Abstract
Leptin exerts its action by binding to and activating the long form of leptin receptors (LEPRb). LEPRb activates JAK2 that subsequently phosphorylates and activates STAT3. The JAK2/STAT3 pathway is required for leptin control of energy balance and body weight. Defects in leptin signaling lead to leptin resistance, a primary risk factor for obesity. Body weight is also regulated by nutrients, including glucose. Defects in glucose sensing also contribute to obesity. Here we report crosstalk between leptin and glucose. Glucose starvation blocked the ability of leptin to stimulate tyrosyl phosphorylation and activation of JAK2 and STAT3 in a variety of cell types. Glucose dose-dependently enhanced leptin signaling. In contrast, glucose did not enhance growth hormone-stimulated phosphorylation of JAK2 and STAT5. Glucose starvation or 2-deoxyglucose-induced inhibition of glycolysis activated AMPK and inhibited leptin signaling; pharmacological inhibition of AMPK restored the ability of leptin to stimulate STAT3 phosphorylation. Conversely, pharmacological activation of AMPK was sufficient to inhibit leptin signaling and to block the ability of glucose to enhance leptin signaling. These results suggest that glucose and/or its metabolites play a permissive role in leptin signaling, and that glucose enhances leptin sensitivity at least in part by attenuating the ability of AMPK to inhibit leptin signaling.
Introduction
Leptin is a metabolic hormone that is required for the maintenance of normal energy balance and body weight [1]. Leptin is secreted into the bloodstream from adipose tissues [2]. It suppresses food intake, increases energy expenditure, and promotes weight loss primarily by binding to and activating LEPRb in the hypothalamus [1]. Genetic deficiency of leptin or LEPRb results in hyperphagia and severe obesity in both rodents and humans [2], [3], [4], [5], [6]. However, circulating leptin levels increase under most obesity conditions [7], [8]; furthermore, diet-induced obesity is associated with a reduced ability of leptin to suppress both food intake and body weight gain [1], [7], [9]. Leptin resistance is believed to be the primary risk factor for obesity [1]; however, the underlying molecular mechanisms for leptin resistance remain largely unknown.
Defects in leptin signaling are likely to be a major contributor to leptin resistance [1]. LEPRb is a cytokine receptor family member that binds to Janus kinase 2 (JAK2), a cytoplasmic tyrosine kinase [1]. Upon leptin binding, LEPRb activates its associated JAK2 that in turn phosphorylates LEPRb at multiple tyrosines, including Tyr985, Tyr1077, and Tyr1138 [10], [11]. Phosphorylated Tyr985 binds to SHP2, leading to activation of the ERK pathway [11], [12], [13]. Brain-specific deletion of SHP2 results in leptin resistance and obesity, suggesting that the LEPRb/SHP2/ERK pathway is required for a full leptin action [14]. Phospho-Tyr985 also binds to SOCS3, which negatively regulates leptin signaling [15]. Phospho-Tyr1077 binds to Signal Transducer and Activator of Transcription (STAT) 5 [16], [17]. Phospho-Tyr1138 recruits STAT3, thus allowing JAK2 to phosphorylate and activate STAT3 [12]. The JAK2/STAT3 pathway is the best-understood pathway in leptin action. Brain specific deletion of STAT3 results in leptin resistance and obesity in mice [18]. Replacement of Tyr1138 with Phe or Ala also results in severe leptin resistance and obesity [19], [20]. These observations indicate that the JAK2/STAT3 pathway is required for leptin regulation of energy balance and body weight.
Energy balance and body weight is also regulated by nutrients, including glucose [21]. Hypothalamic glucopenia (glucose deficit) promotes feeding [22], [23]. Glucose is a metabolic fuel that is metabolized to generate ATP through glycolysis and the tricarboxylic acid cycle. Glucose or its metabolites serve as biosynthetic precursors for lipids and various glycoproteins. Additionally, glucose and its metabolites are also involved in cell signaling. Glucose inhibits KATP channels in hypothalamic neurons, thus increasing cell excitability [24], [25]. Glucose starvation stimulates 5′-AMP-activated protein kinase (AMPK), a molecular energy sensor, in many cell types, including hypothalamic neurons [26], [27]. Glucose-sensing neurons are located in multiple regions in the central nervous system (CNS), including the hypothalamus, brain stem, and amygdala, and hypothalamic glucose sensing is believed to play an important role in energy homeostasis [21], [28]. Glucose directly excites POMC neurons, but inhibits AgRP neurons, in the arcuate nucleus of the hypothalamus [28]. Leptin also stimulates POMC neurons and inhibits AgRP neurons, two subpopulations of hypothalamic neurons that play a key role in the control of energy balance and body weight [1]. Glucose regulates not only the excitability of glucose-sensing neurons but also biochemical events in these cells. In POMC neurons, glucose increases the levels of hypoxia-inducible factors that in turn stimulate POMC expression and reduce body weight [29]. Therefore, hypothalamic neurons are likely to integrate signals from both leptin and glucose, thus controlling energy balance and body weight. In the current study, we show that glucose plays a permissive role in leptin signaling. Glucose promotes leptin pathways at least in part by inhibiting AMPK.
Results
Glucose plays a permissive role in leptin stimulation of STAT3 phosphorylation
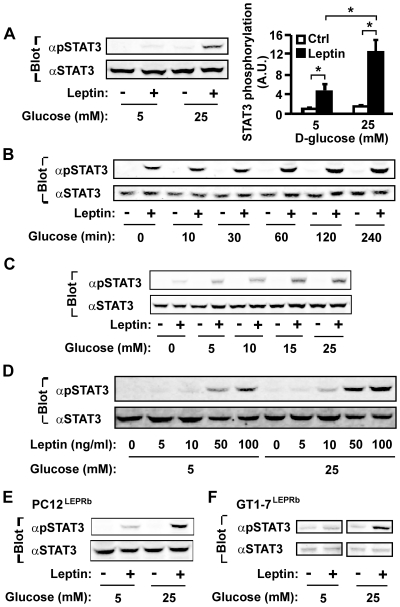
We have generated and characterized human fibrosarcoma (γ2ALEPRb/JAK2) that stably expresses LEPRb and JAK2 [30]. To determine whether glucose levels affect leptin signaling, γ2ALEPRb/JAK2 cells were grown overnight in serum-free medium supplemented with either 5 mM or 25 mM D-glucose and treated with 100 ng/ml leptin for 10 min. Tyrosyl phosphorylation of STAT3 in cell extracts was examined by immunoblotting with anti-phospho-STAT3 (pTyr705) antibody (αpSTAT3). At 5 mM glucose, leptin modestly stimulated STAT3 phosphorylation; in contrast, leptin robustly stimulated STAT3 phosphorylation at 25 mM glucose (Fig. 1A). Glucose enhanced leptin signaling in both time- and dose-dependent manners. Glucose enhancement was detected within 30 min and progressively increased for 4 h (Fig. 1B). Leptin-stimulated STAT3 Tyr705 phosphorylation also increased progressively from 5–25 mM glucose (Fig. 1C). To determine whether glucose increases leptin sensitivity, we examined leptin dose-responses at 5 or 25 mM D-glucose. Glucose increased leptin-stimulated STAT3 Tyr705 phosphorylation at both low (5–10 ng/ml) and high concentrations (50–100 ng/ml) of leptin (Fig. 1D). These data suggest that glucose increases both leptin sensitivity and maximal responses.
Figure 1. Glucose enhances leptin stimulation of STAT3 phosphorylation.
A, γ2ALEPRb/JAK2 cells were grown overnight in serum-free medium supplemented with 5 or 25 mM glucose and treated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with anti-phospho-STAT3 (pTyr705) (αpSTAT3) or αSTAT3 antibodies. The amounts of phospho-STAT3 and total STAT3 were quantified using densitometry, and STAT3 phosphorylation was normalized to the total amount of STAT3. *P<0.05. B, γ2ALEPRb/JAK2 cells were deprived of serum in the presence of 5 mM glucose overnight. Cells were pretreated with 25 mM glucose for 0, 10, 30, 60, 120 or 240 min, and then treated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3. C, γ2ALEPRb/JAK2 cells were deprived of serum overnight in the presence of 0, 5, 10, 15 or 25 mM glucose, and stimulated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3. D, γ2ALEPRb/JAK2 cells were deprived of serum in the presence of 5 or 25 mM glucose overnight, and then stimulated with leptin for 10 min at various concentrations. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3, respectively. E–F, PC12LEPRb neurons (E) and GT1-7LEPRb cells (F) were deprived of serum overnight in 5 or 25 mM glucose and then treated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3.
To determine whether glucose similarly enhances leptin signaling in neurons, PC12LEPRb cells, which stably express LEPRb [31], were differentiated into neurons with nerve growth factor (NGF). The neurons were grown overnight in serum-free medium supplemented with 5 or 25 mM D-glucose and stimulated with 100 ng/ml leptin for 10 min. Leptin stimulated STAT3 Tyr705 phosphorylation modestly at 5 mM glucose but robustly at 25 mM glucose (Fig. 1E). Further reduction in glucose (3 mM) largely abolished leptin signaling (data not shown). To determine whether glucose enhances leptin signaling in hypothalamic neurons, LEPRb was stably introduced into GT1-7 cells (GT1-7LEPRb), a mouse hypothalamic line [32], and leptin signaling was examined at low or high levels of D-glucose. At 5 mM glucose, leptin barely stimulated STAT3 Tyr705 phosphorylation; in contrast, leptin robustly stimulated STAT3 phosphorylation at 25 mM glucose (Fig. 1F). Together, these results demonstrate that glucose plays a permissive role in leptin signaling and dose-dependently enhances leptin signaling.
Glucose enhances leptin stimulation of JAK2
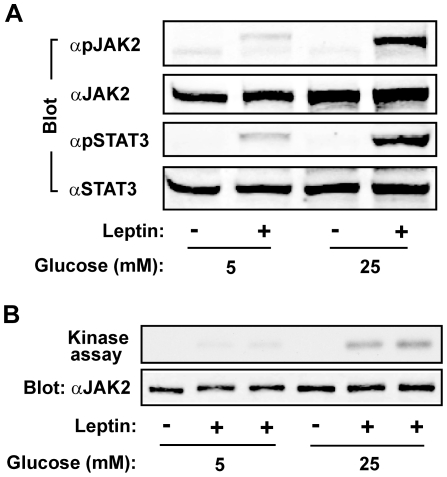
Leptin stimulates STAT3 phosphorylation by both JAK2-dependent and -independent mechanisms [31]. To determine whether glucose is involved in leptin stimulation of JAK2, γ2ALEPRb/JAK2 cells were incubated overnight with either 5 mM or 25 mM D-glucose and treated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with anti-phospho-JAK2 (pTyr1007/1008) antibody. Phosphorylation of Tyr1007 in the activation loop is required for JAK2 activation [33], [34]. 25 mM glucose markedly increased the ability of leptin to stimulate tyrosyl phosphorylation of both JAK2 and STAT3, compared with 5 mM glucose (Fig. 2A). Additionally, JAK2 was immunopurified and its catalytic activity was measured using an in vitro kinase assay. Leptin barely activated JAK2 at 5 mM glucose but robustly stimulated JAK2 at 25 nM glucose (Fig. 2B). These results further support the conclusion that glucose plays a permissive role in leptin signaling.
Figure 2. Glucose enhances leptin stimulation of JAK2.
A, γ2ALEPRb/JAK2 cells were deprived of serum in the presence of 5 or 25 mM glucose overnight and then treated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with anti-phospho-JAK2 (pTyr1007/1008) (αpJAK2), αJAK2, αpSTAT3, or αSTAT3 as designated. B, γ2ALEPRb/JAK2 cells were treated with 5 or 25 mM glucose overnight and then with 100 ng/ml leptin for 10 min. JAK2 in cell extracts was immunoprecipitated with αJAK2 and subjected to an in vitro kinase assay. The same blots were immunoblotted with αJAK2.
Glucose differentially enhances leptin- but not growth hormone (GH)-stimulated JAK2/STAT pathway
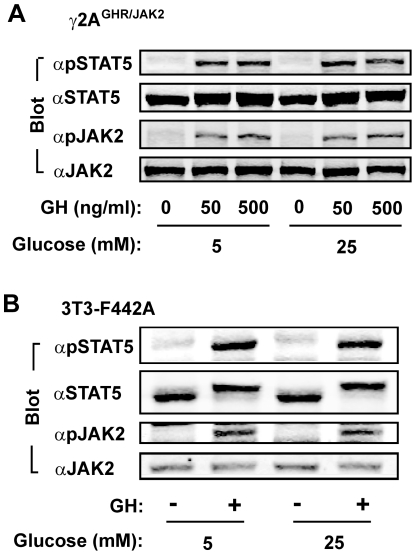
To determine whether glucose similarly enhances the JAK/STAT pathway in response to other hormones, we generated γ2AGHR/JAK2 cells that stably express GH receptors (GHR) and JAK2 [31]. JAK2 binds to GHR and mediates STAT5 tyrosyl phosphorylation and activation in response to GH [35], [36]. γ2AGHR/JAK2 cells were pretreated with 5 mM or 25 mM D-glucose and stimulated with GH for 15 min. Cell extracts were immunoblotted with anti-phospho-STAT5 (pTyr694 of STAT5a or pTyr699 of STAT5b) or anti-phospho-JAK2 (pTyr1007/1008). GH stimulated phosphorylation of both STAT5 and JAK2; surprisingly, glucose did not increase the ability of GH to stimulate phosphorylation of either STAT5 or JAK2 (Fig. 3A). To determine whether glucose enhances endogenous GHR/JAK2/STAT5 pathway, 3T3-F442A cells were grown in the presence of either 5 mM or 25 mM D-glucose and treated with GH. GH stimulated phosphorylation of STAT5 and JAK2 to a similar degree between 5 mM and 25 mM glucose (Fig. 3B). Thus, glucose starvation specifically suppresses leptin stimulation of the JAK2/STAT3 pathway but not GH stimulation of the JAK2/STAT5 pathway. These data also suggest that glucose starvation-induced suppression of leptin signaling is unlikely to be caused by nonspecific inhibition of cell signaling and JAK/STAT pathways.
Figure 3. Glucose does not enhance GH-stimulated phosphorylation of JAK2 and STAT5.
A, γ2AGHR/JAK2 cells were deprived of serum in the presence of 5 or 25 mM glucose overnight and then treated with GH for 15 min. Cell extracts were immunoblotted with αpSTAT5, αSTAT5, αpJAK2 or αJAK2, respectively. B, 3T3-F442A cells were treated with 5 or 25 mM glucose overnight and then with 50 ng/ml GH for 15 min. Cell extracts were immunoblotted with the indicated antibodies.
Glycolysis is required for glucose enhancement of leptin signaling
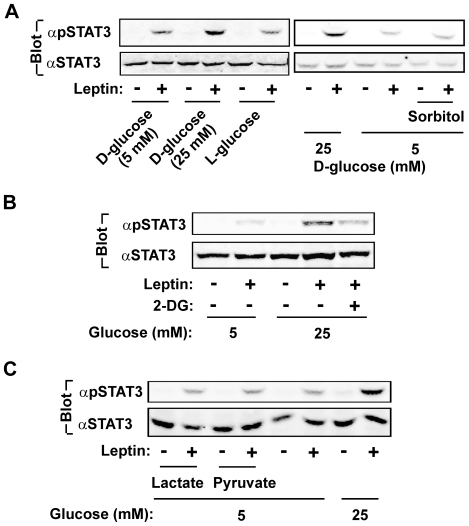
To exclude osmolarity as a contributing factor, γ2ALEPRb/JAK2 cells were grown in 5 mM D-glucose supplemented with additional 20 mM L-glucose, sorbitol, or D-glucose, and stimulated with leptin. D-glucose, but not L-glucose or sorbitol, increased the ability of leptin to stimulate STAT3 Tyr705 phosphorylation (Fig. 4A). L-glucose is unable to be metabolized, raising the possibility that glycolysis is important for glucose to enhance leptin signaling. To test this idea, cells were pretreated with 25 mM D-glucose in the presence or absence of 2-deoxyglucose (2-DG), which blocks glycolysis. 2-DG prevented D-glucose from enhancing leptin stimulation of STAT3 phosphorylation (Fig. 4B). In contrast to D-glucose, neither lactate nor pyruvate enhanced leptin signaling (Fig. 4C). Both lactate and pyruvate are oxidized via the tricarboxylic acid cycle; thus, the tricarboxylic acid cycle is not sufficient to enhance leptin signaling. Therefore, glycolysis is likely to play a permissive role for leptin signaling.
Figure 4. Glycolysis is required for glucose to enhance leptin signaling.
A, γ2ALEPRb/JAK2 cells were grown overnight in serum-free medium supplemented with 25 mM D-glucose, 5 mM D-glucose plus 20 mM L-glucose, or 5 mM D-glucose plus 20 mM sorbitol. Cells were stimulated with 100 ng/ml leptin for 10 min, and cell extracts were immunoblotted with αpSTAT3 or αSTAT3. B, γ2ALEPRb/JAK2 cells were grown overnight in serum-free medium supplemented with 25 mM D-glucose, pretreated with 25 mM 2-DG for 3 h, and then treated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3. C. γ2ALEPRb/JAK2 cells were grown overnight (∼15 h) in serum-free medium containing 5 mM D-glucose plus additional 20 mM lactate, pyruvate, or D-glucose, and stimulated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3.
AMPK mediates glucose regulation of leptin signaling
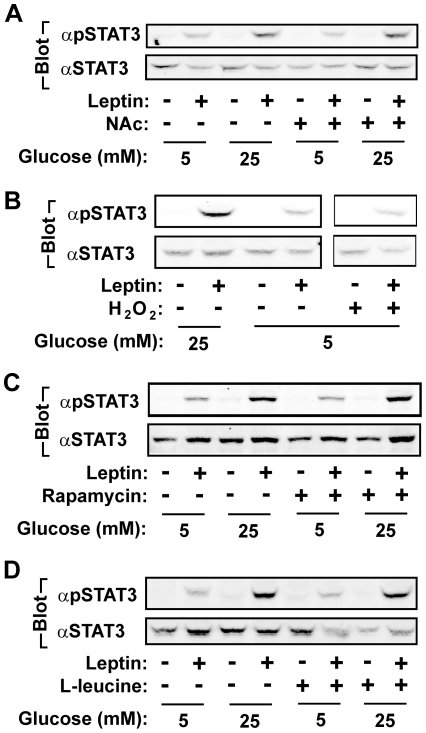
Glucose metabolism links to oxidative stress and the activation of the mTOR pathway. To determine whether reactive oxygen species (ROS) mediate glucose action on leptin signaling, γ2ALEPRb/JAK2 cells were treated with 25 mM D-glucose in the presence of the antioxidant N-acetyl cysteine (NAc) (10 mM). NAc did not inhibit the ability of glucose to enhance leptin-stimulated Tyr705 STAT3 phosphorylation (Fig. 5A). Additionally, H2O2 was unable to mimic glucose action to enhance leptin signaling (Fig. 5B). To examine the mTOR pathway, cells were pretreated with rapamycin, a TORC1 inhibitor. Rapamycin did not decrease the ability of glucose to enhance leptin-stimulated STAT3 phosphorylation (Fig. 5C). In agreement, L-leucine, a potent activator of mTOR, was also unable to enhance leptin signaling (Fig. 5D). These data suggest that oxidative stress and the TORC1 pathway are unlikely to mediate glucose enhancement of leptin signaling.
Figure 5. Oxidative stress, the mTOR pathway, and the p38 MAPK pathway do not mediate glucose enhancement of leptin signaling.
A–B. γ2ALEPRb/JAK2 cells were grown overnight in serum-free medium supplemented with 5 or 25 mM D-glucose, 10 mM NAc (A) or 200 µM H2O2 (B). Cells were stimulated with 100 ng/ml leptin for 10 min, and cell extracts were immunoblotted with αpSTAT3 or αSTAT3. C–D. γ2ALEPRb/JAK2 cells were incubated in 5 or 25 mM glucose overnight, pretreated with 50 nM rapamycin for 1 h (C) or 2 mM L-leucine for 2 h (D), and then stimulated with 100 ng/ml leptin for 10 min. Cell extracts were immunoblotted with αpSTAT3 or αSTAT3.
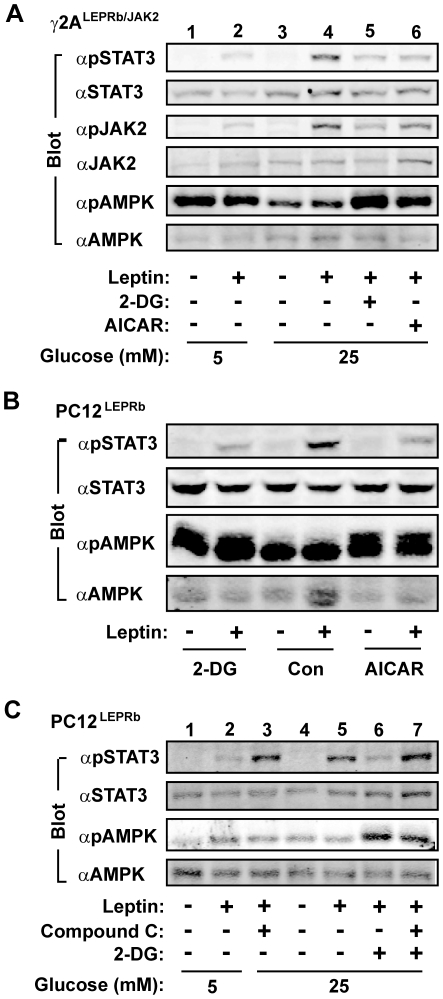
Inhibition of glycolysis by either glucose starvation or 2-DG treatments activates AMPK [37], [38], raising the possibility that AMPK may be involved in D-glucose deficiency-induced suppression of leptin signaling. To test this idea, γ2ALEPRb/JAK2 cells were treated with AICAR, an AMPK activator. As shown in Figures 1A and 2A, 25 mM D-glucose increased leptin-stimulated tyrosyl phosphorylation of STAT3 and JAK2 compared with 5 mM glucose (Fig. 6A, lane 4). Importantly, both 2-DG and AICAR strongly attenuated the ability of glucose to enhance leptin signaling (Fig. 6A, lanes 5–6). Both 2-DG and AICAR stimulated AMPK phosphorylation (pThr172) (Fig. 6A, panel 5); D-glucose deficiency also led to AMPK phosphorylation (Fig. 6A, lanes 1–2, panel 5). AMPK phosphorylation on Thr172 is required for AMPK activity and serves as a marker for estimating its activation [26]. These data suggest that AMPK activation inhibits leptin stimulation of the JAK2/STAT3 pathway, and that glucose provides a permissive condition for leptin signaling at least in part by inhibiting AMPK.
Figure 6. AMPK is involved in glucose enhancement of leptin signaling.
A, γ2ALEPRb/JAK2 cells were deprived of serum overnight (in 25 mM glucose). Cells were treated with 25 mM 2-DG or 2 mM AICAR for 3 h, and then with 100 ng/ml leptin for additional 10 min. Cell extracts were immunoblotted with the indicated antibodies. B, PC12LEPRb neurons were deprived of serum overnight in the presence of 25 mM glucose, and treated with 25 mM 2-DG or 2 mM AICAR for 1 h and then with 100 ng/ml leptin for additional 10 min. Cell extracts were immunoblotted with the indicated antibodies. C, PC12LEPRb neurons were incubated overnight (∼15 h) in the presence or absence of 40 µM compound C. Some cells were pretreated with 2-DG (25 mM) for 1 h as indicated. Cells were stimulated with 100 ng/ml leptin for 10 min, and cell extracts were immunoblotted with the indicated antibodies.
To determine whether AMPK inhibits leptin signaling in neurons, PC12LEPRb cells were differentiated into neurons. Both AICAR and 2-DG suppressed leptin-stimulated tyrosyl phosphorylation of STAT3 at 25 mM glucose in PC12LEPRb neurons (Fig. 6B). To determine whether inhibition of AMPK improves leptin signaling under low glucose conditions, PC12LEPRb neurons were pretreated with compound C, a commonly used AMPK inhibitor, in the presence of 5 mM glucose. Compound C markedly increased the ability of leptin to stimulate STAT3 phosphorylation (Fig. 6C, lanes 2–3). At 25 mM D-glucose, 2-DG suppressed leptin-stimulated phosphorylation of STAT3 (Fig. 6C, lanes 5–6), and compound C reversed 2-DG suppression of leptin signaling (Fig. 6C, lane 7). These data further confirm that AMPK inhibits leptin stimulation of the JAK2/STAT3 pathway, and that glucose enhances leptin signaling at least in part by inhibiting AMPK.
Discussion
Leptin resistance is the primary risk factor for obesity [1]. Upregulation of a number of negative regulators of leptin signaling, including PTP1b and SOCS3, is likely to contribute to leptin resistance [39], [40], [41], [42], [43]. Conversely, downregulation of positive regulators, including SH2B1, may also contribute to leptin resistance [44], [45]. Thus, it is important to identify additional regulators of leptin signaling in order to fully understand energy homeostasis and body weight regulation. Here we have identified glucose and AMPK as new regulators of leptin sensitivity.
Both leptin and glucose, acting on hypothalamic neurons, suppress food intake and weight gain; conversely, leptin deficiency and brain glucopenia promote hyperphagia and weight gain [1], [21]. However, it is unclear whether leptin and glucose exhibit crosstalk in hypothalamic neurons. In this study, we report that glucose is likely to play a permissive role in leptin signaling and to improve leptin sensitivity in a dose-dependent manner. We showed that D-glucose deficiency blocks the ability of leptin to stimulate tyrosyl phosphorylation of JAK2 and STAT3. Additionally, D-glucose enhances leptin signaling in a variety of cell types, including human γ2A fibrosarcoma cells, rat PC12 neurons, and murine GT1-7 hypothalamic neurons. Glycolysis appears to be required for leptin signaling, since blocking glycolysis suppressed leptin signaling and decreased the ability of glucose to increase leptin stimulation of JAK2 and STAT3 phosphorylation. Surprisingly, glucose did not increase the ability of GH to stimulate the JAK2/STAT5 pathway. These observations raise the possibility that glucose or its metabolites may facilitate the coupling between LEPRb and JAK2 but not between GHR and JAK2.
We also identified AMPK as a potential negative regulator of leptin sensitivity. AMPK is a molecular energy sensor that is activated by AMP (associated with low intracellular energy levels) and a variety of hormones and cytokines [26], [27]. Resembling leptin resistance, activation of hypothalamic AMPK increases food intake and body weight in mice; in contrast, blocking hypothalamic AMPK increases the anorexigenic effect of leptin [46], [47]. We observed that at high levels of glucose (25 mM), AMPK activity was suppressed and pharmacological activation of AMPK by either 2-DG or ARCAR markedly reduced the ability of leptin to stimulate tyrosyl phosphorylation of JAK2 and STAT3. Conversely, at low levels of glucose, AMPK was highly activated and leptin signaling was suppressed; pharmacological inhibition of AMPK robustly enhanced leptin signaling. These results suggest that glucose plays a permissive role in the maintenance of normal leptin sensitivity by suppressing AMPK and reversing AMPK inhibition of leptin signaling. Additionally, these observations raise the possibility that AMPK may also mediate crosstalk between leptin and other factors, in addition to glucose, that regulate the AMPK pathway.
In summary, we described novel crosstalk between leptin and glucose. Glycolysis provided a permissive condition for leptin to stimulate the JAK2/STAT3 pathway. We have also identified AMPK as a potential negative regulator of leptin signaling. Glucose enhanced leptin signaling at least in part by inhibiting the ability of AMPK to suppress leptin signaling.
Materials and Methods
Materials
Antibodies against phospho-STAT3(pTyr705), STAT3, STAT5, and AMPKα were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-phospho-AMPK (pThr172) antibody was from Cell Signaling Technology Inc. (Beverly, MA). Anti-JAK2 antibody was from Millipore Corp. (Bedford, MA). Anti-phospho-JAK2 (pTyr1007/1008) and anti-phospho-tyrosine (pY20) antibodies were from Upstate Biotechnology Inc. (Lake Placid, NY). Anti-phospho-STAT5 antibody was obtained from Zymed Labs, Inc. (San Francisco, CA). 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR) and SB203580 were from Calbiochem Bioscience Inc. (La Jolla, CA). Recombinant mouse leptin was from the National Hormone and Peptide Program, NIDDK, National Institutes of Health (Torrance, CA). Compound C and nerve growth factor were from Sigma-Aldrich.
Cell Culture, Neuronal Differentiation, and Immunoblotting
γ2ALEPRb/JAK2, γ2AGHR/JAK2 and PC12LEPRb cell lines have been described previously [31]. GT1-7LEPRb cell lines were derived from mouse hypothalamic tumor cells (GT1-7) [32]. LEPRb was stably introduced into GT1-7 cells via LEPRb retroviral infection [31]. γ2ALEPRb/JAK2 and γ2AGHR/JAK2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 5% heat-inactivated fetal bovine serum (FBS) in the presence of 25 mM glucose, 100 units/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. PC12LEPRb cells were grown on collagen-coated dishes at 37°C in 5% CO2 in DMEM supplemented with 25 mM glucose, 100 units/ml penicillin, 100 µg/ml streptomycin, 10% heat-inactivated horse serum, and 5% FBS. To induce neuronal differentiation, PC12LEPRb cells were cultured for 3 days in DMEM supplemented with 25 mM glucose, 2% horse serum, 1% FBS, 100 ng/ml NGF, 100 units/ml penicillin, and 100 µg/ml streptomycin. For immunoblotting, cells were deprived of serum overnight in DMEM containing 0.6% bovine serum albumin (BSA) in the presence of 5 mM D-glucose supplemented with additional 20 mM D-glucose, L-glucose, sorbitol, pyruvate, or lactate. Cells were pretreated with or without the indicated compounds, and then treated with leptin. Cell extracts were prepared as described previously [48], and immunoblotted with the indicated antibodies. Blots were visualized using Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
In Vitro Kinase Assays
JAK2 in cell extracts was immunoprecipitated with anti-JAK2 antibody and absorbed on protein A-agarose beads. The beads were washed extensively with a kinase reaction buffer (50 mM HEPES, pH 7.6, 10 mM MnCl2, 100 mM NaCl, 0.5 mM dithiothreitol, 1 mM Na3VO4) and then incubated at room temperature for 30 min in the kinase reaction buffer supplemented with [γ-32P]ATP (10 µCi), 20 µM cold ATP, 10 µg/ml aprotinin, and 10 µg/ml leupeptin. The kinase reaction was stopped by washing the beads with lysis buffer. JAK2 was eluted from the beads by boiling for 5 min in the SDS-PAGE sample buffer. JAK2 was resolved by SDS-PAGE and transferred onto nitrocellulose membranes. JAK2 autophosphorylation was detected by autoradiography. The same blots were immunoblotted with anti-JAK2 antibody.
Statistical Analysis
Data are presented as means ± SEM. Differences between groups were analyzed by two-tailed Student's t test. P<0.05 was considered statistically significant.
Acknowledgments
We thank Drs. D. L. Morris and L. Argetsinger for their assistance and discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants RO1-DK065122, RO1-DK073601, and RO1-DK073601 from the National Institutes of Health (NIH); and by a research award 1-09-RA-156 from the American Diabetes Association. This work utilized the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH 5P60 DK20572), the University of Michigan's Cancer Center (funded by NIH 5 P30 CA46592), the University of Michigan Nathan Shock Center (funded by NIH P30AG013283), and the University of Michigan Gut Peptide Research Center (funded by NIH DK34933). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 4.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 6.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 7.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 8.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 9.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–37. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Friedman JM. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. PNAS. 1999;96:9677–9682. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, et al. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci U S A. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang EE, Chapeau E, Hagihara K, Feng G-S. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. PNAS. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 16.Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, et al. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 18.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L, You J, Yu X, Gonzalez L, Yu Y, et al. Tyrosine-dependent and -independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proc Natl Acad Sci U S A. 2008;105:18619–18624. doi: 10.1073/pnas.0804589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campfield LA, Smith FJ. Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol Rev. 2003;83:25–58. doi: 10.1152/physrev.00019.2002. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK, Shin MS, Youn BS, Namkoong C, Gil SY, et al. Involvement of progranulin in hypothalamic glucose sensing and feeding regulation. Endocrinology. 2011;152:4672–4682. doi: 10.1210/en.2011-1221. [DOI] [PubMed] [Google Scholar]
- 23.Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-D-glucose in the rat. Am J Physiol. 1975;229:1438–1447. doi: 10.1152/ajplegacy.1975.229.5.1438. [DOI] [PubMed] [Google Scholar]
- 24.Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, et al. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- 25.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 26.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ruderman NB, Saha AK. Metabolic syndrome: adenosine monophosphate-activated protein kinase and malonyl coenzyme A. Obesity (Silver Spring) 2006;14(Suppl 1):25S–33S. doi: 10.1038/oby.2006.279. [DOI] [PubMed] [Google Scholar]
- 28.Routh VH. Glucose Sensing Neurons in the Ventromedial Hypothalamus. Sensors (Basel) 2010;10:9002–9025. doi: 10.3390/s101009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Zhang G, Gonzalez FJ, Park SM, Cai D. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 2011;9:e1001112. doi: 10.1371/journal.pbio.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Zhou Y, Carter-Su C, Myers MG, Jr, Rui L. SH2B1 Enhances Leptin Signaling by Both Janus Kinase 2 Tyr813 Phosphorylation-Dependent and -Independent Mechanisms. Mol Endocrinol. 2007;21:2270–2281. doi: 10.1210/me.2007-0111. [DOI] [PubMed] [Google Scholar]
- 31.Jiang L, Li Z, Rui L. Leptin Stimulates Both JAK2-dependent and JAK2-independent Signaling Pathways. J Biol Chem. 2008;283:28066–28073. doi: 10.1074/jbc.M805545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Manchon C, Bilezikjian LM, Corrigan AZ, Mellon PL, Vale W. Activin-A modulates gonadotropin-releasing hormone secretion from a gonadotropin-releasing hormone-secreting neuronal cell line. Neuroendocrinology. 1991;54:373–377. doi: 10.1159/000125916. [DOI] [PubMed] [Google Scholar]
- 33.Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, et al. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, et al. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. Embo J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585–2597. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 36.Smit LS, Vanderkuur JA, Stimage A, Han Y, Luo G, et al. Growth hormone-induced tyrosyl phosphorylation and deoxyribonucleic acid binding activity of Stat5A and Stat5B. Endocrinology. 1997;138:3426–3434. doi: 10.1210/endo.138.8.5332. [DOI] [PubMed] [Google Scholar]
- 37.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 38.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 39.Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White CL, Whittington A, Barnes MJ, Wang ZQ, Bray G, et al. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and independent mechanisms. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 42.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 44.Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metabolism. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Ren D, Zhou Y, Morris D, Li M, Li Z, et al. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406. doi: 10.1172/JCI29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 47.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Li Z, Morris DL, Rui L. Identification of SH2B2beta as an inhibitor for SH2B1- and SH2B2alpha-promoted Janus kinase-2 activation and insulin signaling. Endocrinology. 2007;148:1615–1621. doi: 10.1210/en.2006-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]