Abstract
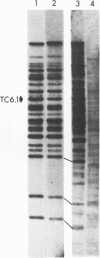
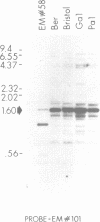
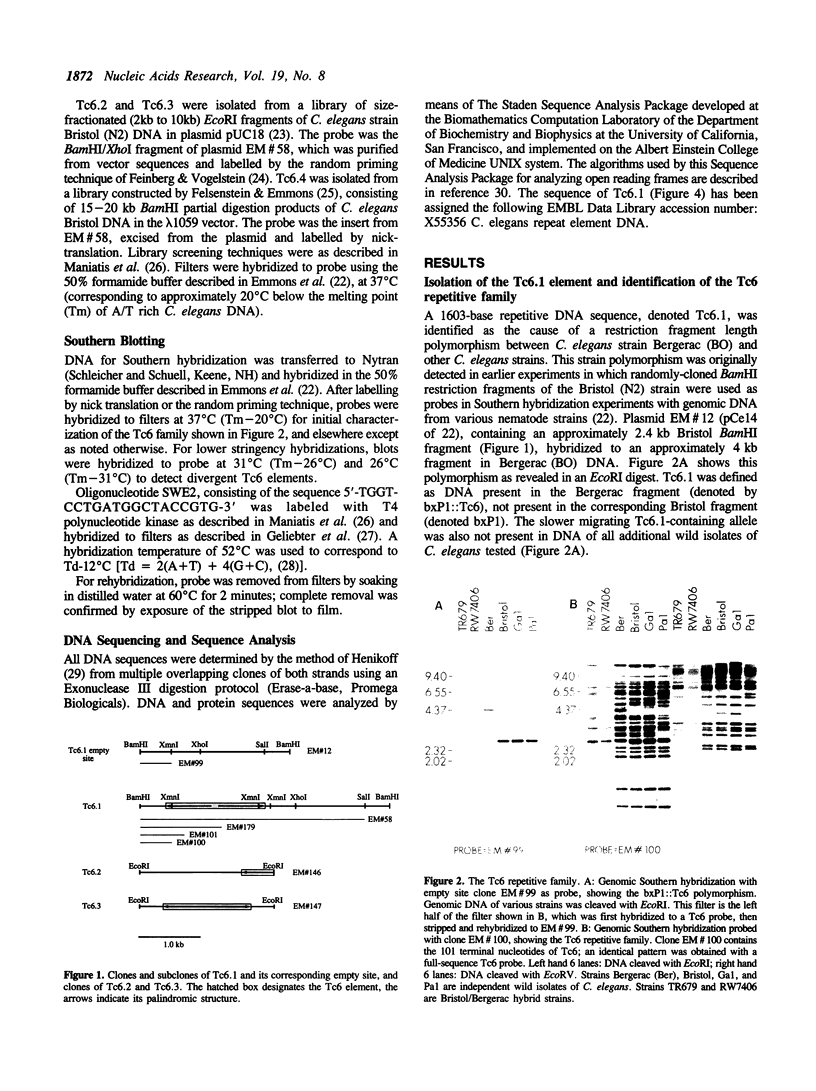
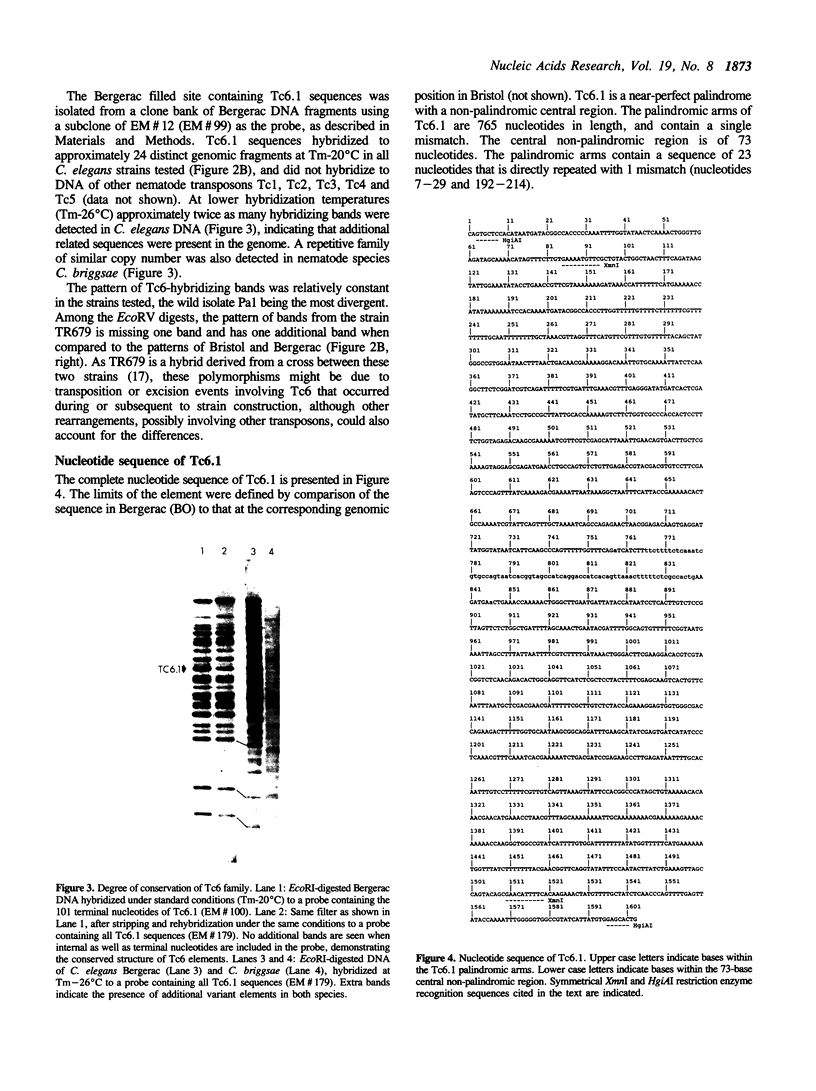
A family of transposon-like sequences in the C. elegans genome is described. This family, termed the Tc6 family, consists mostly of conserved, 1.6 kb elements. Four Tc6 elements or partial elements have been cloned and the DNA sequences of three were determined. One appears to be a complete element of 1603 nucleotides, consisting of a palindrome of 765 nucleotides, with a central, non-palindromic region of 73 nucleotides. Another has an identical structure except for an internal deletion. A third is a partial element terminating at a probable internal restriction site used for cloning. A fourth clone contained portions of the Tc6 sequence juxtaposed to non-Tc6 sequences. All C. elegans strains examined contain 20-30 Tc6 elements. The ends of Tc6 elements are conserved and have sequence similarity to the ends of C. elegans transposons Tc1 and Tc3. The ends of Tc6 elements also have sequence similarity to the heptamer portion of the immunoglobulin and T-cell receptor recombination signal sequence, raising the possibility of wide phylogenetic conservation of the recombination mechanism. Tc6 elements also share sequence motifs with plant-pathogenic viroid RNA's, possibly indicative of a Tc6 RNA replicative phase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezinsky L., Wang G. V., Humphreys T., Hunt J. The transposable element Uhu from Hawaiian Drosophila--member of the widely dispersed class of Tc1-like transposons. Nucleic Acids Res. 1990 Apr 25;18(8):2053–2059. doi: 10.1093/nar/18.8.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley H. L., Potter S. S. Distinct characteristics of loop sequences of two Drosophila foldback transposable elements. Nucleic Acids Res. 1985 Jan 25;13(2):485–500. doi: 10.1093/nar/13.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Forbes E., Anderson P. The Tc3 family of transposable genetic elements in Caenorhabditis elegans. Genetics. 1989 Jan;121(1):47–55. doi: 10.1093/genetics/121.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Saari B., Anderson P. Activation of a transposable element in the germ line but not the soma of Caenorhabditis elegans. Nature. 1987 Aug 20;328(6132):726–728. doi: 10.1038/328726a0. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Dinter-Gottlieb G. Viroids and virusoids are related to group I introns. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6250–6254. doi: 10.1073/pnas.83.17.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L. High-frequency excision of transposable element Tc 1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell. 1984 Mar;36(3):599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L., Ruan K. S., Katzenberg D. Evidence for a transposon in Caenorhabditis elegans. Cell. 1983 Jan;32(1):55–65. doi: 10.1016/0092-8674(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Felsenstein K. M., Emmons S. W. Structure and evolution of a family of interspersed repetitive DNA sequences in Caenorhabditis elegans. J Mol Evol. 1987;25(3):230–240. doi: 10.1007/BF02100016. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Transposable elements in eukaryotes. Int Rev Cytol. 1985;93:281–326. doi: 10.1016/s0074-7696(08)61376-5. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Schulze D. H., Pease L. R., Weiss E. H., Mellor A. L., Flavell R. A., Nathenson S. G. Interaction between Kb and Q4 gene sequences generates the Kbm6 mutation. Mol Cell Biol. 1986 Feb;6(2):645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Baillie D. L., Rose A. M. Sequence identity between an inverted repeat family of transposable elements in Drosophila and Caenorhabditis. Nucleic Acids Res. 1988 Jul 11;16(13):5991–5998. doi: 10.1093/nar/16.13.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Prasad S., Rose A. M. Isolation and sequence analysis of Caenorhabditis briggsae repetitive elements related to the Caenorhabditis elegans transposon Tc1. J Mol Evol. 1990 Apr;30(4):359–369. doi: 10.1007/BF02101890. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Plasterk R. H. Related transposons in C.elegans and D.melanogaster. Nucleic Acids Res. 1988 Jul 11;16(13):6234–6234. doi: 10.1093/nar/16.13.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Owens R. A., Diener T. O. Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6234–6238. doi: 10.1073/pnas.80.20.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D. R., Stahl F. W. Viability of lambda phages carrying a perfect palindrome in the absence of recombination nucleases. 1983 Sep 29-Oct 5Nature. 305(5933):448–451. doi: 10.1038/305448a0. [DOI] [PubMed] [Google Scholar]
- Levitt A., Emmons S. W. The Tc2 transposon in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1989 May;86(9):3232–3236. doi: 10.1073/pnas.86.9.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L. W., Rosenzweig B., Hirsh D. Analysis of a transposable element in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3585–3589. doi: 10.1073/pnas.80.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann D., Hoffman-Liebermann B., Weinthal J., Childs G., Maxson R., Mauron A., Cohen S. N., Kedes L. An unusual transposon with long terminal inverted repeats in the sea urchin Strongylocentrotus purpuratus. Nature. 1983 Nov 24;306(5941):342–347. doi: 10.1038/306342a0. [DOI] [PubMed] [Google Scholar]
- Mori I., Moerman D. G., Waterston R. H. Analysis of a mutator activity necessary for germline transposition and excision of Tc1 transposable elements in Caenorhabditis elegans. Genetics. 1988 Oct;120(2):397–407. doi: 10.1093/genetics/120.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Potter S., Truett M., Phillips M., Maher A. Eucaryotic transposable genetic elements with inverted terminal repeats. Cell. 1980 Jul;20(3):639–647. doi: 10.1016/0092-8674(80)90310-4. [DOI] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]