Abstract
Background
Homeodomain-leucine zipper (HD-ZIP) proteins are plant-specific transcriptional factors known to play crucial roles in plant development. Although sequence phylogeny analysis of Populus HD-ZIPs was carried out in a previous study, no systematic analysis incorporating genome organization, gene structure, and expression compendium has been conducted in model tree species Populus thus far.
Principal Findings
In this study, a comprehensive analysis of Populus HD-ZIP gene family was performed. Sixty-three full-length HD-ZIP genes were found in Populus genome. These Populus HD-ZIP genes were phylogenetically clustered into four distinct subfamilies (HD-ZIP I–IV) and predominately distributed across 17 linkage groups (LG). Fifty genes from 25 Populus paralogous pairs were located in the duplicated blocks of Populus genome and then preferentially retained during the sequential evolutionary courses. Genomic organization analyses indicated that purifying selection has played a pivotal role in the retention and maintenance of Populus HD-ZIP gene family. Microarray analysis has shown that 21 Populus paralogous pairs have been differentially expressed across different tissues and under various stresses, with five paralogous pairs showing nearly identical expression patterns, 13 paralogous pairs being partially redundant and three paralogous pairs diversifying significantly. Quantitative real-time RT-PCR (qRT-PCR) analysis performed on 16 selected Populus HD-ZIP genes in different tissues and under both drought and salinity stresses confirms their tissue-specific and stress-inducible expression patterns.
Conclusions
Genomic organizations indicated that segmental duplications contributed significantly to the expansion of Populus HD-ZIP gene family. Exon/intron organization and conserved motif composition of Populus HD-ZIPs are highly conservative in the same subfamily, suggesting the members in the same subfamilies may also have conservative functionalities. Microarray and qRT-PCR analyses showed that 89% (56 out of 63) of Populus HD-ZIPs were duplicate genes that might have been retained by substantial subfunctionalization. Taken together, these observations may lay the foundation for future functional analysis of Populus HD-ZIP genes to unravel their biological roles.
Introduction
Homeodomain (HD) proteins play fundamental roles in a diverse set of plant developmental processes, from pattern formation to cell type specification [1]. HD proteins constitute a large family of transcription factors with the HD DNA-binding domain at N-termini. In plants, HD proteins can be classified into 14 distinct families based on the sequence similarity of HD domains and their unique codomains [2]. Homeodomain-leucine zipper (HD-ZIP) genes are the most abundant group of HD genes in plants but no HD-ZIP genes present in other eukaryotes. Unique features of HD-ZIP proteins are the presence of a HD domain and an adjacent Leucine Zipper (LZ) motif [3]. The HD domain is responsible for specific DNA binding, whereas the LZ motif mediates protein dimerization [4], [5], [6], [7]. Based on the DNA-binding specificities, additional conserved motifs and their physiological functions, HD-ZIP genes are divided into four subfamilies (HD-ZIP I, II, III and IV) [7], [8].
Arabidopsis HD-ZIP I subfamily has 17 members (ATHB1/HAT5, ATHB3/HAT7, ATHB5–7, ATHB12, ATHB13, ATHB16, ATHB20–23, ATHB40, ATHB51–54) [9]. Arabidopsis HD-ZIP I genes are not only responsive to sugar signaling, abscisic acid (ABA) signaling and abiotic stresses, but also critical to plant embryogenesis and de-etiolation. ATHB13 are potentially regulating sugar signaling [10] and ATHB5, ATHB6, ATHB7, and ATHB12 have been proposed to participate in ABA-dependent and abiotic stress responses [11], [12], [13], [14], [15], [16], of which ATHB6 is a crucial regulator in the ABA signaling pathway [17]. ATHB16 participates not only in blue-light signaling but also in leaf cell expansion [18], whereas ATHB52 regulates photomorphogenesis and de-etiolation [9]. Other HD-ZIP I genes, such as ATHB1, ATHB3, ATHB20, and ATHB23 are involved in cotyledon and leaf development [19], [20] and ATHB1 also responses to de-etiolation of dark-grown seedlings [19]. Recent progress shows that white spruce (Picea glauca) HD-ZIP I proteins also participate in ABA responses [21].
Arabidopsis HD-ZIP II subfamily consists of nine members (ATHB2/HAT4, ATHB4, HAT1–HAT3, HAT9, HAT14, HAT17, and HAT22) [22]. All nine members have a cellular redox status perceptive CPSCE (Cys, Pro, Ser, Cys, and Glu) motif at the downstream of LZ motif [23] and most of these genes are mainly respond to light, shading and auxin as revealed by genetic and biochemical analyses [24], [25], [26], [27]. Both ATHB2/HAT4 and HAT2 regulate auxin-mediated morphogenesis in Arabidopsis. ATHB2/HAT4 mediates red/far-red light effects on leaf cell expansion and shade avoidance intrigued by three distinct phytochromes [24], [27]. HAT2 is also auxin-inducible in Aradibopsis seedlings [26]. Ectopic expression of HAT2 in Arabidopsis, consistent with the typical phenotypes of other auxin-overproducing mutants, produces a variety of phenotypic deviations with long hypocotyls, epinastic cotyledons, long petioles and small leaves [25]. Research on sunflowers also shows that their HD-ZIP II genes act as developmental regulators in response to illumination [28].
Arabidopsis HD-ZIP III subfamily comprises of only five genes, PHABULOSA(PHB)/ATHB14, PHAVOLUTA(PHV)/ATHB9, REVOLUTA (REV)/INTERFASCICULAR FIBERLESS1(IFL1), ATHB8, and CORONA(CNA)/ATHB15/INCURVATA4 (ICU4) [29], but they are the key developmental regulators of Arabidopsis apical embryo patterning, shoot meristem formation, vascular differentiation, organ polarity determination, as well as auxin transportation [29], [30], [31], [32], [33], [34], [35], [36]. Members of Arabidopsis HD-Zip III subfamily have an N-terminal putative steroid/lipid-binding START (STeroidogenic Acute Regulatory protein related lipid Transfer) domain, followed by an adjacent conserved SAD (START-adjacent) domain [37], [38], and a C-terminal PAS-related MEKHLA domain that potentially involves in oxygen redox perception and light signaling [39]. Three closely related Arabidopsis HD-ZIP III genes, REV/IFL1, PHB/ATHB9, and PHV/ATHB14, act antagonistically with KANADI to regulate the establishment of apical meristem, vascular pattern, and adaxial domains in lateral organs [31], [33], [35], [36]. These three HD-ZIP III genes have partially overlapping roles as the single mutants have only minor or even no apparent defects in interfascicular fiber formation as well as floral and lateral meristem initiation [29], but the phb rev and phv reb double mutants enhance these defects [29] and the phb phv rev triple mutants have no apical meristem but substituted by a single radialized cotyledon [29], [31]. Another set of two closely related HD-ZIP III members, ATHB8 and CNA/ATHB15/ICU4, act as regulators of vascular development [30], [32], [34], as athb8 mutants bear no detectable phenotypes but constitutive expression of ATHB8 triggers premature initiations of the secondary growth in xylem cells that eventually leads to the ectopic proliferation of xylem cells [30]. Additional evidences show that ATHB8 also regulates the initiation of procambial development, vein patterning and differentiation [40]. Genetic evidence also show that CNA/ATHB15/ICU4 gene presumably acts as a negative regulator of procambial cell identity or proliferation because ectopic expression of a miRNA-resistant CNA/ATHB15/ICU4 results in moderate dwarfing stature with drastic reduction in xylem and lignified interfascicular tissues, whereas antisense ATHB15 transformants are severely dwarfed with expanded xylem and interfascicular fibers, and ectopic lignified pith [41]. Some other evidences show that the HD-Zip III family transcripts are also specifically targeted and thus negatively regulated by MiRNA165/166 [31], [33], [41], [42], [43], [44], [45].
In Arabidopsis, HD-ZIP IV (also known as HD-GL2) constitutes a large subfamily of genes composed of 16 members: GLABRA2(GL2)/ATHB10, ARABIDOPSIS THALIANA MERISTEM LAYER1(ATML1), ANTHOCYANINLESS2(ANL2), PROTODERMAL FACTOR2 (PDF2), HOMEODOMAIN GLABROUS 1(HDG1)-HDG5, HDG6/FWA, and HDG7–HDG12 [46]. HD-ZIP IV proteins have the similar domain arrangements to those of HD-ZIP III members but only lack of the C-terminal MEHKLA domain, suggesting that class III and IV HD-Zip gene families may share a common ancestor [7], [38]. Genetic analysis shows that HD-ZIP IV proteins play crucial roles in epidermal cell differentiation, trichome formation, root development and anthocyanin accumulation. Three HD-ZIP IV genes, GL2/ATHB10, ATML1, and PDF2, appear to determinate the fate of epidermal layer cells [47], [48], [49], [50], [51]. The GL2 determines trichome outgrowth in shoot epidermal cells as well as root hair cell specification as mutant gl2 has impaired trichome and defected root hair [47], [48], [52]. Loss-of-function mutants of either atml1 or pdf2 mutants also show severe defects in shoot epidermal cell differentiation, and the atml1 pdf2 double mutants fail to differentiate into protoderm during embryogenesis and thus are embryonic lethal [51]. Another set of HD-ZIP IV genes, HDG11 and HDG12, repress trichome outgrowth. hdg11 single mutant leads to excessive trichome branching and hdg12 single mutant has no trichome, while the hdg11 hdg12 double mutants have more excessive trichome branching [46]. ANL2 regulates anthocyanin accumulation in leaf sub-epidermal layer as well as cell identity in root [53].
Compared to the largely investigated functions of Arabidopsis HD-ZIPs, only two Populus HD-ZIP genes (POPREVOLUTA and POPCORONA) have recently been characterized. These two HD-ZIP III genes are largely involved in regulating cell differentiation during secondary growth. Misexpression of POPREVOLUTA (PRE, a Populus orthologue of REV/IFL1) induces cambium initiation in abnormal positions and eventually causes patterning defects in derived secondary vascular tissues, even, to the extent of complete polarity reversals [54]. Overexpression of a miRNA-resistant POPCORONA (PCN, a Populus CNA/ATHB15/ICU4 orthologue) shows delayed lignification of xylem and phloem fibers during secondary growth, whereas synthetic miRNA knockdown of PCN has abnormal lignification in pith cells [55].
Recently, a complete survey and classification of HD genes in ten different plant species from disparate evolutionary groups has already been carried out [2], however, only sequence phylogeny analyses of Populus HD-ZIPs are performed in the previous study and no detailed systematic analysis including genome organization, gene structure and expression compendium has been conducted. In this study, we first performed a genome-wide identification of HD-ZIP genes in Populus to reveal an expanded HD-ZIP family with totally 63 members, and then analyzed the sequence phylogeny, genome organization, gene structure, conserved motifs and expression profiling of these 63 genes. Furthermore, a thorough comparative analysis of Populus HD-ZIP genes to those from seven other plant species was performed. We finally examined the gene expression patterns of 16 Populus HD-ZIP genes to verify the evolution origins of Populus HD-ZIP genes as well as to confirm their tissue-specific expression patterns and inducible expressions under drought and salt stresses. Our results presented here may provide a subset of potential candidate HD-ZIP genes for future engineering modifications of lignocellulosic biomass and stress tolerance characteristics in Populus.
Results and Discussion
Identification of HD-ZIP gene family in Populus and other plant species
To identify putative HD-ZIP genes in Populus, we performed a BLASTP search against Populus genome release v2.1 using HD-ZIP protein sequences in Arabidopsis as queries and the resulting sequences were used as secondary queries. By removing the redundant sequences, 63 HD-ZIP genes were identified in the Populus genome. All HD-ZIP candidates were manually analyzed using InterProScan program (http://www.ebi.ac.uk/Tools/InterProScan/) to verify the presence of HD and LZ domain. In a recently published report, a total of 61 HD-ZIP genes were identified in Populus by a genome-wide bioinformatics survey [2]. In this study, we further revealed three additional HD-ZIP genes in Populus and extended the total member to 63. We designated Populus HD-ZIP genes as PtrHox following the nomenclature proposed in the previous study [56]. The identified HD-ZIP genes in Populus encode proteins ranging from 170 to 855 amino acids (aa) in length with an average of 465 aa. In most cases, there are two or more Populus HD-ZIP genes for the orthologues in Arabidopsis, but in some cases, there are no orthologous Populus HD-ZIP genes in Arabidopsis. The detailed information of HD-ZIP family genes in Populus, including accession numbers and similarities to their Arabidopsis orthologues was listed in Table 1.
Table 1. HD-ZIP gene family in Populus.
| Gene symbol | Gene model (V2.1) | Gene model (V1.1) | Arabidopsis orthologue locus | Arabidopsis locus description | Score | E-value |
| PtrHox1 | POPTR_0001s02030.1 | fgenesh4_pg.C_scaffold_29000247 | AT5G46880.1 | HB7, HDG5 | 775 | 0 |
| PtrHox2 | POPTR_0001s11380.1 | eugene3.00010699 | AT2G46680.1 | ATHB-7 | 131 | 3E-31 |
| PtrHox3 | POPTR_0001s15490.1 | eugene3.00290072 | AT4G16780.1 | ATHB-2, HAT4 | 335 | 2E-92 |
| PtrHox4 | POPTR_0001s18470.1 | fgenesh4_pm.C_LG_I000546 | AT1G79840.1 | GL2 | 941 | 0 |
| PtrHox5/PCN | POPTR_0001s18930.1/HB5 | fgenesh4_pm.C_LG_I000560 | AT1G52150.1 | ATHB-15, CNA | 1467 | 0 |
| PtrHox6 | POPTR_0001s23680.1 | gw1.148.81.1 | AT5G06710.1 | HAT14 | 268 | 4E-72 |
| PtrHox7 | POPTR_0001s38120.1/HB4 | estExt_fgenesh4_pg.C_LG_I2905 | AT2G34710.1 | PHB, ATHB-14 | 1357 | 0 |
| PtrHox8 | POPTR_0001s40540.1 | gw1.146.127.1 | AT5G53980.1 | ATHB-52 | 114 | 2E-26 |
| PtrHox9 | POPTR_0001s44540.1 | gw1.XIII.2898.1 | AT4G04890.1 | PDF2 | 420 | e-117 |
| PtrHox10 | POPTR_0002s10080.1 | estExt_fgenesh4_pm.C_LG_II0461 | AT4G40060.1 | ATHB-16 | 191 | 6E-49 |
| PtrHox11 | POPTR_0002s11410.1 | estExt_fgenesh4_pg.C_LG_II1036 | AT4G37790.1 | HAT22 | 210 | 8E-55 |
| PtrHox12 | POPTR_0002s13720.1 | estExt_fgenesh4_pm.C_LG_II0606 | AT3G60390.1 | HAT3 | 285 | 2E-77 |
| PtrHox13 | POPTR_0002s15610.1 | eugene3.00021438 | AT4G00730.1 | ANL2, AHDP | 1057 | 0 |
| PtrHox14 | POPTR_0002s17680.1 | fgenesh4_pm.C_LG_II000817 | AT2G46680.1 | ATHB-7 | 219 | 1E-57 |
| PtrHox15 | POPTR_0002s23080.1 | estExt_fgenesh4_pm.C_LG_II1004 | AT1G05230.2 | HDG2 | 1154 | 0 |
| PtrHox16 | POPTR_0003s04860.1/HB6 | estExt_fgenesh4_pg.C_LG_III0436 | AT1G52150.1 | ATHB-15, CNA | 1488 | 0 |
| PtrHox17 | POPTR_0003s05100.1 | estExt_fgenesh4_pg.C_LG_III0408 | AT1G79840.1 | GL2 | 948 | 0 |
| PtrHox18 | POPTR_0003s07760.1 | fgenesh4_pm.C_LG_III000187 | AT4G16780.1 | ATHB-2, HAT4 | 320 | 8E-88 |
| PtrHox19 | POPTR_0003s09470.1 | gw1.III.2514.1 | AT5G46880.1 | HB7, HDG5 | 836 | 0 |
| PtrHox20 | POPTR_0003s14700.1 | / | AT2G46680.1 | ATHB-7 | 110 | 5e-25 |
| PtrHox21 | POPTR_0004s01980.1 | fgenesh4_pg.C_LG_IV000079 | AT4G04890.1 | PDF2 | 1186 | 0 |
| PtrHox22 | POPTR_0004s07310.1 | gw1.IV.3966.1 | AT1G73360.1 | HDG11,EDT1 | 631 | 0 |
| PtrHox23/PRE | POPTR_0004s22090.1/HB2 | estExt_Genewise1_v1.C_660759 | AT5G60690.1 | REV, IFL | 1375 | 0 |
| PtrHox24 | POPTR_0005s07290.1 | / | AT4G40060.1 | ATHB-16 | 244 | 4E-65 |
| PtrHox25 | POPTR_0005s12760.1 | estExt_fgenesh4_pg.C_570199 | AT2G18550.1 | HB2, ATHB-21 | 202 | 9E-53 |
| PtrHox26 | POPTR_0005s17390.2 | estExt_Genewise1_v1.C_LG_V4715 | AT4G40060.1 | ATHB-16 | 186 | 9E-48 |
| PtrHox27 | POPTR_0005s19080.1 | gw1.V.4364.1 | AT4G37790.1 | HAT22 | 265 | 2E-71 |
| PtrHox28 | POPTR_0006s07100.1 | fgenesh4_pg.C_LG_VI000532 | AT5G06710.1 | HAT14 | 163 | 8E-41 |
| PtrHox29 | POPTR_0006s11890.1 | gw1.28.964.1 | AT5G03790.1 | ATHB51, LMI1 | 181 | 4E-46 |
| PtrHox30 | POPTR_0006s20820.1 | fgenesh4_pm.C_LG_VI000496 | AT5G06710.1 | HAT14 | 249 | 1E-66 |
| PtrHox31 | POPTR_0006s25390.1/HB8 | estExt_fgenesh4_pm.C_LG_VI0713 | AT4G32880.1 | ATHB-8 | 1404 | 0 |
| PtrHox32 | POPTR_0007s01370.1 | fgenesh4_pg.C_LG_VII000112 | AT4G36740.1 | HB-5, ATHB40 | 78 | 3E-15 |
| PtrHox33 | POPTR_0007s05010.1 | gw1.VII.1686.1 | AT2G22430.1 | ATHB-6 | 248 | 4E-66 |
| PtrHox34 | POPTR_0007s12450.1 | grail3.0019015601 | AT4G36740.1 | HB-5, ATHB40 | 208 | 2E-54 |
| PtrHox35 | POPTR_0007s14590.1 | estExt_Genewise1_v1.C_LG_VII0041 | AT4G37790.1 | HAT22 | 299 | 1E-81 |
| PtrHox36 | POPTR_0008s12840.1 | / | AT2G01430.1 | ATHB-17 | 206 | 1E-53 |
| PtrHox37 | POPTR_0008s14770.1 | estExt_fgenesh4_pg.C_LG_VIII1313 | AT1G69780.1 | ATHB-13 | 333 | 1E-91 |
| PtrHox38 | POPTR_0008s19900.1 | eugene3.00081839 | AT5G15150.1 | ATHB-3, HAT7 | 122 | 4E-28 |
| PtrHox39 | POPTR_0009s01990.1/HB1 | gw1.IX.4748.1 | AT5G60690.1 | REV, IFL | 1367 | 0 |
| PtrHox40 | POPTR_0009s02850.1 | / | AT5G06710.1 | HAT14 | 242 | 3E-64 |
| PtrHox41 | POPTR_0010s10340.1 | estExt_fgenesh4_pg.C_LG_X0852 | AT1G69780.1 | ATHB-13 | 308 | 2E-84 |
| PtrHox42 | POPTR_0010s12300.1 | eugene3.00101057 | AT2G01430.1 | ATHB-17 | 214 | 3E-56 |
| PtrHox43 | POPTR_0011s00520.1 | eugene3.00110213 | AT4G04890.1 | PDF2 | 1164 | 0 |
| PtrHox44 | POPTR_0011s10070.1/HB3 | estExt_fgenesh4_pg.C_2360002 | AT2G34710.1 | PHB, ATHB-14 | 1351 | 0 |
| PtrHox45 | POPTR_0011s11510.1 | gw1.XI.3740.1 | AT5G53980.1 | ATHB-52 | 114 | 4E-26 |
| PtrHox46 | POPTR_0012s03080.1 | gw1.XII.940.1 | AT2G46680.1 | ATHB-7 | 147 | 5E-36 |
| PtrHox47 | POPTR_0012s03550.1 | estExt_fgenesh4_pm.C_LG_XII0033 | AT1G73360.1 | HDG11,EDT1 | 885 | 0 |
| PtrHox48 | POPTR_0012s07270.1 | grail3.0015005801 | AT3G01470.1 | ATHB-1, HAT5 | 145 | 4E-35 |
| PtrHox49 | POPTR_0012s13390.1 | fgenesh4_pg.C_LG_XII001156 | AT4G00730.1 | ANL2, AHDP | 838 | 0 |
| PtrHox50 | POPTR_0014s04460.1 | / | AT3G60390.1 | HAT3 | 263 | 8E-71 |
| PtrHox51 | POPTR_0014s07130.1 | fgenesh4_pg.C_LG_XIV000202 | AT4G00730.1 | ANL2, AHDP | 1045 | 0 |
| PtrHox52 | POPTR_0014s09860.1 | eugene3.00140486 | AT2G46680.1 | ATHB-7 | 237 | 5E-63 |
| PtrHox53 | POPTR_0014s15010.1 | estExt_fgenesh4_pm.C_LG_XIV0411 | AT1G05230.2 | HDG2 | 1174 | 0 |
| PtrHox54 | POPTR_0015s05050.1 | fgenesh4_pg.C_scaffold_122000084 | AT1G73360.1 | HDG11,EDT1 | 917 | 0 |
| PtrHox55 | POPTR_0015s07640.1 | fgenesh4_pm.C_LG_XV000148 | AT3G01470.1 | ATHB-1, HAT5 | 135 | 2E-32 |
| PtrHox56 | POPTR_0015s13340.1 | eugene3.00151195 | AT4G00730.1 | ANL2, AHDP | 828 | 0 |
| PtrHox57 | POPTR_0016s05890.1 | / | AT5G06710.1 | HAT14 | 242 | 2E-64 |
| PtrHox58 | POPTR_0017s01400.1 | gw1.44.578.1 | AT1G73360.1 | HDG11,EDT1 | 606 | e-173 |
| PtrHox59 | POPTR_0017s04820.1 | eugene3.00640210 | AT4G36740.1 | HB-5, ATHB40 | 79 | 2E-15 |
| PtrHox60 | POPTR_0017s11800.1 | gw1.XVII.1044.1 | AT5G15150.1 | ATHB3, HAT7 | 248 | 3E-66 |
| PtrHox61 | POPTR_0018s08110.1/HB7 | fgenesh4_pg.C_LG_XVIII000250 | AT4G32880.1 | ATHB-8 | 1389 | 0 |
| PtrHox62 | POPTR_0018s13300.1 | eugene3.00181192 | AT5G06710.1 | HAT14 | 155 | 2E-38 |
| PtrHox63 | POPTR_0149s00220.1 | gw1.XVI.1869.1 | AT5G06710.1 | HAT14 | 239 | 1E-63 |
Gene loci are from the Phytozome website (http://www.phytozome.net/poplar, release 2.1). A complete list of the coding sequences (CDS), deduced amino acid sequences and genomic DNA sequences is available in Table S1.
In order to gain insights into the evolutionary relationships among plant HD-ZIP proteins, we identified HD-ZIP genes from seven other plant species with whole genome sequences available, including moss (Physcomitrella patens), the monocotyledonous angiosperms Oryza sativa, Sorghum bicolor and Brachypodium distachyon, and the dicotyledonous angiosperms Arabidopsis thaliana, Medicago truncatula and Vitis vinifera. Strikingly, HD-ZIP gene family is apparently land plant-specific. All angiosperm genomes as well as the genome of the moss contained genes encoding HD-ZIP proteins, while no representatives were found in algae. A complete list of all HD-ZIP genes identified in the present study was provided in Table S2.
The number of HD-ZIP genes in Populus (63) is roughly 1.31 fold than that of Arabidopsis (48) and it is only second to its closest woody perennial grape (Vitis vinifera), which possesses 65 HD-ZIP genes. This expansion to more abundant HD-ZIP genes in Populus and grape genome suggests a great need of HD-ZIP genes to participate in more complicated transcriptional regulations of these two woody species.
Phylogenetic analysis of HD-ZIP gene family
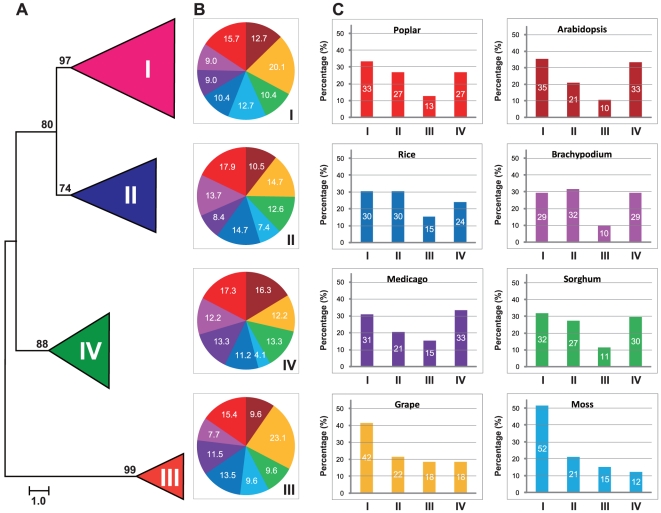
The abundance of Populus HD-ZIP genes to other plant species may derive from multiple gene duplication events, represented by a whole-genome duplication following multiple segmental and tandem duplications [57]. To verify this hypothesis, we first constructed a maximum likelihood phylogenetic tree by PHYML using the full-length HD-ZIP protein sequence alignments of eight different plant species to unveil the evolutionary relationships among plant HD-ZIP proteins. The HD-ZIP proteins of all eight plant species were classified into four well-conserved subfamilies, HD-ZIP I to IV (Fig. 1A), the same as described previously and with significant statistical support [7], [8]. The phylogenetic tree revealed that the plant HD-ZIP sequence distribution predominates with species bias (Fig. 1B). HD-ZIP I genes generally consisted of the largest subfamilies in the plant species except for Brachypodium and Medicago where HD-ZIP II and IV were the largest respectively. In contrast, HD-ZIP III genes composed of the fewest numbers of HD-ZIP members except for moss. It also appears that the numbers of Populus and grape HD-ZIP I and II genes were larger than these of other species. For instance, there were 27 and 21 HD-ZIP I members, 14 and 17 HD-ZIP II members in Populus and grape, respectively. In contrast, only 17 and 14 members in HD-ZIP I subfamily, 10 and 14 members in HD-ZIP II subfamily were present in Arabidopsis and rice, respectively. Species bias was also evident in subfamily IV. Populus and Arabidopsis were predominated with 17 and 16 HD-ZIP IV members but grape had only 12 HD-ZIP IV members nonetheless the number of grape HD-ZIP genes was larger than that of other species. In contrast to the much fewer members in subfamily IV, the number of grape HD-ZIPs showed an overwhelming predominance with 12 members present in subfamily III. Similarly, the number of moss HD-ZIPs in subfamily IV was particularly lower with four members compared to that of other species.
Figure 1. Phylogeny and distribution of HD-ZIP protein from eight plant species.
A. Phylogenetic tree of HD-ZIP proteins from Arabidopsis, rice, Medicago, sorghum, Brachypodium, Populus, Vitis, and moss. Phylogeny was constructed by PhyML using maximum likelihood analysis. Bootstrap support values as percentage, are shown on selected major branches. The scale bar indicates the estimated number of amino acid substitutions per site. B. Percentage representation of HD-ZIP across the eight plant species within each subfamily. Colors correspond to the plant taxa as listed in C. C: Percentage representation of distributions for HD-ZIP within each plant species.
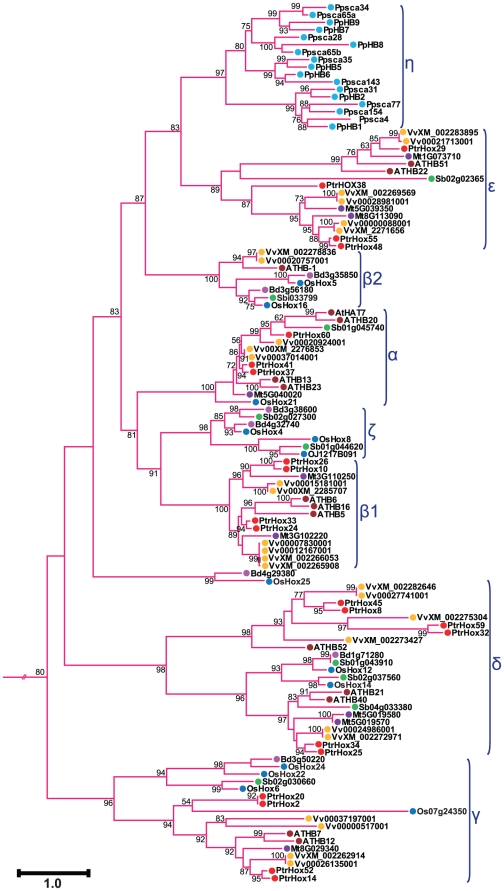
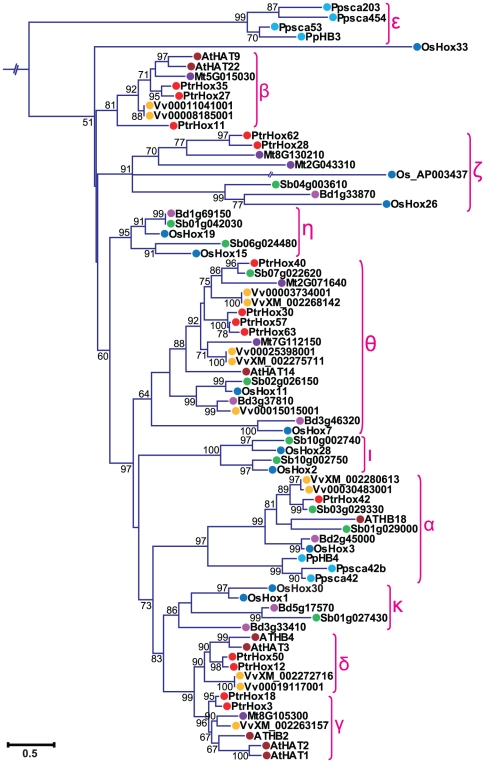
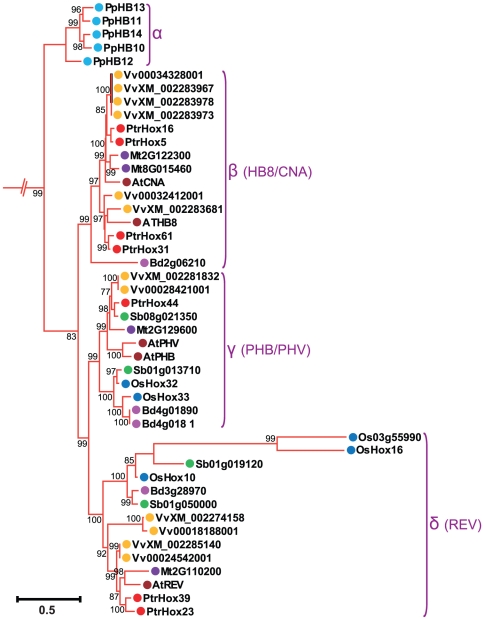
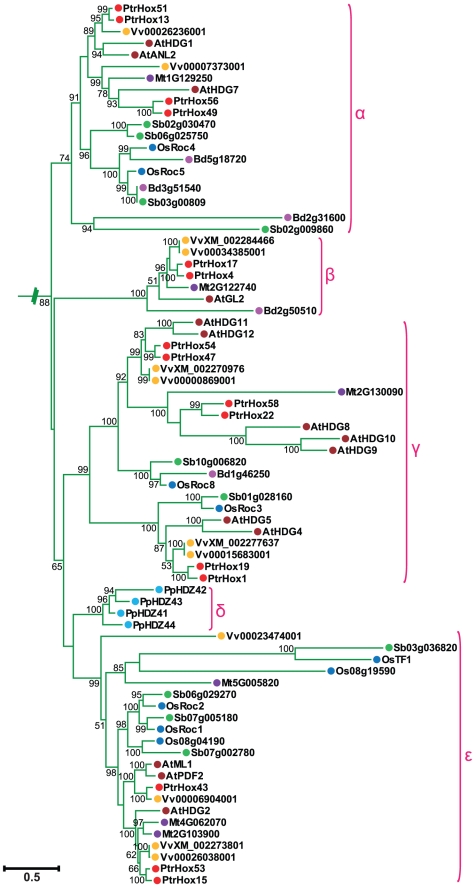
We further examined the subgroups within each HD-ZIP subfamilies. Consistent with the nomenclature in previous studies of Arabidopsis and rice [9], [56], HD-ZIP I subfamily was divided into seven clades designated as clade α, β1, β2, γ, δ, ε, ζ, and η (Fig. 2). Clade η included 17 moss HD-ZIP I members clustered in the most basal clade with high statistical support, whereas clade β1 was exclusively from eudicots (Arabidopsis thaliana, Medicago truncatula, Vitis vinifera, and Populus) and clade ζ entirely from monocots (Oryza sativa, Sorghum bicolor, and Brachypodium distachyon). HD-ZIP II subfamily was divided into ten clades, from α through κ (Fig. 3), with clades α to γ designated in our study corresponding to the four major clades of Arabidopsis HD-ZIP II proteins as previously analyzed [22]. The ε clade constituted a basal grade exclusively containing the moss members. The other three moss HD-ZIPs clustered together with members from eudicots and monocots in clade α. Several clades including β, γ, and δ were composed of HD-ZIP proteins exclusively from eudicots. Similarly, clades η, ι, and κ were exclusively formed of proteins from monocot species. In contrast, clades θ and ζ included HD-ZIP proteins from both monocot and eudicot species. HD-ZIP III subfamily was classified into four clades designated as α, β, γ, and δ (Fig. 4). This classification generally agrees with the definitions of previous studies [29], [58], [59], [60]. Clade α had a basal node exclusively containing five moss members. Clade β included Arabidopsis ATHB8 and ATHB15/CNA as well their orthologues in the other seven plant species, and clade γ contained PHB, PHV and their corresponding members. Subgroup δ corresponded to the REV clade described in previous studies [29], [58], [60] and can be divided into eudicot- and monocot-specific subclades. HD-ZIP IV subfamily was clustered into four individual subgroups, designated clade α, β, γ, δ, and ε as in a previous study [46] (Fig. 5). Most of the HD-ZIP IV members have been functionally characterized in Arabidopsis. Clade α consisted of Arabidopsis ANL2, a regulator of anthocyanin accumulation in leaf sub-epidermal layer and of cell identity in root [53]. Clade β included Arabidopsis GL2 [47], [48], [52] and its orthologues, and Clade γ contained trichome formation genes, HDG4, HDG5, and HDG8–12 [46]. Clade ε was composed of AtML1 and PDF2 responsible for shoot epidermal cell differentiation [51]. Clade δ, placed between clade β and ε, consisted of four HD-ZIP III genes from moss.
Figure 2. Phylogenetic relationship of HD-ZIP I subfamily from eight plant species.
Expanded view of the phylogeny of HD-ZIP I members from Figure 1A. Numbers at each branch indicate bootstrap values and only values higher than 50% are shown. Scale bar corresponds to the estimated number of amino acid substitutions per site. Filled circles represent HD-ZIP proteins from different plant species with colors corresponding to plant taxa as indicated in Figure 1.
Figure 3. Phylogenetic relationship of HD-ZIP II subfamily from eight plant species.
Expanded view of the phylogeny of HD-ZIP II members from Figure 1A. The numbers at the nodes represent the bootstrap values (>50%) from 100 replicates. Scale bar indicates the estimated number of amino acid substitutions per site. Filled circles represent HD-ZIP proteins from different plant species with colors corresponding to plant taxa as indicated in Figure 1.
Figure 4. Phylogenetic relationship of HD-ZIP III subfamily from eight plant species.
Enlarged view of the phylogeny of HD-ZIP III members from Figure 1A. Numbers at each branch indicate bootstrap values and only values higher than 50% are shown. Scale bar corresponds to the estimated number of amino acid substitutions per site. Filled circles represent HD-ZIP proteins from different plant species. Colors correspond to plant taxa as indicated in Figure 1.
Figure 5. Phylogenetic relationship of HD-ZIP IV subfamily from eight plant species.
Enlarged view of the phylogeny of HD-ZIP IV members from Figure 1A. The numbers at the nodes represent the bootstrap values (>50%) from 100 replicates. Scale bar indicates the estimated number of amino acid substitutions per site. Filled circles represent HD-ZIP proteins from different plant species. Colors correspond to plant taxa as indicated in Figure 1.
Tree topology displayed that the majority of HD-ZIP genes tend to cluster in eudicot- and monocot-specific patterns, especially within subgroups harboring large members, i.e., clades β2, γ, and ε in HD-ZIP I subfamily, clades γ and δ in HD-ZIP III, and clades α, γ, and ε in HD-ZIP IV. This substantial lineage-specific pattern suggests that HD-ZIP genes in these subgroups may be expanded and then diversified after the monocot-eudicot radiation so that these genes were acquired or differentially retained in eudicot genomes after their divergences from monocots. Nonetheless, a small number of the HD-ZIP members were presented in both monocots and eudicots in several clades such as clades θ and ζ in HD-ZIP II, which suggests that the expansion of these HD-ZIPs might predate the divergence of eudicots and monocots.
Phylogenetic tree topology further revealed that 28 Populus HD-ZIP pairs at the terminal nodes of each subfamily shared high degrees of sequence similarities and were assigned as paralogous pairs (homologous genes that diverged by gene duplication) (Fig. 2, 3, 4, and 5). These 28 paralogous pairs of HD-ZIP proteins, accounting for more than 89% of the entire HD-ZIP family, had sequence similarity ranging from 62% to 95% (Table S3).
Chromosomal location and gene duplication of Populus HD-ZIP genes
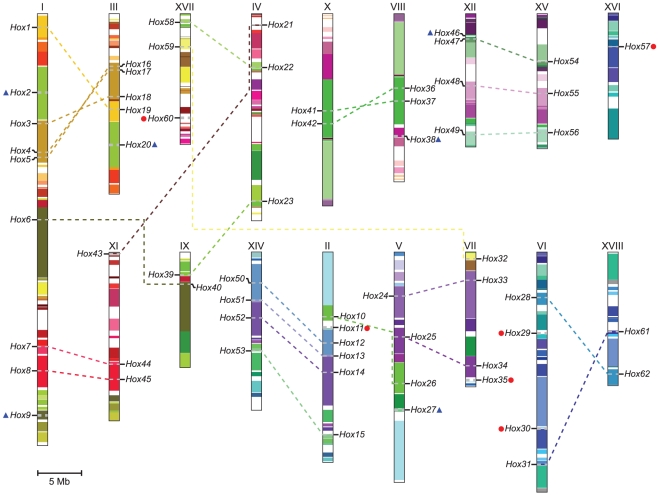
In silico mapping of the gene loci showed that totally 62 Populus HD-ZIP genes were mapped to linkage groups (LG) currently, with only one gene (PtrHox63) remained on as yet unmapped scaffolds (Fig. 6). The 62 Populus HD-ZIP genes were distributed across all LGs, except for LGXIII and IXI. LG I had the largest number of nine HD-ZIP genes followed by six on LG II and five on LG III, respectively. In contrast, only one HD-ZIP gene was found on LG XVI and two HD-ZIP genes on LG IX, XI, and XVIII, respectively. No substantial clustering of Populus HD-ZIP genes was present, even on the LGs with high densities of HD-ZIP genes.
Figure 6. Chromosomal locations and segmental duplication events of Populus HD-ZIP genes.
The schematic diagram of genome-wide chromosome organization arisen from the salicoid genome duplication event in Populus was accomplished based on duplication coordinates from the Populus genome assembly v2.1. Segmental duplicated blocks are indicated with the same colors. The duplicated paralogous pairs of HD-ZIP are connected with dotted lines. Blue triangles indicate HD-ZIPs located on duplicated segments with the corresponding member lost. Red circles represent HD-ZIPs located out of any duplicated regions. Scale represents a 5 Mb chromosomal distance.
Previous studies revealed that Populus genome has undergone at least three rounds of genome-wide duplications followed by multiple segmental duplication, tandem duplication, and transposition events such as retroposition and replicative transposition [57]. Particularly, the segmental duplication associated with the salicoid duplication event occurred 65 million years (MY) ago remarkably contributed to the expansion of many multi-gene families [61], [62], [63], [64], [65], [66]. To determine the possible relationship between the HD-ZIP genes and potential segmental duplications, we mapped Populus HD-ZIPs to the duplicated blocks established in the previous studies [57]. The distributions of HD-ZIP genes relative to the corresponding duplicate blocks were illustrated in Figure 6. Within the identified duplicated blocks associated with the recent salicoid duplication event, about 81% (50 of 62) of Populus HD-ZIPs were preferentially retained duplicates that located in both duplicated regions. Six duplicated blocks only contained HD-ZIPs (PtrHox2, 9, 20, 27, and 38) on one of the blocks and lacked duplicates on the corresponding block, suggesting that dynamic rearrangement may have occurred following the segmental duplication, which led to loss of some of the genes. In contrast, only a small number of six HD-ZIP genes (PtrHox11, 29, 30, 35, 57, and 60) were located outside of any duplicated blocks. None of HD-ZIP genes was represented in distinct tandem duplicate gene clusters, indicating that tandem duplications do not seem to play an important role on the expansion of the HD-ZIP gene family in Populus.
Evidence of salicoid segmental duplications was present in all HD-ZIP subfamilies, particularly in the HD-ZIP III and HD-ZIP IV subfamilies. HD-ZIP III subfamily had the highest rate of segmental duplications among the four subfamilies, with every member impacted by segmental duplications. Similarly, the vast majority genes in HD-ZIP IV except for PtrHox9, accounting for 94.7% of the total subfamily, were impacted by segmental duplications. In comparison, segmental duplications were relatively under-presented in subfamilies HD-ZIP I and II, with rates of 70% (14 out of 20) and 67% (12 out of 18), respectively.
To explore whether other mechanisms contributed towards the expansion of the duplicated genes, we also searched for the presence of transposons and retrotransposons in the flanking genomic sequences of 10-kb upstream and downstream of each HD-ZIP gene. However, there was very limited contribution of transposons/retrotransposons to the expansion of HD-ZIP gene family in Populus (data not shown).
Based primarily on the genomic organization of HD-ZIP genes, we are attempting to speculate that segmental duplications exclusively contributed to the expansion of Populus HD-ZIP gene family. Similarly, segmental duplications have also been shown to contribute to the expansion of other multi-gene families in Populus [61], [62], [63], [64], [65], [66], [67]. Our results indicated that Populus HD-ZIP genes have been preferentially retained at a relatively high rate of 81%. This number is much higher than the average rate following the salicoid genome-wide duplication in the Populus lineage, in which approximately 33% of predicted genes are retained in duplications resulting from the salicoid duplication event on the genome-wide scale [57]. The high retention rates of duplicated genes are also found in other gene families of Populus [62], [63], [68]. These findings corroborates previous findings that genes involved in transcription regulation and signal transduction are preferentially retained following duplications [69], [70], [71]. Another plausible explanation to the relatively high retention rate of duplicate genes in HD-ZIP gene family may lie in the fact that Populus genome has been indicated to evolve at a much slower rate compared to Arabidopsis [57].
As a large proportion of HD-ZIP proteins appear to be paralogous pairs unveiled by the phylogenetic analysis, we further investigated whether traceable genome duplication events have contributed to the expansion of the HD-ZIP family. Of 28 HD-ZIP paralogous pairs we examined, 25 paralogous pairs remained in conserved positions on segmental duplicated blocks (Fig. 6), suggesting that these 25 paralogous pairs may be derived from segmental duplication event during the evolutionary process. No traceable duplication events could be inferred for only three paralogous pairs (PtrHox2/20, PtrHox27/35, and PtrHox30/57). Among them, three genes (PtrHox30, 35, and 57) were located outside of any segmental duplication blocks. Although the other three genes (PtrHox2, 20, and 27) were located on the duplicated blocks, their paralogous counterparts appeared to have lost from the Populus genome.
Duplicated genes may undergo divergent fates such as nonfunctionalization (loss of original functions), neofunctionalization (acquisition of novel functions), or subfunctionalization (partition of original functions) during subsequent evolution [72], [73]. To explore whether Darwinian positive selection was involved in HD-ZIP gene divergence after duplication, the substitution rate ratios of nonsynonymous (dN or Ka) versus synonymous (dS or Ks) mutations (dN/dS or Ka/Ks) were calculated for 28 paralogous pairs. Generally, Ka/Ks = 1 means that the genes are pseudogenes with neutral selection, Ka/Ks<1 indicates the functional constraint with negative or purifying selection of the genes, and Ka/Ks>1 shows the accelerated evolution with positive selection. In this study, the Ka/Ks ratios from 24 segmental duplication pairs were less than 0.4 (Table 2). Only one duplication pair PtrHox32/59 showed the Ka/Ks ratio larger than 0.4 (Table 2). Another paralogous gene pair PtrHox2/20, which were located out of segmental duplication blocks established in current Populus genome assembly (v2.1), had the Ka/Ks ratio slightly larger than 0.5. The relatively higher Ka/Ks ratio of PtrHox2/20 suggests that they may have experienced relatively rapid evolution following duplication. Based on the Ka/Ks analyses, we could conclude that the Populus HD-ZIP gene family have mainly experienced strong purifying selection pressure with limited functional divergence occurred after segmental duplications. However, it still remains unknown whether the duplicated HD-ZIP genes correspond to genetic redundancy or have evolved divergent functions. Based on the divergence rate of 9.1×10−9 synonymous mutations per synonymous site per year as previously proposed for Populus [74], duplications of these 28 paralogous pairs was estimated to have occurred between 9.75 to 24.96 million year (MY) ago (Table 2).
Table 2. The Ka/Ks ratios and estimated divergence time for paralogous HD-ZIP proteins.
| Paralogous pairs | Ka | Ks | Ka/Ks | Duplication date (MY) |
| PtrHox1–PtrHox19 | 0.054 | 0.217 | 0.248 | 11.91 |
| PtrHox10–PtrHox26 | 0.074 | 0.314 | 0.235 | 17.23 |
| PtrHox12–PtrHox50 | 0.046 | 0.207 | 0.221 | 11.39 |
| PtrHox13–PtrHox51 | 0.027 | 0.275 | 0.099 | 15.12 |
| PtrHox14–PtrHox52 | 0.049 | 0.308 | 0.158 | 16.91 |
| PtrHox15–PtrHox53 | 0.029 | 0.284 | 0.102 | 15.62 |
| PtrHox2–PtrHox20 | 0.196 | 0.360 | 0.543 | 19.79 |
| PtrHox21–PtrHox43 | 0.033 | 0.244 | 0.135 | 13.39 |
| PtrHox22–PtrHox58 | 0.116 | 0.296 | 0.393 | 16.27 |
| PtrHox23–PtrHox39 | 0.047 | 0.212 | 0.219 | 11.64 |
| PtrHox24–PtrHox33 | 0.042 | 0.278 | 0.150 | 15.25 |
| PtrHox25–PtrHox34 | 0.083 | 0.219 | 0.379 | 12.03 |
| PtrHox27–PtrHox35 | 0.122 | 0.399 | 0.306 | 21.90 |
| PtrHox28–PtrHox62 | 0.144 | 0.454 | 0.316 | 24.96 |
| PtrHox3–PtrHox18 | 0.040 | 0.367 | 0.110 | 20.15 |
| PtrHox30–PtrHox57 | 0.060 | 0.314 | 0.190 | 17.23 |
| PtrHox31–PtrHox61 | 0.020 | 0.219 | 0.092 | 12.04 |
| PtrHox32–PtrHox59 | 0.097 | 0.197 | 0.492 | 10.83 |
| PtrHox36–PtrHox42 | 0.054 | 0.198 | 0.271 | 10.85 |
| PtrHox37–PtrHox41 | 0.050 | 0.313 | 0.158 | 17.20 |
| PtrHox4–PtrHox17 | 0.040 | 0.191 | 0.208 | 10.50 |
| PtrHox47–PtrHox54 | 0.031 | 0.223 | 0.141 | 12.27 |
| PtrHox48–PtrHox55 | 0.042 | 0.342 | 0.122 | 18.78 |
| PtrHox49–PtrHox56 | 0.063 | 0.178 | 0.357 | 9.75 |
| PtrHox5–PtrHox16 | 0.017 | 0.224 | 0.076 | 12.32 |
| PtrHox6–PtrHox40 | 0.081 | 0.273 | 0.296 | 15.01 |
| PtrHox7–PtrHox44 | 0.025 | 0.190 | 0.131 | 10.43 |
| PtrHox8–PtrHox45 | 0.119 | 0.369 | 0.322 | 20.27 |
Gene structure and conserved motifs of Populus HD-ZIP genes
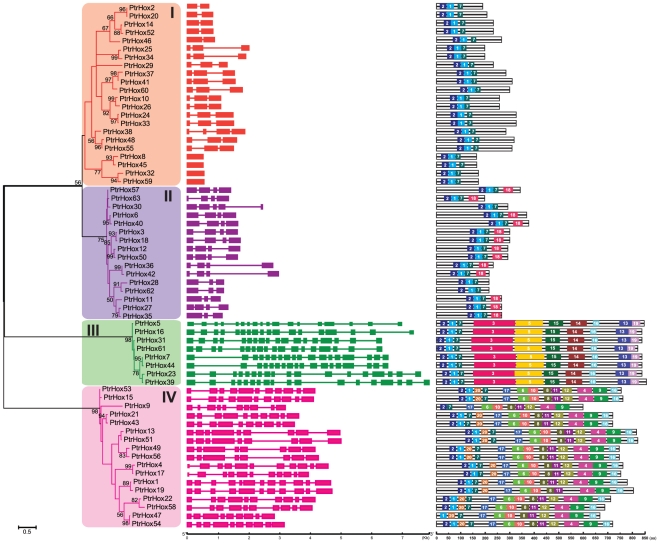
To gain further insights into the structural diversity of Populus HD-ZIP genes, we first constructed a separate phylogenetic tree exclusively using the full-length HD-ZIP protein sequences of Populus. Populus HD-ZIP proteins were also classified into four independent subfamilies as described above (Fig. 7A and Fig. 1A). We then compared the exon/intron organization in the coding sequences of each Populus HD-ZIP genes (Fig. 7B). Most closely related Populus HD-ZIP members within the same subfamilies shared very similar gene structure in terms of either intron numbers or exon lengths (Fig. 7B), i.e, all Populus HD-ZIP I gene had two or three introns, and HD-ZIP III possessed similar number of introns (as many as 17) in their coding sequences. Nonetheless, the gene structures in Populus HD-ZIP subfamilies II and IV appeared to be more variable and displayed the largest number of exon/intron structure variants, i.e., four Populus HD-ZIP II members had no introns in their coding regions but the other HD-ZIP II possessed one to three introns, and HD-ZIP IV members had a large variation from 7 to 11. We also investigated intron phases with respect to codons. Although the intron phases were remarkably well-conserved within the same subfamilies, there were striking distinctions in the arrangement of introns and intron phases among subfamilies of Populus HD-ZIP I–IV (Fig. S1). The conservation of intron phases within Populus HD-ZIP subfamilies and the striking dissimilarity between subfamilies may reciprocally lend supports to the results from phylogenetic analysis and genome duplication.
Figure 7. Phylogenetic relationship, gene structure and motif compositions of Populus HD-ZIP genes.
A. The phylogenetic tree was constructed using full-length protein sequences by the maximum likelihood method with 100 bootstrap replicates. The percentage bootstrap scores higher than 50% are indicated on the nodes. The four major phylogenetic subfamilies designated as I to IV are marked with different color backgrounds. B. Exon/intron structures of HD-ZIP genes from Populus. Exons and introns are represented by green boxes and black lines, respectively. The sizes of exons and introns are proportional to their sequence lengths. C. Schematic representation of the conserved motifs in the HD-ZIP proteins from Populus elucidated by MEME. Each motif is represented by a number in the colored box. The details of individual motif are provided in Table S4.
We further examined the exon/intron organization of 25 paralogous pairs in Populus HD-ZIP genes to inquire the information of traceable intron gain or loss within these genes. Although 22 paralogous pairs showed conserved exon/intron structures in either intron numbers or gene lengths, three paralogous pairs (PtrHox27/35, 30/57, and 47/54) exhibited certain degrees of variations (Fig. 7B). These differences might be derived from the single intron loss or gain event during the structural evolution of HD-ZIP paralogues.
To further reveal the diversification of Populus HD-ZIP genes, we predicted the conserved motifs using MEME motif detection software and 20 distinct motifs were identified (Table S4). The details of the 20 putative motifs were referred in Table S4. Most of the closely related members in the phylogenetic tree shared common motif compositions with each other, suggesting functional similarities among the HD-ZIP proteins within the same subfamily (Fig. 7C). However, the biological significance of most of the putative motifs remains to be elucidated as they do not have homologues when searching against Pfam (http://pfam.sanger.ac.uk/search) and SMART (Simple Modular Architecture Research Tool) databases (http://smart.embl-heidelberg.de). As illustrated in the previous studies, most of the HD-ZIP proteins possessed HD and LZ domains at the N termini. In this study, motif 1 and 2 specifying HD helices and motif 7 corresponding to the ZIP subdomain were present in all of the HD-ZIP family members in Populus. Motif 3 corresponding to the START domain was present in the HD-ZIP III subfamily proteins. The CPSCE motif (motif 18) was found in the majority members in the HD-ZIP II subfamily with the exception of PtrHox28, 57, and 62. The conserved motifs 13 and 19 representing MEKHLA were found to be distributed in the C termini of HD-ZIP III proteins. Members in HD-ZIP I and II possessed significantly reduced number (3–4) of conserved motifs compared to those in HD-ZIP III and IV subfamily (8–10). Particularly, only three motifs (1, 2, and 7) were present in HD-ZIP I proteins, suggesting of high sequence divergence in the other regions of the proteins.
Differential expression profile of Populus HD-ZIP genes
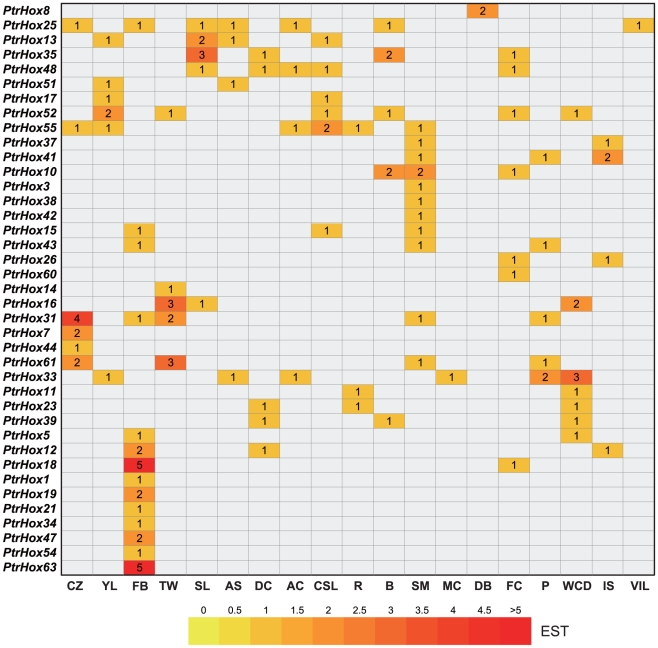
Publicly available Expressed Sequence Tags (ESTs) provide a useful tool to survey gene expression profiles by the means of Digital Northern. We first carried out a preliminary analysis of HD-ZIP gene expression under various growth conditions and across different tissues by counting the frequencies of ESTs in different Populus cDNA libraries (Fig. 8). Completely searching of the digital expression profiles from PopGenIE (http://www.popgenie.org/) [75] yield a total of 39 Populus HD-ZIP genes in the cDNA libraries. Not surprisingly and consistent with the usual transcriptional low-abundances of transcription factors [61], the frequencies of these ESTs were relatively low, and most of the HD-ZIPs were represented by only one single EST in the cDNA libraries. Nevertheless, these expression profiles demonstrated that most of the HD-ZIPs have a broad expression pattern across different tissues.
Figure 8. In sillico EST analysis of Populus HD-ZIP genes.
EST frequency for each gene was calculated by evaluating its EST representation among 19 cDNA libraries available at PopGenIE (http://www.popgenie.org/) [75]. The heatmap was visualized using Heatmapper Plus tool by counting the corresponding ESTs for particular gene in the database. Color bar at bottom represents the frequencies of EST counts. CZ: cambial zone, YL: young leaves, FB: flower buds, TW: tension wood, SL: senescing leaves, AS: apical shoot, DC: dormant cambium, AC: active cambium, CSL: cold stressed leaves, R: roots, B: bark, SM: shoot meristem, MC: male catkins, DB: dormant buds, FC: female catkins, P: petioles, WCD: wood cell death, IS: imbibed seeds, VIS: Virus/fungus-infected leaves.
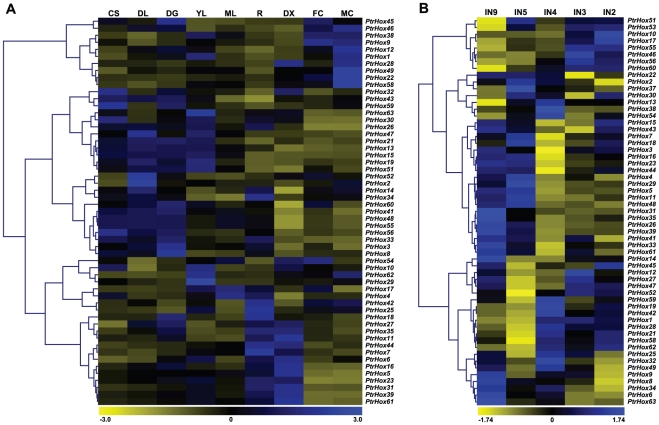
To gain more insights into the expression profiles of HD-ZIP genes, we then re-analyzed the previously published microarray data in Populus. We first investigated the global expression profiles of HD-ZIP genes by examining an Affymetrix (GSE13990) [61] and a Nimblegen (GSE13043) [76] microarray data from Gene Expression Omnibus [77]. Although these two microarray dataset were performed in different platforms, they largely represented the Populus HD-ZIP genes presenting in this study. Fifty-four HD-ZIP genes were included on both GSE13990 and GSE13043, and only three additional genes (PtrHox37, 53, and 55) were solely present on GSE13043 (Table S5). The majority of HD-ZIP genes showed a distinct tissue-specific expression pattern (Fig. 9). Of these 57 HD-ZIPs examined, ten genes presented in both microarray datasets had high transcript accumulation in the differentiating xylems and basal stems (internode 9) undergoing secondary growth. Phylogenetic analysis further showed that eight genes (PtrHox5, 7, 16, 23, 31, 39, 44, and 61) in this subset fell into HD-ZIP III subfamily and the other two genes (PtrHox11 and 35) into HD-ZIP II (Fig. 3). In Arabidopsis and rice, HD-Zip III genes have been functionally well-characterized as developmental regulators of the apical embryo patterning, shoot meristem formation and vascular differentiation [29], [59]. Among them, two closely related members, ATHB8 and CNA/ATHB15/ICU4, have been functionally characterized to regulate vascular patterning and procambial development [30], [32], [36]. In comparison, the eight Populus genes from HD-ZIP III subfamily identified in the present study showed preferentially high expression levels in secondary xylem, indicative of their putative roles in the regulation of secondary growth in Populus. Recently, two orthologous genes of REV (PRE/PtrHox23) and CNA/ATHB15/ICU4 (PCN/PtrHox5) were functionally characterized in Populus respectively, with specific roles in the secondary cell wall formation and cell fate determination [54], [55]. However, the functional roles of the rest HD-ZIP III genes in Populus remain to be elucidated. Besides the large proportion of HD-ZIP genes were highly transcribed in the secondary growth tissues, another 11 genes comprising of four HD-ZIP I genes (PtrHox46, 47, 55, and 60), three HD-ZIP II genes (PtrHox28, 42, and 62) and four HD-ZIP IV genes (PtrHox1, 19, 53, and 56) showed comparatively higher transcript abundances in tissues undergoing primary growth in the upper stem (internode 2 to internode 4) (Fig. 9B). How these Populus HD-ZIPs perform their functional roles in the primary growth and secondary cell wall formation remains to be elucidated and further functional analyses will be required to understand their biological roles in Populus.
Figure 9. Expression profiles of Populus HD-ZIP genes across different tissues.
Background corrected expression intensities were log-transformed and visualized as heatmaps (see Materials and Methods). A. Heatmap showing hierarchical clustering of 54 PtrHox genes across various tissues analyzed. The Affymetrix microarray data were obtained from NCBI Gene Expression Omnibus (GEO) database under the series accession number GSE13990. CL, continuous light-grown seedling; DL, etiolated dark-grown seedling transferred to light for 3 h; DS, dark-grown seedlings; YL, young leaf; ML, mature leaf; R, root; DX, differentiating xylem; FC, female catkins; MC, male catkins. B. Heatmap showing hierarchical clustering of 57 PtrHox genes at different stem development/growth stages. The NimbleGen microarray data were obtained from NCBI GEO database under the series accession number GSE17230. IN2-IN9, stem internodes 2 to stem internodes 9. Color scale represents log2 expression values, yellow represents low level and blue indicates high level of transcript abundances.
Populus HD-ZIP may also involve in other biological processes, such as male and female catkin differentiation, root specification and photosynthetic response. Seventeen HD-ZIP genes were preferentially expressed in male and female catkins (Fig. 9A), of which, 11 genes had the highest transcript abundances in male catkins (MC), and six genes in female catkins (FC). Another subset of 11 HD-ZIP genes, comprising of five HD-ZIP I (PtrHox8, 14, 26, 33, and 34), four HD-ZIP II (PtrHox11, 18, 27, and 35), and two HD-ZIP III genes (PtrHox7 and 44) displayed biased expression in root tissue. In addition, four HD-ZIP genes (PtrHox2, 18, 45, and 47) were differentially expressed in dark-grown etiolated seedlings (DG) and continuous light grown seedlings (CL), suggesting their putative roles in photoperiodic regulation.
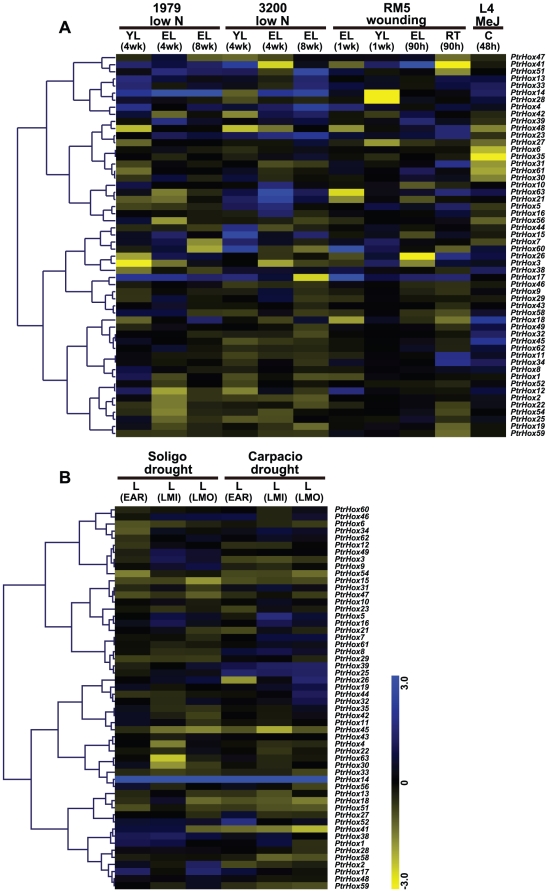
To further investigate the responses of Populus HD-ZIP genes to abiotic stresses, we also examined their expression patterns under abiotic stresses including low nitrogen, mechanical wounding, drought, as well as Methyl Jasmonate (MeJ) treatment. Gene expression of most HD-ZIP genes were induced or suppressed under these abiotic stresses (Fig. 10). PtrHox14 in HD-ZIP I subfamily was commonly up-regulated under both nitrogen deprivation and drought stress treatments in two different Populus genotypes. However, four genes (PtrHox11, 22, 54, and 59) were down-regulated under nitrogen deprivation stress with four-week old young leaves (YL), with four-week and eight-week old expanded leaves (EL) in both genotypes 1979 and 3200 (Fig. 10A).
Figure 10. Differential expression of Populus HD-ZIP genes under different abiotic stresses.
Expression is indicated as fold-change of experimental treatments relative to control samples and visualized in heatmaps (see Materials and Methods). Color scale represents log2 expression values, yellow represents low level and blue indicates high level of transcript abundances. A. Heatmap showing hierarchical clustering of 54 PtrHox genes across various tissues and genotypes analyzed. Microarray data under the series accession number GSE16786 was obtained from NCBI GEO database. Genotypes analyzed included: P. fremontii×angustifolia clones 1979, 3200, and RM5, P. tremuloides clones 271 and L4, and Populus deltoids clones Soligo and Carpaccio. Tissues analyzed included: YL, young leaves; EL, expanding leaves; ML, mature leaves; RT, root tips; C, suspension cell cultures. Stress treatments included: low N, nitrogen limitation; MeJ, Methyl Jasmonate elicitation; wounding, sampled either one week or 90 hours after wounding. B. Heatmap showing hierarchical clustering of 54 PtrHox genes under short-term and long-term water deficit. Microarray data under the series accession number GSE17230 was obtained from NCBI GEO database. EAR, early response (EAR) to water deficit by 36 hours; LMI, long-term (10-day) response to mild stress with soil relative extractable water (REW) at 20–35%; LMO, long-term (10-day) response to moderate stress with soil relative extractable water (REW) at 10–20%.
The responses of HD-ZIP genes to nitrogen deficit stress differ between the two Populus genotypes examined. For instance, three genes namely PtrHox1, PtrHox8 and PtrHox12 were significantly up-regulated at four-week old leaves in genotype 1979, whereas no distinctive expression patterns were observed in genotype 3200 (Fig. 10A). Mechanical wounding stress caused commonly up-regulation of two genes (PtrHox17 and 23) and down-regulation of PtrHox27 at 90 h and/or one week after wounding in young leaves, expanding leaves and root tips. In addition, a subset of genes showed up- or down-regulation only under mechanical wounding stress with young leaves. Similarly, transcripts of a considerable proportion of genes were either enhanced or repressed following mechanical wounding in expanded leaves or root tips, respectively (Fig. 10A). In response to MeJ feeding in cell culture, two genes (PtrHox18 and 45) were shown to be significantly up-regulated, whereas the transcripts of seven genes (PtrHox6, 30, 31, 35, 48, 56, and 61) showed down-regulation (Fig. 10A). In responses to drought stress, eight genes including three genes from HD-ZIP I (PtrHox33, 45, and 54), one gene from HD-ZIP II (PtrHox6) as well as four genes in HD-ZIP IV subfamilies (PtrHox15, 47, 51, and 58) were shown to be down-regulated in both genotypes under all drought conditions tested, which included an early response (EAR) to water deficit by 36 hours and long-term (10-day) response to mild stress (LMI) and moderate stress (LMO) (Fig. 10B).
The high proportion of segmental duplication of HD-ZIP genes and the preferential retention of duplicates raises the question about their functional redundancy. Duplicate genes may have different evolutionary fates: nonfunctionalization, neofunctionalization, or subfunctionalization, which may be indicated with divergence in their expression patterns. Of the 28 paralogous pairs of HD-ZIP genes, five genes do not have corresponding probe sets in the microarray datasets and thus were excluded in further analysis. Twenty-one pairs out of the remaining 23 paralogous pairs were located onto duplicated blocks. Five paralogous pairs (PtrHox5/16, PtrHox7/44, PtrHox13/51, PtrHox23/39, and PtrHox31/61) derived from segmental duplications shared almost identical expression patterns with respect to different tissues and various stresses. In contrast, the expression patterns of three paralogous pairs (PtrHox8/45, PtrHox21/43, and PtrHox22/58) diversified significantly, indicating substantial neofunctionalization during subsequent evolution processes. For instance, PtrHox8 gene showed the highest transcript abundances in dark-grown etiolated seedlings and roots, and the least expressions in male and female catkins, whereas its duplicated counterpart PtrHox45 was preferentially expressed in female catkins and dark-grown etiolated seedlings. Although the expression patterns of the rest of duplicate genes were partially redundant, distinct pattern shifts can be discerned with respect to the microarray datasets investigated, suggests that they might have undergone subfunctionalization. These findings indicated that expression profiles of HD-ZIPs have diverged substantially after segmental duplications, thus we are attempting to speculate that the HD-ZIP genes in Populus are likewise to have been retained by substantial subfunctionalization during the evolutionary processes.
Examination of HD-ZIP gene expressions by qRT-PCR
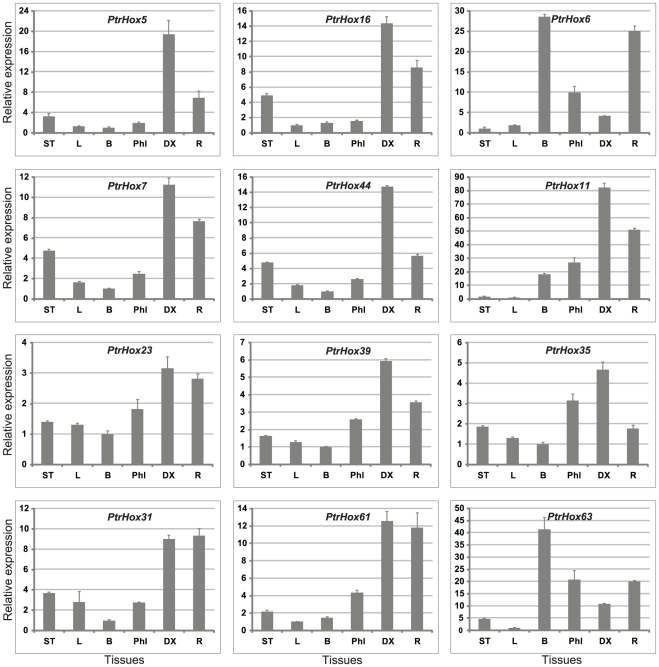
To verify the expression profiles of Populus HD-ZIP genes obtained by the microarray analysis, qRT-PCR analysis was performed on six different tissues for 12 selected HD-ZIP genes, including four paralogous pairs (PtrHox5/16, PtrHox7/44, PtrHox23/39, and PtrHox31/61) that were presumably highly expressed in developing xylem based on microarray analysis. Sequence-specific primers were used to distinguish the amplicons of the paralogous pairs. The gene expression pattern detected by qRT-PCR was roughly in consistency with the microarray analysis and had very distinct tissue-specific expression pattern (Fig. 11). A subset of ten genes were highly expressed in differentiating xylem and weakly expressed in cortex and leaves. Among them, PtrHox5 (PCN) and PtrHox23 (PRE) have been recently functionally characterized [54], [55].
Figure 11. Expression analysis of 12 selected HD-ZIP genes using qRT-PCR.
The relative mRNA abundance of 12 selected HD-ZIP genes was normalized with respect to two reference genes UBQ10 and UKN1 (Populus orthologue of Arabidopsis AT4G33380) in six different tissues. Bars represent standard deviations (SD) of three technical replicates. ST, shoot tips; L, leaves from 4–6 stem internodes; Phl, phloem; DX, differentiating xylem; R, roots; B, bark.
Paralogous pairs with divergent expression patterns could represent the events of sub- or neo-functionalization that may lead to evolutionary diversifications of gene functionalities. To test this hypothesis, we first investigated the divergence of gene expression in the duplicated PtrHox gene pairs to reveal the role of segmental duplications. Four paralogous gene pairs we examined showed similar tissue-specific expression patterns. PtrHox5/16 and PtrHox7/44 were most abundantly expressed in differentiating xylems, followed by roots and shoot tips, and were least expressed in cortex and leaves tissues. PtrHox31/PtrHox61 genes showed the highest transcript abundances in both differentiating xylems and roots with much lower expressions in cortex and leaf tissues. PtrHox23/PtrHox39 genes were expressed much higher in differentiating xylems and roots than the other tissues. Meanwhile, Ka/Ks ratios of these four paralogous pairs were relatively low with ratios 0.076 for PtrHox5/16, 0.092 for PtrHox31/61, 0.131 for PtrHox7/44 and 0.219 for PtrHox23/39 (Table 2). This tissue-specific expression pattern, combined with the Ka/Ks analysis (Table 2), suggests that the functions of Populus HD-ZIP III genes may have been retained with a relatively low divergence.
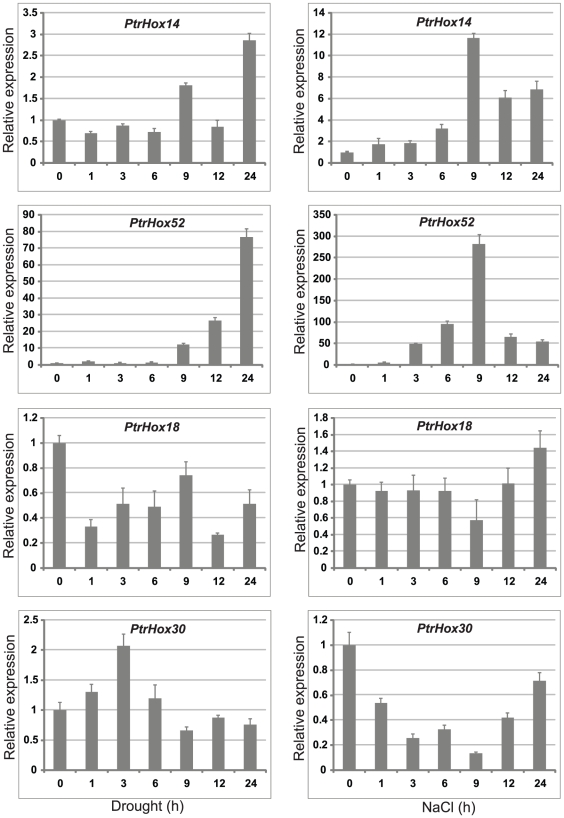
Besides the tissue-specific gene expression pattern, the response of four HD-ZIP genes to drought and salinity stresses was also analyzed by qRT-PCR and the results were broadly consistent with those of the microarray data (Fig. 12 and Fig. 10). Response of PtrHox18 gene was significantly decreased in leaves at different time-points following dehydration stress, while no obvious differential expressions were observed in a 24-hour time-course following salinity stress treatment. PtrHox30 gene was significantly induced in the first three hours following dehydration stress and then decreased gradually at four time points thereafter, whereas the expression of PtrHox30 decreased significantly following NaCl treatment with the lowest expression at nine hours after treatment. Similar to microarray analysis, the transcript abundances of paralogous pair PtrHox14/52, orthologous to Arabidopsis ATHB7 involving in ABA-related and abiotic stress responses [13], [15], [16], were both significantly induced in Populus leaves 24 hours after dehydration stress and both showed the highest accumulation in leaves nine hours after the treatment of high salinity stress.
Figure 12. Expression analysis of four selected HD-ZIP genes under drought and salinity stresses using qRT-PCR.
The relative mRNA abundance of four selected HD-ZIP genes was normalized with respect to two reference genes UBQ10 and PP2a in drought and salinity stress treatments. Bars represent standard deviations (SD) of three technical replicates. X-axis is time courses of stress treatments for each gene.
Taken all of the evidences together, the expression patterns of Populus HD-ZIP genes detected by qRT-PCR are generally consistent with microarray analyses nonetheless two different Populus genotypes (Populus deltoids and Populus×euramericana cv) were used.
Materials and Methods
Ethics Statement
No specific permits were required for the described field studies. No specific permissions were required for these locations and activities. The location is not privately-owned or protected in any way and the field studies did not involve endangered or protected species.
Database search and sequence retrieval
Sequences of Arabidopsis HD-ZIP proteins were obtained from the Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org/, release 10.0). Rice HD-ZIP gene sequences were downloaded from rice genome annotation database (http://rice.plantbiology.msu.edu/, release 5.0). Sequences of Populus, Medicago, Sorghum, Brachypodium, Vitis, and Physcomitrella patens were downloaded from Phytozome (http://www.phytozome.net/). Local blast was performed using Arabidopsis HD-ZIP protein as queries for the identification of the HD-ZIP genes from Populus and seven other plant species. For the misannotated genes, manual reannotation was performed using online web server FGENESH (http://linux1.softberry.com/berry.phtml). Then, all the sequences were further manually analyzed to confirm the presence of HD and LZ domain using InterProScan program (http://www.ebi.ac.uk/Tools/InterProScan/).
Phylogenetic analysis
Multiple sequence alignments of the full-length protein sequences were performed by MAFFT (v6.843b) program. The maximum likelihood (ML) phylogenetic tree was constructed using PhyML (v3.0) under the Jones-Taylor-Thornton (JTT) amino acid substitution model, with 100 replicates of bootstrap analysis, estimated proportion of invariable sites, four rate categories, estimated gamma distribution parameter, and optimized starting BIONJ tree [78], [79]. The phylogenetic trees were displayed using MEGA (v5.0) with 50% threshold of branch value [80].
Chromosomal location and gene duplication
Genes were mapped on chromosomes by identifying their chromosomal position provided in the Phytozome database. Identification of segmental duplications resulting from salicoid genome-wide duplications was accomplished based on duplication coordinates from the Populus genome assembly v2.1. Blocks in the same colors represent the homeologous chromosomal segments.
To search for retrotransposons, BioMart online server at Phytozome website (http://www.phytozome.net/) was used to extract the flanking genomic sequences of 10-kb upstream and downstream of each HD-ZIP gene. Sequences were subjected to BLASTX searches against the GenBank non-redundant protein database at National Center for Biotechnology Information (NCBI) using E-value cutoff set to 1e−10. The output file was manually inspected for the presence of transposable elements including retrotransposons (LTR or non-LTR-related) and transposon (MULE, CACTA, hAT, and Heliton).
Calculation of Ka/Ks Values
Amino acid sequences from segmentally duplicated pairs were aligned first by Clustal X v1.83 and the aligned sequences were subsequently transferred into original cDNA sequences using the PAL2NAL program (http://www.bork.embl.de/pal2nal/) [81], which uses the CODEML program of PAML [82] to estimate synonymous (Ks) and nonsynonymous (Ka) substitution rates. Divergence time (T) was calculated using a synonymous mutation rate of λ substitutions per synonymous site per year as T = Ks/2λ (λ = 9.1×10−9 for Populus) [74].
Gene structure analysis
The exon/intron organization for individual HD-ZIP gene was illustrated with Gene structure display server (GSDS) program (http://gsds.cbi.pku.edu.cn/) [83] by alignment of the cDNAs with their corresponding genomic DNA sequences from Phytozome (http://www.phytozome.net/poplar, release 2.1).
Identification of conserved motifs
The program MEME (v4.3.0) (http://meme.sdsc.edu) was used for the elucidation of motifs in 63 deduced Populus HD-ZIP protein sequences. The following parameters were used: number of repetitions - any, maximum number of motifs - 20, and the optimum motif widths were constrained to between 6 and 200 residues. Structural motif annotation was performed using the SMART (http://smart.embl-heidelberg.de) and Pfam (http://pfam.sanger.ac.uk) databases.
EST profiling and microarray analysis
The expression profile for each gene was obtained by evaluating its EST representation among 19 cDNA libraries derived from different tissues and/or developmental stages available at PopGenIE (http://www.popgenie.org/) [75]. The heatmap was visualized using Heatmapper Plus tool at the Bio-Array Resource for Plant Functional Genomics (http://bar.utoronto.ca/ntools/cgi-bin/ntools_heatmapper_plus.cgi) [84].
The microarray data for various tissues/organs and developmental stages available at NCBI Gene Expression Omnibus (GEO) database [77] under the series accession numbers GSE13990 and GSE13043 were used for the tissue-specific expression analysis. The series GSE13990 includes Affymetrix microarray data from nine different tissue samples representing three biological replicates [61], whereas series GSE13043 contains NimbleGen microarray data from five stem internodes from the apical bud to the base of the shoot (internode 2 to internode 5, and internode 9) in two biological replicates [76]. For Affymetrix microarray data GSE13990, the Affymetrix CEL files representing nine tissues/organs as well as photoperiodic treatments were downloaded from GEO database at NCBI and imported into GeneSpring GX (V11.5) software (Agilent Technologies) for further analysis. The data was normalized by the Gene Chip Robust Multiarray Analysis (GCRMA) algorithm followed by log transformation and average calculation. After normalization and log transformation of data for all the Populus genes present on the chip, the log signal intensity values for Populus probe IDs corresponding to HD-ZIP gene model (v1.1) (Table 1 and Table S5) were extracted as a subset for further analyses. The tab-delimited files for the average log signal intensity values were imported into Genesis program (v1.75) to generate heatmaps [85]. Hierarchical clustering was performed based on Pearson coefficients with average linkage rule. NimbleGen array data GSE13043 were normalized using the NimbleGen microarray data processing pipeline (NMPP) [86]. Each gene model is represented by three replicated 60 mer isothermal probes on the array. Background hybridization intensity for determining expressed genes was estimated using signal intensity of negative control probes on the array. Gene-level analyses were performed using the mean normalized fluorescence values for all probes and replicates. The log signal intensity values for Populus probe IDs corresponding to HD-ZIP gene model (v1.1) (Table 1 and Table S5) were extracted as a subset for further analyses. The tab-delimited files for the average log signal intensity values were imported into Genesis program (v1.75) to generate heatmaps [85]. Clustering of gene expression was performed using hierarchical algorithm based on Pearson correlations.
For abiotic and hormone treatments, Affymetrix microarray data available at NCBI GEO database under the series accession numbers GSE17230 (drought stress) and GSE17686 were analyzed [87], [88]. GSE17686 is composed of the following five subset series: GSE14893 (nitrogen limitation, genotype 1979), GSE14515 (nitrogen limitation, genotype 3200), GSE16783 (one week after leaf wounding), GSE16785 (90 hours after leaf wounding) and GSE16773 (methyl jasmonate-elicited suspension cell cultures). The Affymetrix CEL files representing different abiotic and hormone treatments were downloaded from GEO database at NCBI and preprocessed by using GeneSpring GX (V11.5) software (Agilent Technologies). The data was normalized by GCRMA algorithm followed by log transformation and average calculation. After normalization and log transformation of data for all the Populus genes present on the chip, the log signal intensity values for Populus probe IDs corresponding to HD-ZIP gene model (v1.1) (Table 1 and Table S5) were extracted as a subset for further analyses. Expression was indicated as fold change of experimental treatments relative to control samples. The tab-delimited files for the average log signal intensity values were imported into Genesis program (v1.75) to generate heatmaps [85]. Hierarchical clustering was performed based on Pearson coefficients with average linkage rule.
Probe sets corresponding to HD-ZIP genes were identified using an online Probe Match tool available at POParray (http://aspendb.uga.edu/poparray). For probe sets matching several Populus HD-ZIP gene models, only those exhibited the highest hybridization signals consistently across multiple samples were considered. The list of probe sets corresponding to Populus HD-ZIP genes was provided in Table S5.
Plant material and growth conditions
Plant material was collected from clonally propagated one-year-old Populus deltoides grown in the growth camber under long day conditions (16 h light/8 h dark) at 25–28°C. Shoot tip from stem internodes 1–3, young leaves from stem internodes 4–6, and root tissues were separately collected. Bark with phloem attached from the basal internodes was peeled off of the stem, phloem was scraped from the inside of the bark, and developing xylem was scraped from the outer layers of the wood. All samples were immediately frozen in liquid nitrogen and stored at −80°C until RNA isolation.
The clonally propagated six-month-old Nanlin 895 (Populus×euramericana cv) plants were used in stress treatments. Salt stress was conducted by watering plants with sodium chloride (NaCl) solution at concentration of 200 mM to saturation. For drought treatment, the intact root systems of plants were removed from the pots, washed gently with water to remove soil and then laid down on filter paper with 70–80% humility at 25°C under dime light. Two biological replicates were performed for each stress treatment. After exposure to stresses after 0, 1, 3, 6, 9, 12, and 24 hours, young leaves from three different plants were harvested at various time points, flash frozen in liquid nitrogen, and stored at −80°C for further analysis.
RNA isolation and qRT-PCR
Total RNA from shoot tip, leaf, differentiating xylem, and phloem was extracted using TRIzol reagent (Invitrogen, Ca, USA) according to manufacturer's instructions. Alternatively, total RNA from bark and roots was isolated by CTAB method with minor modifications [89]. RNA integrity was verified by 2% agar gel electrophoresis. Before cDNA synthesis, RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI, USA) according to the manufacturer's instructions to ensure no DNA contamination, and then the first-strand cDNA synthesis was carried out with approximately 2 µg RNA using the RevertAid First Strand cDNA Synthesis Kit (MBI, Fermentas) and oilgo-dT primers according to the manufacturer's procedure. Primers were designed using Beacon Designer v7.0 (Premier Biosoft International, California, USA) with melting temperatures 58–60°C, primer lengths 20–25 bp and amplicon lengths 50–200 bp. All the primer sequences were listed in Table S6.
qRT-PCR was conducted on LightCycler® 480 Detection System (Roche, Penzberg, Germany) using SYBR Premix Ex Taq (TaKaRa, Toyoto, Japan). Reactions were prepared in a total volume of 20 µl containing: 10 µl of 2×SYBR Premix, 2 µl of cDNA template, 0.4 µl of each specific primer to a final concentration of 200 nM. The reactions were performed as the following conditions: initial denaturation step of 95°C for 10 s followed by two-step thermal cycling profile of denaturation at 95°C for 5 s, and combined primer annealing/extension at 60°C for 1 min for 40 cycles. No-template controls were included for each primer pair and each PCR reaction was performed in triplicate. To verify the specificity of the amplicon for each primer pair, a melting curve analysis was performed ranging from 60°C to 95°C with temperature increasing steps of 0.06°C/s (5 acquisitions per °C) at the end of each run. Baseline and threshold cycles (Ct) were automatically determined using the LightCycler® 480 Software release 1.5.0. Relative expression was calculated by the ΔΔCt method [90] using the geometric mean of two reference genes: UBQ10 and UKN1(Populus orthologue of Arabidopsis AT4G33380) for different tissues, UBQ10 and PP2a for abiotic stress treatments. The normalization factor (NF) of two reference genes was calculated and the relative abundance of target genes was analyzed using the geNorm (V3.5) software package [91].
Supporting Information
Exon/intron organization of Populus HD-ZIP genes. Exons and introns are represented by green boxes and black lines, respectively. The numbers indicate the splicing phases of the HD-ZIP genes, 0 refers to phase 0, 1 to phase 1, and 2 to phase 2.
(EPS)
A complete list of Populus HD-ZIP gene sequences identified in the present study. The list comprises of 63 HD-ZIP sequences identified in this study. Amino acid sequences were deduced from their corresponding coding sequences and genomic DNA sequences were obtained from Phytozome (http://www.phytozome.net/poplar, release 2.1).
(XLS)
A list of HD-ZIP protein sequences identified from eight plant species in this study.
(TXT)
Pairwise identities between paralogous pairs of HD-ZIP genes from Populus . Pairwise identities and sequence alignments of the 28 paralogous pairs identified from Populus HD-ZIP gene family.
(XLS)
Sequence logos for the conserved motifs of Populus HD-ZIP proteins. Conserved motifs and the sequence logos were generated using the MEME search tool. Numbers on the horizontal axis represent the sequence positions in the motifs and the vertical axis represents the information content measured in bits. Motif 1 and 2 represents the homeodomain (HD), motif 7 represents the Leucine-Zip domain (LZ), motif 3 represents the START domain, motif 13 and 19 represents MEKHLA, and motif 18 represents the CPSCE.
(XLS)
A list of probes corresponding to Populus HD-ZIP for microarray analysis.
(XLS)
A list of primer sequences of the 16 selected HD-ZIP genes for qRT-PCR analysis.
(XLS)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the National High-Tech Research and Development Program of China (“863” program, 2009AA10Z101 and 2011AA100209), the National Basic Research Program of China (“973” program, 2012CB114501), the National Natural Science Foundation of China (30901157 and 31000311), the Program of 100 Distinguished Young Scientists of the Chinese Academy of Sciences, the Promotive Research Fund for Young and Middle-aged Scientists of Shandong Province (BS2011SW055), and the Qingdao Municipal Science and Technology Plan Project (11-2-4-8-(2)-jch). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, et al. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee K, Brocchieri L, Burglin TR. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol. 2009;26:2775–2794. doi: 10.1093/molbev/msp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruberti I, Sessa G, Lucchetti S, Morelli G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 1991;10:1787–1791. doi: 10.1002/j.1460-2075.1991.tb07703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sessa G, Morelli G, Ruberti I. The Athb-1 and -2 HD-Zip domains homodimerize forming complexes of different DNA binding specificities. EMBO J. 1993;12:3507–3517. doi: 10.1002/j.1460-2075.1993.tb06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank W, Phillips J, Salamini F, Bartels D. Two dehydration-inducible transcripts from the resurrection plant Craterostigma plantagineum encode interacting homeodomain-leucine zipper proteins. Plant J. 1998;15:413–421. doi: 10.1046/j.1365-313x.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 6.Johannesson H, Wang Y, Engstrom P. DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro. Plant Mol Biol. 2001;45:63–73. doi: 10.1023/a:1006423324025. [DOI] [PubMed] [Google Scholar]
- 7.Sessa G, Steindler C, Morelli G, Ruberti I. The Arabidopsis Athb-8, -9 and -14 genes are members of a small gene family coding for highly related HD-ZIP proteins. Plant Mol Biol. 1998;38:609–622. doi: 10.1023/a:1006016319613. [DOI] [PubMed] [Google Scholar]
- 8.Aso K, Kato M, Banks JA, Hasebe M. Characterization of homeodomain-leucine zipper genes in the fern Ceratopteris richardii and the evolution of the homeodomain-leucine zipper gene family in vascular plants. Mol Biol Evol. 1999;16:544–552. doi: 10.1093/oxfordjournals.molbev.a026135. [DOI] [PubMed] [Google Scholar]
- 9.Henriksson E, Olsson AS, Johannesson H, Johansson H, Hanson J, et al. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 2005;139:509–518. doi: 10.1104/pp.105.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson J, Johannesson H, Engstrom P. Sugar-dependent alterations in cotyledon and leaf development in transgenic plants expressing the HDZhdip gene ATHB13. Plant Mol Biol. 2001;45:247–262. doi: 10.1023/a:1006464907710. [DOI] [PubMed] [Google Scholar]
- 11.Soderman E, Hjellstrom M, Fahleson J, Engstrom P. The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol Biol. 1999;40:1073–1083. doi: 10.1023/a:1006267013170. [DOI] [PubMed] [Google Scholar]
- 12.Johannesson H, Wang Y, Hanson J, Engstrom P. The Arabidopsis thaliana homeobox gene ATHB5 is a potential regulator of abscisic acid responsiveness in developing seedlings. Plant Mol Biol. 2003;51:719–729. doi: 10.1023/a:1022567625228. [DOI] [PubMed] [Google Scholar]
- 13.Soderman E, Mattsson J, Engstrom P. The Arabidopsis homeobox gene ATHB-7 is induced by water deficit and by abscisic acid. Plant J. 1996;10:375–381. doi: 10.1046/j.1365-313x.1996.10020375.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Oh HS, Cheon CI, Hwang IT, Kim YJ, et al. Structure and expression of the Arabidopsis thaliana homeobox gene Athb-12. Biochem Biophys Res Commun. 2001;284:133–141. doi: 10.1006/bbrc.2001.4904. [DOI] [PubMed] [Google Scholar]
- 15.Hjellstrom M, Olsson ASB, Engstrom P, Soderman EM. Constitutive expression of the water deficit-inducible homeobox gene ATHB7 in transgenic Arabidopsis causes a suppression of stem elongation growth. Plant, Cell & Environment. 2003;26:1127–1136. [Google Scholar]
- 16.Olsson AS, Engstrom P, Soderman E. The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol. 2004;55:663–677. doi: 10.1007/s11103-004-1581-4. [DOI] [PubMed] [Google Scholar]
- 17.Himmelbach A, Hoffmann T, Leube M, Hohener B, Grill E. Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J. 2002;21:3029–3038. doi: 10.1093/emboj/cdf316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Henriksson E, Soderman E, Henriksson KN, Sundberg E, et al. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Dev Biol. 2003;264:228–239. doi: 10.1016/j.ydbio.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Aoyama T, Dong CH, Wu Y, Carabelli M, Sessa G, et al. Ectopic expression of the Arabidopsis transcriptional activator Athb-1 alters leaf cell fate in tobacco. Plant Cell. 1995;7:1773–1785. doi: 10.1105/tpc.7.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YK, Son O, Kim MR, Nam KH, Kim GT, et al. ATHB23, an Arabidopsis class I homeodomain-leucine zipper gene, is expressed in the adaxial region of young leaves. Plant Cell Rep. 2007;26:1179–1185. doi: 10.1007/s00299-007-0340-9. [DOI] [PubMed] [Google Scholar]
- 21.Tahir M, Belmonte MF, Elhiti M, Flood H, Stasolla C. Identification and characterization of PgHZ1, a novel homeodomain leucine-zipper gene isolated from white spruce (Picea glauca) tissue. Plant Physiol Biochem. 2008;46:1031–1039. doi: 10.1016/j.plaphy.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, et al. The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant Mol Biol. 2008;68:465–478. doi: 10.1007/s11103-008-9383-8. [DOI] [PubMed] [Google Scholar]
- 23.Tron AE, Bertoncini CW, Chan RL, Gonzalez DH. Redox regulation of plant homeodomain transcription factors. J Biol Chem. 2002;277:34800–34807. doi: 10.1074/jbc.M203297200. [DOI] [PubMed] [Google Scholar]
- 24.Carabelli M, Morelli G, Whitelam G, Ruberti I. Twilight-zone and canopy shade induction of the Athb-2 homeobox gene in green plants. Proc Natl Acad Sci U S A. 1996;93:3530–3535. doi: 10.1073/pnas.93.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J. 1998;14:603–611. doi: 10.1046/j.1365-313x.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 26.Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, et al. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J. 2002;32:1011–1022. doi: 10.1046/j.1365-313x.2002.01488.x. [DOI] [PubMed] [Google Scholar]
- 27.Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, et al. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- 28.Dezar CA, Giacomelli JI, Manavella PA, Re DA, Alves-Ferreira M, et al. HAHB10, a sunflower HD-Zip II transcription factor, participates in the induction of flowering and in the control of phytohormone-mediated responses to biotic stress. J Exp Bot. 2011;62:1061–1076. doi: 10.1093/jxb/erq339. [DOI] [PubMed] [Google Scholar]
- 29.Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, et al. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, et al. The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001;126:643–655. doi: 10.1104/pp.126.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Green KA, Prigge MJ, Katzman RB, Clark SE. CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell. 2005;17:691–704. doi: 10.1105/tpc.104.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, et al. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi-Ito K, Fukuda H. HD-zip III homeobox genes that include a novel member, ZeHB-13 (Zinnia)/ATHB-15 (Arabidopsis), are involved in procambium and xylem cell differentiation. Plant Cell Physiol. 2003;44:1350–1358. doi: 10.1093/pcp/pcg164. [DOI] [PubMed] [Google Scholar]
- 35.Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001;25:223–236. doi: 10.1046/j.1365-313x.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhong R, Ye ZH. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- 38.Schrick K, Nguyen D, Karlowski WM, Mayer KF. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 2004;5:R41. doi: 10.1186/gb-2004-5-6-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee K, Burglin TR. MEKHLA, a novel domain with similarity to PAS domains, is fused to plant homeodomain-leucine zipper III proteins. Plant Physiol. 2006;140:1142–1150. doi: 10.1104/pp.105.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, et al. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;121:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Jung JH, Reyes JL, Kim YS, Kim SY, et al. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005;42:84–94. doi: 10.1111/j.1365-313X.2005.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong R, Ye ZH. Regulation of HD-ZIP III Genes by MicroRNA 165. Plant Signal Behav. 2007;2:351–353. doi: 10.4161/psb.2.5.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MC. microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature. 2004;428:84–88. doi: 10.1038/nature02363. [DOI] [PubMed] [Google Scholar]
- 44.Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, et al. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5′ region. EMBO J. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McHale NA, Koning RE. MicroRNA-directed cleavage of Nicotiana sylvestris PHAVOLUTA mRNA regulates the vascular cambium and structure of apical meristems. Plant Cell. 2004;16:1730–1740. doi: 10.1105/tpc.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura M, Katsumata H, Abe M, Yabe N, Komeda Y, et al. Characterization of the class IV homeodomain-Leucine Zipper gene family in Arabidopsis. Plant Physiol. 2006;141:1363–1375. doi: 10.1104/pp.106.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rerie WG, Feldmann KA, Marks MD. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994;8:1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- 48.Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, et al. The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J. 1996;10:393–402. doi: 10.1046/j.1365-313x.1996.10030393.x. [DOI] [PubMed] [Google Scholar]
- 49.Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, et al. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- 50.Ohashi Y, Oka A, Ruberti I, Morelli G, Aoyama T. Entopically additive expression of GLABRA2 alters the frequency and spacing of trichome initiation. Plant J. 2002;29:359–369. doi: 10.1046/j.0960-7412.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- 51.Abe M, Katsumata H, Komeda Y, Takahashi T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 2003;130:635–643. doi: 10.1242/dev.00292. [DOI] [PubMed] [Google Scholar]
- 52.Shen B, Sinkevicius KW, Selinger DA, Tarczynski MC. The homeobox gene GLABRA2 affects seed oil content in Arabidopsis. Plant Mol Biol. 2006;60:377–387. doi: 10.1007/s11103-005-4110-1. [DOI] [PubMed] [Google Scholar]
- 53.Kubo H, Peeters AJ, Aarts MG, Pereira A, Koornneef M. ANTHOCYANINLESS2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. Plant Cell. 1999;11:1217–1226. doi: 10.1105/tpc.11.7.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robischon M, Du J, Miura E, Groover A. The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiol. 2011;155:1214–1225. doi: 10.1104/pp.110.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du J, Miura E, Robischon M, Martinez C, Groover A. The Populus Class III HD ZIP transcription factor POPCORONA affects cell differentiation during secondary growth of woody stems. PLoS One. 2011;6:e17458. doi: 10.1371/journal.pone.0017458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agalou A, Purwantomo S, Overnas E, Johannesson H, Zhu X, et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol Biol. 2008;66:87–103. doi: 10.1007/s11103-007-9255-7. [DOI] [PubMed] [Google Scholar]
- 57.Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 58.Prigge MJ, Clark SE. Evolution of the class III HD-Zip gene family in land plants. Evol Dev. 2006;8:350–361. doi: 10.1111/j.1525-142X.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 59.Itoh J, Hibara K, Sato Y, Nagato Y. Developmental role and auxin responsiveness of Class III homeodomain leucine zipper gene family members in rice. Plant Physiol. 2008;147:1960–1975. doi: 10.1104/pp.108.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cote CL, Boileau F, Roy V, Ouellet M, Levasseur C, et al. Gene family structure, expression and functional analysis of HD-Zip III genes in angiosperm and gymnosperm forest trees. BMC Plant Biol. 2010;10:273. doi: 10.1186/1471-2229-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkins O, Nahal H, Foong J, Provart NJ, Campbell MM. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009;149:981–993. doi: 10.1104/pp.108.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barakat A, Bagniewska-Zadworna A, Choi A, Plakkat U, DiLoreto DS, et al. The cinnamyl alcohol dehydrogenase gene family in Populus: phylogeny, organization, and expression. BMC Plant Biol. 2009;9:26. doi: 10.1186/1471-2229-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalluri UC, Difazio SP, Brunner AM, Tuskan GA. Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 2007;7:59. doi: 10.1186/1471-2229-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu R, Qi G, Kong Y, Kong D, Gao Q, et al. Comprehensive Analysis of NAC Domain Transcription Factor Gene Family in Populus trichocarpa. BMC Plant Biol. 2010;10:145. doi: 10.1186/1471-2229-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barakat A, Choi A, Yassin NB, Park JS, Sun Z, et al. Comparative genomics and evolutionary analyses of the O-methyltransferase gene family in Populus. Gene. 2011;479:37–46. doi: 10.1016/j.gene.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Zhuang J, Cai B, Peng RH, Zhu B, Jin XF, et al. Genome-wide analysis of the AP2/ERF gene family in Populus trichocarpa. Biochem Biophys Res Commun. 2008;371:468–474. doi: 10.1016/j.bbrc.2008.04.087. [DOI] [PubMed] [Google Scholar]
- 67.Tuominen LK, Johnson VE, Tsai CJ. Differential Phylogenetic Expansions in BAHD Acyltransferases Across Five Angiosperm Taxa and Evidence of Divergent Expression Among Populus Paralogues. BMC Genomics. 2011;12:236. doi: 10.1186/1471-2164-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindsey KTuominen, Virgil EJohnson, Tsai C-J. Differential Phylogenetic Expansions in BAHD Acyltransferases Across Five Angiosperm Taxa and Evidence of Divergent Expression Among Populus Paralogues. BMC Genomics. 2011;12:236. doi: 10.1186/1471-2164-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanc G, Wolfe KH. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell. 2004;16:1679–1691. doi: 10.1105/tpc.021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanc G, Wolfe KH. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 2004;16:1667–1678. doi: 10.1105/tpc.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maere S, De Bodt S, Raes J, Casneuf T, Van Montagu M, et al. Modeling gene and genome duplications in eukaryotes. Proc Natl Acad Sci U S A. 2005;102:5454–5459. doi: 10.1073/pnas.0501102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prince VE, Pickett FB. Splitting pairs: the diverging fates of duplicated genes. Nat Rev Genet. 2002;3:827–837. doi: 10.1038/nrg928. [DOI] [PubMed] [Google Scholar]
- 73.Vandepoele K, Simillion C, Van de Peer Y. Evidence that rice and other cereals are ancient aneuploids. Plant Cell. 2003;15:2192–2202. doi: 10.1105/tpc.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 75.Sjodin A, Street NR, Sandberg G, Gustafsson P, Jansson S. The Populus Genome Integrative Explorer (PopGenIE): a new resource for exploring the Populus genome. New Phytol. 2009;182:1013–1025. doi: 10.1111/j.1469-8137.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- 76.Dharmawardhana P, Brunner AM, Strauss SH. Genome-wide transcriptome analysis of the transition from primary to secondary stem development in Populus trichocarpa. BMC Genomics. 2010;11:150. doi: 10.1186/1471-2164-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barrett T, Edgar R. Gene expression omnibus: microarray data storage, submission, retrieval, and analysis. Methods Enzymol. 2006;411:352–369. doi: 10.1016/S0076-6879(06)11019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 79.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 80.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011 doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609–612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 83.Guo AY, Zhu QH, Chen X, Luo JC. [GSDS: a gene structure display server]. Yi Chuan. 2007;29:1023–1026. [PubMed] [Google Scholar]
- 84.Toufighi K, Brady SM, Austin R, Ly E, Provart NJ. The Botany Array Resource: e-Northerns, Expression Angling, and promoter analyses. Plant J. 2005;43:153–163. doi: 10.1111/j.1365-313X.2005.02437.x. [DOI] [PubMed] [Google Scholar]
- 85.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, He H, Li L, Chen R, Deng XW, et al. NMPP: a user-customized NimbleGen microarray data processing pipeline. Bioinformatics. 2006;22:2955–2957. doi: 10.1093/bioinformatics/btl525. [DOI] [PubMed] [Google Scholar]
- 87.Cohen D, Bogeat-Triboulot MB, Tisserant E, Balzergue S, Martin-Magniette ML, et al. Comparative transcriptomics of drought responses in Populus: a meta-analysis of genome-wide expression profiling in mature leaves and root apices across two genotypes. BMC Genomics. 2010;11:630. doi: 10.1186/1471-2164-11-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan Y, Chung JD, Fu X, Johnson VE, Ranjan P, et al. Alternative splicing and gene duplication differentially shaped the regulation of isochorismate synthase in Populus and Arabidopsis. Proc Natl Acad Sci U S A. 2009;106:22020–22025. doi: 10.1073/pnas.0906869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang S, Puryear J, C J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:113–116. [Google Scholar]
- 90.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 91.geNorm website. Available: [ http://medgen.ugent.be/~jvdesomp/genorm/]. Accessed 2011 May 20.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exon/intron organization of Populus HD-ZIP genes. Exons and introns are represented by green boxes and black lines, respectively. The numbers indicate the splicing phases of the HD-ZIP genes, 0 refers to phase 0, 1 to phase 1, and 2 to phase 2.
(EPS)
A complete list of Populus HD-ZIP gene sequences identified in the present study. The list comprises of 63 HD-ZIP sequences identified in this study. Amino acid sequences were deduced from their corresponding coding sequences and genomic DNA sequences were obtained from Phytozome (http://www.phytozome.net/poplar, release 2.1).
(XLS)
A list of HD-ZIP protein sequences identified from eight plant species in this study.
(TXT)
Pairwise identities between paralogous pairs of HD-ZIP genes from Populus . Pairwise identities and sequence alignments of the 28 paralogous pairs identified from Populus HD-ZIP gene family.
(XLS)
Sequence logos for the conserved motifs of Populus HD-ZIP proteins. Conserved motifs and the sequence logos were generated using the MEME search tool. Numbers on the horizontal axis represent the sequence positions in the motifs and the vertical axis represents the information content measured in bits. Motif 1 and 2 represents the homeodomain (HD), motif 7 represents the Leucine-Zip domain (LZ), motif 3 represents the START domain, motif 13 and 19 represents MEKHLA, and motif 18 represents the CPSCE.
(XLS)
A list of probes corresponding to Populus HD-ZIP for microarray analysis.
(XLS)
A list of primer sequences of the 16 selected HD-ZIP genes for qRT-PCR analysis.
(XLS)