Abstract
Neuropeptide Y is one of the most abundant neurotransmitters in the myocardium, and is known to influence cardiovascular remodeling. We hypothesized that diabetic neuropathy could possibly be associated with altered neuropeptide Y and its receptor expression levels in myocardium and plasma. Plasma neuropeptide Y levels in diabetic (n = 24, HgbA1c 7.9 ± 1.1%) and non-diabetic (n = 27, HgbA1c 5.8 ± 0.5%) patients undergoing cardiac surgery utilizing cardiopulmonary bypass were analyzed. Right atrial tissue of these patients was used to determine the expression of neuropeptide Y, the receptors 1–5, and leptin by immunoblotting, real-time PCR and immunofluorescence. Apoptosis signaling and endostatin and angiostatin were measured to determine the effects of leptin.
Plasma neuropeptide Y levels were significantly increased in patients with Type II diabetes mellitus as compared to non-diabetic patients (P = 0.026). Atrial tissue neuropeptide Y mRNA levels were lower in diabetic patients (P = 0.036). There was a significant up-regulation of myocardial Y2 and Y5 receptors (P = 0.009, P = 0.01 respectively) in the diabetic patients. Leptin, involved with apoptosis and angiogenesis, was down regulated in diabetic patients (P = 0.05). The levels of caspase-3, endostatin and angiostatin were significantly elevated in diabetic patients (P = 0.003, P = 0.008, P = 0.01 respectively). Y1 receptors were more likely to be localized within the nuclei of cardiomyocytes and vascular smooth muscle cells.
Neuropeptide expression is altered differentially in the serum and myocardium by diabetes. Altered regulation of this system in diabetics may be in part responsible for the decreased angiogenesis, increased apoptosis, and increased vascular smooth muscle proliferation leading to coronary artery disease and heart failure in this patient population.
Keywords: Neuropeptide Y, Neuropeptide Y receptor, Type II diabetes mellitus, Cardiovascular disease, Angiogenesis, Apoptosis, Leptin
1. Introduction
Neuropeptide Y is one of the most abundant neurotransmitters in the nervous and cardiovascular systems (Jonsson-Rylander et al., 2003; Onuoha et al., 1999; Uddman et al., 2002; Zukowska et al., 2003a; Zukowska et al., 2003b). It is known to modulate a variety of neuro-endocrine and metabolic functions in the body. These modulations include, but are not limited to energy homeostasis, peripheral vascular tone, and vascular remodeling in response to stress (Abe et al., 2007; Abe et al., 2010). Recently, neuropeptide Y itself has been shown to be regulated by leptin in the hypothalamus and peripheral autonomic nervous system. In the cardiovascular system neuropeptide Y is co-localized with the classic sympathetic neurotransmitter noradrenaline, present in sympathetic nerves innervating coronary arteries (Maturi et al., 1989), cardiomyocytes, and endothelium (Jonsson-Rylander et al., 2003). It has been shown to act by multiple G coupled receptors (Y1–Y5) on vascular endothelial cells (Uddman et al., 2002), vascular smooth muscle (VSMC), cardiomyocytes (Nicholl et al., 2002), immune and fat cells. The neuropeptide Y1 and Y2 receptors appear to be involved with the development of cardiomyopathy (Chottova Dvorakova et al., 2008). Increased levels of Y1 receptor, in particular, have been implicated in the pathogenesis of cardiomyocyte hypertrophy in the rat myocardium by both decreased protein degradation and increased protein synthesis through activation of P13K/p70 pathways (Protas et al., 2003) (Millar et al., 1994). Neuropeptide via Y2 receptor promotes angiogenesis as demonstrated in vitro and in vivo studies.
Plasma levels of neuropeptide Y have been shown to be increased in diabetes mellitus in animal and human studies (Ilhan et al., 2010; Milewicz et al., 2000; Satoh et al., 1999). In diabetic rats alterations in tissue levels of neuropeptide Y have been seen in the myocardium, along with up-regulation of its receptors (Chottova Dvorakova et al., 2008). The alterations of Y1–5 receptor levels in the chronic diabetic rat myocardium suggest that it could be playing a fundamental role in the pathogenesis of diabetic cardiomyopathy. The assessment of neuropeptide Y receptor concentration in human myocardium has been limited to cadaveric studies and has demonstrated the presence of similar receptors on cardiomyocytes, endocardium, and nerve endings (Jonsson-Rylander et al., 2003). Due to the particular localization of receptors in cardiac tissue and its known trophic effects, it is possible that neuropeptide Y plays a similar role in the etiology and pathogenesis of diabetic cardiomyopathy in humans. To demonstrate an association between diabetes and neuropeptide Y receptor concentration in human myocardium, we conducted this study to analyze human atrial tissue excised during cardiac surgery for the neuropeptide Y receptor concentration and localization. We hypothesized that patients with diabetes mellitus will demonstrate increased serum levels of neuropeptide Y and altered expression of its receptors, leading to changes in cardiac structure and function.
2. Materials and methods
2.1. Human subjects and tissue harvesting
After Institutional Review Board approval and obtaining written informed consent, patients undergoing elective cardiac surgery utilizing cardiopulmonary bypass were enrolled in this study. After induction of general anesthesia and median sternotomy, right atrial tissue excised during right atrial cannulation was obtained for analysis prior to institution of cardiopulmonary bypass. Atrial tissue was snap frozen in liquid nitrogen for RT-PCR and immunoblot analysis. Tissue for immunofluorescence staining was fixed in 10% buffered formalin for 24 h followed by being paraffin embedded and sectioned into 5-µm slices. After placement of radial artery cannula in the preoperative holding area 10 ml of blood was obtained and was centrifuged immediately at 10,000 g for 6 min for extraction of plasma, which was immediately frozen (−80 °C) for ELISA assays.
2.2. Neuropeptide Y Elisa
Neuropeptide Y plasma levels were measured using a competitive peptide enzyme immunoassay according to the manufacturer procedural recommendations (Peninsula Laboratories, San Carlos, CA). As per the manufacturer the specificity and sensitivity of the assay was 99% for neuropeptide Y.
2.3. Western blotting
Frozen atrial tissue was homogenized in RIPA buffer consisting of 50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS (Boston Bioproducts, Worcester, MA), phosphatase inhibitor cocktails I and II at 1:100 (Sigma, St. Louis, MO), one-half tablet of Complete EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN) and 64 mM NaF (Fisher Scientific, Pittsburgh, PA). Protein concentration was quantified using the Micro BCA Protein Assay Kit (Pierce, Rockford, Illinois) and equal amounts of protein (40ug) were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, Bellerica, MA). Membranes were stained with Ponceau S to ensure equal protein transfer and separation. Membranes were blocked in 5% blotting-grade milk (Bio-Rad, Hercules, CA), washed in TBS with 0.05% Tween-20 (Boston Bioproducts), followed by incubations in primary and secondary antibodies and visualized using chemiluminescence (Supersignal West Pico Chemiluminescent kit, Pierce) with X-ray films. X-ray films optical density values were obtained using a flat-bed scanner and ImageJ 1.4 software (National Institutes of Health, USA). All calculated densities were normalized to Ponceau S staining intensity.
2.4. Antibodies
Primary antibodies for Western blots were obtained from Alpha Diagnostic International (San Antonio, TX), and anti-rabbit HRP was obtained from Cell Signaling (Beverly, MA). For immunofluorescence studies, primary antibodies were anti-neuropeptide Y and anti-α smooth muscle actin obtained from Abcam (Cambridge, MA), anti-NPY1R from Imgenex (San Diego, CA), anti-Y2 from Neuromics (Edina, MN), and anti-PGP 9.5 from Cedarlane (Burlington, NC). The other primary antibodies for Western blots were endostatin (Millipore), angiostatin (Calbiochem), caspase-3, BCL-2, and MCl-1 (Cell Signaling Apoptosis Ab kit).
2.5. RT-PCR
Total RNA from frozen atrial tissue was extracted using the TRI Reagent kit (Sigma, St. Louis, MO). RNA integrity was verified by electrophoresis on a 1% agarose gel, and quantified by spectrophotometric analysis at 260/280 nm using the NanoDrop 1000 (Thermo Scientific, Wilmington, DE). Equal amounts of total RNA were amplified using a one-step TaqMan RT-PCR system (Applied Biosystems, Foster City, CA) with primers pairs for neuropeptide Y, Y1, Y2, DPPIV, Y5, and 18S rRNA (Applied Biosystems). Each sample was analyzed in duplicate and relative differences in gene expression were calculated using the 2−DDCT methods by normalizing the CT values for each gene to the CT values for 18S rRNA.
2.6. Confocal microscopy
Paraffin sections of atrial tissue from patients were subjected to immunofluorescence for localization and expression of neuropeptide Y, Y1 and Y2 receptor (Abcam) Sections were deparaffinized in xylene and rehydrated in graded ethanol and distilled water. Antigens were unmasked by boiling in sodium citrate (10 mM, pH 6.0) for 10 min, followed by a phosphate-buffered saline (PBS) wash and blocking in 1% bovine serum albumin for 1 h. Primary antibodies were added according to the manufacturer's recommendation at 4 °C overnight. Sections were washed in PBS and incubated with the appropriate AlexaFluoro antibodies (Molecular Probes, Eugene, OR) for 30 min. Sections were washed in PBS, mounted in Vectashield plus DAPI (Vector Labs, Burlingame, CA), and slides were viewed under a Zeiss LSM510 confocal microscope (Carl Zeiss MicroImaging Inc, Thornwood, NY). Negative controls were prepared by adding only the secondary antibody to the slides and pre blocking the receptors with peptide before staining with primary antibody.
2.7. Statistical analysis
Demographic and baseline data are presented as mean ± standard deviation or incidence of group, as appropriate. Comparisons for outcomes were made between type II diabetic patients and non-diabetic patients. Outcome data is presented as mean ± 95% confidence intervals (CI). Relative protein levels and relative RNA expression values were calculated by normalizing raw data from the experimental group to the average of the controls. Comparisons were made between groups using t-test when normally distributed, or Mann–Whitney test when not. Assessment of the normality of the data was performed using Shapiro–Wilkes test. Comparison of dichotomous variables was performed using Fisher's exact test. Significant differences were assessed at the p ≤ 0.05 values. Data were analyzed using GraphPad Prism 4 (GraphPad Software, La Jolla, CA).
3. Results
3.1. Patient characteristics
A total of 51 patients were enrolled for this study between August 2008 and February 2009. Of these, there were 27 patients who were non-diabetic and 24 patients with type II diabetes mellitus. All patients in the diabetic group had a primary diagnosis of type II diabetes, and were on oral hypoglycemics or insulin. Both groups were similar in demographic characteristics, except the levels of HbA1C (5.8 ± 0.5% in non-diabetic group and 7.9 ± 1.1% in the type II diabetes mellitus group, P ≤ 0.05), and the levels of creatinine were significantly higher in diabetic patients (1.0 ± 0.3 non-diabetic vs. 1.5 ± 1.1 diabetic, P ≤ 0.05). There was no difference in the BMI between the groups (28 ± 3.6 non-diabetic 29 ± 5.4, (P = 0.6) (Table 1).
Table 1.
Baseline characteristics of non-diabetic and diabetic groups.
| Demographics | Non-diabetic (n = 27) | Diabetic (n = 24) |
|---|---|---|
| Age (years) | 67 (±11) | 71 (±9) |
| Male | 20 (74%) | 22 (92%) |
| HbA1C (mg/dl) | 5.8 (±0.5) | 7.9 (±1.1)a |
| Insulin | N/A | 4 (17%)a |
| Oral hypoglycemic agent | N/A | 20 (80%)a |
| Hypertension | 24 (88%) | 21 (88%) |
| Congestive heart failure | 5 (18%) | 4 (17%) |
| Coronary artery bypass graft | 25 (92%) | 21 (88%) |
| Creatinine (mg/dl) | 1.0 (±0.3) | 1.5 (±1.1)a |
| Smoking | 15 (55%) | 13 (54%) |
| BMI (body mass index) | 28 (±3.6) | 29 (±5.4) |
Data are presented as mean (SD), or total number (n) with percentage of cohort (%).
denotes P < 0.05.
CHF: Congestive Heart Failure.
CABG: Coronary Artery Bypass Graft.
BMI: Body Mass Index.
3.2. Levels of neuropeptide Y in plasma and tissue
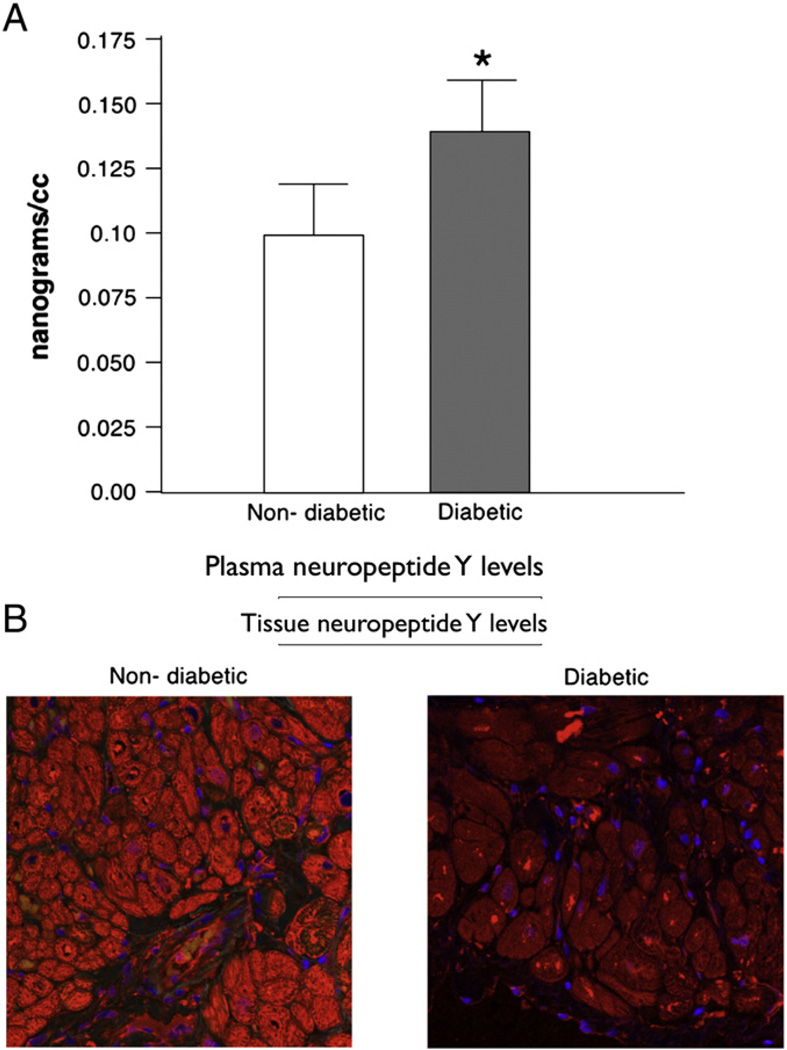
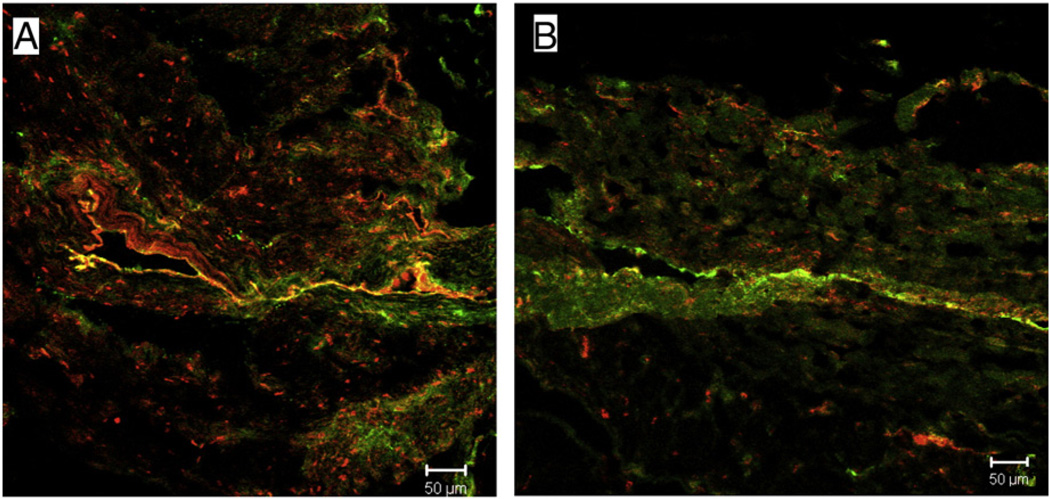
Plasma neuropeptide Y levels were significantly increased (0.192 vs. 0.053 ng/ml, P = 0.026) in patients with diabetes mellitus (Fig. 1A). On immunohistochemistry the neuropeptide Y expression levels were significantly decreased in the atrial tissue from diabetic patients (Fig. 1B). Co-staining for nerves with PGP9.5 showed decreased levels of this neurotransmitter at the nerve endings in diabetic tissue as compared to non-diabetic atrial tissue (Fig. 2).
Fig. 1.
(A) Plasma levels of neuropeptide Y in non-diabetic with HbA1c levels <5.8% versus diabetic with HbA1c levels >6.8% patients as measured by ELISA immunoassay. The plasma levels were significantly elevated in diabetic patients as compared to non-diabetic patients (P=0.026). (B) Immunohistochemistry staining with neuropeptide Y showing decreased levels of neuropeptide Y in diabetic tissue as compared to non-diabetic atrial tissue.
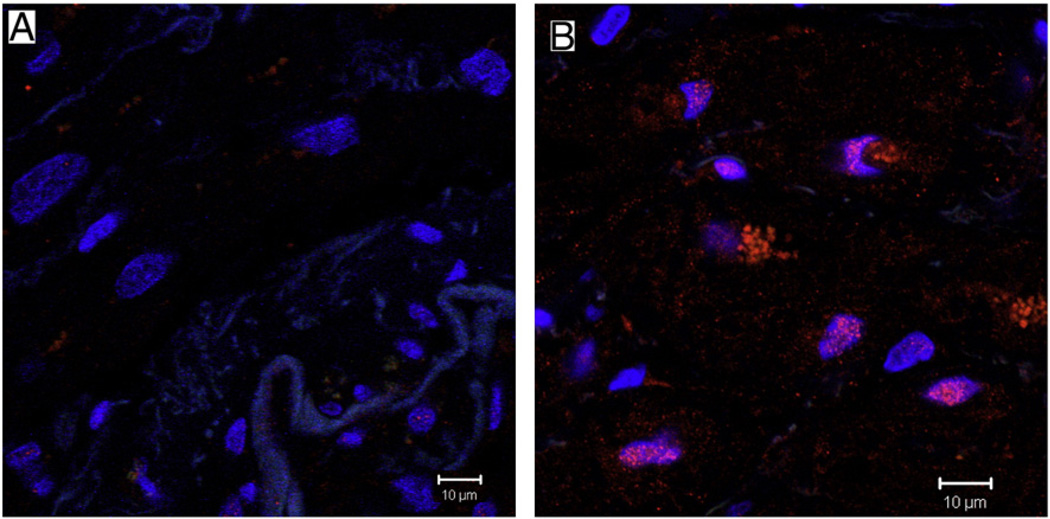
Fig. 2.
Representative confocal microscopy. neuropeptide Y (red) co-stained with the general nerve marker protein gene product 9.5 (PGP 9.5, green), showing neuropeptide Y staining in perivascular nerves in non-diabetic and diabetic patient atrial tissue. There is decreased neuropeptide Y co-staining with PGP 9.5 in diabetic atrial tissue (B) as compared to non-diabetic tissue (A).
3.3. Immunoblotting
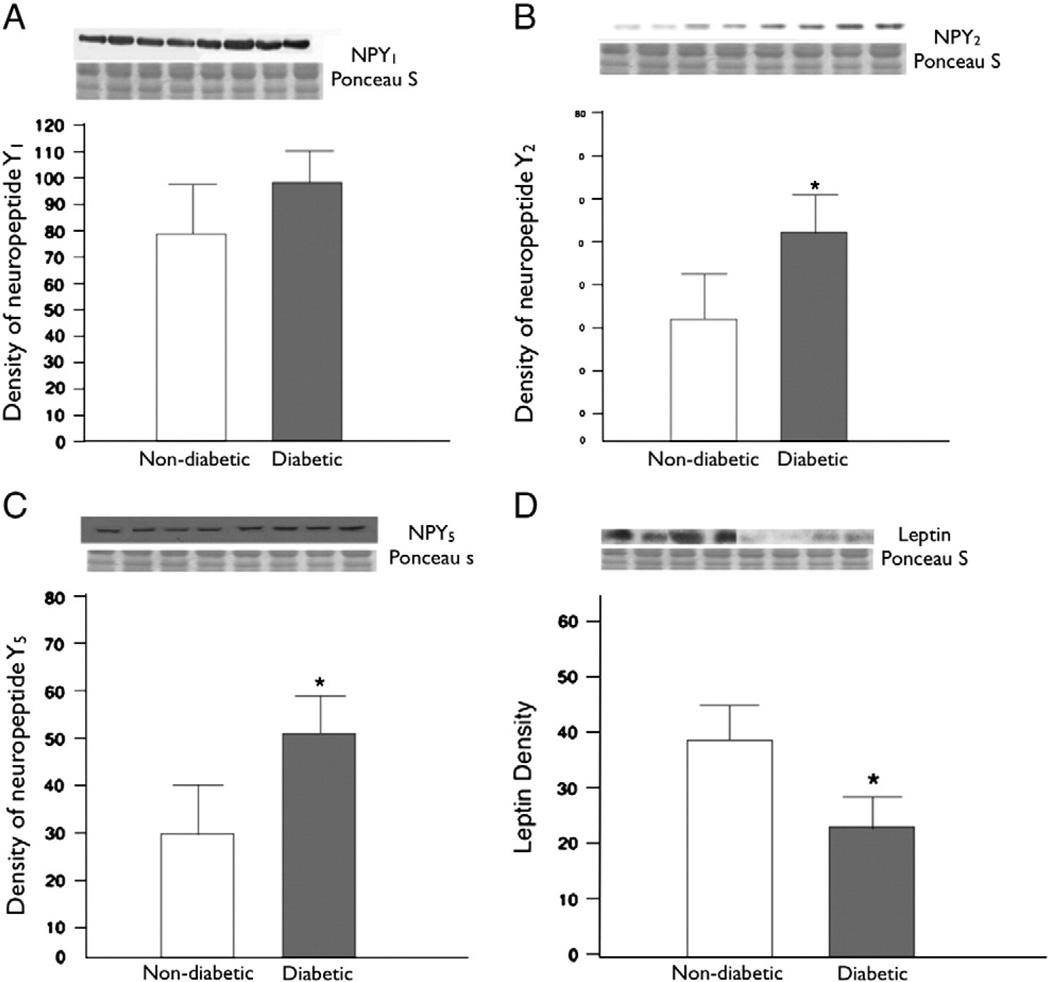
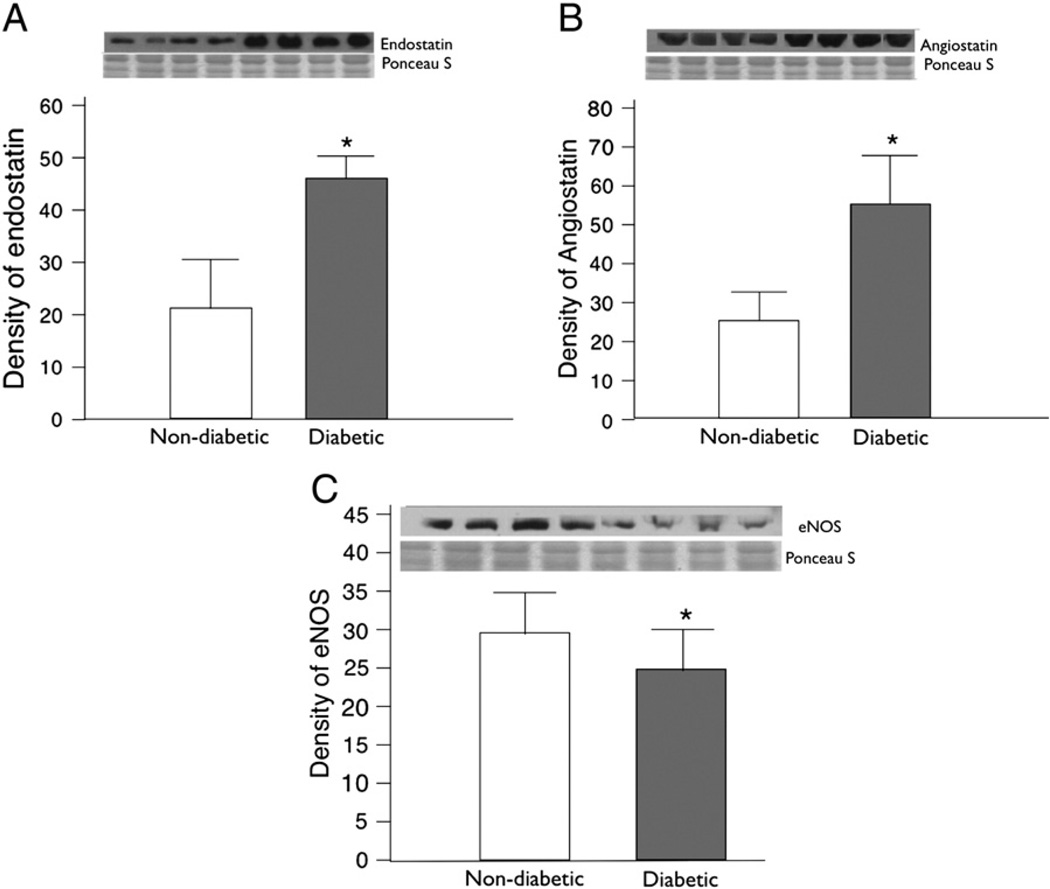
Immunoblotting experiments were carried out in total 30 patients (non-diabetic n = 15, diabetic n = 15). The levels of Y2 and Y5 receptors were significantly up regulated in atrial tissue from diabetic as compared to non-diabetic patients (Y2 receptor P = 0.009, Y5 receptor P = 0.01) (Fig. 3B, C). The total levels of the neuropeptide Y1 receptor protein in atrial tissue were slightly higher in patients with diabetes mellitus, but this difference was not significant (P = 0.2) (Fig. 3A). The expression levels of leptin were significantly decreased in diabetic patients as compared to non-diabetic patients (P = 0.05) (Fig. 3D). The levels of anti-angiogenic factors endostatin and angiostatin were also significantly elevated in diabetic tissue as compared to non-diabetic atrial tissue (endostatin P = 0.008, angiostatin P = 0.01) (Fig. 4A, B). We assessed the levels of eNOS as it is associated with angiogenesis and vasodilation along with neuropeptide Y, and found significantly decreased levels of eNOS in diabetic tissue (P = 0.01) (Fig. 4C).
Fig. 3.
Protein expression was assessed in the atrial tissue of non-diabetic and diabetic patients. (A) neuropeptide Y1 levels were elevated but not significantly, in diabetic patients than in non-diabetic patients (P=0.2). (B) neuropeptide Y2 were significantly elevated in diabetic patients than in non-diabetic patients (P=0.008). (C) neuropeptide Y5 were significantly elevated in diabetic patients than in non-diabetics (P=0.01). (D) Leptin protein was significantly reduced in diabetic patients as compared to non-diabetics, (P=0.05).
Fig. 4.
(A) Endostatin levels were assessed in the atrial tissue, the endostatin levels were significantly elevated in non-diabetic as compared to diabetic (P=0.008). (B) The levels of angiostatin were significantly elevated in diabetic as compared to non-diabetic patients (P=0.01). (C) The expression of eNOS was decreased in diabetic as compared to non-diabetic (P=0.01).
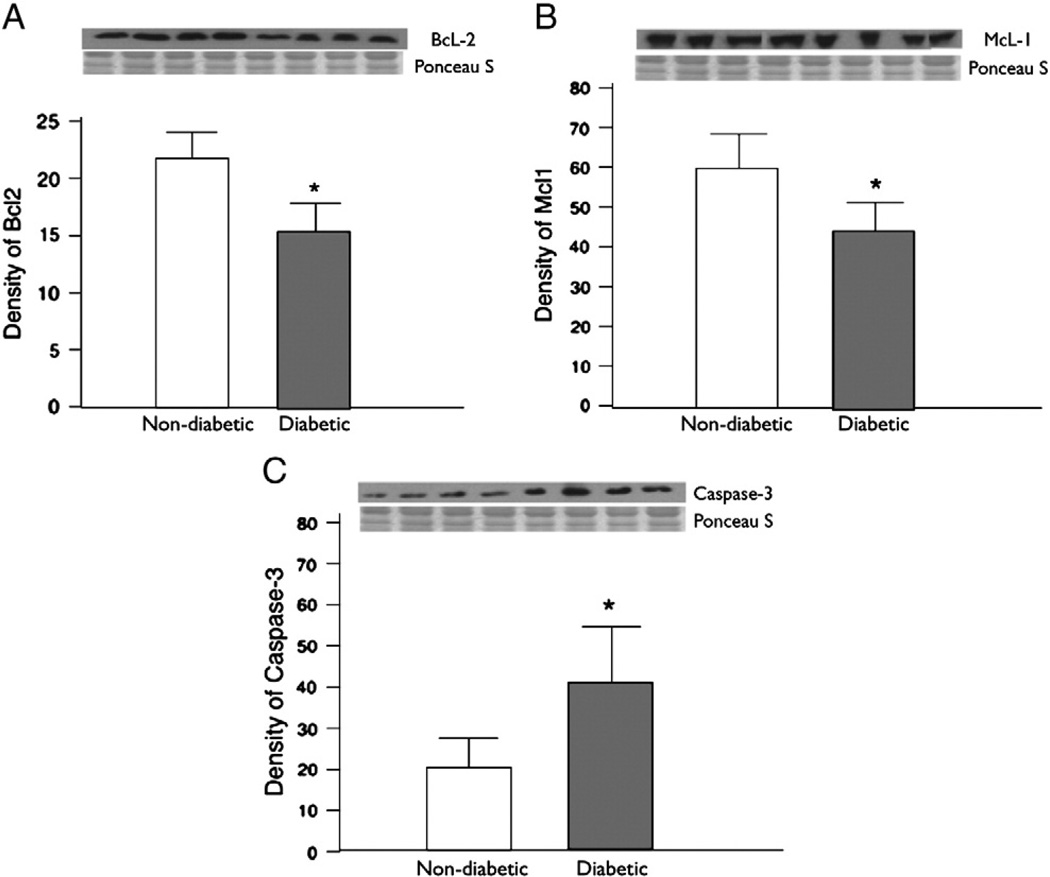
There were significantly elevated levels of caspase-3 (P = 0.003) suggesting increased apoptosis signaling. While the pro-survival factors BcL-2 and McL-1 were significantly decreased in patients with diabetes mellitus as compared to non-diabetic patients (BcL-2 P = 0.003, McL-1 P = 0.003) (Fig. 5).
Fig. 5.
(A) The levels of pro-survival factor BCl-2 were decreased in diabetic as compared to non-diabetic (P=0.003) (B) The levels of another pro-survival factor MCl-1 were significantly decreased in diabetic as compared to non-diabetic (P=0.003) (C) There were significantly elevated levels of caspase-3 in diabetic as compared to non-diabetic (P=0.003).
3.4. RT-PCR
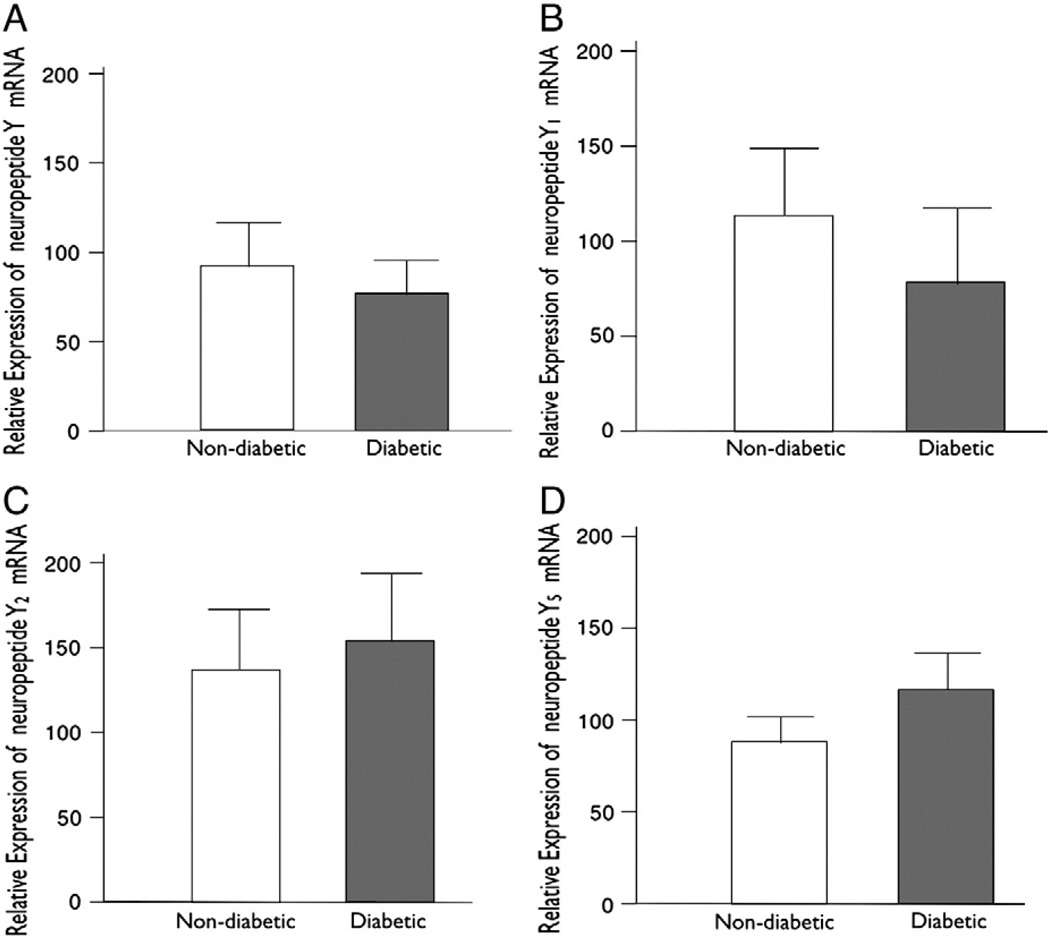
RT-PCR for mRNA was carried out in total 24 patients with n = 12 in each group. No significant differences were observed between the diabetic and non-diabetic groups with respect to total myocardial levels of the NPY, Y1 receptor, Y2 receptor, and Y5 receptor mRNA as measured by RT-PCR (neuropeptide Y (Fig. 6A) mRNA P = 0.036, Y1 receptor (Fig. 6B) P = 0.7, Y2 receptor (Fig. 6C) P = 0.67, Y5 receptor (Fig. 6D) P = 0.5) in the right atrial tissue (Fig. 6).
Fig. 6.
(A) Myocardial levels of NPY mRNA in non-diabetic and diabetic patients as measured by semi-quantitative real-time PCR (P=0.36). (B) Myocardial levels of neuropeptide Y1 mRNA in non-diabetic (n=12) versus diabetic (n=12) patients as measured by real-time PCR (P=0.7). (C) Relative expression of neuropeptide Y2 in diabetic patients as compared to non-diabetic patients (P=0.67). (D) Relative expression of neuropeptide Y5 in diabetic patients as compared to non-diabetics (P=0.5).
3.5. Immunohistochemistry
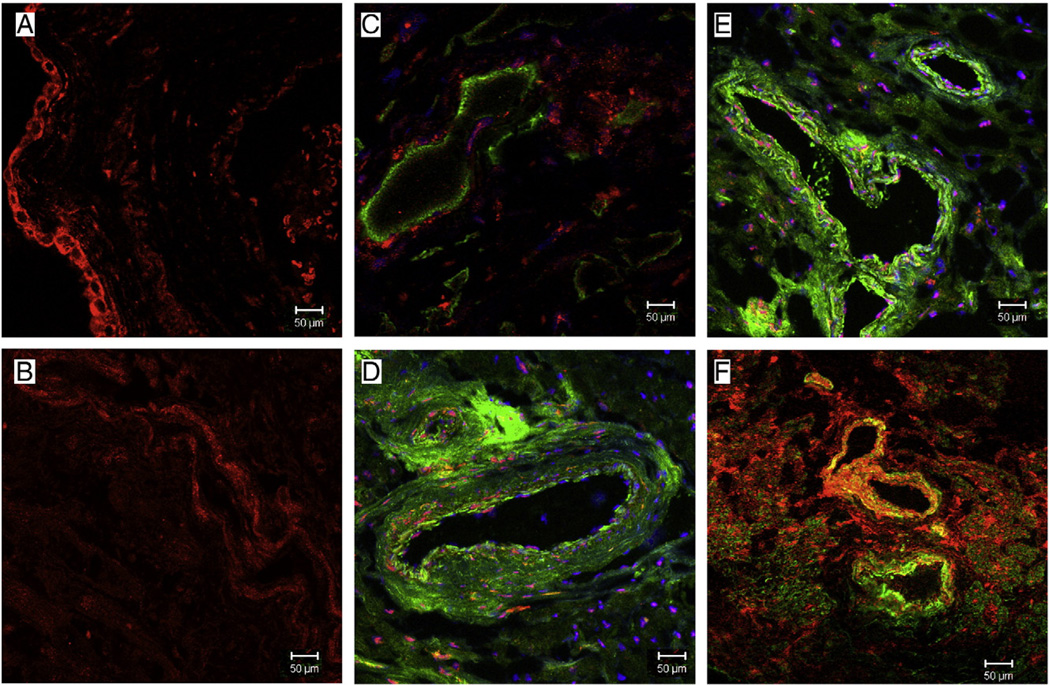
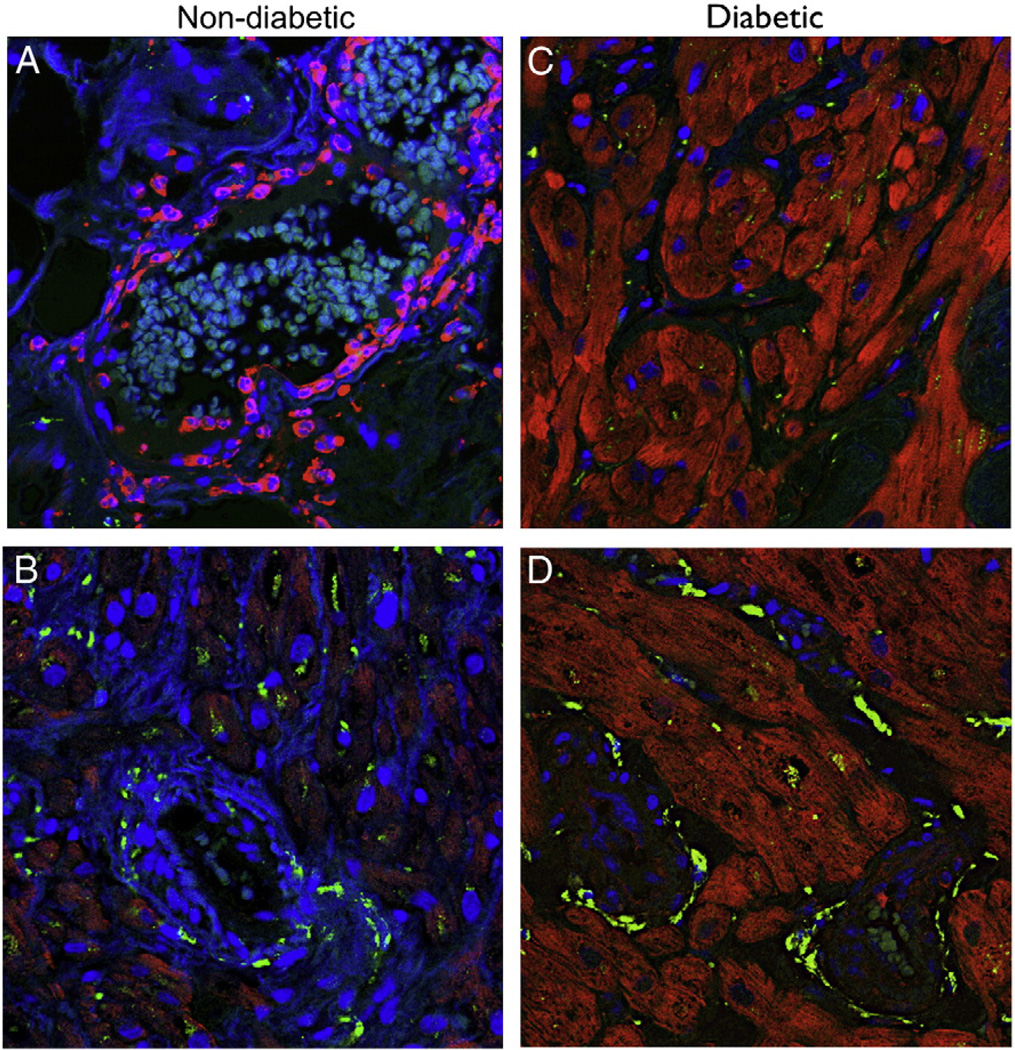
We used tissue from total 18 patients n = 9 in each group to do immunohistochemistry staining. On immunohistochemistry, staining for NPY demonstrated decreased levels in diabetic right atrial tissue as compared to non-diabetic (Fig. 1B). The concentration of Y1 receptor was more localized in the endothelium of non-diabetic patients as compared to diabetic patients (Fig. 7A, B). Co-labeling vascular smooth muscle cells with α-smooth muscle actin showed the diabetic group exhibited hypertrophic vessels compared to the non-diabetic group as well as intense vascular smooth muscle staining for Y1 (Fig. 7C and E non-diabetic, D and F diabetic).
Fig. 7.
Representative immunofluorescence staining for Y1 (red) in sections co-stained with the vascular smooth muscle marker -smooth muscle actin (green) in atrial tissue from Non-diabetic and Diabetic patients. (A and B) There is staining of Y1 receptor in the endothelium of non-diabetic tissue, while the staining is decreased in the endothelium of diabetic tissue. (D and F) There are increased staining of Y1 receptor around the arteries in the atrial tissue of diabetic patients as compared to non-diabetic patients (C and E).
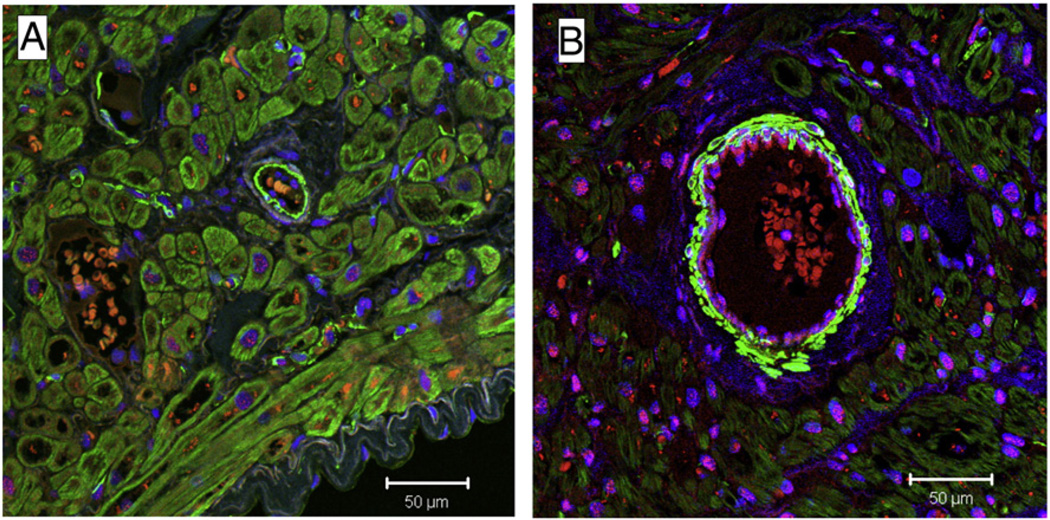
After immunofluorescence staining for the Y1 and nuclei co-staining with 4’ 6’-diamidino-2-phenylindole (DAPI) in the human myocardium there was intense Y1 staining in the cardiomyocyte nuclei of diabetic patients (Fig. 8). The number of cardiomyocytes with nuclear staining was determined by hand counting multiple regions of multiple slides. The numbers of cardiomyocytes with nuclear staining in the diabetic group were statistically significant (P ≤ 0.05) as compared to non-diabetic group (Fig. 9). To confirm the sensitivity of Y1 antibody, we were able to block the Y1 receptor nuclear staining after pre incubating the tissue with the blocking peptide. The IHC staining for Y5 and nuclear co-staining with DAPI showed dense staining in the cardiomyocytes of diabetic tissue (C&D) as compared to tissue from non-diabetic patients (A&B) (Fig. 10).
Fig. 8.
Immunofluorescence images. Representative slides of atrial tissue from non-diabetic and diabetic patients. Sections were co-stained with 4′,6′-diamidino-2-phenylindole (DAPI, blue) to demarcate the nucleus, green for alpha smooth muscle actin and red for Y1 receptor. There is increased Y1 receptor expression around the arteries and in the cardiomyocyte nuclei in diabetic patient. Magnification for both images is 20×.
Fig. 9.
Sections were co-stained with 4′,6′-diamidino-2-phenylindole (DAPI, blue) to demarcate the nucleus. Magnification for all images is 63×. The number of nuclei staining with Y1 nuclear staining+cells/mm2) is statistically significant in diabetic patients (B) as compared to non-diabetics (A) (P=0.04).
Fig. 10.
Immunofluorescence images. Representative slides of atrial tissue from non-diabetic and diabetic patients. Sections were co-stained with 4′,6′-diamidino-2-phenylindole (DAPI, blue) to demarcate the nucleus and Y5 receptors. Magnification for all images is 40×. There is increased staining for Y5 receptors in diabetic tissue cardiomyocytes (C and D) as compared to non-diabetic tissue where the localization of Y5 receptors is around blood vessel. (A and B).
4. Discussion
The results of our study have demonstrated that there are significant changes in the concentration and distribution of neuropeptide Y and its receptors in the right atrial tissue of patients with type II diabetes mellitus (HbA1C> 6.8%), as compared to atrial tissue from non-diabetics. Our study raises the possibility that chronic diabetes mellitus determines ultra-structural myocardial changes in humans. Our finding of increased neuropeptide Y plasma levels in diabetic patients is consistent with previous studies (Ilhan et al., 2010; Milewicz et al., 2000; Satoh et al., 1999). However, our demonstration of decreased levels of neuropeptide Y in right atrial tissue of diabetics is a novel finding, as neuropeptide Y is abundant in the myocardial tissue and stored in the sympathetic nerve endings under normal conditions (Gu et al., 1984; Sternini and Brecha, 1985). There could be multiple explanations for this observation. It may be due to an absolute reduction in the number of sympathetic nerve endings in the myocardium or possibly a decreased secretion of neuropeptide Y from the nerve endings, as suggested by the findings of our study.
In our study, the up-regulation of neuropeptide Y receptors in the presence of decreased tissue neuropeptide Y levels may be interpreted as counter-regulatory up-regulation in the presence of decreased agonist. The findings of neuropeptide Y receptors up-regulation in myocardial tissue have also been reported in animal models of chronic hyperglycemia (Chottova Dvorakova et al., 2008; Protas and Robinson, 1999). Whereas in humans with diabetes, similar observations are limited to neuropeptide Y and Y1 receptor level alterations in subcutaneous tissue in patients with peripheral neuropathy (Chottova Dvorakova et al., 2008; Kuncova et al., 2005; Levy et al., 1989) (Kuo et al., 2007). Our study demonstrates that such a correlation exits in human myocardial tissue as well.
Greater localization of Y1 receptors in the nuclei of cardiomyocytes of diabetic patients is also a novel finding. The nuclear localization of these receptors has been demonstrated in cardiac endothelial cells in humans and cardiomyocytes in animal studies (Abe et al., 2007). However, this was not in the context of comparison of myocardial changes in normal and chronic diabetes. The Y1 receptor in the cardiomyocyte is known to be involved in multiple enzymatic regulatory processes e.g. adenyl cyclase, calcium ion channel dynamics and hence contractile function (Nicholl et al., 2002; Nie and Selbie, 1998; Shigeri and Fujimoto, 1993). Increased expression of neuropeptide Y receptors in animal studies has also been shown to cause protein turnover by up-regulation of fetal gene expression leading to pathological cardiomyocyte hypertrophy (Fang et al., 2004; Jacques et al., 2003; Jacques et al., 2006; Lebeche et al., 2008; Nicholl et al., 2002). The cellular structural changes observed in our study raise the possibility that a similar mechanism may be involved in the histological changes observed in the myocardium of chronic diabetes in humans.
We found significantly elevated protein levels of Y2 and Y5 receptors in the heart tissue from diabetic patients as compared to non-diabetic patients. The increased localization and up-regulation of Y5 receptors probably explains the involvement of these receptors in cardiomyocyte hypertrophy in diabetic patients. Although, some of the neuropeptide Y mediated effects require both Y1 and Y2 receptors, the Y2 receptor effects predominate in protecting the heart from excessive stress by improving perfusion, through cellular migration, proliferation, and angiogenesis. We have shown in a swine model of chronic myocardial ischemia that up-regulation of Y2 and Y5 receptors with local infiltration of pro-angiogenic NPY3–36 leads to significantly increased angiogenesis (Robich et al., 2010). However, in the current work we demonstrated elevated levels of anti-angiogenic factors (endostatin and angiostatin) along with increased apoptosis signaling in the myocardium of patients with chronic diabetes. Interestingly, we also found levels of leptin to be significantly decreased in the diabetic tissue. Leptin has been shown to be an anti-apoptotic factor by increasing BCl-2 levels and promoting angiogenesis by decreasing anti-angiogenic factors. We showed decreased levels of pro-survival factors BCl-2 and Mcl-1 while there are significantly higher levels of Caspase-3, a marker of apoptosis. Significantly decreased levels of eNOS in diabetic tissue are important to note as not only eNOS is independently angiogenic, neuropeptide Y also causes angiogenesis through eNOS as has been shown in multiple studies. Thus, despite increased levels of Y2 receptors, which are involved in neo-vasularization, there are also higher levels of anti-angiogenic factors, higher levels of pro-apoptotic factors, and lower levels of pro-survival factors in the diabetic heart.
Our study has demonstrated that the myocardial expression of neuropeptide Y receptors is altered in type II diabetes mellitus. High levels of neuropeptide Y receptors may lead to structural changes in the myocardium, and at the cardiomyocyte level promoting cell hypertrophy and possibly leading to changes in calcium signaling. Whether these changes in neuropeptide Y ligand and its receptors represent the initial etiology of disease in the myocardium needs to be seen (Protas and Robinson, 2008; Schwertfeger et al., 2004) (Herring et al., 2008).
4.1. Limitations
There are limitations to the current study. Our study is limited to analysis of right atrial tissue only, and thus cannot ascertain whether these observations apply to other myocardial compartments in humans. Our study was observational, and conducted on a complicated patient population with multiple co-morbidities and treatment overlaps. Whether these changes were a direct cause or effect of diabetes and its complications has yet to be discovered.
4.2. Conclusion
We have demonstrated higher levels of neuropeptide Y in the plasma, decreased levels of neuropeptide Y in the myocardium with increased expression of the associated receptors in patients with chronic type II diabetes (HbA1C> 6.8%). Simultaneously, there were significantly increased levels of anti-angiogenic factors and pro-apoptotic proteins in the myocardium of diabetic patients along with decreased levels of leptin, pro-survival proteins, and eNOS in the cardiac tissue. The altered levels of neuropeptide Y, its receptors, and leptin may be involved in cardiac remodeling. Altered regulation of the neuropeptide Y system in diabetes may be in part responsible for decreased angiogenesis, increased apoptosis, and increased vascular smooth muscle proliferation that is responsible for coronary artery disease and heart failure in this patient population (Fig. 11). Thus, the changes in neuropeptide Y and its receptors may be the consequence of chronic uncontrolled type II diabetes and may represent myocardial neuropathy.
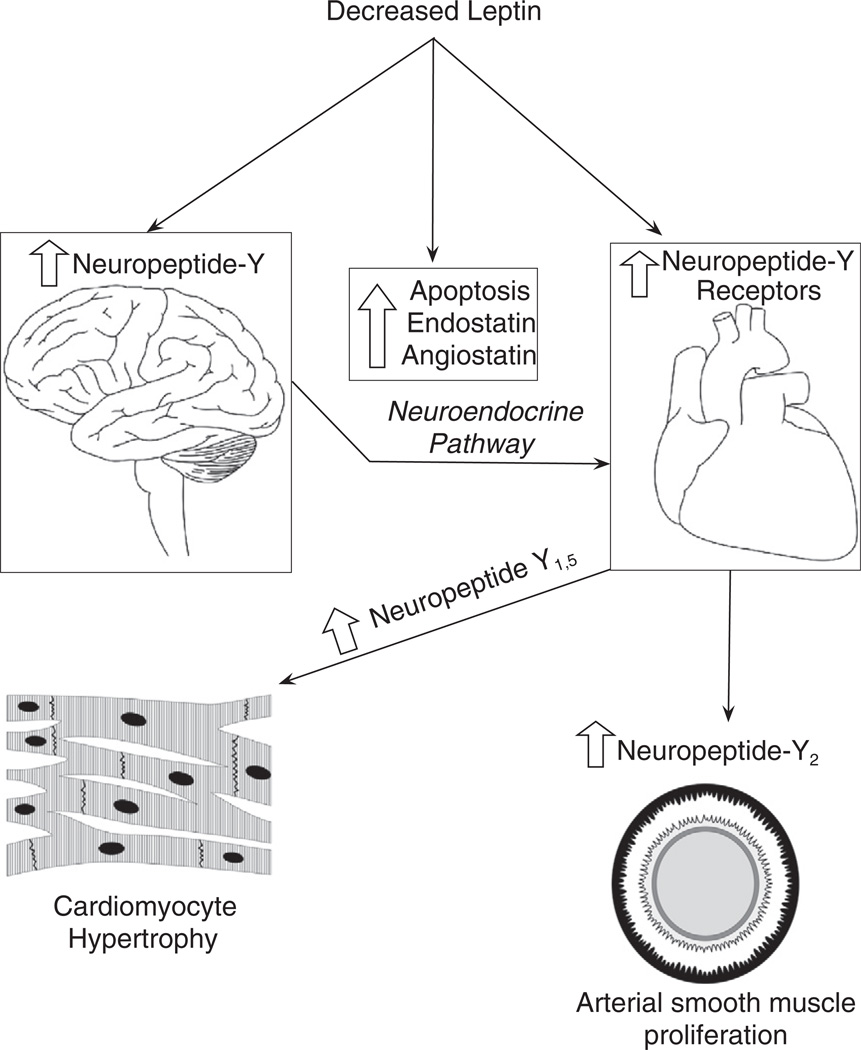
Fig. 11.
Schematic diagram of leptin interaction and neuropeptide Y. In diabetes mellitus there is either decreased leptin or leptin resistance that affects neuropeptide Y levels in the hypothalamus and peripheral autonomic nervous system. This altered regulation of neuropeptide Y leads to changes in neuropeptide Y receptor concentration. Decreased leptin itself causes increased apoptosis and increased anti-angiogenic factors. The neuropeptide Y receptorsmay be involved in cardiomyocytes hypertrophy and arterial smoothmuscle proliferation.
Acknowledgements
We would like to thank Debbie Bennett and Bejan Abaspour for their efforts in obtaining samples for our study. We would like to thank Faraz Mahmood for his efforts in making figures and tables for the paper.
Funding. The Department of Anesthesia, Critical Care, and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, MA provided funding to R.M. Funding for this project was provided to F.W.S. by NHLBI, RO1HL46716, RO1HL69024, and RO1HL85647 (F.W.S.), NIH 5T32-HL0074 (M.P.R.), and the Irving Bard Memorial Fellowship (M.P.R., L.M.C.).
Disclosures. Dr. Frank W. Sellke has research support from Ikaria (Clinton, NJ) and Orthologic (Tempe, AZ), and is a consultant for Novo Nordisk (Princeton, NJ), and Cubist Pharmaceuticals (Lexington, MA). Dr. Sellke is a consultant for the law firms representing Pfizer (Princeton, NJ) in the Bextra/Celebrex litigation.
References
- Abe K, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr. Top. Med. Chem. 2007;7:1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- Abe K, Kuo L, Zukowska Z. Neuropeptide Y is a mediator of chronic vascular and metabolic maladaptations to stress and hypernutrition. Exp. Biol. Med. (Maywood) 2010;235:1179–1184. doi: 10.1258/ebm.2010.009136. [DOI] [PubMed] [Google Scholar]
- Chottova Dvorakova M, Wiegand S, Pesta M, Slavikova J, Grau V, Reischig J, Kuncova J, Kummer W. Expression of neuropeptide Y and its receptors Y1 and Y2 in the rat heart and its supplying autonomic and spinal sensory ganglia in experimentally induced diabetes. Neuroscience. 2008;151:1016–1028. doi: 10.1016/j.neuroscience.2007.07.069. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr. Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- Gu J, Polak JM, Allen JM, Huang WM, Sheppard MN, Tatemoto K, Bloom SR. High concentrations of a novel peptide, neuropeptide Y, in the innervation of mouse and rat heart. J. Histochem. Cytochem. 1984;32:467–472. doi: 10.1177/32.5.6546942. [DOI] [PubMed] [Google Scholar]
- Herring N, Lokale MN, Danson EJ, Heaton DA, Paterson DJ. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. J. Mol. Cell. Cardiol. 2008;44:477–485. doi: 10.1016/j.yjmcc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Ilhan A, Rasul S, Dimitrov A, Handisurya A, Gartner W, Baumgartner-Parzer S, Wagner L, Kautzky-Willer A, Base W. Plasma neuropeptide Y levels differ in distinct diabetic conditions. Neuropeptides. 2010;44(6):485–489. doi: 10.1016/j.npep.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Jacques D, Sader S, Perreault C, Fournier A, Pelletier G, Beck-Sickinger AG, Descorbeth M. Presence of neuropeptide Y and the Y1 receptor in the plasma membrane and nuclear envelope of human endocardial endothelial cells: modulation of intracellular calcium. Can. J. Physiol. Pharmacol. 2003;81:288–300. doi: 10.1139/y02-165. [DOI] [PubMed] [Google Scholar]
- Jacques D, Sader S, Perreault C, Abdel-Samad D, Provost C. Roles of nuclear NPY and NPY receptors in the regulation of the endocardial endothelium and heart function. Can. J. Physiol. Pharmacol. 2006;84:695–705. doi: 10.1139/y05-162. [DOI] [PubMed] [Google Scholar]
- Jonsson-Rylander AC, Nordlander M, Svindland A, Ilebekk A. Distribution of neuropeptide Y Y1 and Y2 receptors in the postmortem human heart. Peptides. 2003;24:255–262. doi: 10.1016/s0196-9781(03)00041-x. [DOI] [PubMed] [Google Scholar]
- Kuncova J, Sviglerova J, Tonar Z, Slavikova J. Heterogenous changes in neuropeptide Y, norepinephrine and epinephrine concentrations in the hearts of diabetic rats. Auton. Neurosci. 2005;121:7–15. doi: 10.1016/j.autneu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Abe K, Zukowska Z. Stress, NPY and vascular remodeling: Implications for stress-related diseases. Peptides. 2007;28:435–440. doi: 10.1016/j.peptides.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat. Clin. Pract. Cardiovasc. Med. 2008;5:715–724. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- Levy DM, Karanth SS, Springall DR, Polak JM. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia. 1989;32:427–433. doi: 10.1007/BF00271262. [DOI] [PubMed] [Google Scholar]
- Maturi MF, Greene R, Speir E, Burrus C, Dorsey LM, Markle DR, Maxwell M, Schmidt W, Goldstein SR, Patterson RE. Neuropeptide-Y. A peptide found in human coronary arteries constricts primarily small coronary arteries to produce myocardial ischemia in dogs. J. Clin. Invest. 1989;83:1217–1224. doi: 10.1172/JCI114004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz A, Mikulski E, Bidzinska B. Plasma insulin, cholecystokinin, galanin, neuropeptide Y and leptin levels in obese women with and without type 2 diabetes mellitus. Int. J. Obes. Relat. Metab. Disord. 2000;24 Suppl 2:S152–S153. doi: 10.1038/sj.ijo.0801310. [DOI] [PubMed] [Google Scholar]
- Millar BC, Schluter KD, Zhou XJ, McDermott BJ, Piper HM. Neuropeptide Y stimulates hypertrophy of adult ventricular cardiomyocytes. Am. J. Physiol. 1994;266:C1271–C1277. doi: 10.1152/ajpcell.1994.266.5.C1271. [DOI] [PubMed] [Google Scholar]
- Nicholl SM, Bell D, Spiers J, McDermott BJ. Neuropeptide Y Y(1) receptor regulates protein turnover and constitutive gene expression in hypertrophying cardiomyocytes. Eur. J. Pharmacol. 2002;441:23–34. doi: 10.1016/s0014-2999(02)01440-1. [DOI] [PubMed] [Google Scholar]
- Nie M, Selbie LA. Neuropeptide Y Y1 and Y2 receptor-mediated stimulation of mitogen-activated protein kinase activity. Regul. Pept. 1998;75–76:207–213. doi: 10.1016/s0167-0115(98)00070-6. [DOI] [PubMed] [Google Scholar]
- Onuoha GN, Nicholls DP, Alpar EK, Ritchie A, Shaw C, Buchanan K. Regulatory peptides in the heart and major vessels of man and mammals. Neuropeptides. 1999;33:165–172. doi: 10.1054/npep.1999.0017. [DOI] [PubMed] [Google Scholar]
- Protas L, Robinson RB. Neuropeptide Y contributes to innervation-dependent increase in I(Ca, L) via ventricular Y2 receptors. Am. J. Physiol. 1999;277:H940–H946. doi: 10.1152/ajpheart.1999.277.3.H940. [DOI] [PubMed] [Google Scholar]
- Protas L, Robinson RB. Dissecting the NPY signaling cascade between cardiac sympathetic and parasympathetic nerves. J. Mol. Cell. Cardiol. 2008;44:470–472. doi: 10.1016/j.yjmcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Protas L, Qu J, Robinson RB. Neuropeptide y: neurotransmitter or trophic factor in the heart? News Physiol. Sci. 2003;18:181–185. doi: 10.1152/nips.01437.2003. [DOI] [PubMed] [Google Scholar]
- Robich MP, Matyal R, Chu LM, Feng J, Xu SH, Laham RJ, Hess PE, Bianchi C, Sellke FW. Effects of neuropeptide Y on collateral development in a swine model of chronic myocardial ischemia. J. Mol. Cell. Cardiol. 2010;49(6):1022–1030. doi: 10.1016/j.yjmcc.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh C, Satoh F, Takahashi K, Murakami O, Sone M, Totsune K, Yabe T, Ohneda M, Fukuda M, Sugimura K, Ogawa S, Nagakubo H, Sato T, Mouri T. Elevated plasma immunoreactive neuropeptide Y concentrations and its increased urinary excretion in patients with advanced diabetic nephropathy. Endocr. J. 1999;46:139–146. doi: 10.1507/endocrj.46.139. [DOI] [PubMed] [Google Scholar]
- Schwertfeger E, Klein T, Vonend O, Oberhauser V, Stegbauer J, Rump LC. Neuropeptide Y inhibits acetylcholine release in human heart atrium by activation of Y2-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:455–461. doi: 10.1007/s00210-004-0930-9. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Fujimoto M. Neuropeptide Y stimulates DNA synthesis in vascular smooth muscle cells. Neurosci. Lett. 1993;149:19–22. doi: 10.1016/0304-3940(93)90337-k. [DOI] [PubMed] [Google Scholar]
- Sternini C, Brecha N. Distribution and colocalization of neuropeptide Y- and tyrosine hydroxylase-like immunoreactivity in the guinea-pig heart. Cell Tissue Res. 1985;241:93–102. doi: 10.1007/BF00214630. [DOI] [PubMed] [Google Scholar]
- Uddman R, Moller S, Nilsson T, Nystrom S, Ekstrand J, Edvinsson L. Neuropeptide Y Y1 and neuropeptide Y Y2 receptors in human cardiovascular tissues. Peptides. 2002;23:927–934. doi: 10.1016/s0196-9781(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Zukowska Z, Grant DS, Lee EW. Neuropeptide Y: a novel mechanism for ischemic angiogenesis. Trends Cardiovasc. Med. 2003a;13:86–92. doi: 10.1016/s1050-1738(02)00232-3. [DOI] [PubMed] [Google Scholar]
- Zukowska Z, Pons J, Lee EW, Li L. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can. J. Physiol. Pharmacol. 2003b;81:89–94. doi: 10.1139/y03-006. [DOI] [PubMed] [Google Scholar]