Abstract
Continuously erupting teeth have associated with them a continuously regenerating periodontal ligament, but the factors that control this amazing regenerative potential are unknown. We used genetic strategies to show that the periodontal ligament arises from the cranial neural crest. Despite their histological similarity, the periodontal ligament of continuously erupting incisor teeth differs dramatically from the periodontal ligament of molar teeth. The most notable difference was in the distribution of Wnt responsive cells in the incisor periodontal ligament, which coincided with regions of periodontal ligament cell proliferation. We discuss these findings in the context of dental tissue regeneration.
Keywords: bone, periodontal ligament, tooth, Wnt, regeneration, incisor, molar
Introduction
For centuries, biologists have been fascinated by the dentition. Not only are teeth a good model system for studying patterning and morphogenesis during embryonic development (Miletich and Sharpe, 2003), they also provide a window into evolution. For example, the open-rooted dentition of Fruitafossor windscheffeli, a chipmunk-sized mammal from the Jurassic period, is shared by present day armadillos and rodents (Luo and Wible, 2005). The wide opening of the roots allows for the rapid replacement of dentin, and demonstrates that the animal's teeth grew continuously throughout life (Luo and Wible, 2005). This continuous eruption is made possible by the development of a periodontium.
The periodontium is composed of three tissues: the cementum which covers the tooth root; the alveolar bone which surrounds and supports the tooth root; and the periodontal ligament which spans these two hard tissues and functions as a kind of hammock in which the tooth is nestled (Noble, 1969). Not all teeth have a periodontium, however. The extinct reptile Captorhinus aguti had teeth that were ankylosed into a deep socket with no intervening space for a periodontal ligament, or any other soft tissue (Bolt and Demar, 1975). Likewise, the teeth of most teleosts, amphibians, and reptiles attach to the alveolar bone via ankylosis (McIntosh et al., 2002). This arrangement, it has been noted, is incompatible with the continuous growth of teeth (Edmund, 1960).
Despite a strong association between a functional periodontium and tooth eruption, there is very little known about how the periodontal ligament actually regulates the process at a mechanistic or a molecular level (Berkovitz and Thomas, 1969; Wise et al., 2002). Removal of the dental follicle, from which the periodontal ligament arises, halts tooth eruption (Cahill and Marks, 1980; Marks and Cahill, 1984). Precisely why this results in a cessation of tooth eruption is not known; removal of the dental follicle might cause ankylosis but it is also possible that the dental follicle serves as a source of molecular or mechanical signals that instigate tooth eruption. There is some data to suggest that the periodontal ligament generates mechanical forces that “push” the tooth into the oral cavity, but this idea has been discounted in recent years (Wise and King, 2008). Another theory proposes that changes in the pattern of collagen cross-linking within the periodontal ligament contribute to tooth eruption (Taverne, 1993).
Continuously erupting teeth have associated with them a continuously regenerating periodontal ligament (Melcher, 1967; Klodzinski, 1978; Beertsen, 1979). The periodontal ligament remodels normally (Silva et al., 2004) and appears to do so through a pluripotent cell population that gives rise to cementoblasts, osteoblasts, and periodontal fibroblasts (Amar and Chung, 1994; Lin et al., 2008). The embryonic origin(s) of these periodontal progenitor cells, and the molecular mechanisms that regulate their postnatal proliferation and differentiation are unknown. To address these questions, we undertook a study comparing the periodontal ligament of continually erupting adult incisor teeth with the periodontal ligament of slowly erupting molar teeth. We chose to evaluate these different periodontia in mice because of the availability of numerous transgenic lines, the abundance of molecular and cellular reagents, and the fact that their incisors erupt continuously but their molars do not (Marks and Schroeder, 1996; Melcher, 1967). In fact, the mouse incisors, unlike the molars, grow at an astonishing rate of ~0.3mm/day (Zegarelli, 1944). Thus, we compared the periodontium surrounding the incisors and molars within each individual animal to effectively eliminate inter- and intra-species variations. In doing so, we gained new insights into the mechanisms that regulate continual regeneration of the mammalian periodontal ligament, which may provide clues into therapeutic strategies to regenerate diseased periodontal tissues (Foster and Somerman, 2005).
Results
Organization of incisor and molar periodontal ligaments are similar
Murine incisors erupt continuously throughout life while their molar counterparts do not (Melcher, 1967). Therefore, we evaluated the periodontal ligaments around both types of teeth for differences in their organization that might be associated with these tooth-specific eruption rates. Histological analyses, however, failed to reveal any notable changes in the general architecture of the two periodontal ligaments (Fig. 1A, B). Picrosirius red staining is one method for assessing the organization of collagen fibers: Under polarized light, well-aligned fibrillar collagen appears red whereas unaligned or immature collagen appears green (Whittaker et al., 1994). We evaluated picrosirius red staining and found that incisor and molar periodontal ligaments exhibited the same general patterns of birefringence (Fig. 1C, D). Thus, the dramatic difference in eruption rates of the incisor and molar were not reflected by an overt histologic distinction nor in a difference of collagen organization within their associated periodontal ligaments.
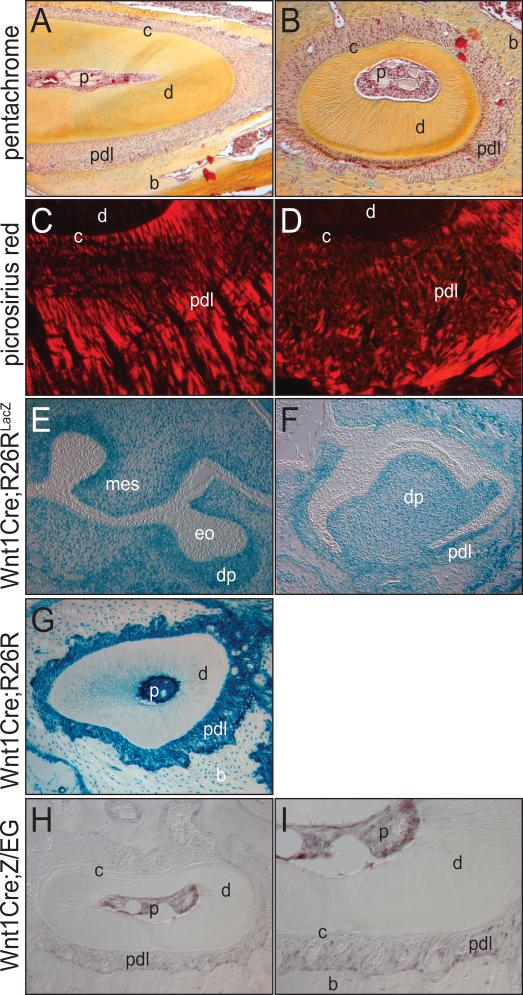
Figure 1.
Histology, collagen distribution and embryonic origin of the periodontal ligament. (A) Incisor, pentachrome histology. (B) Molar root, pentachrome histology. (C) Collagen layout of incisor periodontal ligament (PDL) shown through picrosirius red. (D) Collagen organization in molar PDL shown through picrosirius red staining and polarized light. (E) e13.5 Wnt1Cre;R26RLacZ molar tooth bud, showing Xgal staining in dental mesenchyme. (F) e17.5 Wnt1Cre;R26RLacZ molar tooth development where Xgal staining indicates distribution of neural crest cells. (G) Adult Wnt1Cre;R26RLacZ molar tissue stained with Xgal demonstrates the PDL arises from neural crest. (H, I) GFP expression in the molar of Wnt1Cre;Z/EG mice. p, pulp; c, cementum; d, dentin; pdl, periodontal ligament; b, bone; mes, mesenchyme; eo, enamel organ; dp, dental papilla.
The periodontium is derived from the neural crest
We next examined the embryonic origins of the periodontium in an effort to understand more about the early development of the periodontal ligament. The craniofacial complex has a dual embryonic origin: most of the facial bones and cartilages are derived from cranial neural crest while the mesoderm contributes to more posterior regions of the pharyngeal skeleton and skull, and to most of the facial muscles (Le Douarin, 1973; Le Douarin et al., 2004). These fate maps were established using avian embryos as a model system, which precluded investigation into the embryonic origins of the periodontium. We used a genetic approach to label neural crest derivatives by crossing Wnt1-Cre mice (Jiang et al., 2000) with R26R mice to produce Wnt1Cre;R26RLacZ embryos. Wnt1Cre drives the expression of Cre recombinase in derivatives of the neural crest, while R26R mice express the LacZ gene under transcriptional control of the ubiquitously expressed ROSA26 locus (Soriano, 1999). Thus, in Wnt1Cre;R26R mice the pattern of beta galactosidase activity reflects the distribution of neural crest cells.
We examined embryos at a stage when the future periodontium was first detectable. At e13.5, highly specific Xgal staining was apparent in the mesenchyme surrounding the developing molar tooth bud but was absent from the enamel organ (Fig. 1E), consistent with its ectodermal origin (Sharpe and Ferguson, 1988). On the other hand, the dental papilla, which gives rise to the pulp, was Xgal positive (Fig. 1E), in agreement with its neural crest origin (Slavkin et al., 1988). All of the mesenchyme surrounding the tooth bud, some of which will contribute to the periodontium, was also Xgal positive (Fig. 1E).
We next examined the developing molar tooth bud at e17.5, when the dental follicle is evident (Fig. 1F). The dental follicle gives rise to the periodontal ligament (Noble, 1969) and at this stage of development both the dental papilla and the future periodontal ligament exhibited strong beta galactosidase activity (Fig. 1F). We also examined the molar periodontium in adult Wnt1Cre;R26R mice and found that alveolar bone and dental pulp cells and the periodontal ligament retained the strong beta galactosidase activity (Fig. 1G). These data strongly indicate that the periodontium originates from the neural crest.
We used a second genetic strategy to confirm the neural crest origin of the periodontium. In this case, floxed Z/EG mice (Novak et al., 2000) were crossed with Wnt1Cre mice to produce Wnt1Cre;Z/EG embryos. This strategy results in GFP labeling of Wnt1-expressing neural crest derivatives while their cell types, including mesodermal derivatives, express beta galactosidase (Leucht et al., 2008a). We performed GFP immunostaining and found that the dental pulp and the periodontal ligament were both immunopositive (Fig. 1H, I). Taken together, both genetic labeling strategies indicate that the periodontal ligament is derived from neural crest mesenchyme, and does not originate from cells of an ectodermal origin.
THE DISTRIBUTION OF PROLIFERATING AND DIFFERENTIATING CELLS DIFFER BETWEEN INCISOR AND MOLAR PERIODONTAL LIGAMENTS
Rodent incisors continuously erupt, which necessitates that the periodontal ligament must continually remodel to accommodate this function (Marks and Schroeder, 1996). We reasoned that the molar periodontal ligament would be relatively static due to the lack of molar eruption. We first assessed the distribution of alkaline phosphatase activity as a marker of differentiation (Anderson, 1984). The incisor periodontal ligament showed a gradation in alkaline phosphatase activity with the highest level of activity near the alveolar bone and the lowest level of activity close to the cementum (Fig. 2A, 3G, H). The molar periodontal ligament, on the other hand, exhibited equivalent levels of alkaline phosphatase activity across the entire periodontal ligament (Fig. 2B, 3I).
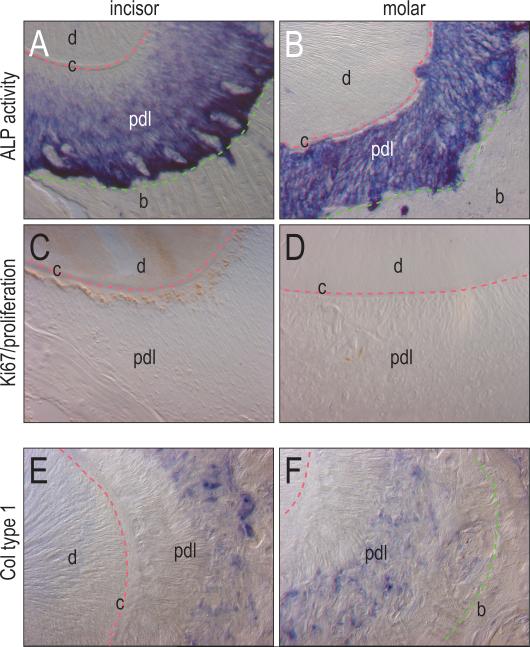
Figure 2.
Differential distribution of proliferation and differentiation within the PDL of incisors and molars. (A) Alkaline phosphatase activity is present in the PDL of the incisor with a gradient of minimal activity next to the cementum, and strong activity adjacent to the bone. (B) Alkaline activity in the molar PDL is more consistent throughout the periodontal ligament. (C) Cell proliferation of the PDL of the incisor shown through Ki67 immunostaining is evident adjacent to the cementum, but lacking in the rest of the PDL. (D) Cell proliferation is minimal in the PDL of the molar without the heavy proliferation next to the cementum as seen in the incisor. (E, F) Collagen type 1 is found in the PDL of the molar next to the bone, but not adjacent to the cementum. c, cementum; d, dentin; pdl, periodontal ligament; b, bone.
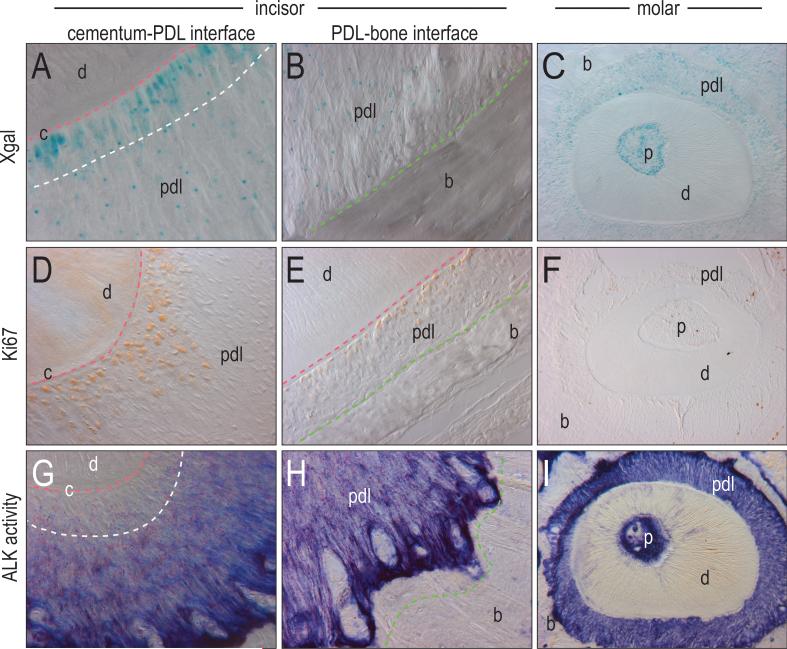
Figure 3.
Graded distribution of cell activities with in the periodontium. (A, B) Xgal activity shows cells in the PDL of the incisor display strong Wnt responsiveness adjacent to the cementum, but is sparsely distributed throughout the rest of the PDL. The same strong signal is not found adjacent to the bone of the PDL. (C) Xgal activity shows cells in the PDL of the molar are Wnt responsive, but do not display the same localization next to the cementum as seen in the incisors. (D, E) Cell proliferation of the incisor, shown through Ki67 immunostaining, is significant adjacent to the cementum but not adjacent to the bone. (F) Cell proliferation shown through Ki67 immunostaining is minimal in the PDL of the molar. (G, H) The distribution of alkaline phosphatase activity is shown to be minimal adjacent to the cementum and strongest adjacent to the bone. (I) Alkaline phosphatase activity in the PDL of the molar is more evenly distributed throughout the periodontal ligament. p, pulp; c, cementum; d, dentin; pdl, periodontal ligament; b, bone.
The gradation in alkaline phosphatase activity we observed in the incisor periodontal ligament suggested that the cells closest to the tooth surface were not as mature as those near the alveolar bone surface. We used Ki67 immunostaining to identify cells that were in a less mature, proliferative state (Iatropoulos and Williams, 1996). We found that the region of diminished differentiation in the incisor periodontal ligament coincided with increased cell proliferation (Fig. 2C). Molar periodontal ligaments showed only minimal signs of Ki67 immunostaining (Fig. 2D).
Thus far, these data suggest that within the periodontal ligament itself there was a balance between proliferation and differentiation. To further test the validity of this hypothesis we evaluated the pattern of collagen type I expression as a marker of early differentiation towards a mineralized phenotype (Lane et al., 1986). Cells closest to the tooth surface did not express collagen type I while cells closer to the alveolar bone showed high collagen type I expression (Fig. 2E, F). From these data we conclude that periodontal ligament cells closest to the tooth surface continue to proliferate while periodontal ligament cells closest to the alveolar bone undergo differentiation. Our next experiments focused on understanding on the molecular mechanisms that controlled these cell states.
Areas of cell proliferation coincide with regions of heightened Wnt responsiveness within the periodontal ligament
In the embryonic facial prominences, domains of cell proliferation coincide with regions of strong Wnt responsiveness (Brugmann et al., 2007); we wondered whether this same relationship existed in the periodontal ligament. To address this possibility we employed a strain of transgenic mice in which a beta galactosidase reporter gene is inserted into the Axin2 locus. Since Axin2 is a direct target of Wnt signaling (Jho et al., 2002; Lustig et al., 2002), the pattern of beta galactosidase activity accurately reflects the pattern of Wnt responsiveness (Yu et al., 2005; Liu et al., 2007).
We first focused on the incisor periodontal ligament because within this single tissue, regions of cell proliferation were clearly demarcated from regions of differentiation. In the adult Axin2LacZ+/- incisor periodontal ligament we found that Xgal staining was highest in cells approximating the Axin2LacZ+/- tooth surface (i.e., the cementum-PDL interface; Fig. 3A). In sharp contrast, periodontal ligament cells approximating the alveolar bone (i.e., the PDL-alveolar bone interface) showed only low beta galactosidase activity (Fig. 3B). This low level of beta galactosidase activity was equivalent to that observed throughout the molar periodontal ligament (Fig. 3C).
The graded distribution of Wnt responsiveness in the periodontal ligament closely coincided with the distribution of proliferating cells. Using Ki67 immunostaining on adjacent sections we found that the domain of Ki67-immunopositivity was restricted to the Wnt-responsive cells located at the cementum-PDL interface (Fig. 3D). On the other hand, cells at the incisor PDL-alveolar bone interface showed no signs of Ki67 immunostaining (Fig. 3E), equivalent to the pattern of Ki67 immunostaining in the molar periodontal ligament (Fig. 3F). Thus, Wnt responsiveness in the periodontal ligament was coincident with regions of active cell proliferation.
Vascularization and matrix remodeling are highest at the PDL-bone interface
In order for incisors to continually erupt there must be continual remodeling of the periodontal ligament and its associated bone (see, for example, Melcher, 1967; Klodzinski, 1978; Beertsen, 1979). We evaluated the periodontium for the distribution of bone remodeling osteoclasts using tartrate-resistant acid phosphatase activity as a marker (James et al., 1996). While there was no evidence of TRAP activity near the cementum–PDL interface, there was robust TRAP activity at the PDL-bone interface (Fig. 4A, B).
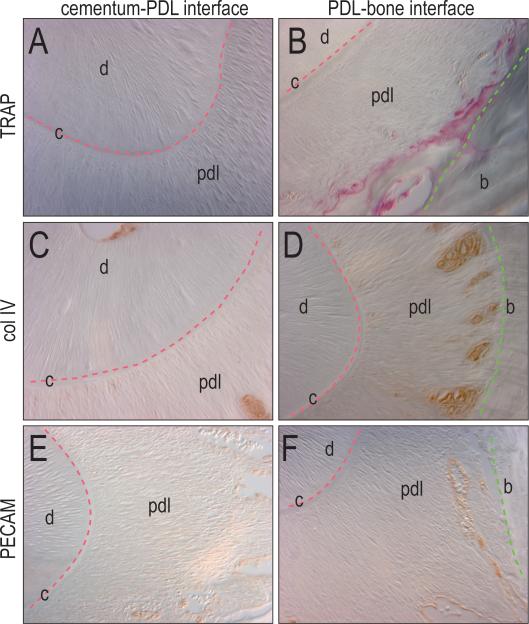
Figure 4.
Differentiation is highest at PDL-bone interface. (A, B) Osteoclast activity is shown through TRAP staining adjacent to the bone, but not to the cementum. (C, D) Collagen type IV is shown adjacent to the bone, but not to the cementum. (E, F) Vascularization is displayed through PECAM immunostaining with blood vessels near the bone but not near the cementum. p, pulp; c, cementum; d, dentin; pdl, periodontal ligament; b, bone.
We also evaluated the distribution of blood vessels within the periodontal ligament. Using collagen type IV as a marker of pericytes (Schlingemann et al., 1991) we found immunostaining restricted to the PDL-bone interface with no immunopositive cells near the cementum-PDL interface (Fig. 4C, D). Furthermore, using platelet endothelial cell adhesion molecule (PECAM) as a marker for endothelial cells (Albelda et al., 1991), we detected immunostaining in the PDL-bone interface, and no staining at the cementum-PDL zone (Fig. 4E, F).
Thus, there is a clear distribution of cell activities within the periodontal ligament: periodontal ligament cells close to the tooth surface are both Wnt responsive and highly proliferative. In the middle region of the periodontal ligament, cells begin to express collagen type I and secrete a mineralized matrix. Closer to the alveolar bone, periodontal ligament cells show the highest rate of collagen type I expression and differentiation; this is also the region of highest remodeling and vascularization.
Discussion
A current theory in the field of stem cell biology posits that during development, a cellular repository is created within each tissue that houses a subset of embryonic cells. These cells, rather than differentiating, remain in a quiescent state until disease or injury activates them. Upon activation, the theory goes, these adult, tissue-specific stem cells are activated and contribute to the process of tissue repair and/or regeneration.
It has long been thought that the periodontal ligament contained such a stem cell repository. For example, the apical region of the tooth contains proliferating cells that contribute to the periodontal ligament (Perera and Tonge, 1981). Some label-retaining cells (slowly cycling cells) have been identified within the periodontal ligament and were thought to indicate a stem cell phenotype (McCulloch, 1985). More recent studies, however, show that stem cells can be highly proliferative (van der Flier and Clevers, 2008). More recently, cells have been isolated from the periodontal ligament that retain the ability to differentiate into chondrocytes, osteoblasts, and adipocytes in vitro (Gay et al., 2007). Whether these cells actually represent periodontal stem cells, however, is not certain. Such a distinction is reserved for cells that retain the ability to differentiate into multiple cell types upon transplantation, as well as the ability to self-renew. Nonetheless, the possibility that stem cells exist within the adult periodontal ligament raises a number of interesting questions. For example, where is the periodontal stem cell niche? Do stem cells contribute to the regenerating periodontal ligament that surrounds continually erupting teeth? If so, what molecular signals regulate the self-renewal, versus the proliferation and differentiation of these cells? Could these same molecular stimuli be used to enhance periodontal ligament regeneration following disease?
What is the developmental potential of the periodontium?
Remarkably little is known about the origins and development of the periodontal ligament. Our data indicate that this tissue arises from the embryonic neural crest, a population of pluripotent cells that has the capacity to produce cartilage, bone, nerves, muscle, and adipose tissues (Le Douarin et al., 2004). Yet whether there exists a repository of neural crest-derived stem cells in the periodontium is not known. We reasoned that if such a population existed it would be most readily detectable in the periodontium associated with teeth that undergo continual, rapid eruption. The mouse incisor, which grows continuously throughout life, is such a tooth. As the incisor advances into the oral cavity, its associated periodontal ligament must undergo equivalent, continual remodeling to maintain the architecture of the periodontium. The factors that regulate this remarkable regenerative potential may still be a mystery, but the availability of transgenic mice in which incisor tooth eruption is disturbed may be particularly informative.
Wnt signaling in the periodontal ligament
There is virtually no information available on the role of Wnt signaling in the development or maintenance of the periodontium. There is, however, a wealth of data regarding the function of Wnt signaling in mediating the behaviors of other types of stem cells. For example, in embryonic mesenchymal cells a Wnt signal is simultaneously responsible for maintaining a self-renewing, proliferative state, while repressing differentiation in the same cell population (ten Berge et al., 2008). As proliferation continues in this population of mesenchymal cells, the developmental field expands and that leads to two simultaneous events: Cells that are at the greatest distance from the Wnt source escape its effects and the repression on cell differentiation is relieved. As a result, cells at a distance from the Wnt source initiate their differentiation while cells close to the Wnt source continue to proliferate (Leucht et al., 2008c).
These data from an embryonic system are closely mirrored by our findings in the adult periodontium. Cells showing the highest level of Wnt responsiveness also show the greatest proliferation (Fig. 3). Conversely, cells with the lowest levels of Wnt responsiveness show the greatest amount of cell differentiation (Fig. 4), but it is still unclear whether Wnt signaling directly regulates this cell proliferation within the periodontium. Inactivating all Wnt signaling in an embryo leads to early lethality, and given the fact that there are at least 19 Wnt ligands a gene knock-out approach is not a realistic strategy with which to address this question. An alternative approach would to provisionally inhibit Wnt signaling by over-expression of a Wnt antagonist such as Dkk. We have employed this approach to block Wnt signaling during skeletal tissue repair (Kim et al., 2007; Leucht et al., 2008b); perhaps a similar strategy could be used to temporarily block Wnt signaling, in order to evaluate the effects on periodontal remodeling and incisor tooth eruption.
The periodontium as a regenerating tissue
Every adult tissue harbors stem cells, which potentially could be used to regenerate damaged or diseased tissues (Wagers and Weissman, 2004; Moore and Lemischka, 2006). But there are problems: adult stem cells are usually found in low abundance, and their response to stress and aging typically diminishes their ability to self-renew and proliferate (Orford and Scadden, 2008). If we understood the regulatory pathways that control stem cell self-renewal, proliferation, and differentiation then theoretically we could expand a limited population of adult stem cells, which would differentiate in response to cues from their local environment (Fuchs et al., 2004; Moore and Lemischka, 2006). Such a “biomimetic” (mimicking nature) approach would avoid the inherent complications associated with the introduction of exogenous stem cells, but still reach the end goal of restoring damaged or diseased tissues. The challenge is now to adapt insights gained from developmental biology to the field of dental regenerative medicine. We envision a strategy where the molecular signals that regulate self-renewal and proliferation of periodontal cells in a continuously erupting tooth could be exploited to enhance the proliferation of adult periodontal stem cells following disease.
Experimental Procedures
Tissue collection, processing, histology and Xgal staining
Maxillas and mandibles were dissected, decalcified in 19% EDTA for 72 hours and processed for paraffin embedding. Sections (8 μm ) were collected on Superfrost-plus slides (Fisher Scientific, Pittsburgh, PA) for histology using a modification of Movat's Pentachrome staining (Movat, 1955). Matrix organization was visualized by staining tissues with the acidic dye, picrosirius red (0.1% Direct Red 80 in saturated picric acid [Sigma]), to discriminate tightly packed and aligned collagen molecules. For Xgal staining, maxillas and mandibles were fixed in 0.4% paraformaldehyde (PFA) overnight prior to being decalcified with 19% EDTA for 72 hours and infused with 30% sucrose for 24 hours. Samples were then embedded in OCT medium and cryosectioned. Xgal staining performed as previously described (Brugmann et al., 2007).
Animals
All experiments were performed in accordance with Stanford University Animal Care and Use Committee guidelines. Transgenic mice, expressing Cre recombinase under the control of the Wnt1 promoter (Jiang et al., 2002) were mated to Rosa26RLacZ and Z/EG mice (Novak et al., 2000) to get Wnt1Cre;R26R LacZ/+ and Wnt1Cre;Z/EG heterozygous mice, respectively. Axin2 LacZ/+ mice were obtained from Dr. Walter Birchmeier, Germany.
Immunohistochemistry
Immunohistochemical assays conducted as previously described (Leucht et al., 2007). Antibodies included Ki67 (NeoMarkers), PECAM (BD Pharmingen), Col IV (abcam), and GFP (abcam).
TRAP ACTIVITY
TRAP staining of osteoclasts was performed as described (Colnot et al., 2005) using Leukocyte acid phosphate kit (Sigma) with fast red violet.
Alkaline phosphatase activity
For alkaline phosphatase (ALP) staining, slides were preincubated overnight at 4°C in ALP buffer containing 100 mM Tris (pH 9.5), 50 mM MgCl2, 100 mM NaCl, and 0.1% Tween 20. Slides were then incubated in B M-purple solution (Roche Diagnostic Corporation, Indianapolis, IN) overnight at 4°C until a dark purple color reaction appeared.
In situ hybridization
In situ hybridization with tissue section conducted as previously described (Albrecht et al., 1998) with a Col1a1 probe.
Acknowledgments
We would like to acknowledge the contributions of Li Chen, who initiated this study while an undergraduate student in the laboratory, and the contribution of Philipp Leucht, who served as her supervisor during that period. Funding for this study was provided by NIH grant number 2R01EB000504-05 and CIRM grant number TR1-01249 to JAH.
References
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Lu H-C, Revelli J-P, Xu X-C, Lotan R, Eichele G. Studying gene expression on tissue sections using in situ hybridization. CRC Press, Inc.; Boca Raton, FL: 1998. pp. 93–120. [Google Scholar]
- Amar S, Chung KM. Clinical implications of cellular biologic advances in periodontal regeneration. Curr Opin Periodontol. 1994:128–140. [PubMed] [Google Scholar]
- Anderson HC. Mineralization by matrix vesicles. Scan Electron Microsc. 1984:953–964. [PubMed] [Google Scholar]
- Beertsen W. Remodelling of collagen fibers in the periodontal ligament and the supra-alveolar region. Angle Orthod. 1979;49:218–224. doi: 10.1043/0003-3219(1979)049<0218:ROCFIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK, Thomas NR. Unimpeded eruption in the root-resected lower incisor of the rat with a preliminary note on root transection. Arch Oral Biol. 1969;14:771–780. doi: 10.1016/0003-9969(69)90168-x. [DOI] [PubMed] [Google Scholar]
- Bolt JR, Demar R. An Explanatory Model of the Evolution of Multiple Rows of Teeth in Captorhinus aguti. Journal of Paleontology. 1975;49:815–832. [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Cahill DR, Marks SC., Jr Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Colnot C, Romero DM, Huang S, Helms JA. Mechanisms of action of demineralized bone matrix in the repair of cortical bone defects. Clin Orthop Relat Res. 2005:69–78. doi: 10.1097/00003086-200506000-00012. [DOI] [PubMed] [Google Scholar]
- Edmund AG. Tooth replacement phenomena in the lower vertebrates, Royal Ontario Mus. Life Science Div. 1960;52:1–190. [Google Scholar]
- Foster BL, Somerman MJ. Regenerating the periodontium: is there a magic formula? Orthod Craniofac Res. 2005;8:285–291. doi: 10.1111/j.1601-6343.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149–160. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol. 1996;48:175–181. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]
- James IE, Dodds RA, Lee-Rykaczewski E, Eichman CF, Connor JR, Hart TK, Maleeff BE, Lackman RD, Gowen M. Purification and characterization of fully functional human osteoclast precursors. J Bone Miner Res. 1996;11:1608–1618. doi: 10.1002/jbmr.5650111104. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kim JB, Leucht P, Lam K, Luppen C, Ten Berge D, Nusse R, Helms JA. Bone regeneration is regulated by Wnt signaling. J Bone Miner Res. 2007;22:1913–1923. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- Klodzinski S. [Dr. Tadeusz Kowalski]. Przegl Lek. 1978;35:211–214. [PubMed] [Google Scholar]
- Lane JM, Suda M, von der Mark K, Timpl R. Immunofluorescent localization of structural collagen types in endochondral fracture repair. J Orthop Res. 1986;4:318–329. doi: 10.1002/jor.1100040308. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. A biological cell labelling technique and its use in experimental embryology. Developmental Biology. 1973;30:217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim J-B, Amasha RR, Girod SA, Helms JA. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008a doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Helms JA. Beta-catenin-dependent Wnt signaling in mandibular bone regeneration. J Bone Joint Surg Am. 2008b;90(Suppl 1):3–8. doi: 10.2106/JBJS.G.01136. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Wazen R, Currey JA, Nanci A, Brunski JB, Helms JA. Effect of mechanical stimuli on skeletal regeneration around implants. Bone. 2007;40:919–930. doi: 10.1016/j.bone.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA. Translating insights from development into regenerative medicine: The function of Wnts in bone biology. Semin Cell Dev Biol. 2008c doi: 10.1016/j.semcdb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Lin NH, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Putative stem cells in regenerating human periodontium. J Periodontal Res. 2008;43:514–523. doi: 10.1111/j.1600-0765.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Yu HM, Hsu W. Craniosynostosis caused by Axin2 deficiency is mediated through distinct functions of beta-catenin in proliferation and differentiation. Dev Biol. 2007;301:298–308. doi: 10.1016/j.physletb.2003.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZX, Wible JR. A Late Jurassic digging mammal and early mammalian diversification. Science. 2005;308:103–107. doi: 10.1126/science.1108875. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SC, Jr., Cahill DR. Experimental study in the dog of the non-active role of the tooth in the eruptive process. Arch Oral Biol. 1984;29:311–322. doi: 10.1016/0003-9969(84)90105-5. [DOI] [PubMed] [Google Scholar]
- Marks SC, Jr., Schroeder HE. Tooth eruption: theories and facts. Anat Rec. 1996;245:374–393. doi: 10.1002/(SICI)1097-0185(199606)245:2<374::AID-AR18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- McCulloch CA. Progenitor cell populations in the periodontal ligament of mice. Anat Rec. 1985;211:258–262. doi: 10.1002/ar.1092110305. [DOI] [PubMed] [Google Scholar]
- McIntosh JE, Anderton X, Flores-De-Jacoby L, Carlson DS, Shuler CF, Diekwisch TG. Caiman periodontium as an intermediate between basal vertebrate ankylosis-type attachment and mammalian “true” periodontium. Microsc Res Tech. 2002;59:449–459. doi: 10.1002/jemt.10222. [DOI] [PubMed] [Google Scholar]
- Melcher AH. Remodelling of the periodontal ligament during eruption of the rat incisor. Arch Oral Biol. 1967;12:1649–1651. doi: 10.1016/0003-9969(67)90199-9. [DOI] [PubMed] [Google Scholar]
- Miletich I, Sharpe PT. Normal and abnormal dental development. Hum Mol Genet 12 Spec No. 2003;1:R69–73. doi: 10.1093/hmg/ddg085. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Movat HZ. Demonstration of all connective tissue elements in a single section; pentachrome stains. AMA Arch Pathol. 1955;60:289–295. [PubMed] [Google Scholar]
- Noble HW. The evolution of the mammalian periodontium. Academic Press; New York: 1969. pp. 1–26. [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Perera KA, Tonge CH. Fibroblast cell proliferation in the mouse molar periodontal ligament. J Anat. 1981;133:77–90. [PMC free article] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991;138:1335–1347. [PMC free article] [PubMed] [Google Scholar]
- Sharpe PM, Ferguson MW. Mesenchymal influences on epithelial differentiation in developing systems. Journal of Cell Science. Supplement. 1988;10:195–230. doi: 10.1242/jcs.1988.supplement_10.15. [DOI] [PubMed] [Google Scholar]
- Silva TA, Rosa AL, Lara VS. Dentin matrix proteins and soluble factors: intrinsic regulatory signals for healing and resorption of dental and periodontal tissues? Oral Dis. 2004;10:63–74. doi: 10.1111/j.1601-0825.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- Slavkin HC, MacDougall M, Zeichner-David M, Oliver P, Nakamura M, Snead ML. Molecular determinants of cranial neural crest-derived odontogenic ectomesenchyme during dentinogenesis. Am J Med Genet Suppl. 1988;4:7–22. doi: 10.1002/ajmg.1320310508. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Taverne AA. Collagen responsible for tooth eruption? A study of the eruption of rat incisors. Aust Orthod J. 1993;12:199–206. [PubMed] [Google Scholar]
- ten Berge D, Brugmann SA, Helms JA, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu Rev Physiol. 2008 doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- Wise GE, Frazier-Bowers S, D'Souza RN. Cellular, molecular, and genetic determinants of tooth eruption. Crit Rev Oral Biol Med. 2002;13:323–334. doi: 10.1177/154411130201300403. [DOI] [PubMed] [Google Scholar]
- Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H-MI, Jerchow B, Sheu T-J, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegarelli EV. Adamantoelastomas in the Slye stock of mice. American Journal of Pathology. 1944;20:23–87. [PMC free article] [PubMed] [Google Scholar]