Abstract
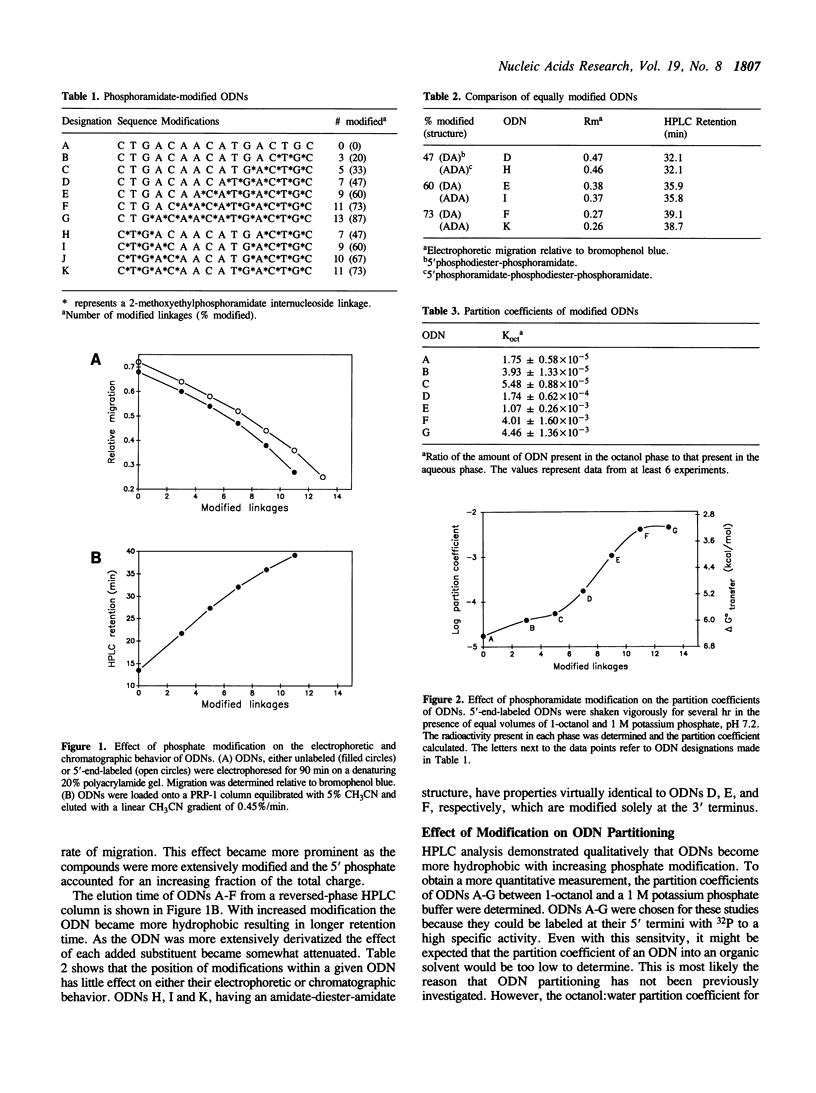
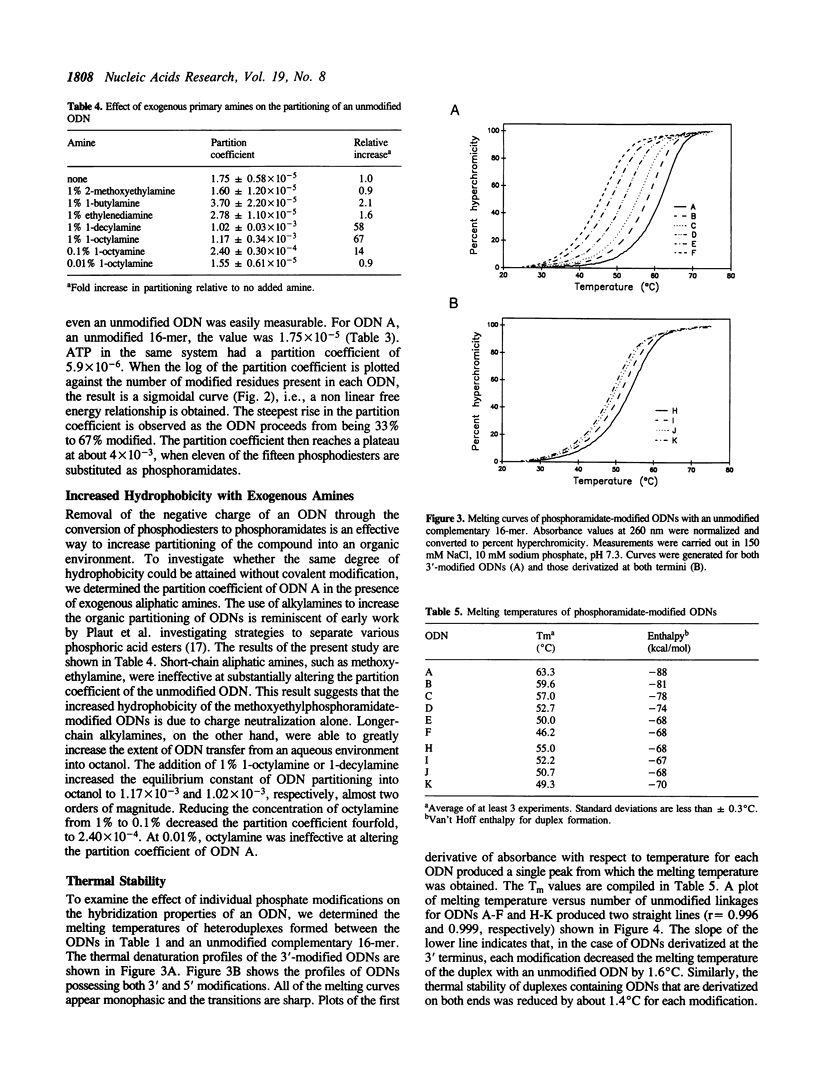
Because of their nuclease resistance and ability to form substrates for RNase H, antisense oligodeoxynucleotides (ODNs) possessing several methoxyethylphosphoramidate linkages at both termini have proven effective at targeting the degradation of specific mRNAs in Xenopus embryos. The efficacy of these compounds subsequently observed in tissue culture focused our attention on the issue of cellular uptake. To investigate the extent to which phosphate backbone modifications may increase the lipophilicity of ODNs, and thereby increase passive uptake by cells, the partitioning of a series of phosphoramidate-modified compounds between aqueous and organic phases was examined. The octanol:water partition coefficient of an unmodified, mixed-sequence 16-mer was 1.75 x 10(-5). The log of the partition coefficient increased in a sigmoidal manner with the number of methoxyethylphosphoramidate internucleoside linkages, indicating a nonlinear free energy relationship. The highest level of partitioning demonstrated was approximately 4 x 10(-3) (a 230-fold increase), attained when 11 of the 15 phosphodiesters were modified. An increase in hydrophobicity was also attained with C8 and C10 alkylamines acting as phase-transfer agents. The melting temperatures of heteroduplexes formed between a phosphoramidate-modified ODN and a complementary unmodified DNA strand decreased by approximately 1.5 degrees C for every phosphate group modification. ODNs can thus be extensively derivatized without substantially compromising duplex formation under physiological conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Mayrand S. H., Zamecnik P. C., Pederson T. Site-specific excision from RNA by RNase H and mixed-phosphate-backbone oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1401–1405. doi: 10.1073/pnas.87.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Walder J. A., Weeks D. L. Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis. Nucleic Acids Res. 1990 Aug 25;18(16):4751–4757. doi: 10.1093/nar/18.16.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagle J. M., Weeks D. L., Walder J. A. Pathways of degradation and mechanism of action of antisense oligonucleotides in Xenopus laevis embryos. Antisense Res Dev. 1991 Spring;1(1):11–20. doi: 10.1089/ard.1991.1.11. [DOI] [PubMed] [Google Scholar]
- Froehler B., Ng P., Matteucci M. Phosphoramidate analogues of DNA: synthesis and thermal stability of heteroduplexes. Nucleic Acids Res. 1988 Jun 10;16(11):4831–4839. doi: 10.1093/nar/16.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. P., Jordan J. C., Mandel P. Coefficients de partage d'oligoribonucléotides dans les systèmes solvants salins. J Chromatogr. 1972 May 3;67(2):277–290. doi: 10.1016/s0021-9673(01)91230-0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Barrett J. C., Ts'o P. O. Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid. Biochemistry. 1974 Nov 19;13(24):4887–4896. doi: 10.1021/bi00721a003. [DOI] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]
- PLAUT G. W. E., KUBY S. A., LARDY H. A. Systems for the separation of phosphoric esters by solvent distribution. J Biol Chem. 1950 May;184(1):243–249. [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]



