An 18-year-old man with a three-year history of well-controlled systemic lupus erythematosus experienced new-onset fatigue, malaise and diffuse myalgia before leaving on a camping trip to the midwestern United States. These symptoms began about one week before leaving on the trip and coincided with changes to his maintenance dose of mycophenolate. When the diagnosis was originally made at the age of 14 years, the patient had arthralgia, rash, nephritis, nasal ulcers and multiple biochemical markers consistent with systemic lupus erythematosus. He was initially given immunosuppressants, and his disease had been in remission for about three years with treatment with mycophenolate.
Over a period of about 48 hours, the patient experienced ascending numbness and flaccid paralysis in both legs, accompanied by thoracolumbar pain, malar rash, fever and rigor. Rectal incontinence and urinary retention followed, prompting emergent transportation to a large tertiary care centre.
On admission, the patient had no fever and was alert, with normal vital signs. He had a malar rash, but no actively inflamed joints, identifiable insect bites or visual symptoms. He had flaccid leg paralysis and areflexia, with paresthesia below the level of T11, and an upgoing left plantar response. The results of initial blood work, including a complete blood count, liver enzymes levels, total bilirubin level, coagulation profile, renal function and urinalysis, were all within normal ranges. The erythrocyte sedimentation rate and C-reactive protein level were within the reference ranges, whereas the serum levels of C3 (0.41 [normal 0.73–1.73] g/L) and C4 (0.113 [normal 0.13–0.52] g/L) were low. Results of tests for antiphospholipid antibodies, including anticardiolipin and anti–β2-glycoprotein, were negative.
The results of a lumbar puncture showed an opening pressure of 10 (normal 5–20) cm H2O, a leukocyte count of 160 (normal 0–5) × 106/L, with 90% neutrophils, 8% lymphocytes and 2% monocytes (normal 70% lymphocytes and 30% monocytes), a glucose level of 2.01 (normal 1.5–4.0) mmol/L and a total protein level of 1.27 (normal 0.18–0.58) g/L. The patient’s cerebrospinal fluid sample was negative for bacterium and syphilis antibody testing. The results of viral serology of the cerebrospinal fluid for enterovirus, HIV, herpes simplex virus, cytomegalovirus and West Nile virus were also negative. Tests for antineuromyelitis optica antibodies were negative, and there was no evidence of oligoclonal banding.
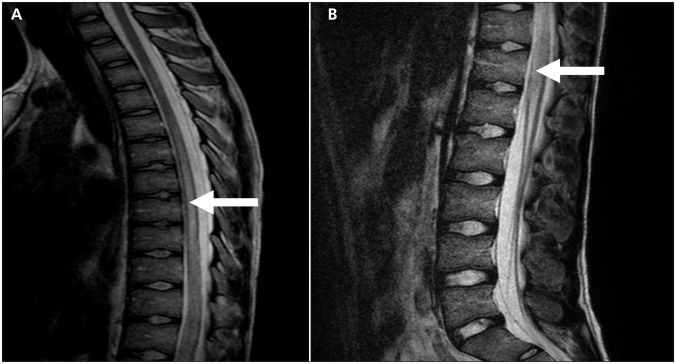
Magnetic resonance imaging (MRI) of the patient’s spine indicated heterogeneous abnormal T2-weighted hyperintensive cord signal beginning at the level of T3 and extending caudally to the conus medullaris (Figure 1). These findings were consistent with a diagnosis of longitudinally extensive transverse myelitis.
Figure 1:
T2-weighted sagittal magnetic resonance image of (A) the upper thoracic region and (B) the lower thoracic and lumbar region of an 18-year-old man with systemic lupus erythematosus and weakness, showing heterogenous hyperintensity (arrow) in keeping with central spinal cord edema and longitudinally extensive transverse myelitis.
The patient was originally given antibiotics and antiviral agents, which were stopped when no infectious agents could be identified. Given the patient’s history, physical findings, and biochemical and radiographic test results, longitudinally extensive transverse myelitis secondary to an acute lupus flare was diagnosed. Pulse methylprednisolone daily for five days was begun with a single dose of cyclophosphamide given intravenously. His condition stabilized, and he was transferred to our institution, where he received five cycles of plasmapheresis and oral prednisone daily, with pulse cyclophosphamide administered intravenously every two weeks. Intensive physical therapy was started when he arrived, and he was transferred to a spinal-cord rehabilitation unit for comprehensive rehabilitation.
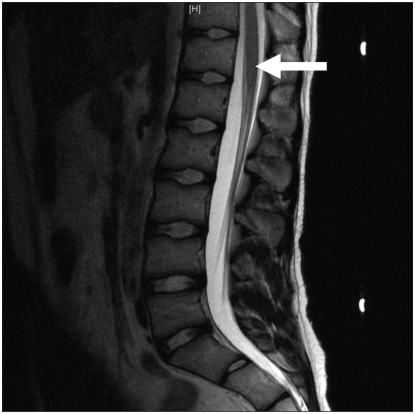
An MRI obtained three months after therapy was started showed complete resolution of all inflammation of the patient’s spinal cord (Figure 2). He had regained all bowel and bladder function and some function of his legs, but he was unable to walk. Twelve months after the flare-up, the patient remained on maintenance mycophenolate, and he was actively involved in physical rehabilitation. Thirty months after the flare-up, he remained on maintenance therapy, and he had no substantial residual neurologic or functional deficits.
Figure 2:
T2-weighted sagittal magnetic resonance image obtained three months after therapy was started. There is almost complete resolution of hyperintense signal (arrow) within the spinal cord, which is consistent with the marked clinical improvement observed.
Discussion
Systemic lupus erythematosus is a multisystem autoimmune disorder of the connective tissue, which can manifest as a variety of neurologic presentations, ranging from mild headache and vision changes to acute psychosis, seizures and stroke. Transverse myelitis (inflammation across one or more segments of the spinal cord) has been reported in 1%–2% of people with systemic lupus erythematosus.1
One of the most devastating types of transverse myelitis is longitudinally extensive transverse myelitis, in which involvement of the central nervous system extends beyond four vertebrae on neuroimaging, with a corresponding extensive level of neurologic sequelae. The incidence of this subtype is unknown; however, a recent systematic review of the literature between 1966 and 2008 included 22 published cases of longitudinally extensive transverse myelitis associated with systemic lupus erythematosus.2 Catastrophic presentations such as urinary and fecal incontinence with para- or tetraparesis are more typical of longitudinally extensive transverse myelitis than of more mild forms of transverse myelitis associated with systemic lupus erythematosus.2 It has also been associated with other autoimmune conditions, including neuromyelitis optica3 and neurosarcoidosis.4
Although the precipitating factors have not been fully identified, there is evidence that the onset of transverse myelitis in patients with systemic lupus erythematosus seems to follow a temporal pattern, with a mean time of occurrence of about three years after the initial diagnosis.5 Our patient’s presentation coincides with this time frame, although it also coincided with alterations to his maintenance regimen of immunosuppressants and a flare-up of his systemic lupus erythematosus. These factors may have contributed to the onset of the transverse myelitis.
Diagnosing transverse myelitis
Magnetic resonance imaging is the diagnostic modality of choice for longitudinally extensive transverse myelitis,6,7 allowing compressive lesions such as hematomas or intramedullary tumours to be ruled out. Evidence of increased signal on T2-weighted images of the spinal cord extending beyond four vertebrae is considered diagnostic of this condition.8 Our patient had widespread disease, with heterogeneous involvement of the spinal cord from the level of T3 to the conus.
Infectious causes of myelitis, such as varicella, can present with similar radiographic involvement of the spinal cord. These causes must be ruled out by sampling the cerebrospinal fluid before a diagnosis of autoimmune longitudinally extensive transverse myelitis is considered and intensive immunosuppression can be started.
Treatment
The cornerstone of treatment for autoimmune-related transverse myelitis is the concomitant administration of high doses of corticosteroid and intensive immunosuppressive therapy (e.g., cyclophosphamide).9 In a small case series involving 13 patients with transverse myelitis (but not longitudinally extensive transverse myelitis) related to systemic lupus erythematosus, all patients who received corticosteroids and immunosuppressants showed some measure of clinical improvement.10 This treatment paradigm has been extended to the more extreme presentation of longitudinally extensive transverse myelitis, although the results have been less satisfactory.2
A systematic review of the treatment of acute transverse myelitis suggests additional benefit when mycophenolate is added to the treatment plan, as was the case with our patient.11 Currently, there are no randomized trials to guide therapeutic decisions in cases of longitudinally extensive transverse myelitis related to systemic lupus erythematosus.
Improving the prognosis
The prognosis for patients with longitudinally extensive transverse myelitis is typically poor, although retrospective studies suggest that a similar proportion of patients will experience neurologic improvement with treatment as do those with milder types of acute transverse myelitis.2,11 In the recent systematic review by Espinosa and colleagues,2 some patients (14%) recovered completely but most had residual neurologic sequelae or no recovery at all.
The clinical outcome for our patient was excellent for a condition that usually does not respond well to therapy. Several key factors have been identified that are associated with a positive clinical response in patients with transverse myelitis and may also hold true for longitudinally extensive transverse myelitis.
Despite the delay in the initial diagnosis and treatment for several days because of inaccessibility, our patient received prompt diagnostic investigation and treatment after transfer to a tertiary care centre. One case series has indicated that starting treatment early may significantly affect the outcome of transverse myelitis related to systemic lupus erythematosus,12 with earlier treatment more likely to result in a positive outcome. The optimal therapeutic window may be up to one week, after which clinical efficacy is considerably lower.12
The speed at which neurologic symptoms develop has prognostic significance. Hyperacute presentations, typically evolving over several hours, are associated with the worst prognosis.13 Our patient had a less acute onset of neurologic symptoms, with a time frame of about two days, which is consistent with patients who experience a more favourable outcome. It is also possible that the use of mycophenolate may have influenced a positive outcome, as has recently been suggested.11
Patients with longitudinally extensive transverse myelitis associated with systemic lupus erythematosus often test positive for antiphospholipid antibodies. The pathophysiology of longitudinally extensive transverse myelitis may be causally related to the presence of antiphospholipid antibodies, with acute microvascular thrombus formation in the spinal vessels precipitating neuronal ischemia and cell death. Although initial reports have suggested that the presence of these antibodies is correlated with poor outcomes in acute transverse myelitis,10,14 a recent systematic review of this condition in patients with systemic lupus erythematosus that included those with longitudinal extensive disease did not support these initial findings.11 The results of tests for antiphospholipid antibodies in our patient on presentation and again on transfer were negative.
There is limited evidence that patients with longitudinal extensive transverse myelitis who test positive for antiphospholipid antibodies and receive anticoagulation or antiplatelet therapy, even without a documented thrombus, may experience better clinical outcomes.10 However, a recent systematic review did not support this finding, and anticoagulation or antiplatelet therapy was not associated with superior outcomes.11
Currently there are no randomized trials to dictate the role of anticoagulation or antiplatelet therapy in patients with transverse myelitis who are positive for antiphospholipid antibodies but who have no documented thrombus. Those with a documented venous or arterial thrombus in this situation should be considered for anticoagulation therapy, as they would potentially fulfill the criteria for antiphospholipid antibody syndrome.
Given the risks associated with systemic anticoagulation and the apparent lack of benefit, the utility of anticoagulation or antiplatelet therapy in those with acute transverse myelitis who are positive for antiphospholipid antibody but without a documented thrombus is unclear. However, at least one major rheumatology organization has indicated that anticoagulation therapy should be considered as treatment for patients with acute myelopathy associated with systemic lupus erythematosus who fall into this category.15 More data are needed to help determine which patients, if any, would benefit from anticoagulation or antiplatelet therapy.
Conclusion
Longitudinally extensive transverse myelitis is a potentially catastrophic presentation of systemic lupus erythematosus and is typically associated with poor clinical outcomes despite aggressive immunosuppressive therapy. Given the association between early treatment and favourable outcomes, any acute widespread neurologic deterioration in a patient with systemic lupus erythematosus should arouse suspicion for longitudinally extensive transverse myelitis, especially if the clinical presentation involves urinary or fecal incontinence and para- or tetraparesis.2 With rapid initiation of immunosuppressive therapy, such as a high-dose corticosteroid, pulse cyclophosphamide and perhaps mycophenolate, as well as rigorous physical rehabilitation, some patients may experience substantial clinical recovery.
Key points
Acute widespread neurologic deterioration, particularly involving urinary and fecal incontinence and para- or tetraparesis in a patient with systemic lupus erythematosus should arouse suspicion for longitudinally extensive transverse myelitis.
Before immunosuppression therapy is started, infectious causes of transverse myelitis need to be ruled out.
Early and intense immunosuppression offers the best opportunity for a positive clinical outcome.
Testing for the presence of antiphospholipid antibodies may help guide prognosis and therapeutic interventions.
Editor’s note.
The American Academy of Neurology has published a new guideline on clinical evaluation and treatment of transverse myelitis. The guideline recommends that plasma exchange be considered as rescue therapy when there is an inadequate response to corticosteroid treatment.
The guideline is available at www.neurology.org/content/early/2011/12/07/WNL.0b013e31823dc535.full/pdf+html
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors made substantial contributions to the conception and design of the article and the drafting and revising of the article for important intellectual content. All of the authors approved the final version submitted for publication.
References
- 1.Kovacs B, Lafferty TL, Brent LH, et al. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis 2000;59:120–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinosa G, Mendizábal A, Mínguez S, et al. Transverse myelitis affecting more than 4 spinal segments associated with systemic lupus erythematosus: clinical, immunological, and radiological characteristics of 22 patients. Semin Arthritis Rheum 2010;39: 246–56 [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–9 [DOI] [PubMed] [Google Scholar]
- 4.Dolhun R, Sriram S. Neurosarcoidosis presenting as longitudinally extensive transverse myelitis. J Clin Neurosci 2009;16:595–7 [DOI] [PubMed] [Google Scholar]
- 5.Vinken PJ, Bruyn GW, Klawans HL, editors. Nervous system involvement in systemic lupus erythematosus including the antiphospholid antibody syndrome. In: Handbook of Clinical Neurology. Vol. 27(71): systemic disease part III. Amsterdam: Elsevier Science B.V.; 1998. p. 35–58 [Google Scholar]
- 6.Merine D, Wang H, Kumar AJ, et al. CT myelopathy and MRI imaging of acute transverse myelitis. J Comput Tomogr 1987;11: 606–8 [DOI] [PubMed] [Google Scholar]
- 7.Brinar VV, Habek M, Zadro I, et al. Current concepts in the diagnosis of transverse myelopathies. Clin Neurol Neurosurg 2008; 110:919–27 [DOI] [PubMed] [Google Scholar]
- 8.Deodhar AA, Hochenedel T, Bennett RM. Longitudinal involvement of the spinal cord in a patient with lupus related transverse myelitis. J Rheumatol 1999;26:446–9 [PubMed] [Google Scholar]
- 9.Kaplin AI, Krishnan C, Deshpande DM, et al. Diagnosis and management of acute myelopathies. Neurologist 2005;11:2–18 [DOI] [PubMed] [Google Scholar]
- 10.D’Cruz DP, Mellor-Pita S, Joven B, et al. Transverse myelitis as the first manifestation of systemic lupus erythematosus or lupus-like disease: good functional outcome and relevance of antiphospholipid antibodies. J Rheumatol 2004;31:280–5 [PubMed] [Google Scholar]
- 11.Katsiari CG, Giavri I, Mitsikostas DD, et al. Acute transverse myelitis and antiphospholipid antibodies in lupus. No evidence for anticoagulation. Eur J Neurol 2011;18:556–63 [DOI] [PubMed] [Google Scholar]
- 12.Harisdangkul V, Doorenbos D, Subramony SH. Lupus transverse myelopathy: better outcome with early recognition and aggressive high-dose intravenous corticosteroid pulse treatment. J Neurol 1995;242:326–31 [DOI] [PubMed] [Google Scholar]
- 13.Ropper AH, Poskanzer DC. The prognosis of acute and subacute transverse myelopathy based on early signs and symptoms. Ann Neurol 1978;4:51–9 [DOI] [PubMed] [Google Scholar]
- 14.Téllez-Zenteno JF, Remes-Troche JM, Negrete-Pulido RO, et al. Longitudinal myelitis associated with systemic lupus erythematosus: clinical features and magnetic resonance imaging of six cases. Lupus 2001;10:851–6 [DOI] [PubMed] [Google Scholar]
- 15.Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010;69:2074–82 [DOI] [PubMed] [Google Scholar]