Abstract
Background
We investigated the contractile response of human coronary microvasculature to thromboxane A-2 (TXA-2), with and without the blockade of TXA-2 receptors or the inhibition of phospholipase-C (PLC) or of protein kinase C-α (PKC-α) in the human coronary microvasculature before and after cardioplegia, followed by reperfusion (CP/Rep). Protein/gene expression and localization of TXA-2 receptors, TXA-2 synthase, PLC, and other TXA-2–related proteins was also examined.
Methods
Right atrial tissue was harvested before and after cold blood cardioplegia, followed by about 10 minutes of reperfusion, from 28 patients undergoing cardiac operations. Coronary arterioles (90 to 170 µm in diameter) were dissected from the harvested tissue.
Results
The post-CP/Rep contractile response of coronary arterioles to TXA-2 analog U-46619 was significantly impaired vs pre-CP/Rep (p < 0.05). The TXA-2 receptor antagonist SQ-29548 (10−6 M) prevented the contractile response to U-46619 (p < 0.05). Pretreatment with the PLC inhibitor U73122 (10−6 M) significantly inhibited the U-46619–induced contractile response (p < 0.05). Administration of the PKC-α inhibitor safingol failed to affect U-46619–induced contraction. Total protein levels and gene expression of TXA-2 receptors, TXA-2 synthase, PLC-β3, phospho–PLC-β3, PLC-γ1, and phospho–PLC-γ1 were not altered after CP/Rep. Confocal microscopy showed no significant differences in the expression of TXA-2 receptors or PLC-β3 in the microcirculation. TXA-2 receptors and PLC-β3 were both present in smooth muscle and endothelium.
Conclusions
Cardioplegia/Rep decreases the contractile response of human coronary arterioles to TXA-2 soon after cardiac operations. The contractile response to the TXA-2 analog U-46619 is through activation of TXA-2 receptors and PLC.
Cardioplegia, followed by reperfusion (CP/Rep) during cardiopulmonary bypass (CPB), is often associated with vasomotor dysfunction of the human coronary microcirculation after cardiac operations [1–4]. Thromboxane A-2 (TXA-2) production in systemic and coronary circulation is significantly increased after cardioplegia and CPB [5–7]. TXA-2 is thought to be responsible for a prothrombotic milieu, myocardial dysfunction, and arrhythmias after CP/Rep and CPB due to its biologic activities, including vasoconstriction, and stimulating platelet aggregation. In addition, the administration of prostacyclin, prostaglandin E-1, TXA-2 receptor antagonist, and TXA-2 synthase inhibitor have been reported to reduce thrombosis and preserve cardiac function during CP/Rep or CPB, or both [1, 5–7].
TXA-2 is generated from arachidonic acid and prostaglandin H2 by cyclooxygenase and TXA-2 synthase [7, 8]. In smooth muscle cells, activation of G protein–coupled TXA-2 receptors induces vascular smooth muscle contraction through the initial activation of phospholipase-C (PLC), with production of 1,4,5-inositol phosphate, release of intracellular calcium stores, and phosphorylation of myosin light chain [8, 9]. However, the role of TXA-2 in CP/Rep-related microvascular vasomotor dysfunction in human coronary arterioles and the molecular mechanisms underlying TXA-2–induced vasomotor response after CP/Rep remain to be elucidated. Thus, this study was designed to examine the effect of CP/Rep on the myogenic responses of human coronary arterioles to a TXA-2 analog, with and without inhibition of TXA-2 receptor, a PLC or protein kinase C-α (PKC-α), and to relate these responses to possible alterations in expression and localization of TXA-2 receptors, TXA-2 synthase, PLC, and other TXA-2–related protein and gene expression in human atrial tissue.
Material and Methods
Tissue harvesting and all procedures were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center and Harvard Medical School. Informed consent was obtained from all enrolled patients as required by the Institutional Review Board.
Patients and Tissue Harvesting
Samples of right atrial appendage were harvested from patients undergoing coronary artery bypass grafting (CABG) before and after exposure of the heart to blood CP/Rep under conditions of CPB. Samples were handled in a nontraumatic fashion. Double 3-0 polypropylene purse-string sutures (Ethicon, Somerville, NJ) were placed in the atrial appendage. The first sample of atrial appendage was harvested (pre-CP/Rep) during placement of the venous cannula. The superior suture was tightened to secure the venous cannula. The inferior suture remained loose to allow this portion of the atrium to be exposed to blood cardioplegia and reperfused (post-CP/Rep) after removal of the aortic cross clamp.
An initial 600 to 800 mL of cold-blood (0° C to 4°C) hyperkalemic (15 mmol/L K+) cardioplegic solution was delivered antegrade into the aortic root. This method was followed at 8-minute to 15-minute intervals with 200 to 300 mL of cold cardioplegic solution (15 mmol/L K+). The composition of cardioplegic solutions have been previously described in detail [2, 4].
The second sample of atrial tissue (post-CP/Rep) was harvested between the pursestrings during removal of the venous cannula. Sections of atrial samples were immediately frozen in liquid nitrogen for immunoblotting, fixed in 10% formalin for 24 hours, followed by paraffin embedding and sectioning into 5-µm slices for immunofluorescent staining, or were placed in cold (5° to 10°C) Krebs-Henseleit buffer (KHB) for microvascular studies.
Microvessel Reactivity
Microvessel studies were performed by in vitro organ bath videomicroscopy, as described previously [2–4]. Coronary microvessels (90 to 170 µm internal diameter) were dissected from harvested human atrial tissue by the use of a dissecting microscope (Olympus Optical, Tokyo, Japan). The microvessels were placed in an organ bath (University of Iowa Medical Instrumentation, Iowa City, IA) and cannulated with 10-0 nylon monofilament suture (Ethicon). Oxygenated (95% O2/5% CO2) KHB solution warmed to 37°C was continuously circulated through the organ bath. The microvessels were pressurized to 40 mm Hg in a nonflow state filled with KHB solution. An inverted microscope (Olympus Optical) connected to a video camera was used to project the vessel image through a television monitor. An electronic dimension analyzer (Living System Instrumentation, Burlington, VT) was used to measure the internal diameter, and the measurement was recorded.
After 60 minutes of equilibration, pre-CP/CPB and post-CP/CPB microvessels were exposed to the TXA-2 analog U-46619 (10−9 to 10−5 mol/L). Selected vessels were pretreated with TXA-2 receptor antagonist SQ-29548 (10−6 mol/L; Sigma-Aldrich, St. Louis, MO) 5 minutes before U-46619 (10−9 to 10−5 mol/L) administration [9]. Other microvessels were preincubated with PLC inhibitor U-73122 (10−6 M) or PKC-α inhibitor safingol (2 × 10−5 mmol/L; Avanti Polar Lipids, Alabaster, AL) 20 minutes before U-46619 (10−9 to 10−5 mol/L) administration.
The baseline diameter was defined as the diameter measured after cannulation and equilibration in the aerated KHB solution. Internal diameter was measured after treatment with U-46619 and normalized to the baseline diameter (% contraction). The microvessels were washed with the KHB solution and allowed to equilibrate 15 to 30 minutes between pharmacologic interventions. All chemicals were applied randomly on each vessel.
Immunoblot
Human atrial tissue (n = 6) collected from pre-CP/CPB and post-CP/CPB was analyzed for levels of total TXA-2 receptor proteins, TXA-2 synthase, and PLC by immunoblotting. The total protein (40 µg) was fractionated on an 4% to 20% sodium dodecyl sulfate polyacrylamide gel by electrophoresis and transferred to Immobilon-P, a poly-vinylidene difluoride membrane (Millipore Corp, Bedford, MA), as described previously [2–4]. The membranes were incubated overnight at 4°C with 1:500 dilutions of individual polyclonal rabbit primary antibodies to TXA-2 receptor (Cayman Chemical, Ann Arbor, MI), TXA-2 synthase (Abcam, Cambridge, MA), PLC-β3 (Abcam), phosphor-PLC-β3 (Santa Cruz Biotechnology Inc, Santa Cruz, CA), PLC-γ1, and phosphor-PLC-γ1 (Cell Signaling Technology Inc, Danvers, MA).
The membranes were incubated for 1 hour with horse-radish peroxidase–conjugated secondary antiimmunoglobulin, washed three times in Tris saline buffer, and processed for chemiluminescent detection (Pierce, Rockford, IL). Band intensity was measured by densitometric analysis of the digitalized images (G:Box, Chemi HR16, Syngene, Frederick, MD) using Image J 1.43 software (National Institutes of Health, Bethesda, MD).
Immunofluorescence Staining and Confocal Microscopy
Atrial tissue sections from 6 patients were deparaffinized in xylene, rehydrated in graded ethanol and phosphate-buffered saline (PBS) solution, and antigen unmasked with sodium citrate (10 mmol/L; pH, 6.0), followed by PBS wash and blocking with 2% bovine serum albumin in PBS at room temperature for 2 hours. After PBS wash, overnight incubation with individual polyclonal rabbit or mouse antibodies to TXA-2 receptors (1:200, Cayman Chemical) or PLC-β3 (1:50, Cell Signaling Technology Inc), were performed at 4°C. Antimouse smooth muscle α-actin (1:200; Sigma-Aldrich) was used to detect microvascular smooth muscle.
Sections were washed in PBS and incubated with the appropriate Alexa fluor secondary antibody and mounted using fluorescent mounting medium (Vector Laboratories, Burlingame, CA). The tissue was visualized using a confocal microscope system Nikon C1SI (Nikon, Tokyo, Japan). Tissue labeling with secondary antibodies alone served as negative controls [2–4].
RNA Isolation and Microarray Processing
Total RNA was isolated from 200 mg of tissue samples with a Trizol-based method, following the manufacturer’s protocol (Gibco BRL, Invitrogen, Carlsbad, CA). For microarray analysis after the quantitative and qualitative assessment of extracted total RNA, single-stranded, followed by double-stranded complimentary DNA (cDNA) synthesis was performed. Biotin-labeled cRNA was obtained by in vitro transcription of double-stranded cDNA using the AFFI kit (Affymetrix, Santa Clara, CA). The cRNA was further purified, fragmented, hybridized overnight onto Affymetrix gene chips, and washed in streptavidin, followed by array scanning, as previously described [4].
Microarray Analysis
Transcriptional profiling was performed on HG-U95Av2 Affymetrix chips, with matched pre-bypass samples serving as controls. Quality control, normalization, differential gene expression, and statistical analysis of microarray data were performed using JMP Genomics software (SAS Institute, Cary, NC). After quality control analysis, one outlier sample was eliminated from the analysis. Chips were normalized using the Robust Multichip average, and gene expression in post-CPB samples was compared with pre-CPB samples using one-way analysis of variance. A post hoc false detection rate algorithm with α = 0.05 was applied to control for false-positive results. Fold changes < 2 or > −2 were considered real changes, and values at −log10 p > 3.83 were considered significant. Gene ontology and canonical pathway analyses were done using Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA).
Chemicals
U-46619 and U-73122 were obtained from Sigma. U-46619 was dissolved in ethanol and prepared on the day of the study. SQ-29548 was purchased from Cayman Chemical. Safingol was purchased from Avanti Polar Lipids. U-73122, and SQ-29548 were dissolved in dimethylsulfoxide to make a stock solution. Safingol was dissolved in ethanol to make a stock solution. All stock solutions were stored at −20°C. All dilutions were prepared fresh daily.
Data Analysis
The data are presented as the mean and standard error of the mean. Repeated measures analysis of variance and the Student t test were used to compare variables among or between vessels. The treatment effects were statistically examined by paired or independent two-tailed Student t tests. Statistical significance was taken at a value of p < 0.05.
Results
Patient characteristics are listed in Table 1. All patients with preoperative hypertension were receiving antihypertensive medication, including β-blocker, aspirin, calcium channel blocker, or angiotensin-converting enzyme inhibitor. All patients with hypercholesterolemia were receiving statin treatment.
Table 1.
Characteristics for Patients Undergoing Coronary Artery Bypass Grafting
| Variable | Value |
|---|---|
| Patients, No. | 28 |
| Age, mean ± SD, years | 68 ± 3 |
| Sex, No. | |
| Male | 20 |
| Female | 8 |
| Hypertension, No. | 22 |
| Hypercholesterolemia, No. | 24 |
| Diabetes mellitus, No. | 6 |
| CPB duration, mean ± SD, min | 85 ± 6 |
| Cross-clamp time, mean ± SD, min | 74 ± 5 |
| Atrial fibrillation, No. | 4 |
| Grafts performed, median (range) | 3 (3–4) |
| Redo case, No. | 0 |
CPB = cardiopulmonary bypass; SD = standard deviation.
Microvascular Reactivity
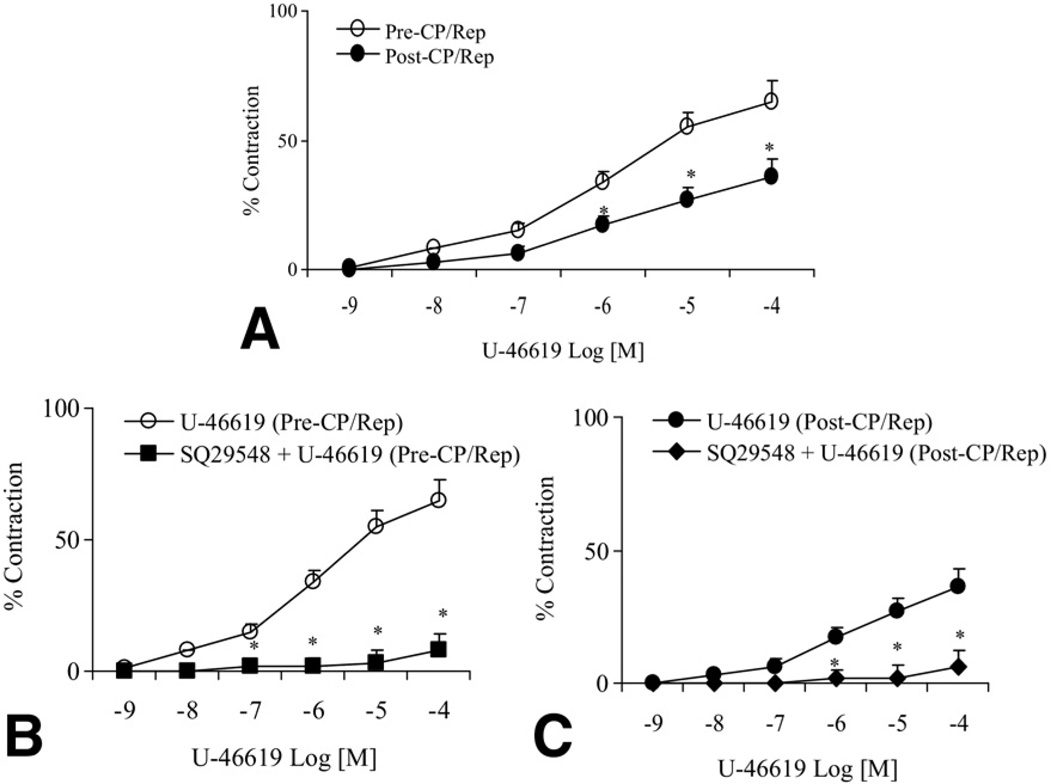
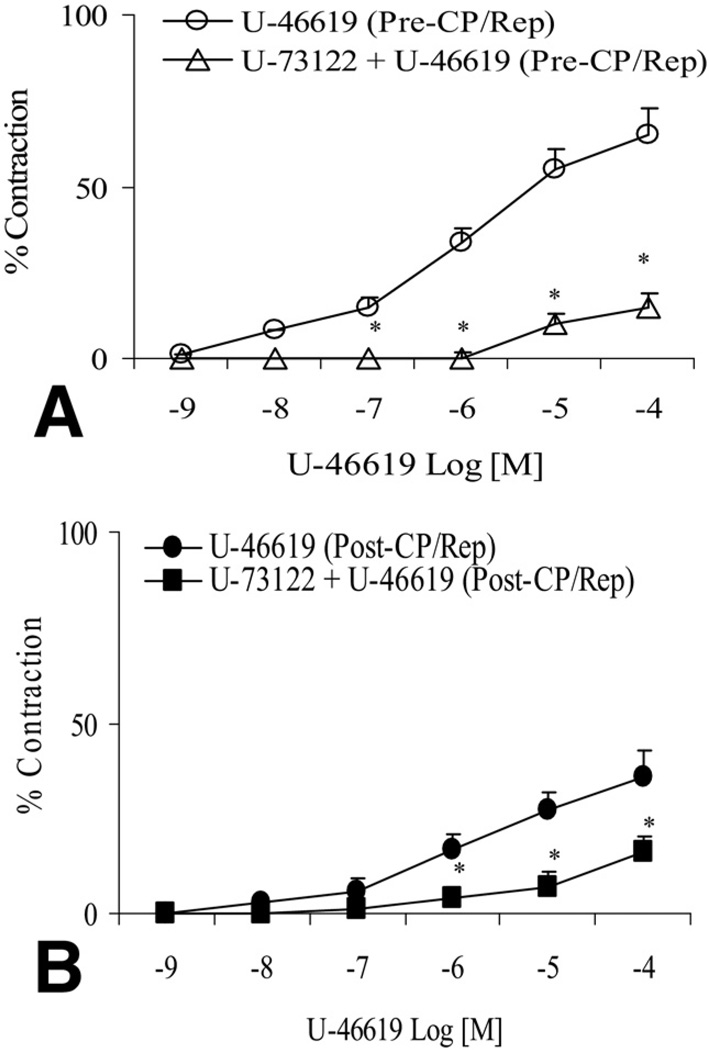
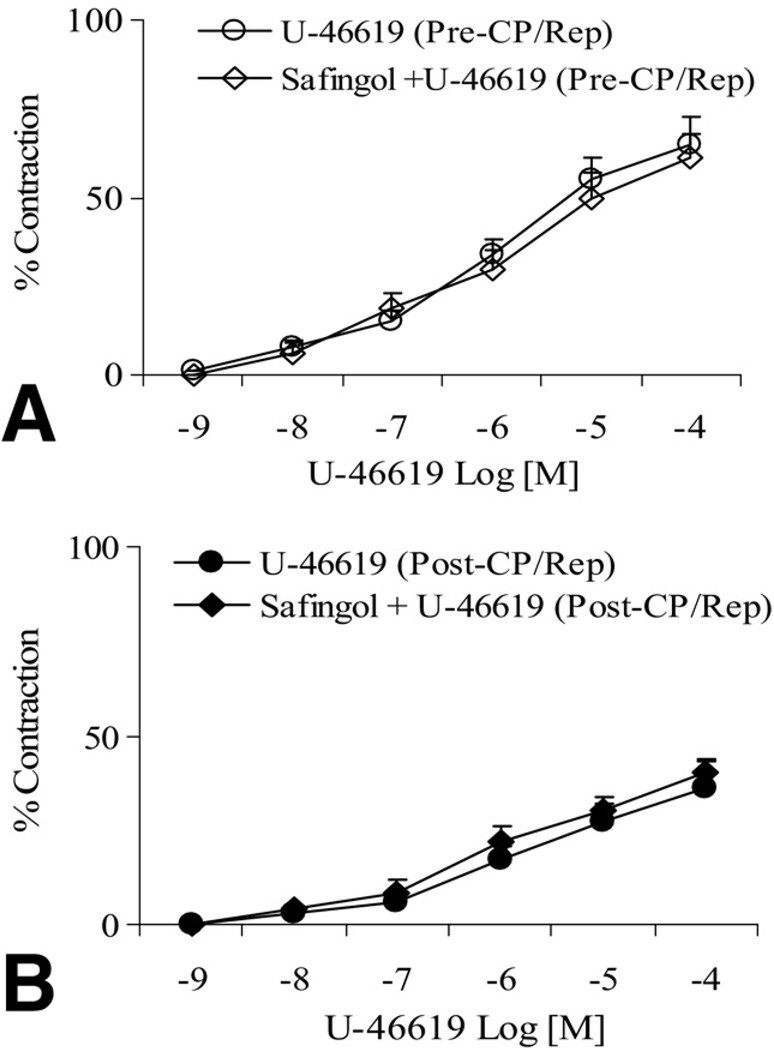
There was no significant difference in the baseline diameter of the microvessels between the pre-CP/Rep (137 ± 7 µm) and post-CP/Rep groups (144 ± 6 µm, p > 0.05). The post-CP/Rep contractile response of skeletal muscle arterioles to TXA-2 analog U-46619 was significantly decreased compared with pre-CP/Rep responses (p < 0.05, Fig 1A). Pretreatment of pre-CP/Rep coronary arterioles with the TXA-2 receptor antagonist SQ-29548 (10−6M) abolished the U-46619-induced vasoconstriction (p < 0.05, Fig 1B). Incubation of post-CP/Rep coronary arterioles with SQ-29548 before addition of U-46619 also inhibited vasoconstriction (p < 0.05, Fig 1C). In addition, the presence of the PLC inhibitor U-73122 (10−6M) reduced U-46619–induced contraction in pre-CP/Rep and post-CP/Rep arterioles (p < 0.05, respectively, Fig 2A and B). In contrast, addition of the PKC-α inhibitor safingol (2 × 10−5M) to pre-CP/Rep or post-CP/Rep arterioles failed to affect U-46619–induced vasoconstriction (p > 0.05, Fig 3).
Fig 1.
(A) Coronary arteriolar vasoconstriction in response to the thromboxane A (TXA)-2 analog U-46619 before vs after cardioplegic ischemia, followed by reperfusion (pre-CP/Rep [white circles] vs post-CP/Rep [black circles]); *p < 0.05 vs pre-CP/Rep. (B) Administration of U-46619 with or without TXA-2 antagonist SQ-29548 to microvessels of pre-CP/Rep (U-46619 [pre-CP/Rep, white circles] or SQ-29548 + U-46619 [pre-CP/Rep, black squares]); *p < 0.05 vs U-46619 (pre-CP/Rep). (C) Infusion of U-46619, with or without the TXA-2 antagonist SQ-29548, to post-CP/Rep vessels (U-46619 [post-CP/Rep, black circles] or SQ-29548 + U-46619 [post-CP/Rep, black diamonds]); p < 0.05 vs U-46619 (post-CP/Rep); n = 6 to 8/group. The bars show the standard error of the mean.
Fig 2.
(A) Administration of U-46619 to coronary arteriole before cardioplegic ischemia, followed by reperfusion (pre-CP/Rep), with or without the presence of the phospholipase-C (PLC) inhibitor U-73122 (U-46619 [Pre-CP/Rep, white circles] or U-73122 + U-46619 [pre-CP/Rep, white triangles]), *p < 0.05 vs U-46619 (pre-CP/Rep). (B) Perfusion of U-46619 to post-CP/Rep coronary arterioles, with or without the presence of the PLC inhibitor U-73122 (U-46619 [post-CP/Rep, black circles] or U-73122 + U-46619 [post-CP/Rep, black squares]),*p < 0.05 vs. U-46619 (post-CP/Rep); n = 6 to 8/group. The bars show the standard error of the mean.
Fig 3.
(A) Addition of U-46619 to the vessels from samples before cardioplegic ischemia, followed by reperfusion (pre-CP/Rep), in the absence or presence of the protein kinase C (PKC) inhibitor safingol (U-46619 [pre-CP/Rep, white circles] or safingol + U-46619 [pre-CP/Rep, white diamonds]). (B) Addition of U-46619 to the vessels from post-CP/Rep samples in the absence or presence of PKC inhibitor safingol (U-46619 [post-CP/Rep, black circles] or safingol + U-46619 [post-CP/Rep, black diamonds]); n = 6 to 8/group. The bars show the standard error of the mean.
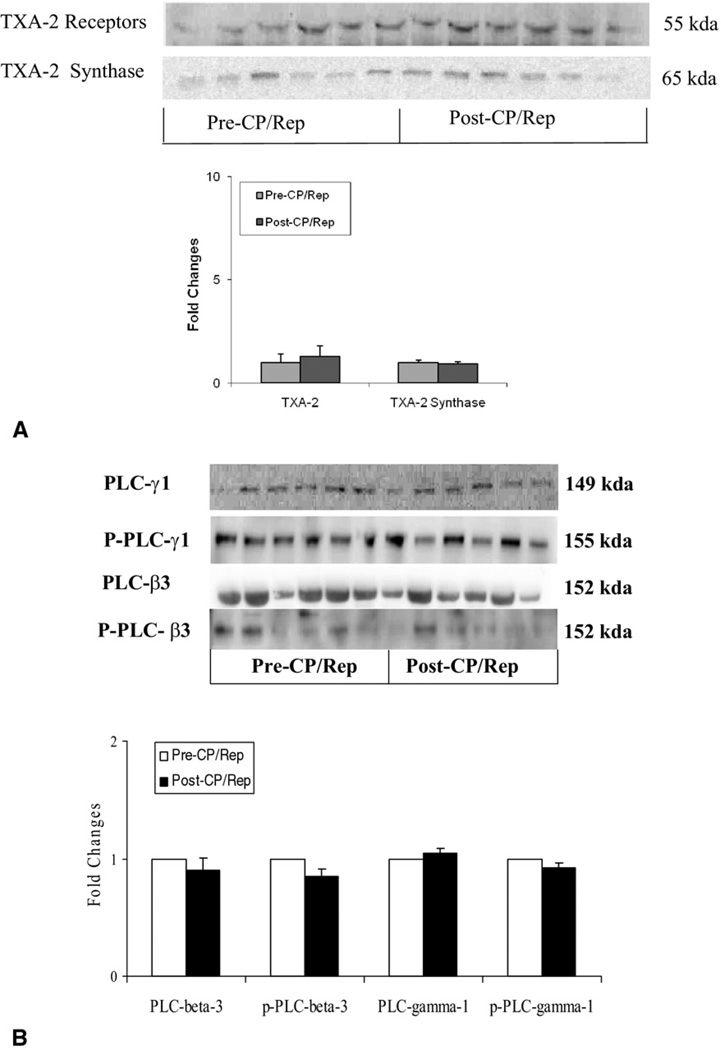
Effect of CP/Rep on Levels of TXA-2 Receptor, TXA-2 Synthase, and PLC Polypeptides
Atria tissue levels of the TXA-2 receptor, TXA-2 synthase (Fig 4A), PLC-β3; phosphor–PLC-β3, PLC-γ1, and phosphor–PLC-γ1 polypeptides (Fig 4B) were unchanged after CP/Rep, as detected by immunoblot.
Fig 4.
Representative immunoblot of human atria tissue. Lanes 1 to 12, loaded with 40 µg protein, were developed for (A, top) thromboxane A (TXA)-2 receptor and TXA-2 synthase (n = 6) and (B, top) phospholipase-C (PLC)-β3, phospho–PLC-β3, PLC-γ1, and phosphor–PLC-γ1 polypeptides (n = 6). Immunoblot band intensity shows unaltered levels of TXA-2 receptor, TXA-2 synthase, PLC-γ1, and PLC-β3 polypeptides after cardioplegic ischemia, followed by reperfusion (CP/Rep; bottom of Fig 4A and B), respectively. The bars show the standard error of the mean.
CP/Rep-Induced TXA-2 Receptor and PLC Microvessel Localization
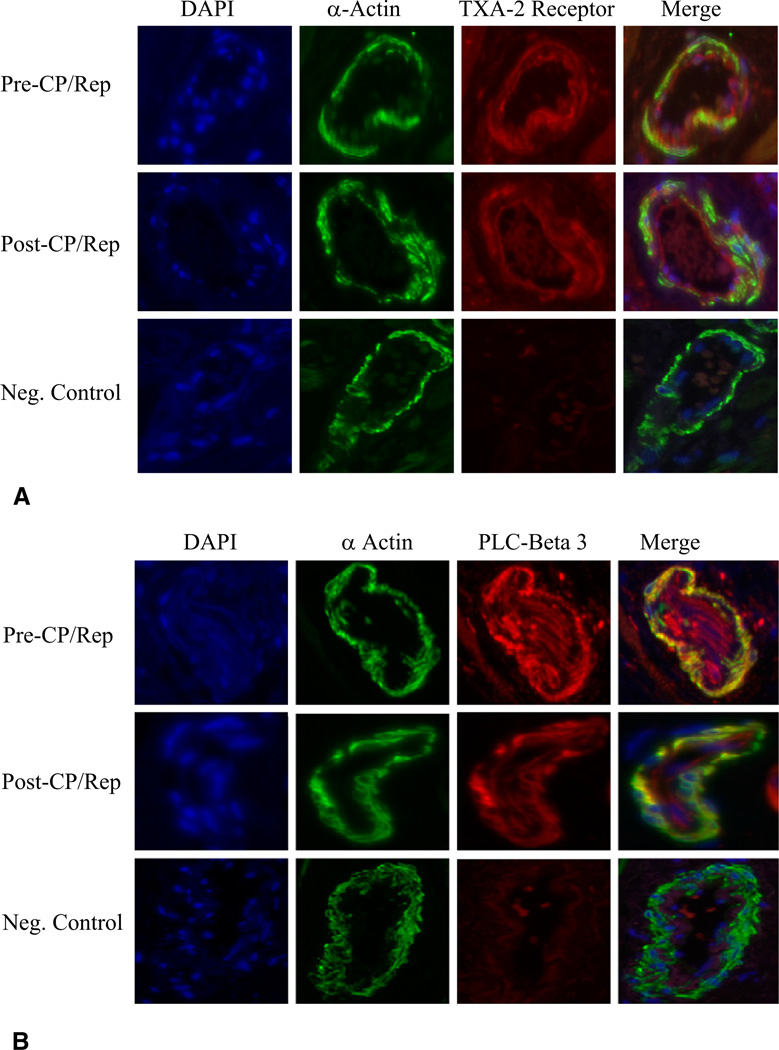
Confocal microscopy revealed TXA-2 receptors (Fig 5A) and PLC-β3 (Fig 5B) localized to coronary microvessel (red). Immunofluorescent double staining demonstrated that TXA-2 receptor and PLC-β3 localized to coronary smooth muscle (orange) and endothelium (red). There were no significant differences in TXA-2 receptor or PLC-β3 signal in vessels between tissue before and after CP/Rep. Negative controls documented a low level of background fluorescence (red) and a strong signal of smooth muscle α-actin staining specifically in smooth muscle (green).
Fig 5.
Immunolocalization of thromboxane A (TXA)-2 receptor, phospholipase-C (PLC)-γ1, and PLC-β3 polypeptides in human coronary microvessels (n = 6). Vessels were costained for smooth muscle α-actin with (A) TXA-2 receptor antibody or (B) PLC-β3. Matched negative controls are displayed below each row of primary antibody. (DAPI = 4’,6-diamidino-2-phenylindole.)
Effect of CP/Rep on the Gene Expression of TXA-2 Receptors, TXA-2 Synthase, and Other Related Proteins
The data obtained from microarray analysis are summarized in Table 2. The post-CP/Rep fold-changes of gene expression of TXA-2 receptors and TXA-2 synthase were less than onefold, respectively, suggesting no significant changes had occurred in TXA-2 receptors and TXA-2 synthase gene expression. Other potential TXA-2–related signaling cascades, such as G protein–coupled receptor kinase, glycogen synthase kinase, PLC, phospholipases A2, phospholipase D, Rho guanine nucleotide exchange factor, mitogen-activated protein kinase, and PKC also were not significantly altered in post-CP/Rep samples.
Table 2.
Data From Microarray Analysisa
| Variable | Fold Changes |
−Log10 (p Value) | Variable | Fold Changes |
−Log10 (p Value) |
|---|---|---|---|---|---|
| PLA2-G1B | 0.20025 | 0.51986 | PLC-β1 | −0.14719 | 0.35611 |
| PLA2-G2A | −0.38263 | 0.85158 | PLC-β2 | 0.108125 | 0.71506 |
| PLA2-G4A | 0.05025 | 0.38109 | PLC-β3 | −0.0045 | 0.5079 |
| PLA2-G4C | 0.150875 | 0.41136 | PLC-β4 | 0.15325 | 0.47148 |
| PLA2-G5 | −0.23550 | 0.26525 | PLC-γ1 | 0.036625 | 0.46310 |
| PLA2-G6 | −0.00167 | 0.7964 | PLC-γ2 | −0.05863 | 0.41421 |
| PLA2-G7 | 0.092375 | 0.4245 | PLC-δ1 | −0.12919 | 0.601 |
| PLA2-G10 | 0.032313 | 0.7037 | PLC-ε1 | −0.2055 | 0.38971 |
| PLA2-G15 | 0.218188 | 0.63171 | PLC-η1 | 0.05125 | 0.38601 |
| PLA2-G16 | 0.051313 | 0.29672 | PLC-η2 | 0.046625 | 0.40614 |
| TXA-2 receptor | −0.02581 | 0.59576 | TXA-2 synthase | 0.066 | 0.72305 |
| MAPK-1 | 0.578313 | 0.88625 | Rho-GEF-1 | −0.03756 | 0.514944 |
| MAPK-3 | −0.41356 | 0.87362 | Rho-GEF-2 | 0.215 | 0.531000 |
| MAPK-4 | 0.057438 | 0.50662 | Rho-GEF-4 | −0.0745 | 0.645063 |
| MAPK-6 | 0.452125 | 0.81766 | Rho-GEF-5 | −0.20275 | 0.554031 |
| MAPK-7 | 0.1885 | 0.55187 | Rho-GEF-7 | 0.190938 | 0.895394 |
| MAPK-8 | −0.03033 | 0.46150 | Rho-GEF-10 | 0.087813 | 0.359363 |
| MAPK-9 | 0.4345 | 0.75439 | Rho-GEF-11 | 0.228375 | 0.710306 |
| MAPK-10 | 0.176938 | 0.45802 | Rho-GEF-12 | 0.042 | 0.545938 |
| MAPK-11 | 0.058563 | 0.38492 | Rho-GEF-15 | 0.0495 | 0.45661 |
| MAPK-12 | −0.0325 | 0.45474 | Rho-GEF-16 | −0.02125 | 0.63001 |
| MAPK-13 | −0.07294 | 0.44960 | Rho-GEF-17 | 0.063563 | 0.40668 |
| MAPK-14 | 0.3535 | 0.73609 | Rho-GEF-18 | −0.5015 | 0.94140 |
| MTOR | 0.33968 | 0.67460 | Rho-A | 0.183625 | 0.44555 |
| PKC-α | 0.159375 | 0.44953 | PLD-1, PS | −0.0155 | 0.460233 |
| PKC-β | 0.130625 | 0.45263 | PLD-2 | 0.01825 | 0.442563 |
| PKC-γ | 0.0925 | 0.44668 | PLD-3 | 0.635625 | 0.887557 |
| GRK-1 | 0.50405 | 0.148125 | GSK-β | 0.253125 | 0.456617 |
| GRK-4 | 0.55180 | −0.05544 | CaM-1-PK-δ | −0.09713 | 0.630019 |
| GRK-5 | 0.68997 | 0.074125 | CaM-3-PK-δ | 0.142563 | 0.406688 |
| GRK-6 | 0.57186 | −0.13606 | Caldesmon 1 | 0.784933 | 0.941400 |
Microarray analysis showed no significant changes in the gene expression of TXA-2 receptors, TXA-2 synthase, PLC-γ1, or PLC-β3 after cardioplegic ischemia and reperfusion.
CaM-PK = calmodulin phosphorylase kinase; GRK = G protein–coupled receptor kinase; GSK = glycogen synthase kinase; MAPK = mitogen-activated protein kinase; MTOR = mammalian target of rapamycin; PKC = protein kinase C; PLA = phospholipase A; PLC = phospholipase C; PLD = phospholipase D; Rho-A = Ras homolog gene family, member A; Rho-GEF = Rho guanine nucleotide exchangefactor; TXA = thromboxane A.
Comment
We and others have previously shown that CP/Rep results in vasomotor dysfunction with altered contractile responses of the human coronary microvasculature to phenylephrine, vasopressin, serotonin and endothelin-1, and other substances [1–4]. The present study demonstrates a similarly diminished contractile response of human coronary microvessels to the TXA-2 agonist U-46619 after CP/Rep. U-64419–induced constriction was prevented by the TXA-2 receptor antagonist SQ29548, proving that U-46619 selectively activates TXA-2 receptors. TXA-2 receptors and TXA-2 synthase were detected by immunoblot of extracts from human atrial tissues. The signals from confocal microscopy and costaining show that TXA-2 receptors were largely detected in coronary smooth muscle and endothelium. No significant change occurred in TXA-2 receptor–polypeptide localization between pre-CP/Rep and post-CP/Rep samples, confirming that CP/Rep did not affect the quantity of total TXA-2 receptor protein.
The human TXA-2 receptor is a typical Gq protein–coupled receptor with seven transmembrane segments [8]. Activation of PLC is an initial step for TXA-2–induced cell signaling. At least seven families of PLCs have been identified: PLC-α, PLC-β, PLC-γ, PLC-δ, PLC-ε, PLC-ξ, and PLC-η [10, 11]. The PLC-β subfamily includes four members: PLC-β1, -β2, -β3, and -β4. The PLC-γ subfamily also includes four members: PLC-γ1, -γ2, -γ3, and -γ4. In the present study, PLC-β3, phospho–PLC-β3, PLC-γ1, and phospho–PLC-γ1 were detected in human atrial tissue. No significant changes of PLC-β3, phospho–PLC-β3, PLC-γ1, and phospho–PLC-γ1 were observed between pre-CP/Rep and post-CP/Rep samples. Consistent with the protein analysis, the signals from the immuno-staining also indicate no significant differences of the PLC-β3 between pre-CP/Rep and post-CP/Rep.
Our results show that PLC-β3 and PLC-γ1 are present in human coronary microvasculature. The signals from costaining and confocal microscopy also indicate PLC-β3 is present in smooth muscle and endothelium. The responses of pre-CP/Rep and post-CP/Rep vessels to U-46619 were significantly reduced after the administration of the PLC inhibitor U-73122, suggesting PLC is involved in TXA-2–induced signaling at the human coronary microvasculature. Furthermore, no significant changes of TXA-2 receptors, TXA-2 synthase, or PLCs gene expression after CP/Rep were obtained from microarray analysis, supporting the findings from the protein analysis. In addition, other TXA-2–related gene expressions were also not altered after CP/Rep.
Taken together, these results suggest that a period of intraoperative hypothermic ischemia and reperfusion may modify the functional state of TXA-2 receptor–dependent signaling rather than the steady-state levels of their proteins. This may partially explain why the contractile response to TXA-2 was diminished after CP/Rep. To specifically explain why post-CP/Rep vessels demonstrated a reduced response to TXA-2, the activities of TXA-2 receptors, PLC, and PKC-α need to be studied further.
Protein kinase C-α appears to be the predominant conventional PKC isoform present in the human microcirculation [2–4]. We recently found that phenylephrine and endothelin-1–induced vasoconstriction in the human microvasculature is partially mediated by PKC-α and that CP/Rep results in reduced PKC-α activity in the human coronary microcirculation [2–4]. However, the present study indicates that U-46619–induced vasoconstriction in the human coronary arterioles acts by mechanisms other than PKC-α, suggesting that TXA-2–induced signaling pathways are different from those of phenylephrine and endothelin-1.
The current study has several limitations: First, the findings are not specific to cardioplegia and reperfusion. Other factors cannot be ruled out, such as exposure to anesthetic agents, the operation itself, volume shifts, temperature changes, heparin, mechanical trauma, and stretch. However, the observed findings are a real reflection of what happens in patients during an operation. The major effect is believed due to exposure to cardioplegic arrest and reperfusion.
Second, the contractile responses were examined after only a brief period of reperfusion. The responses might have been different at 1 or 2 hours after cross-clamp removal compared with those observed.
Finally, this study does not address whether the responses to TXA-2 differ between patients with or without diabetes and other common comorbidities. We plan to address this topic in the future.
In conclusion, CP/Rep decreases the contractile response of human coronary arterioles to the TXA-2 analog U-46619 soon after termination of CPB. This contractile response requires activation of TXA-2 receptors and PLC, but not PKC-α. CP/Rep does not change the expression of TXA-2 receptors or TXA-2 synthase, nor does it alter TXA-2 receptor localization to coronary microvessels. CP/Rep does not alter the expression of PLC-β3, phosphor–PLC-β3, PLC-γ1, or phosphor–PLC-γ1 polypeptides, nor does it affect PLC-β3 localization. These results provide novel mechanisms for TXA-2–induced contraction in vasomotor dysfunction of coronary microvasculature soon after cardiac surgical procedures and may offer targets to mitigate the detrimental consequences of hyperkalemic cardioplegic arrest.
Acknowledgments
This research was supported in part by the National Heart, Lung and Blood Institute Grants HL-69024, HL-085647, and HL-46716 to Dr Sellke. This research was also supported by National Institutes of Health Grafts 5T32-HL76130-05 to Dr Liu and to Dr Robich, 5T32-HL0074 to Dr Robich, and the Irving Bard Memorial Fellowship to Dr Chu and Dr Robich.
Abbreviations and Acronyms
- CABG
coronary artery bypass grafting
- CaM PK
calmodulin phosphorylase kinase
- CPB
cardiopulmonary bypass
- CP/Rep
cardioplegia followed by reperfusion
- DAPI
4′,6-diamidino-2-phenylindole
- GRK
G protein-coupled receptor kinase
- GSK
glycogen synthase kinase
- KHB
Krebs-Henseleit buffer
- MAPK
mitogen-activated protein kinase
- MTOR
mammalian target of rapamycin
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- PLA
phospholipase A
- PLC
phospholipase C
- PLD
phospholipase D
- RhoA
Ras homolog gene family, member A
- Rho-GEF
Rho guanine nucleotide exchange factor
- SD
standard deviation
- TXA-2
thromboxane A-2
References
- 1.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Feng J, Liu YH, Clements RT, et al. Calcium-activated potassium channels contribute to human coronary micro-vascular dysfunction after cardioplegia arrest. Circulation. 2008;118 suppl I:S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Chu LM, Robich M, et al. Endothelin-1 induced contraction and signaling in the human skeletal muscle microcirculation. Circulation. 2010;122:S150–S155. doi: 10.1161/CIRCULATIONAHA.109.928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Liu YH, Khabbaz KR, et al. Endothelin-1 induced contractile responses of human coronary arterioles via endothelin-A receptors and PKC-α signaling pathways. Surgery. 2010;147:798–804. doi: 10.1016/j.surg.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erez E, Erman A, Snir E, et al. Thromboxane production in human lung during cardiopulmonary bypass: beneficial effect of aspirin? Ann Thorac Surg. 1998;65:101–106. doi: 10.1016/s0003-4975(97)01040-0. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, Liu RY, Wu GZ, Tang SB. PGE1 reduces cardiac-derived TXA2 in ischemic arrest in isolated working rat heart. Intern J Cardiol. 1996;55:265–270. doi: 10.1016/0167-5273(96)02715-5. [DOI] [PubMed] [Google Scholar]
- 7.Byrne JG, Appleyard RF, Sun SC, et al. Cardiac-derived thromboxane A2. An initiating mediator of reperfusion injury? J Thorac Cardiovasc Surg. 1993;105:689–693. [PubMed] [Google Scholar]
- 8.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Ogletree ML, Harris DN, Greenberg R, Haslanger MF, Nakane M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J Pharmacol Exp Ther. 1985;234:435–441. [PubMed] [Google Scholar]
- 10.Bleasdale JE, Thakur NR, Gremban RS, et al. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 11.Craven PA, Studer RK, DeRubertis FR. Thromboxane/prostaglandin endoperoxide-induced hypertrophy of rat vascular smooth muscle cells is signaled by protein kinase C-dependent increases in transforming growth factor-β. Hypertension. 1996;28:169–176. doi: 10.1161/01.hyp.28.2.169. [DOI] [PubMed] [Google Scholar]