the following phase by drug condition comparisons: Baseline phase PL vs. Baseline phase ZOL; Chronic phase PL vs. Chronic phase ZOL; Baseline phase PL vs. Chronic phase PL; Baseline phase ZOL vs. Chronic phase ZOL. Tolerance to ZOL-related performance impairment was defined as: a drug condition by phase interaction for which follow-up testing revealed significantly better performance in Chronic phase ZOL relative to Baseline phase ZOL. Acute abstinence/withdrawal was similarly defined as: a drug condition by phase interaction for which follow-up testing revealed significantly worse performance in Chronic phase PL relative to Baseline phase PL.
Figure 1.
Mean divided attention accuracy (cross hair/cursor overlap; tracking) as a function of drug condition (PL and ZOL) and phase (baseline and chronic) across testing time points. The asterisk (*) indicates a significant main effect of drug condition at the peak time point.
Results
At-Home Dosing Compliance
There were 18 instances (out of 359 possible) where participants did not call the voicemail system to report taking their nightly dose. Six of these instances (3 participants total) represented a missed nightly dose (1.7% of all possible nightly doses). Two participants forgot to take one dose each and one participant missed 4 doses (2 consecutive nights due to illness, 2 non-consecutive nights due to forgetting). The range of nightly dosing between sessions 2 and 3 (Baseline/ZOL to Chronic/ZOL) was 22 to 30 days (mean = 26 days) with variability due to scheduling constraints and missed doses. All but two participants completed 25 days or more of nightly dosing during this time period; one participant completed 22 days of dosing (scheduled for 26; missed 4 doses as described above), while the other participant completed 24 days of dosing (scheduled for 25; missed one dose). For the time period between sessions 3 and 4 (Chronic/ZOL to Chronic/PL), participants’ daily at home dosing ranged from 5 to 8 days (mean = 6 days), again with variability due to scheduling constraints and missed doses (only one participant was confirmed to miss a dose during this time period).
Outcome Measures
Table 2 shows data from the peak performance battery. Table 3 shows data associated with polysomnographic sleep recordings for sleep periods 1 and 2. Trends towards significance (.05 < p ≤ .10) are noted when present, but not indicated in Tables 2 and 3.
Table 3.
Descriptive statistics for sleep outcomes across Period 1 and Period 2 of sleep.
| Placebo | Zolpidem | Placebo | Zolpidem | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline phase | Baseline phase | Chronic phase | Chronic phase | Significance | |||||
| Mean | (SE) | Mean | (SE) | Mean | (SE) | Mean | (SE) | ||
| Sleep Period 1 | |||||||||
| Initial sleep latency (min) | 12.04 | 3.72 | 8.29 | 2.42 | 12.45 | 2.28 | 8.06 | 1.46 | - |
| Wake after sleep onset (min) | 1.14 | 0.43 | 2.33 | 2.05 | 5.64 | 2.37 | 0.72 | 0.41 | - |
| Total sleep time (min) | 42.55 | 3.00 | 44.74 | 2.35 | 38.55 | 3.68 | 45.91 | 2.59 | - |
| Sleep efficiency* | 0.78 | 0.06 | 0.82 | 0.04 | 0.69 | 0.06 | 0.84 | 0.03 | Z>P |
| Stage 1 (%)* | 0.16 | 0.06 | 0.07 | 0.02 | 0.18 | 0.05 | 0.07 | 0.01 | Z<P |
| Stage 2 (%)+ | 0.33 | 0.04 | 0.43 | 0.06 | 0.50 | 0.06 | 0.44 | 0.07 | 1<2 |
| slow wave sleep (%) | 0.51 | 0.06 | 0.48 | 0.07 | 0.32 | 0.07 | 0.47 | 0.06 | - |
| REM sleep (%) | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.02 | 0.02 | - |
| Sleep Period 2 | |||||||||
| Initial sleep latency (min)* | 10.58 | 2.29 | 2.71 | 0.70 | 22.04 | 9.43 | 2.31 | 0.61 | Z<P |
| Wake after sleep onset (min) | 28.84 | 8.15 | 26.13 | 6.80 | 32.71 | 7.64 | 35.94 | 9.56 | - |
| Total sleep time (min) | 387.30 | 8.18 | 393.13 | 8.22 | 372.12 | 10.34 | 389.53 | 9.47 | - |
| Sleep efficiency | 0.91 | 0.02 | 0.93 | 0.02 | 0.87 | 0.03 | 0.91 | 0.02 | - |
| Stage 1 (%)* | 0.09 | 0.02 | 0.05 | 0.01 | 0.09 | 0.02 | 0.06 | 0.01 | Z<P |
| Stage 2 (%)* | 0.49 | 0.02 | 0.53 | 0.03 | 0.50 | 0.02 | 0.54 | 0.02 | Z>P |
| slow wave sleep (%)+ | 0.13 | 0.02 | 0.13 | 0.02 | 0.10 | 0.02 | 0.11 | 0.02 | 1>2 |
| REM sleep (%) | 0.30 | 0.02 | 0.29 | 0.02 | 0.31 | 0.02 | 0.29 | 0.01 | - |
| REM latency (min) | 55.29 | 10.03 | 68.77 | 11.80 | 58.30 | 9.53 | 56.40 | 10.84 | - |
Note. SE = standard error. Significance: P = Placebo, Z = Zolpidem, 1 = baseline phase, 2 = chronic phase.
Symbols:
dash (-) = not significant;
asterisk (*) = main effect of drug condition;
plus sign (+) = main effect of phase.
Episodic Memory
A significant main effect of drug condition was observed for hit rate and d’ (sensitivity in discriminating between old and new words on the recognition memory test) for Tests 2 (study/test peak) and 3 (study peak/test next morning) [F values (1, 14) = 33.5 and 30.6, respectively]. For both outcomes, performance was impaired in the ZOL condition relative to PL for both Tests, independent of phase, suggesting tolerance did not develop to the memory impairing effects of ZOL (see Table 2). In addition, a significant main effect of drug condition was observed for false alarm rate on Test 2 and a marginally significant effect for Test 3 [p = 0.051; F values (1, 14) = 24.7 and 4.6, respectively], and the pattern of means for both Tests indicated that false alarms were increased in the ZOL condition relative to PL. In addition, a significant main effect of drug condition was observed for C on Test 3 [F = (1, 14) = 5.5], but not Test 2, indicating that participants responded more conservatively in the ZOL condition relative to PL (see Table 2). Lastly, a significant main effect of drug condition was observed for gamma correlations for Tests 2 and 3 [Fs = (1, 14) = 10.3 and 8.3, respectively]. Mean correlation values were significantly smaller in the ZOL condition relative to PL, suggesting impaired metamemory performance, independent of phase (i.e., tolerance did not develop).
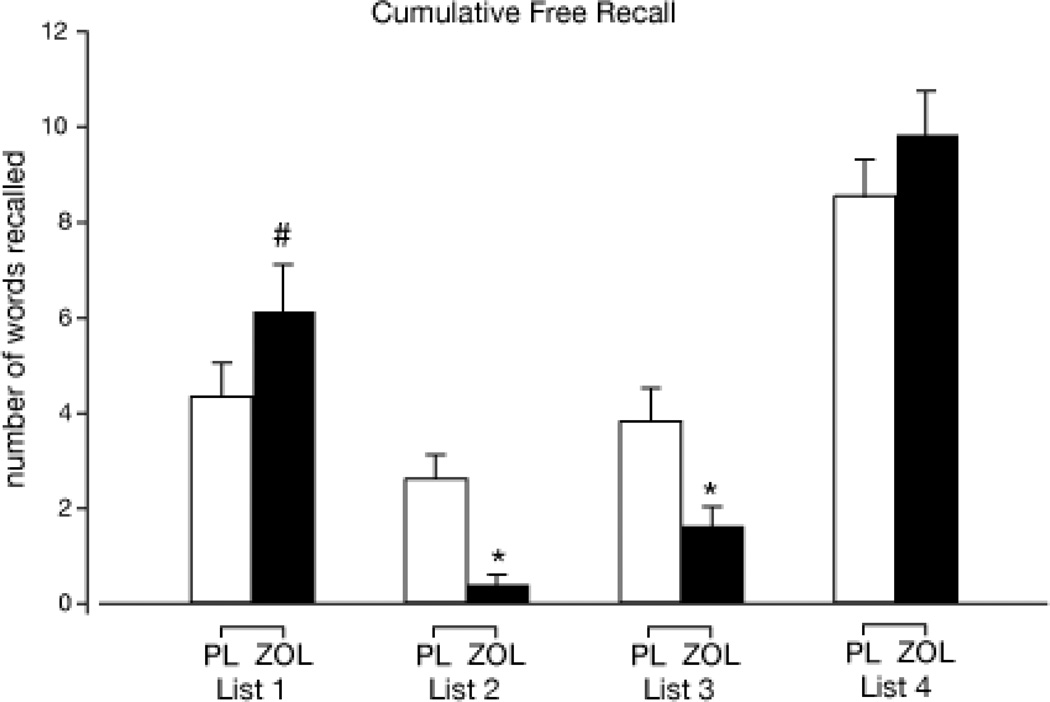
Impairment of free recall was observed for Tests 2 and 3 as well [F values (1, 14) 17.5 and 9.2, respectively; see Table 2). Number of words recalled was significantly lower in the ZOL condition relative to PL, independent of phase, suggesting that tolerance did not develop to the ZOL-related memory impairments on this outcome. For the cumulative free recall test (Test 5; next morning battery; see Table 1), a significant interaction of drug condition and list (1, 2, 3, or 4) was observed [F (3, 42) = 10.1; see Figure 3]. Simple effects tests revealed that performance was significantly worse in the ZOL condition relative to PL for lists 2 and 3 (study at peak for both), but not different between drug conditions for list 4 (study next morning). A simple effects test with a trend towards significance (p = .06) was observed for list 1 (study pre-drug) such that the number of words recalled from this list was greater in the ZOL condition relative to PL, suggestive of retrograde memory facilitation.
Figure 3.
Cumulative free recall (Test 5; next morning battery) (mean + 1 SE) as a function of drug condition and study list. The asterisk (*) indicates a significant difference from PL within the respective list and the pound sign (#) represents a trend towards significance (p = .06) between PL and ZOL.
Psychomotor
A significant main effect of drug condition was observed for balance [F (1,14) = 74.2]. Balance was significantly impaired in the ZOL condition, independent of phase, suggesting tolerance did not develop to ZOL’s impairing effects (see Table 2). In addition, a significant phase by drug condition interaction [F (1,14) = 5.4] was observed for circular lights, and simple effects tests revealed that performance was lower in the ZOL condition relative to PL in the Baseline phase (see Table 2); performance did not differ significantly between ZOL/Baseline phase and ZOL/Chronic phase suggesting that, similar to balance, tolerance did not develop to ZOL’s effect on circular lights. In addition, inspection of the means revealed that the number of correct responses for circular lights was lower in PL/Chronic relative to PL/Baseline, suggesting a possible acute abstinence effect following ZOL discontinuation (see Table 2) [although the simple effects test (p = 0.04) for PL/Baseline vs. PL/Chronic was not significant with the Bonferroni corrected alpha level of 0.0125].
Attention
DSST
Significant main effects of drug condition were observed for number attempted and proportion correct on the DSST [F (1,14) = 82.2 and 15.4, respectively]. For both outcomes, peak values were significantly lower in the ZOL condition relative to PL, independent of phase, suggesting that tolerance did not develop to these acute impairing effects of ZOL (see Table 2). The main effect for number attempted was qualified by a significant phase by drug condition interaction [F (1,14) = 9.4; Table 2]; although simple effects tests revealed no significant differences among means, a trend towards significance (p = 0.04; not significant after Bonferroni correction) indicated that mean number attempted was lower in the PL/Chronic phase relative to PL/Baseline phase condition suggesting a possible acute abstinence effect, as observed for circular lights.
Divided attention
Both divided attention outcomes, accuracy (tracking portion of task) and reaction time (monitoring portion of task), included significant main effects of drug condition: performance in the ZOL condition was significantly impaired relative to PL and these effects did not differ as a function of phase (i.e., tolerance did not develop; see Figure 1 and Table 2).
Working Memory
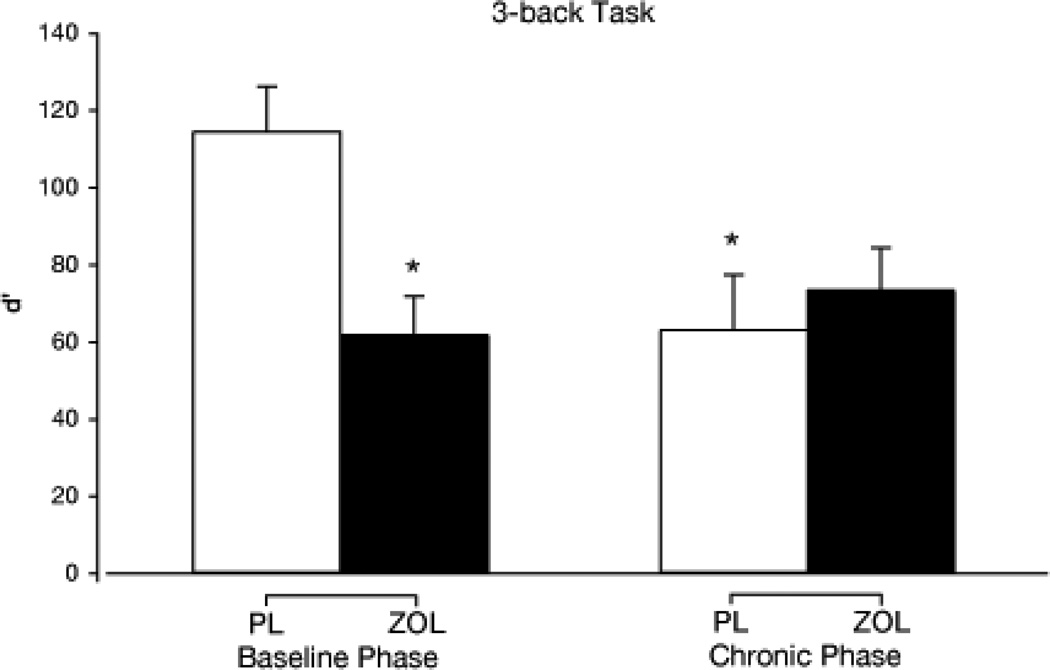
N-back task
A significant phase by drug condition by task condition interaction was observed for d’ [discrimination between letters shown N-back in the series relative to letters not N-back; F (1,14) = 4.6] with the 0-back condition entered as a covariate. Simple effects tests revealed that d’ on the 3-back task was not significantly different between ZOL conditions (Baseline v. Chronic) suggesting that tolerance did not develop to this impairment. Mean d’ was significantly lower in the ZOL condition relative to PL in the Baseline phase but not in the Chronic phase, and was significantly lower in the PL/Chronic phase relative to PL/Baseline phase, suggesting possible acute abstinence effects after ZOL discontinuation (see Figure 2). In contrast, simple effects tests did not reveal significant differences among means for d’ for the 1- or 2-back tasks. A drug condition by task condition interaction was also observed for median response time. Simple effects tests revealed that response time was longer in the ZOL condition relative to PL on the 1- and 2-back tasks. The lack of an interaction with phase suggests that tolerance did not develop to the slowing effects of ZOL. There were no significant simple effects tests for 3-back indicating that ZOL did not slow response time for this condition.
Figure 2.
3-back working memory d’ at peak time point (% pre-drug; mean +1 SE) as a function of drug condition and phase. The asterisk (*) indicates a significant difference from the PL baseline phase condition.
Modified Sternberg task
A significant main effect of drug condition was observed for median response time [F (1, 14) = 5.0], but not proportion correct [F (1, 14) = 1.7] on the Sternberg task with the non-memory control condition entered as a covariate (see Table 2). Independent of phase and collapsed across delay condition, median response time was significantly higher (i.e., slower) for ZOL relative to PL. As observed for the N-back task, these findings suggest that tolerance did not develop to the slowing effects of ZOL.
Performance Estimation
Although performance estimates were collected for DSST, N-back, Sternberg, and recognition memory, performance estimate data from the N-back and Sternberg tasks will not be presented due to the absence of significant ZOL-induced impairment in the corresponding ‘actual’ performance measures across task conditions (i.e., accuracy was not impaired across task conditions in the N-back and Sternberg tasks; see above). For recognition memory, performance estimate data will not be presented from Test 3 because the longer retention interval for Test 3 (overnight) relative to pre-drug (Test 1; 60 min) makes the use of percentage of predrug scores in the calculation of performance estimate values (see Outcome Measures section, above) inappropriate.
Main effects of drug condition were observed for DSST speed (participant estimate of speed minus actual number attempted) and recognition memory performance (participant estimate of accuracy minus d’) Test 2 [Fs (1, 14) = 30.4 and 38.4, respectively], but not for DSST proportion correct [participant estimate of accuracy minus actual proportion correct; F (1,14) = 2.3]. For both significant performance estimate outcomes, mean values (collapsed across time of rating) were significantly larger in the ZOL condition relative to PL (see Table 2) indicating that performance was overestimated relative to actual performance (i.e., over confidence in the ZOL condition).
Subjective
A significant main effect of drug condition was observed for ratings of several subjective states (e.g., feel drug effect, feel good effects, difficulty concentrating; data not shown) with higher values always in the ZOL condition relative to PL. These effects were independent of phase (i.e., Baseline v. Chronic), suggesting tolerance did not develop to the subjective effects of ZOL.
Polysomnographic sleep recordings
For sleep period 1 (the period from initial lights out to lights on prior to the peak battery), sleep efficiency was increased and percent Stage 1 sleep decreased in the ZOL condition relative to PL [main effects of drug condition: sleep efficiency, F (1, 14) = 4.9 and percent Stage 1 sleep, F (1, 14) = 9.1; see Table 3]. Numerically, % sleep in other sleep stages was larger in the ZOL condition relative to PL, thus accounting for a decrease in Stage 1 sleep and suggesting a shift towards increases in deeper sleep (as would be expected with ZOL). In addition, a main effect of phase was observed for percent Stage 2 sleep [F (1, 14) = 21.8] such that more Stage 2 sleep took place in the Chronic phase (M = 47.0, SE = 4.3) relative to the Baseline phase (M = 38.4, SE = 3.8), independent of drug condition. There was also a trend towards significance for phase by drug condition interactions for wake after sleep onset, % Stage 2 sleep, and % SWS (Fs (1, 14) ≤ 4.4, ps ≤ .08; see Table 3). The pattern of means indicated that wake after sleep onset and % Stage 2 sleep were increased, and % SWS was decreased in the PL/Chronic condition relative to PL/Baseline, suggesting possible acute abstinence effects following ZOL discontinuation.
Overall, for sleep period 2 (the period from lights out after the peak battery to lights on the next morning), expected ZOL-related changes in sleep parameters were observed (i.e., decreased initial sleep latency and % Stage 1 sleep; increased % Stage 2 sleep) as reflected in main effects of drug condition [sleep latency, F (1, 14) = 8.1; % Stage 1 sleep, F (1, 14) = 6.1; % Stage 2 sleep, F (1, 14) = 8.8; see Table 3]. In addition, a trend towards significance was observed for the main effect of drug condition [F (1, 14) = 4.5), p = 0.053] for sleep efficiency with larger mean values in the ZOL condition relative to PL (see Table 3), as observed for sleep period 1 (see above). Lastly, a main effect of phase was observed for % SWS [F (1, 14) = 6.5] such that mean values were lower in the Chronic phase relative to the Baseline phase.
Discussion
The present study examined whether tolerance develops to the acute performance-impairing effects of ZOL after repeated nightly dosing across a wide range of outcomes. As expected, bedtime administration of ZOL was associated with changes in sleep architecture (e.g., increased sleep efficiency and % Stage 2 sleep; decreased % Stage 1 sleep and initial sleep latency), as well as impairment across all performance domains during nighttime testing (psychomotor function, attention, working memory, episodic memory) with no residual/next morning impairment (as reported elsewhere: DeClerk & Bisserbe; 1998; Mintzer et al., 1997; 1999a; Otmani et al., 2008; Stoops & Rush, 2003; Verster et al., 2002). To our knowledge, this is the first study to test performance during forced middle of the night awakening following bedtime ZOL administration in young healthy adults; we confirmed that performance impairments previously observed in daytime laboratory studies are also observed under these clinically relevant conditions. Importantly, tolerance did not develop to any of the performance impairments observed in the present study after 22–30 days of nightly dosing. This lack of tolerance is striking as it spanned a wide range of performance domains, as well as physiological and subjective measures. The degree to which tolerance might develop to these effects after longer periods of time (e.g., months or years) is unknown.
The use of a complex battery of cognitive assessments in the present study allows for the examination of effects of ZOL on specific cognitive processes. First, we observed that ZOL impaired encoding on the episodic memory tasks (impairment on Tests 2 and 3 and Test 5/cumulative free recall for Lists 2 & 3; see Table 2 and Figure 3), replicating previous findings (e.g., Danjou et al., 1999; Mintzer et al., 1997; 1999a; Otmani et al., 2008; Verster et al., 2002). Second, we also replicated previous work (Fillmore, Kelly, Rush, & Hays, 2001; Hinrichs et al., 1984; Mintzer & Griffiths, 1999a) demonstrating ZOL-related retrograde facilitation; more words were recalled in the ZOL condition relative to PL from List 1 (studied during the predrug battery) during Test 5/cumulative free recall (although only a trend towards significance was observed for this effect).
An additional novel finding in the present study was the observation that metacognition (i.e., participants’ awareness of their performance) was disrupted by ZOL such that recognition memory gamma correlations (relationship between confidence and accuracy) were significantly lower in the ZOL condition relative to PL for both Tests 2 (study/test peak) and 3 (study peak/test next morning). In addition, volunteers were overconfident when rating their performance on the recognition memory task and on the DSST (performance estimate outcomes). In combination, these metacognitive findings suggest that volunteers were not attuned to the magnitude of their performance impairments, including when their memory was tested the following morning for words studied during nighttime awakening (recognition memory Test 3). These findings are consistent with Stoops and Rush (2003) observation that participant ratings of performance increased after multiple ZOL exposures despite actual performance remaining impaired across time (i.e., overconfidence in performance). Furthermore, findings correspond to previous research suggesting that benzodiazepines produce overconfidence in performance (Bacon et al., 1998; Massin-Krauss, Bacon, & Danion, 2002; Mintzer & Griffiths, 2003; Roache et al., 1993; Roache & Griffiths, 1985, 1987). Interestingly, participants were also more conservative in their responding on the recognition memory task the morning after drug administration (Test 3; C/response bias) such that they were more likely to respond “new” in the ZOL condition relative to PL. The adoption of a more conservative response bias during Test 3 suggests that volunteers may have had some awareness that their nighttime performance was impaired, though the metacognitive data suggest that this awareness was not enough to permit accurate assessments of objective performance.
Importantly, the observed ZOL-related impairments could significantly impact safety if the reason for nighttime awakening required proper functioning (e.g., responding to an emergency). Furthermore, concerns over the safe use of ZOL among individuals that work in critical life or death situations, such as military personnel (e.g., pilots) and emergency medical personnel, have been noted and are particularly relevant to the present findings as these jobs often require proper functioning during unexpected middle of the night awakening (Caldwell & Caldwell, 2005; McBeth et al., 2009; Schultz & Miller, 2004). The problematic nature of these impairments is further highlighted by findings from episodic memory outcomes. First, significantly impaired memory for lists studied during nighttime awakening (Lists 2 and 3 as reflected in impairment on Tests 2 and 3 and Test 5/cumulative free recall for Lists 2 & 3; see Table 2 and Figure 3) in the ZOL condition may be considered a laboratory equivalent of recent case reports of individuals completing complex behaviors following bedtime hypotic use without recollection of such behaviors the following morning (Gustavsen et al., 2008; Siddigui et al., 2008). Test 3 and Test 5 are particularly relevant to these reports given that memory was tested the following morning after the drug effects had subsided. Second, we observed that ZOL significantly increased false alarm rate on the recognition memory task during Test 2 (study and test during nighttime awakening). This finding suggests that ZOL not only impairs accuracy, but also might increase impulsivity during the period of peak effects as false alarms/commission errors have been previously correlated with increased impulsivity (Dougherty et al., 1999). ZOL-related increases in impulsivity, as well as impairments in metacogntion (described above), increase the likelihood that individuals under the influence of ZOL could place themselves in risky or dangerous situations (e.g., driving a car). Future research aimed at exploring these impairments with more refined tasks (e.g., driving simulator; standardized measures of impulsivity) would be valuable given such safety concerns.
An additional point to be highlighted with regards to patient safety is that the magnitude of ZOL-related impairments on the balance task in the present study are on par, if not worse, than impairments observed in a separate study conducted in our laboratory using an identical balance task with a dose of alcohol (0.8 g/kg; ingested in a 10 min window) that yielded a mean peak breath alcohol level above the legal limit for driving (BAL = 0.096; Kleykamp et al., 2010). In that study, balance performance after alcohol was reduced by 63.9% relative to a pre-drug assessment. Similarly, in the present study, balance performance was reduced by 69.5% and 64.0% in each of the ZOL conditions (Baseline and Chronic, respectively) relative to a pre-drug assessment. Thus, the degree of performance impairment associated with ZOL during middle of the night awakening is not negligible and might be similar to what an individual would experience after drinking 3–4 alcohol beverages within a relatively brief period of time. Impaired balance is a particularly salient issue with older adults who may be more prone to fall during routine activities such as getting out of bed or walking down stairs during middle of the night awakening. Indeed, reports suggest that ZOL and other benzodiazepine receptor agonists are associated with increased falls and hip fractures in older adults (Allain, Bentue-Ferrer, Polard, Akwa, & Patat, 2005; Rhalimi, Helou, & Jaecker, 2009).
A secondary outcome of interest in the present study was the extent to which volunteers experienced abstinence/withdrawal effects on the first night of medication discontinuation (Session 4; PL/Chronic). Although we observed evidence of acute abstinence effects for some performance outcomes (i.e., worse performance in the PL/Chronic phase relative to the PL/Baseline phase on circular lights, DSST attempted, and 3-back outcomes), these findings were not robust (with only trends towards significance observed for circular lights and DSST). Nevertheless, the detection of any performance withdrawal effects is novel as we are aware of no research to date that has examined the acute withdrawal effects of ZOL on performance outcomes. Further, it is possible that the magnitude of these effects might increase with higher doses or more extended use (e.g., multiple months or years of nightly administration). In addition to performance, time awake after sleep onset and % Stage 2 sleep (sleep period 1) were higher, while mean values for % SWS (sleep period 1) were lower in the PL/Chronic phase relative to the PL/Baseline phase, again suggesting possible abstinence effects. However, because the study used a fixed order design the possibility exists that any differences between PL conditions for sleep outcomes could be due to time and experience with the study protocol. For example, the increase in wake after sleep onset observed in Session 4 (PL/Chronic) during the first sleep period could be explained by participants being restless or “on alert” for forced awakening due to experience in previous sessions. This explanation for the wake after sleep onset findings is reinforced by the absence of a similar effect in the second sleep period when participants were not anticipating a forced awakening. In contrast to sleep outcomes, the fixed session order and potential influence of task experience was likely not a concern for performance outcomes as performance data were analyzed relative to pre-drug performance within each experimental session. Overall, these data suggest that the discontinuation effects of ZOL warrant further study given that patients might continue to take ZOL despite wanting to stop use, in an effort to avoid abstinence effects.
There are additional methodological limitations associated with the present study. First, the study design did not include objective verification of ZOL exposure during the course of the study (e.g., blood levels of ZOL) to confirm adherence to at-home medication ingestion. Despite the absence of biochemical verification, several precautions were taken to guard against noncompliance, including asking participants to: 1) report medication administration daily on a voicemail system, 2) report to the laboratory weekly to pick up medication and drop off old packages, and 3) submit urine/BAL samples for testing at all visits for other psychoactive substances (all samples were negative). Given that there were relatively few instances of missed calls/doses (see At-Home Dosing Compliance) and that participants returned the unused capsules after missing a dose in every instance, we believe our procedures for enforcing compliance were effective. An additional methodological concern in the present study is our homogenous sample of young male participants with normal sleep patterns. Although we feel this constraint was necessary to provide control over variables known to influence the effects of ZOL (e.g., sex hormones, age; Cubala et al., 2010; Olubodun et al., 2003), we also note that this constraint limits the generalizability of our study findings, especially for populations that are most likely to be prescribed ZOL (e.g., insomnia patients, older adults; Rhalimi, Helou, Jaecker, 2009).
In sum, the present study demonstrates that the acute impairing effects of ZOL are also present during nighttime awakening across a range of performance outcomes, including metacognitive outcomes, and that tolerance does not develop to such impairments after a month of nightly use. A tentative finding in the present study was the presence of acute abstinence effects associated with cessation of ZOL use, although these findings warrant further study. Thus, clinicians who prescribe ZOL might consider informing patients of the magnitude and persistence of these ZOL-related impairments across time given the possibility that patients could incorrectly assume that the absence of a treatment duration limit for ZOL extended-release indicates that impairments would subside over time.
Acknowledgements
This project was supported by National Institute on Drug Abuse grants DA-11936 and DA-07209, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR-054871, and grant M01RR-02719 from the Clinical Research Unit Program of the National Center of Research Resources, National Institutes of Health.
The authors thank Crystal Barnhouser, Jared Saletin, and Jenna Cohen for protocol management and technical assistance, John Yingling for computer programming assistance and technical support, and Paul Nuzzo for assistance with the data analysis.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha
Disclosures
All authors contributed in a significant way to this manuscript and all authors have read and approved the final manuscript.
None of the authors have any conflicts of interest to report.
Portions of these data were presented at the 72nd annual meeting of the College on Problems of Drug Dependence.
Contributor Information
Bethea A. Kleykamp, National Institutes of Health, National Institute on Drug Abuse
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Una D. McCann, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Michael T. Smith, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Miriam Z. Mintzer, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
References
- Allain H, Bentué-Ferrer D, Polard E, Akwa Y, Patat A. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: a comparative review. Drugs Aging. 2005;22(9):749–765. doi: 10.2165/00002512-200522090-00004. [DOI] [PubMed] [Google Scholar]
- Berlin I, Warot D, Hergueta T, Molinier P, Bagot C, Puech AJ. Comparison of the effects of zolpidem and triazolam on memory functions, psychomotor performance, and postural sway in healthy subjects. Journal of Clinical Psychopharmacoly. 1993;13(2):100–106. [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. Journal of Psychosom Research. 2004;56(2):239–243. doi: 10.1016/S0022-3999(03)00067-9. [DOI] [PubMed] [Google Scholar]
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18(1):37–48. doi: 10.2165/00023210-200418010-00004. [DOI] [PubMed] [Google Scholar]
- Bacon E, Danion JM, Kauffmann-Muller F, Schelstraete MA, Bruant A, Sellal F, Grange D. Confidence level and feeling of knowing for episodic and semantic memory: an investigation of lorazepam effects on metamemory. Psychopharmacology. 1998;138:318–325. doi: 10.1007/s002130050677. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Caldwell JL. Fatigue in military aviation: An overview of U.S. Military-Approved Pharmacological Countermeasures. Aviation, Space, and Environmental Medicine. 2005;76(7):C39–C51. [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology. 2009;206(1):141–154. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Collins R, Fletcher S, Kee SC, Woods B, Iliffe S. Older adults and withdrawal from benzodiazepine hypnotics in general practice: Effects on cognitive function, sleep, mood and quality of life. Psychological Medicine. 2003;33(7):1223–1237. doi: 10.1017/s0033291703008213. [DOI] [PubMed] [Google Scholar]
- Cubała WJ, Wiglusz M, Burkiewicz A, Gałuszko-Wegielnik M. Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as a differentiating factor. Eur J Clin Pharmacol. 2010;66(9):955. doi: 10.1007/s00228-010-0854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Bond A, O’Sullivan G, Bruce M, Marks I, Lelliot P, Shine P, Lader M. Memory functions, alprazolam and exposure therapy: a controlled longitudinal study of patients with agoraphobia and panic disorder. Psychological Medicine. 1994;24:969–976. doi: 10.1017/s0033291700029056. [DOI] [PubMed] [Google Scholar]
- Danjou P, Paty I, Fruncillo R, Worthington P, Unruh M, Cevallos W, Martin P. A comparison of the residual effects of zaleplon and zolpidem following administration 5 to 2 h before awakening. British Journal of Clinical Pharmacology. 1999;48(3):367–374. doi: 10.1046/j.1365-2125.1999.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClerk AC, Bisserbe JC. Short-term safety profile of zolpidem: objective measures of cognitive effects. European Journal of Psychiatry. 1998;12 Suppl 1:15s–20s. doi: 10.1016/s0924-9338(97)80016-8. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Moeeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increased commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23(8):1342–1351. [PubMed] [Google Scholar]
- Fillmore MT, Kelly TH, Rush CR, Hays L. Retrograde facilitation of memory by triazolam: effects on automatic processes. Psychopharmacology. 2001;158(3):314–321. doi: 10.1007/s002130100873. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Mewaldt SP, Berie JL, Hinrichs V. Memory and performance effects of single and 3 week administration of diazepam. Psychopharmacology. 1981;73:147–151. doi: 10.1007/BF00429206. [DOI] [PubMed] [Google Scholar]
- Gustavsen I, Bramness JG, Skurtveit S, Engeland A, Neutel I, Mørland J. Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam. Sleep Medicine. 2008;9(8):818–822. doi: 10.1016/j.sleep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross classifications. Journal of the American Statistical Association. 1954;49:732–764. [Google Scholar]
- Hinrichs JV, Ghoneim MM, Mewaldt SP. Diazepam and memory: retrograde facilitation produced by interference reduction. Psychopharmacology. 1984;84(2):158–162. doi: 10.1007/BF00427439. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Patat A, Stanley N, Paty I, Rigney U. Residual effects of zaleplon and zolpidem following middle of the night administration five hours to one hour before awakening. Human Psychopharmacology. 2001;16(2):159–167. doi: 10.1002/hup.282. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuorscience. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Experimental and Clinical Psychopharmacology. 2010;18(1):1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Loube DL, Owens J, Pancer JP, Wise M. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- Massin-Krauss M, Bacon E, Danion JM. Effects of the benzodiazepine lorazepam on monitoring and control processes in semantic memory. Consciousness and Cognition. 2002;11(1):123–137. doi: 10.1006/ccog.2001.0538. [DOI] [PubMed] [Google Scholar]
- McBeth BD, McNamara RM, Ankel FK, Mason EJ, Ling LJ, Flottemesch TJ, Asplin BR. Modafinil and zolpidem use by emergency medicine residents. Acad Emerg Med. 2009;16(12):1311–1317. doi: 10.1111/j.1553-2712.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavioral Research Methods and Instruments. 1982;14:463–466. [Google Scholar]
- Mintzer MZ, Allen RP, Griffiths RR. Investigation of preference for nightly triazolam versus placebo in moderate social alcohol drinkers. Journal of Psychopharmacology. 2001;15:3–8. doi: 10.1177/026988110101500101. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: A comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behavioural Pharmacology. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. Journal of Psychopharmacology. 1999a;13:18–31. doi: 10.1177/026988119901300103. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Triazolam and zolpidem: Effects on human memory and attentional processes. Psychopharmacology. 1999b;144:8–19. doi: 10.1007/s002130050971. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: A single-dose comparison of effects on human memory and attentional processes. Experimental and Clinical Psychopharmacology. 2003;11(1):56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Drugs, memory, and metamemory: a dose-effect study with lorazepam and scopolamine. Exp Clin Psychopharm. 2005;13(4):336–347. doi: 10.1037/1064-1297.13.4.336. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 2002;67(1):41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Otmani S, Demazières A, Staner C, Jacob N, Nir T, Zisapel N, Staner L. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Human Psychopharmacology. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- Olubodun JO, Ochs HR, von Moltke LL, Roubenoff R, Hesse LM, Harmatz JS, Shader RI, Greenblatt DJ. Pharmacokinetic properties of zolpidem in elderly and young adults: possible modulation by testosterone in men. Br J Clin Pharmacol. 2003;56(3):297–304. doi: 10.1046/j.0306-5251.2003.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physicians Desk Reference. 57 edition. Montvale: Thomson PDR; 2003. [Google Scholar]
- Rhalimi M, Helou R, Jaecker P. Medication use and increased risk of falls in hospitalized elderly patients: a retrospective, case-control study. Drugs Aging. 2009;26(10):847–852. doi: 10.2165/11317610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Roache JD, Cherek DR, Bennett RH, Schenkler JC, Cowan KA. Differential effects of triazolam and ethanol on awareness, memory, and psychomotor performance. Journal of Clinical Psychopharmacology. 1993;13:3–15. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Comparison of triazolam and pentobarbital: performance impairment, subjective effects and abuse liability. Journal of Pharmacology and Experimental Therapeutics. 1985;234(1):120–133. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Lorazepam and meprobamate dose effects in humans: Behavioral effects and abuse liability. The Journal of Pharmacology and Experimental Therapeutics. 1987;243:978–988. [PubMed] [Google Scholar]
- Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18(1):49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- Schultz D, Miller J. Fatigue and use of GO/NO GO pills in F-16 pilots subjected to extraordinarily long combat sorties. Human effectiveness directorate, Brooks city Base. 2004 AFRL Report No AFRL-HE-BR-TR-2004- 0014.
- Siddiqui F, Osuna E, Chokroverty S. Writing emails as part of sleepwalking after increase in Zolpidem. Sleep Med. 2009;10(2):262–264. doi: 10.1016/j.sleep.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Differential effects in humans after repeated administrations of zolpidem and triazolam. The American Journal of Drug and Alcohol Abuse. 2003;29(2):281–299. doi: 10.1081/ada-120020513. [DOI] [PubMed] [Google Scholar]
- Tata PR, Rollings J, Collins M, Pickering A, Jacobson RR. Lack of cognitive recovery following withdrawal from long-term benzodiazepine use. Psychological Medicine. 1994;24:203–213. doi: 10.1017/s0033291700026969. [DOI] [PubMed] [Google Scholar]
- Thorndike EL, Lorge I. The Teacher’s Word Book of 30, 000 Words. New York: Teacher’s College, Columbia University; 1944. [Google Scholar]
- Verster JC, Volkerts ER, Schreuder AH, Eijken EJ, van Heuckelum JH, Veldhuijzen DS, Verbaten MN, Paty I, Darwish M, Danjou P, Patat A. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. Journal of Clinical Psychopharmacology. 2002;22(6):576–583. doi: 10.1097/00004714-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R manual. New York: Psychological Corporation; 1981. [Google Scholar]