Abstract
Mitochondria are crucial in different intracellular pathways of signal transduction. Mitochondria are capable of decoding a variety of extracellular stimuli into markedly different intracellular actions, ranging from energy production to cell death. The fine modulation of mitochondrial calcium (Ca2+) homeostasis plays a fundamental role in many of the processes involving this organelle. When mitochondrial Ca2+ homeostasis is compromised, different pathological conditions can occur, depending on the cell type involved. Recent data have shed light on the molecular identity of the main proteins involved in the handling of mitochondrial Ca2+ traffic, opening fascinating and ambitious new avenues for mitochondria-based pharmacological strategies.
Keywords: Mitochondria, Calcium, Aging, Neurodegeneration, Diabetes, Cardiovascular and mitochondrial disorders
1. Introduction
It is now firmly established that mitochondria are key players in different pathophysiological contexts (Duchen, 2004). Indeed, they are very efficient machines for decoding intracellular signals and in particular the calcium (Ca2+) one (Clapham, 2007).
The mitochondrial respiratory chain, by pumping protons across the ion-impermeable inner membrane, establishes a gradient (ΔμH), composed of an electrical potential (Δψ) and a concentration ratio (ΔpH), according to the Nernst equation: ΔμH = zFΔψ + RT ln[H+]i/[H+]o. This has major implications for Ca2+ transport and distribution. In actively respiring mitochondria, considering that the buffering capacity is mostly provided by weak acids, the gradient is supposed to be maintained mostly as an electrical gradient across the inner membrane (~ 180 mV). This implies a strong thermodynamic force in favor of the accumulation of cations (Rizzuto et al., 2000).
Ca2+ import across the outer mitochondrial membrane (OMM) occurs through the voltage-dependent anion channels (VDAC) (Simamura et al., 2008). VDAC is as a large voltage-gated channel, fully opened with high-conductance and weak anion-selectivity at low transmembrane potentials (< 20–30 mV), but switching to cation selectivity and lower conductance at higher potentials (Colombini, 2009; De Pinto et al., 2008; Shoshan-Barmatz et al., 2010). The precise mechanisms of VDAC conductance are however still under debate.
Ca2+ mitochondrial traffic across the inner mitochondrial membrane (IMM) takes place essentially through two pathways: i) an electrophoretic “uniporter” that transports Ca2+ down the electrical gradient established by the respiratory chain, and ii) a Na+/Ca2+ exchanger, mostly expressed in excitable cells (muscle and brain), and a H+/Ca2+ exchanger, that represents the prevailing route in most other tissues (see Fig. 1). These electroneutral antiporters prevent the attainment of an electrochemical equilibrium (Rizzuto et al., 2000).
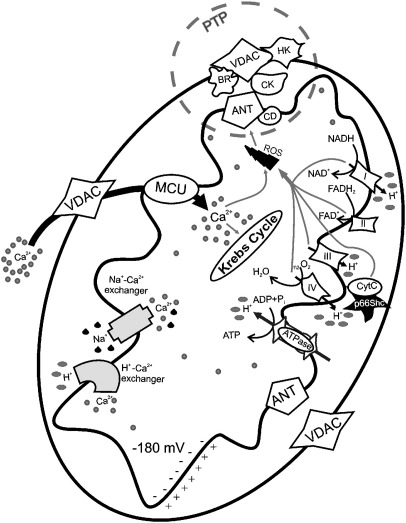
Fig. 1.
Mitochondrial physiology and mitochondrial Ca2+ handling. Ca2+ enters in the mitochondrial matrix via a low affinity uniporter (MCU) and through a high electronegative potential (− 180 mV). Extrusion of Ca2+ takes two major routes, one driven by the Na+/Ca2+ exchanger, and another pathway which involves H+–Ca2+ exchanger. In the matrix, Ca2+ stimulates the activity of three Ca2+-sensitive dehydrogenases of the Krebs cycle. The gradient across the inner membrane is important for the mitochondrial state and is established by the activity of the mitochondrial respiratory chain. VDAC: voltage-dependent anion channel, ANT: adenosine nucleoside transporter, HK: hexokinase, CD: cyclophilin D, CK: creatine kinase, BR: benzodiazepine receptor, PTP: permeability transition pore.
Despite intense investigations on the mitochondrial Ca2+ uniporter (MCU) for almost 50 years, the molecular identity of this gated channel is still a matter of debate. Among the early candidates for the MCU were mitochondria-localized RyR1 (Beutner et al., 2005) and the uncoupling proteins (UCP) 2 and 3 (Trenker et al., 2007), but such a role for these proteins is still controversial and further work will be needed to definitively elucidate the molecular identity of the channels or carriers involved. The identity of the MCU, as the product of the ccdc109a gene, has recently been reported by two recent publications (Baughman et al., 2011; De Stefani et al., 2011). In that respect, the report that the IMM protein leucine-zipper-EF hand-containing transmembrane region (Letm1), previously described as a K+/H+ exchanger (Nowikovsky et al., 2004), could be a Ca2+/H+ antiporter, catalyzing the uptake of Ca2+ into mitochondria was received with great interest (Jiang et al., 2009). These results remain a subject of intense discussion because they diverge in several issues from previous studies; in particular, the effect of Letm1 down-regulation/over-expression on mitochondrial Ca2+ transport could be a secondary effect of a decreased K+/H+ exchange activity. Finally, it is important to mention the recent description of an IMM Ca2+-binding protein named mitochondrial calcium uptake 1 (MICU1), which appears essential for mitochondrial Ca2+ uptake (Perocchi et al., 2010). MICU1 is a single-pass transmembrane protein and thus does not seem to participate in channel pore formation. It is possible that MICU1 is part of a complex with the mitochondrial Ca2+ channel, or functions as Ca2+ buffer, or as a Ca2+-dependent regulatory protein acting as a Ca2+ sensor (through a pair of Ca2+-binding EF-hand domains present in its sequence, the mutation of which eliminates the mitochondrial Ca2+ uptake).
About the system for mitochondrial Ca2+ extrusion, the mitochondrial Na+/Ca2+ exchanger (mNCX) and H+/Ca2+ exchanger (mHCX), have well known kinetic characteristics, are present in the IMM (Bernardi, 1999b). Recently, strong evidence has been provided that the Na+/Ca2+ exchanger isoform NCLX is the long-sought protein responsible for the mitochondrial Na+-dependent Ca2+ efflux (Palty et al., 2010).
Moreover, great attention has been drawn to the potential role of a large-conductance channel, commonly referred to as the permeability transition pore (PTP). It is supposed to be a multiprotein complex activated in various pathophysiological conditions (e.g., mitochondrial Ca2+ overload) (see Fig. 1). Its role in mitochondrial Ca2+ homeostasis, however, remains elusive (Bernardi and Forte, 2007).
In a variety of cell systems (ranging from epithelial cells to neurons), the cytosolic [Ca2+] ([Ca2+]c) rises evoked by physiological stimulations are always paralleled by rapid mitochondrial [Ca2+] ([Ca2+]m) increases, that reach values well above those of the bulk cytosol (up to ~ 500 μM in chromaffin cells) (Giorgi et al., 2009).
A Ca2+ signal originating in the cytoplasm that is propagated to mitochondria permits the transmission of an activatory signal to the energy powerhouse of the cell. Here, three key metabolic enzymes (pyruvate, α-ketoglutarate, and isocitrate dehydrogenases) are activated by Ca2+. Thus, in conjunction with the triggering of energy-consuming processes in the cytosol (contraction, secretion, etc.), mitochondrial dehydrogenases are stimulated, adapting aerobic metabolism to the increased energy needs of an active cell (Rimessi et al., 2008).
A completely different role for mitochondrial Ca2+ uptake is now well recognized. The alteration of the Ca2+ signals that reach mitochondria in association with different pathological conditions (e.g., oxidative stress) can induce a profound alteration of organelle structure and function. As a consequence, the cell may be driven to its death (Giorgi et al., 2008).
Here, we review some human diseases associated with perturbations in mitochondrial homeostasis with particular emphasis on the role of Ca2+ signals.
2. Mitochondria and aging
Aging is not considered as an adaptative response, but rather as the price of other evolutionary selected functions, currently not completely specified (Kirkwood and Austad, 2000). This degeneration is characterized by a progressive loss of organ function, proposed to be at the basis of several degenerative disorders, promoted by a progressive occurrence of apoptosis in non-proliferating cells. It was recognized that mitochondrial ATP synthesis is associated with reactive oxygen species (ROS) production, including primarily superoxide (O2−) formation by the respiratory chain complexes I and III (Balaban et al., 2005; Raha and Robinson, 2000). Subsequently, superoxide leads to the formation of diverse oxidant products, such as peroxynitrite, hydrogen peroxide (H2O2) and the highly reactive hydroxyl radical (OH). Mitochondria are considered the major source of ROS and the extent of oxidative damage to macromolecules is generally thought to be the random consequence of physiological functions, as exemplified by lipid peroxidation, protein oxidation, and mitochondrial DNA mutations (Cadenas and Davies, 2000). Different works on the molecular routes of apoptosis have revealed the important role of mitochondria in decoding of oxidative insults, with the release of proteins such as cytochrome c (cyt c) or Smac/Diablo, which act as co-factors of effector caspases. Ca2+ overload, through a still controversial mechanism, can also lead to the opening of the PTP and swelling of the organelle in what appears to be a pivotal pathway (Duchen, 2000; Ravagnan et al., 2002).
One important implication of recent findings is that these phenomena are genetically determined at least in part and biochemically controlled by a stress-induced signal transduction pathway involving the 66-kDa isoform of Shc, p66Shc. The relationship between mitochondria and p66Shc emerged once the protein was effectively localized to the organelle and by the observation that, upon oxidative stress, part of the cytosolic pool of p66Shc translocates to mitochondria (Orsini et al., 2004). The protein lacks a conventional mitochondrial targeting sequence, but its association with the mitochondrial TOM/TIM import complex and the mitochondrial heat shock protein mtHsp70 has been reported (Orsini et al., 2004). Within mitochondria, in particular in the mitochondrial intermembrane space, p66Shc binds cyt c and acts as a redox-enzyme, generating H2O2, which, in turn, induces the opening of the PTP and apoptosis. ROS production by p66Shc appears to be a specialized function whereby electrons are diverted from the mitochondrial electron transport chain to catalyze the partial reduction of molecular oxygen (Giorgio et al., 2005). Furthermore, the CH2 domain of p66Shc (a specific N-terminus domain that distinguishes p66Shc from the shorter Shc isoforms, p46 and p52) seems to form a redox module responsible for apoptosis initiation, and can be activated through reversible tetramerization by forming two disulfide bonds (Donoghue et al., 1990). The redox activity of p66Shc is likely to be the explanation of the decrease in ROS levels typical of p66Shc knockout cells (Migliaccio et al., 2006), as well as an altered mitochondrial metabolism, characterized by lower oxygen consumption under basal conditions (Nemoto et al., 2006). To exert its functions, p66Shc has to be phosphorylated at Serine 36 (Migliaccio et al., 1999): the responsible kinase was identified as PKCβ (Pinton et al., 2007). This phosphorylation of Ser36 induces translocation of phosphorylated p66Shc to mitochondria, and both H2O2 challenge and PKCβ activation promote binding of p66Shc to Pin1, causing this translocation (Pinton et al., 2007). The enzymatic activity of Pin1 is fundamental because the phosphorylated sites, once isomerized by Pin1, become targets for dephosphorylation by PP2A (Wulf et al., 2005). This solves the apparent paradox showing the importance of p66Shc phosphorylation and the dephosphorylated state of p66Shc within mitochondria. Thus, the signaling pathway involving PKCβ, Pin1 and PP2A controls the mitochondrial import of p66Shc where the translocated protein can exert its oxidoreductase activity, generating H2O2 and inducing the opening of the PTP. All these events are finely linked to an altered mitochondrial Ca2+ homeostasis, revealing a primary mitochondrial perturbation both in structure and function. In fact, oxidative stress causes a drastic reduction of [Ca2+]m in MEF cells, whereas knockouts for p66Shc or Pin1 are mostly insensitive to H2O2 treatment. Moreover, the simple over-expression of PKCβ induces a strong decrease of mitochondrial Ca2+ uptake, underlining how mitochondrial Ca2+ alteration is one of the first events of oxidoreductase activity of p66Shc.
Translocation of p66Shc to mitochondria is a characteristic event of some kinds of tumors, such as prostate cancer. MEF cells from p66Shc knockout mice were more resistant to PEITC (an apoptosis-inhibitor) and the same treatment increased both Ser36 phosphorylation and p66Shc-Pin1 binding in PC-3 and LNCaP human cancer prostate cells (Xiao and Singh, 2010). Moreover, growth stimulation of prostate cancer cells with 5α-dihydrotestosterone (DHT) is accompanied by increased p66Shc levels, ROS production, translocation of p66Shc into mitochondria and an augmented interaction with cyt c (Veeramani et al., 2008).
3. Mitochondria and neurodegenerative diseases
Among other deleterious consequences described in this article, mitochondrial impairment has also been associated with the pathogenesis of some neurodegenerative and neuroinflammatory disorders. Many lines of evidence suggest that both mitochondrial DNA mutations and increased oxidative stress are major contributors in aging — a process rendering the organism prone to neurodegeneration. Particularly interesting, it has been demonstrated in many of the most frequently occurring diseases that mitochondria interact with proteins crucial to the development the pathology. In these interactions, Ca2+ homeostasis often plays a key role (see Fig. 2). This brings hope for effective therapies that target mitochondrial processes and interaction sites of mitochondria and disease-related proteins.
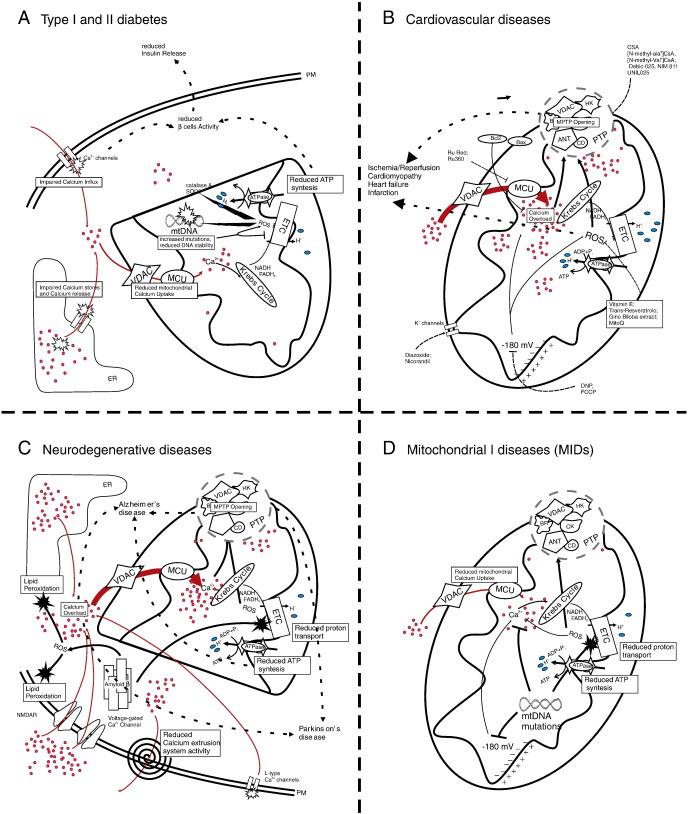
Fig. 2.
Schematic representation of mitochondrial dysfunctions in some common disease. A. In pancreatic β cells, generalized reduction of mitochondrial Ca2+ uptake (due to reduced extracellular Ca2+ influx or Ca2+ release from stores) impairs Krebs cycle and electron transport chain (ETC) activity. Anomalous ROS production can generate mitochondrial DNA mutations, leading to further malfunctioning of ETC. The resulting impairment in ATP production brings to reduced β cell activity and reduced insulin release. B. Excessive mitochondrial Ca2+ uptake induces opening of PTP leading to well known toxic effects. Further excessive ion concentration impairs ETC efficiency promoting ROS generation with consequent lipid perodxation and enhancement the probability of PTP opening. C. Ca2+ overload induce cell death in neurons. In Alzheimer's disease the presence of β-amyloid leads to ROS generation in neurons, causing cytosolic Ca2+ accumulation by different mechanisms. This homeostasis deregulation brings to mitochondrial Ca2+ overload and PTP opening. Mitochondrial Ca2+ overload is also responsible for dopaminergic neurons death in Parkinson's disease. D. Neuronal severe dysfunctions can be generated by reduced mitochondrial Ca2+ uptake in mitochondrial primary diseases (MIDs). Mutations in mtDNA are able to affect ETC and mitochondrial membrane potential and ROS generation, leading to impaired ATP synthesis and reduced Ca2+ accumulation. Because of the Ca2+ dependency of some enzymes of Krebs cycle a positive feedback progressively reduced the ability of mitochondria to synthetize ATP affecting cell physiology and survival.
The most frequent age-related neurodegenerative disorder – Alzheimer's disease (AD) – is manifested by an impairment in cognitive abilities, particularly responsible for memory functions. At the molecular level, AD pathogenesis is associated with the accumulation of a number of proteins, including reelin and presenilins, which leads to the overproduction of β-amyloid (Aβ). Aggregated amyloid plaques and neurofibrillary tangles are considered a sign of AD. The β-amyloid toxicity is further supported by the inability of neurons to properly regulate intracellular Ca2+ levels (Mattson, 2007; Thibault et al., 2007). Harmful consequences of Aβ activity include increased Ca2+ influx into neurons, rendering them prone to excitotoxicity and apoptosis (Bezprozvanny and Mattson, 2008). Also the interaction of Aβ with other cations, such as Fe2+ and Cu2+, leads to the generation of OH which causes membrane lipid peroxidation and, in turn, impairs the function of plasma membrane Ca2+ ATPase (PMCA), a Ca2+ pump responsible for Ca2+ extrusion from the cell interior to the extracellular compartment. Moreover, lipid peroxidation due to destabilization of membrane composition causes depolarization of plasma membrane and leads to the opening of NMDAR and voltage-gated Ca2+ channels, additionally intensifying an influx of toxic amounts of Ca2+ into the cytoplasm (Berridge, 2010). Finally, mutants of presenilins lower the [Ca2+] of intracellular store by increasing Ca2+ leakage and reducing Ca2+ uptake (Brunello et al., 2009).
Ca2+ homeostasis can also be affected by ROS due to the deterioration of membranes forming the intracellular Ca2+ stores (e.g., endoplasmic reticulum, ER). It has also been demonstrated that Aβ accumulates in mitochondria decreasing the activity of complexes III and IV of the respiratory chain (Anandatheerthavarada and Devi, 2007). Through such a decrease in mitochondrial function, Aβ propels a vicious cycle in which an elevation in cytosolic Ca2+ levels, oxidative stress, and decreased ATP synthesis, all induce a further Ca2+ overload and an even higher oxidative stress (Lin and Beal, 2006). Taken together these findings indicate that suppression or blocking of Ca2+ influx could in effect promote the survival of degenerating neurons. Several preliminary trials of L-type Ca2+ and NMDAR channel blockers have been carried out with some success; however, crucial obstacles still need to be overcome before the therapy can be considered successful (Mattson, 2007).
The most frequent movement disorder – Parkinson's disease (PD) – is caused by widespread neurodegeneration in the brain and a significant selective loss of nigrostriatal dopaminergic neurons (Thomas and Beal, 2010). Processes underlying PD pathogenesis, especially in its non-familial occurrence, remain unclear. In both cases, PD pathogenesis is associated with mitochondrial degeneration. The engagement of L-type Ca2+ channels is crucial in spontaneous pacemaking (Hausser et al., 2004), while at the same time it may contribute to elevated toxicity of mitochondrial toxins to nigrostriatal dopaminergic neurons. In this way, Ca2+ homeostasis is a regulator of their selective vulnerability (Chan et al., 2009; Surmeier et al., 2010). It has been proposed that the reduction in expression of the transient receptor potential cation (TRPC1) channel may be involved in dopaminergic neurodegeneration. TRPC1 could also be neuroprotective against PD-inducing agents (Bollimuntha et al., 2005).
A progressive neurodegenerative disorder – Huntington's disease (HD) – is characterized by severe motor, cognitive, and psychiatric symptoms. The genetic etiology of HD has been established to be a CAG-triplet mutation within the huntingtin gene, inherited in an autosomal dominant fashion. Possible links between mitochondrial dysfunctions and changes in Ca2+ homeostasis and HD pathology have been recently reviewed (Celsi et al., 2009).
Another frequent neurological disorder potentially associated with mitochondrial dysfunctions and Ca2+ signaling is epilepsy. A number of authors report that this disease, prevalently initiated by neurological insults, is related to shifts in Ca2+ homeostasis. An increase in extracellular glutamate concentration sustains long-term distortions in Ca2+ signaling, a characteristic recognized as the epileptic phenotype (Delorenzo et al., 2005; Nagarkatti et al., 2009). A number of mitochondrial, as well as nuclear, DNA mutations have been implicated with epileptic phenotypes. Changes in electron transport chain activity increase metabolic demand in neurons and lead to distortions in Ca2+ homeostasis increasing its dendritic level.
4. Mitochondrial diseases
The number and distribution of mitochondria varies from one tissue to another, depending from the tissue's dependence on oxidative phosphorylation for energy provision. For this reason, Mitochondrial Diseases (MIDs) predominantly manifest themselves in tissue/organs with high-energy requirements, and are aggravated by fever, infection, stress, toxic agents, or certain drugs (Finsterer, 2006).
MIDs are mainly due to mutations in mitochondrial or nuclear DNA (mtDNA and nDNA, respectively), resulting in the altered function of the respiratory chain or oxidative phosphorylation (OXPHOS) (Reinecke et al., 2009).
The respiratory chain machinery consists of approximately 80 proteins, of which 13 are encoded by mtDNA and all others are encoded by nDNA (DiMauro, 2004; Finsterer, 2010).
Interplay between the mitochondrial and nuclear genomes is evident in disorders of nuclear- and mtDNA-encoded subunits of the OXPHOS complexes (Smeitink et al., 2001). Deficiencies in OXPHOS result in immediate and downstream metabolic, structural, and functional effects. These effects are closely associated with mitochondrial dysfunction. ATP production, and consequently ATP/ADP homeostasis, is disturbed in OXPHOS deficiencies (Smeitink et al., 2006).
The mitochondrial genome possesses a very high mutation rate, 10- to 17-fold higher than that observed in nDNA. Protective histones are also lacking, and although mtDNA repair systems do exist (de Souza-Pinto et al., 2009), they are not sufficient to counteract the oxidative damage sustained by the mitochondrial genome (Tuppen et al., 2010). At the same time, pathogenic nDNA mutations are likely to be more numerous than pathogenic mtDNA mutations (Finsterer, 2010). Point mutations have been correlated with numerous diseases, such as Leigh syndrome, Barth syndrome, neuropathies, cardiomyopathies, hepatopathy, nephropathy, multisystemic disease, and neurodegenerative diseases, including PD, AD and amyotrophic lateral sclerosis. (DiMauro, 2004; Finsterer, 2008; Guy et al., 2002).
Given the multi-systemic feature of mitochondrial cytopathies, different pharmacological approaches are required, but most of the proposed therapies are usually of scarce effectiveness (Zaffanello and Zamboni, 2005).
Non-specific drug therapy consists of different pharmacological agents, divided according to the type of action into antioxidants and lactate lowering agents (which remove noxious metabolites), electron transfer mediators (which bypass the defective site), alternative energy providers, cofactors, and other agents (Finsterer, 2010), such as Coenzyme Q10, ascorbic acid, vitamin E, lipoic acid, riboflavin, thiamin, niacin, and vitamin K (phylloquinone and menadione). The general objective is to increase mitochondrial ATP production and to slow (or arrest) the progression of the clinical symptoms (Marriage et al., 2003).
In order to correct the energy imbalance in MIDs, it would be interesting to introduce drugs able to act on intracellular Ca2+ homeostasis since a strong relationship exists between Ca2+ and mitochondrial ATP synthesis (see Fig. 2). Briefly, Ca2+ is stored at a high concentration within the ER. This Ca2+ content is actively maintained by the sarcoplasmic/endoplasmic Ca2+ ATPase (SERCA) located on membranes of the ER that constantly pump cytosolic Ca2+ into the lumen, at the expense of ATP. This ATP is mostly provided by mitochondria through the close contact sites between ER and mitochondria (Giorgi et al., 2009).
It has been reported, especially for defects on respiratory complex I, that cells from patients with mitochondrial diseases show reduced mitochondrial membrane potential together with reduced ATP synthesis. This causes a reduced mitochondrial Ca2+ uptake under physiological stimulations (Visch et al., 2006). Since Ca2+ uptake by mitochondria promotes Krebs cycle activity and ATP synthesis (Jouaville et al., 1999), it could be hypothesized that a negative feedback is created that maintains low intracellular Ca2+ levels and ATP contents in cells derived from MIDs patients. In this scenario, it would be interesting to study the molecules capable of increasing ER or mitochondrial Ca2+ levels. It has already been proposed that the pharmacological inhibition of the mitochondrial sodium Ca2+ exchanger (that extrudes Ca2+ from mitochondria after physiological stimulation, recovering the basal [Ca2+]) is able to restore mitochondrial ATP synthesis in cells harboring mitochondrial DNA mutations (Brini et al., 1999; Visch et al., 2004). Mitochondrial Ca2+ uptake is also a signaling event in the apoptotic cascade, especially in oxidative stress conditions. Indeed, sustained mitochondrial Ca2+ elevations are able to induce the opening of the PTP, almost without stimulating the Krebs cycle.
Since oxidative stress appears to be a common feature in many MIDs, therapeutical approaches based on Ca2+ handling should be considered together with antioxidant drugs or blockers of the PTP. The latter, especially Cyclosporin A (CsA), are able to inhibit cytochrome c release induced by a number of apoptotic stimuli (Ow et al., 2008; Walter et al., 1998) and also increase the mitochondrial membrane potential.
Cyclosporins are used as immunosuppressants (probably independently from their mitochondrial activity) and diverse studies support a protective role for CsA in diseases with associated mitochondrial dysfunctions. Myoblasts from patients affected by Ullrich congenital muscular dystrophy display several mitochondrial alterations and a higher susceptibility to apoptosis due to the inappropriate opening of the PTP. In these conditions, it has been observed that CsA inhibits apoptosis engagement (Merlini et al., 2008).
5. Mitochondria as drug targets in the treatment of cardiovascular diseases
Cardiovascular disease is the leading cause of morbidity and mortality in the developed world. A multitude of recent studies suggest that mitochondria play critical roles in both the life and death of terminally differentiated cardiac myocytes. Mitochondria malfunction has been implicated in various cardiac pathologies, including ischemia–reperfusion (I/R) injury, cardiomyopathy, and congestive heart failure (Gustafsson and Gottlieb, 2008).
In healthy myocytes, mitochondria occupy a large portion of the cell and are located between the myofibrils and just below the sarcolemma (Andrienko et al., 2003). Pathophysiological conditions that can open the PTP are characteristically present during myocardial I/R (Di Lisa and Bernardi, 2006) (see Fig. 2). As such, an increasing number of pharmacological approaches are currently being developed to try to interfere with these parameters. One approach is based on the inhibition of Ca2+ overload. Cardioprotective effects of strategies that limit mitochondrial Ca2+ accumulation, have been demonstrated (Miyamae et al., 1996). For instance, treatment of hearts with ruthenium red (RR) or Ru360, inhibitors of the Ca2+ uniporter, reduced infarct size in hearts subjected to I/R (Garcia-Rivas Gde et al., 2006; Park et al., 1990). In addition, Ru360 treatment dramatically decreased [Ca2+]m and inhibited PTP opening, which was associated with improved recovery of heart performance (Goldberg and Bursey, 1992). Mitochondrial membrane potential is another critical regulator of Ca2+ accumulation: depolarization reduces the driving force for Ca2+ uptake by mitochondria and thereby prevents Ca2+ overload; this prompted researchers to consider the decrease of the potential as a possible mechanism of cellular protection. This objective is attained with uncoupler agents such as 2-4-dinitrophenol (DNP) and carbonyl cyanide p-(trifluoromethoxy)-phenylhydrazone (FCCP) (Brennan et al., 2006), and mitochondrial ATP-sensitive K+ (mitoKATP) channel openers, such as diazoxide (Garlid et al., 1997; Iwai et al., 2000) or nicorandil, (Carreira et al., 2008; Iwai et al., 2002), which showed a cardioprotective effect in different models of I/R (Ishii et al., 2005; Kitakaze et al., 2007; Liu et al., 1998). Pharmacological openers of the mitoKATP channel have been developed for the treatment of angina pectoris (nicorandil) and hypertension (cromakalim and pinacidil) (Grover et al., 2001; Yang and Yu, 2010).
Cardiac mitoKATP channels were also identified as important mediators of protection during ischemic preconditioning (IPC), the most potent method for reducing reperfusion injury (Fryer et al., 2000; Murry et al., 1986), and thus represent a promising drug target.
Oxidative stress has also been associated with loss of cells in heart failure, I/R injury, and doxorubicin-induced cardiomyopathy, whereas reducing ROS production is cardioprotective (Gustafsson and Gottlieb, 2008). Moreover, decreased antioxidant capacity and increased oxidative stress are both related to heart failure. There is an increased production of ROS in failing hearts after myocardial infarction and consequently lipid peroxidation, decrease of mtDNA copy number, and a reduced oxidative capacity due to lower activity of electron transfer enzymes (Ide et al., 2001). Antioxidants, such as Vitamin E (Sethi et al., 2000), Ginkgo biloba extract (Seif-El-Nasr and El-Fattah, 1995), Trans-resveratrol (Goh et al., 2007; Ray et al., 1999), that remove radicals from cells, protect against I/R injury (Reeve et al., 2005). The use of novel mitochondria-targeted antioxidants has gained much interest (Victor and Rocha, 2007). Mitochondrial targeting is feasible by conjugating an antioxidant to a lipophilic cation. MitoQ, based on the endogenous mitochondrial ubiquinone coenzyme Q, is such a compound; a recent study demonstrated that feeding rats with MitoQ significantly limited cardiac reperfusion injury (Adlam et al., 2005).
Moreover, inhibition of the PTP through direct targeting of its putative components (i.e., the adenine nucleotide translocator (ANT) and Cyclophilin D (CyP-D)) may represent an effective therapeutic approach in protecting the heart against various cardiac pathologies (reviewed in (Bernardi and Forte, 2007; Halestrap and Pasdois, 2009)). CsA has been demonstrated to be cardioprotective in isolated cardiomyocytes subjected to anoxia and reoxygenation (Griffiths et al., 2000; Nazareth et al., 1991), in Langendorff-perfused hearts subjected to global I/R (Griffiths and Halestrap, 1993), as well as in animals subjected to coronary artery ligation and reperfusion (Hausenloy et al., 2003). One problem with the use of CsA is that it can induce additional effects through inhibition of the Ca2+-regulated protein phosphatase calcineurin, that has direct effects on heart function (Periasamy, 2002) and also undesirable immunosuppressive activity (Schreiber and Crabtree, 1992). To overcome this, other cyclosporine derivatives, such as the immunosuppressant sanglifehrin A (SfA), as well as non-immunosuppressants [N-methyl-ala6]CsA, [N-methyl-Val4]CsA, Debio 025, NIM 811 and UNIL025, were found to exhibit similar properties in terms of blocking pore opening, while being devoid of calcineurin inhibitory activity (Clarke et al., 2002; Gomez et al., 2007; Javadov et al., 2009). In addition, studies on isolated mitochondria and cultured cardiomyocytes have confirmed a protective role of the ANT inhibitor bongkrekic acid, via inhibition of pore opening (Akao et al., 2003). Recently, Schaller et al., (2010) have discovered a new mitochondrial-targeted cardioprotective drug, TRO40303 (3,5-Seco-4-nor-cholestan-5-one oxime-3-ol), that binds specifically to the mitochondrial translocator protein (TSPO, formerly known as Peripheral Benzodiazepine Receptor) and inhibits PTP opening triggered by oxidative stress. Another interesting result was obtained with the free radical scavenger edavarone, which was shown to inhibit PTP opening and to prevent cardiac reperfusion injury (Tsujita et al., 2006).
Finally, the Bcl-2 family proteins, which control mitochondrial membrane permeabilization and Ca2+ homeostasis, also play a central role in regulating apoptosis in the cardiovascular system (Gustafsson and Gottlieb, 2007). Many of the pro-apoptotic Bcl-2 proteins have been implicated in the pathogenesis of various cardiac diseases, including myocardial hypertrophy, infarction, and heart failure (Condorelli et al., 1999; Jung et al., 2001). As such, anti-apoptotic interventions targeting the Bcl-2 protein family provide opportunities for possible anti-ischemic therapies (Reed, 2001), either through a gain of anti-apoptotic function or loss of pro-apoptotic function. The pharmacological strategy inhibiting mitochondrial outer membrane permeability, and thus apoptosis, by manipulation of the Bcl-2 family to protect the myocardium against I/R injury is very recent but has already provided interesting results (Hetz et al., 2005), supporting the idea that clinical benefits may be obtained in the near future.
From the above-described evidence, therapeutic interventions designed to prevent mitochondrial damage hold major promise as a novel strategy for reducing cardiac injury, the leading cause of death in western societies. Effective therapies developed along these lines will thus represent a major advance in health care.
6. Mitochondria, Ca2+ dyshomeostasis and diabetes
Diabetes mellitus is a disorder resulting from defects of both insulin secretion and action. To date, the cellular pathophysiology of diabetes-induced impairments, remain controversial. Conceptually, the leading mechanism driving cellular pathology is believed to be associated with the accumulation of extracellular glucose, and generation of ROS, thereby triggering pathological outcomes. There is abundant evidence suggesting a central role of mitochondria in diabetes, insulin resistance, insulin secretion and in complications derived from diabetes (Patti and Corvera, 2010). However, it is still difficult (and controversial) to establish whether the alterations in mitochondrial function are the cause or the effect of the disease. Typically, the number and size of mitochondria is reduced in the muscle of diabetics, there is reduced mitochondrial biogenesis and switching of metabolic substrate use that is linked directly to mitochondrial enzymes (Chabi et al., 2005). Mitochondria are an integral part of the insulin system found in the islet cells of the pancreas. Ultrastructural examination of β-cells has suggested that mitochondria are often in close proximity to the secretory insulin granules. This may facilitate metabolism–secretion coupling, as ATP is a major permissive factor for movement of insulin granules and for priming of exocytosis (Maechler and Wollheim, 2001). When blood glucose is high, the rate of glycolysis in β-cells will increase and, as a consequence, the ATP production is accelerated to provide the energy for insulin exocytosis. Glucose-stimulated insulin release from β-cells involves a complex series of signaling pathways. As in other exocytotic fusion events, Ca2+ is an essential trigger of insulin granule exocytosis (Rorsman and Renstrom, 2003). The Ca2+ signal resulting from mitochondrial activation completes the chain of events between glucose uptake into β-cells and insulin exocytosis. A common motif in these cascades is the elevation of intracellular Ca2+ both in the cytosol and within the mitochondria.
Mitochondria take up Ca2+ through the mitochondrial Ca2+ uniporter in response to the glucose-induced rise in cytoplasmic Ca2+, and release Ca2+ through the mNCX (Bernardi, 1999a).
[Ca2+]c rises may originate from Ca2+ influx at the plasma membrane or Ca2+ mobilization from the ER. Local Ca2+ influx or mobilization creates microdomains, local increases of Ca2+ exceeding the bulk [Ca2+] in the cytosol (Giorgi et al., 2009). For both pathways, the amplitude and the duration of the [Ca2+]c rise may determine to what extent β-cell mitochondria are activated. Depending on the combination of secretagogues, the number and localization of such Ca2+ domains most certainly vary (Rizzuto and Pozzan, 2006; Rutter et al., 2006).
The diabetic state is related to mitochondrial dysfunction and generally characterized by insufficient supply of energy or defects in the insulin signaling pathway.
It has been shown that a reduction in mitochondrial Ca2+ accumulation is responsible for a reduction in insulin secretion (Wiederkehr and Wollheim, 2008), suggesting that modulation of mitochondrial Ca2+ may be a therapeutic target.
Restoration of the mitochondrial Ca2+ signal using an inhibitor of the mNCE was shown to improve ATP generation and insulin secretion (Lee et al., 2003; Visch et al., 2004), highlighting the exchanger as a potential new target for diabetes drug discovery.
Among the most convincing links between mitochondrial dysfunction and diabetes are the observed point mutation in mtDNA. Indeed, diabetes mellitus is very common in patients affected by mitochondrial diseases (also referred to as mitochondrial encephalomyopathies, characterized by genetic disorders of mtDNA, such as MERRF and MELAS), while and a maternally-inherited form of diabetes is associated to mtDNA mutations (Ballinger et al., 1992). Accordingly, with the above suggested pivotal role of Ca2+ homeostasis in the cellular pathogenesis of diabetes, agonist-induced Ca2+ rises and ATP generation were blunted and could be restored by applying an inhibitor of the mitochondrial Ca2+ efflux (Brini et al., 1999).
Finally, but not less important, an abnormal Ca2+ homeostasis and impaired mitochondrial function have also been linked to neurological complications associated with diabetes. Diabetic conditions affect ER Ca2+ homeostasis in sensory neurons by lowering the ER Ca2+ content due to reduced SERCA pump activity, inducing a mild condition of ER stress that results in the decrease of nerve conductance velocity (Verkhratsky and Fernyhough, 2008).
In conclusion, the signaling mechanisms that decode changes in nutrient supply are complex and not completely understood, but a control in intracellular [Ca2+], in particular at the mitochondrial level, appears to be of great significance for further research.
7. Conclusions
Mitochondria are the powerhouse of the cell being the place where oxidative metabolism takes place, rendering mitochondria crucial both in health and disease. Many (if not all) of the mitochondrial activities are driven in a Ca2+-dependent manner. The biochemical properties of the proteins involved in mitochondrial Ca2+ homeostasis have been known for over four decades, but only recently have we begun to unravel the dynamics of this cation at the molecular and mechanistic levels (Hajnoczky and Csordas, 2010). Currently, specific drugs that act on mitochondrial Ca2+ homeostasis are being developed and these may open the way to new biochemically designed therapeutic approaches in the treatment of several disorders.
Acknowledgements
This research was supported by: the Ministry of Science and Higher Education, Poland, grant NN407 075 137 to MRW, the Italian Association for Cancer Research (AIRC), Telethon (GGP09128), local funds from the University of Ferrara, the Italian Ministry of Education, University and Research (COFIN), the Italian Cystic Fibrosis Research Foundation and Italian Ministry of Health to P.P. SM was supported by a FIRC fellowship; AB was supported by a research fellowship FISM — Fondazione Italiana Sclerosi Multipla — Cod. 2010/B/1; SP was supported by a training fellowship FISM — Fondazione Italiana Sclerosi Multipla — Cod. 2010/B/13; JMS was also supported by PhD fellowship from The Foundation for Polish Science (FNP), UE, European Regional Development Fund and Operational Programme “Innovative Economy”.
References
- Adlam V.J., Harrison J.C., Porteous C.M., James A.M., Smith R.A., Murphy M.P., Sammut I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia–reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- Akao M., O'Rourke B., Kusuoka H., Teshima Y., Jones S.P., Marban E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ. Res. 2003;92:195–202. doi: 10.1161/01.res.0000051862.16691.f9. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada H.K., Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer's disease. Neuroscientist. 2007;13:626–638. doi: 10.1177/1073858407303536. [DOI] [PubMed] [Google Scholar]
- Andrienko T., Kuznetsov A.V., Kaambre T., Usson Y., Orosco A., Appaix F., Tiivel T., Sikk P., Vendelin M., Margreiter R., Saks V.A. Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. J. Exp. Biol. 2003;206:2059–2072. doi: 10.1242/jeb.00242. [DOI] [PubMed] [Google Scholar]
- Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Ballinger S.W., Shoffner J.M., Hedaya E.V., Trounce I., Polak M.A., Koontz D.A., Wallace D.C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat. Genet. 1992;1:11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., Koteliansky V., Mootha V.K. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10234. (Electronic publication ahead of print). doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P., Forte M. The mitochondrial permeability transition pore. Novartis Found. Symp. 2007;287:157–164. discussion 164–159. [PubMed] [Google Scholar]
- Berridge M.J. Calcium hypothesis of Alzheimer's disease. Pflügers Arch. 2010;459:441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- Beutner G., Sharma V.K., Lin L., Ryu S.Y., Dirksen R.T., Sheu S.S. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation–metabolism coupling. Biochim. Biophys. Acta. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimuntha S., Singh B.B., Shavali S., Sharma S.K., Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J. Biol. Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J.P., Southworth R., Medina R.A., Davidson S.M., Duchen M.R., Shattock M.J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc. Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Brini M., Pinton P., King M.P., Davidson M., Schon E.A., Rizzuto R. A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nat. Med. 1999;5:951–954. doi: 10.1038/11396. [DOI] [PubMed] [Google Scholar]
- Brunello L., Zampese E., Florean C., Pozzan T., Pizzo P., Fasolato C. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J. Cell. Mol. Med. 2009;13:3358–3369. doi: 10.1111/j.1582-4934.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Carreira R.S., Monteiro P., Kowaltowski A.J., Goncalves L.M., Providencia L.A. Nicorandil protects cardiac mitochondria against permeability transition induced by ischemia–reperfusion. J. Bioenerg. Biomembr. 2008;40:95–102. doi: 10.1007/s10863-008-9133-2. [DOI] [PubMed] [Google Scholar]
- Celsi F., Pizzo P., Brini M., Leo S., Fotino C., Pinton P., Rizzuto R. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim. Biophys. Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B., Adhihetty P.J., Ljubicic V., Hood D.A. How is mitochondrial biogenesis affected in mitochondrial disease? Med. Sci. Sports Exerc. 2005;37:2102–2110. doi: 10.1249/01.mss.0000177426.68149.83. [DOI] [PubMed] [Google Scholar]
- Chan C.S., Gertler T.S., Surmeier D.J. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Clarke S.J., McStay G.P., Halestrap A.P. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J. Biol. Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- Colombini M. The published 3D structure of the VDAC channel: native or not? Trends Biochem. Sci. 2009;34:382–389. doi: 10.1016/j.tibs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Condorelli G., Morisco C., Stassi G., Notte A., Farina F., Sgaramella G., de Rienzo A., Roncarati R., Trimarco B., Lembo G. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation. 1999;99:3071–3078. doi: 10.1161/01.cir.99.23.3071. [DOI] [PubMed] [Google Scholar]
- De Pinto V., Reina S., Guarino F., Messina A. Structure of the voltage dependent anion channel: state of the art. J. Bioenerg. Biomembr. 2008;40:139–147. doi: 10.1007/s10863-008-9140-3. [DOI] [PubMed] [Google Scholar]
- de Souza-Pinto N.C., Mason P.A., Hashiguchi K., Weissman L., Tian J., Guay D., Lebel M., Stevnsner T.V., Rasmussen L.J., Bohr V.A. Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA Repair (Amst) 2009;8:704–719. doi: 10.1016/j.dnarep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10230. (Electronic publication ahead of print). doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo R.J., Sun D.A., Deshpande L.S. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol. Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F., Bernardi P. Mitochondria and ischemia–reperfusion injury of the heart: fixing a hole. Cardiovasc. Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- DiMauro S. Mitochondrial diseases. Biochim. Biophys. Acta. 2004;1658:80–88. doi: 10.1016/j.bbabio.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Donoghue D.J., Perez F.M., Diamante B.S., Malamed S., Scanes C.G. Influence of catecholamines, prostaglandins and thyroid hormones on growth hormone secretion by chicken pituitary cells in vitro. Domest. Anim. Endocrinol. 1990;7:35–42. doi: 10.1016/0739-7240(90)90052-2. [DOI] [PubMed] [Google Scholar]
- Duchen M.R. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 2000;529(Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M.R. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Overview on visceral manifestations of mitochondrial disorders. Neth. J. Med. 2006;64:61–71. [PubMed] [Google Scholar]
- Finsterer J. Leigh and Leigh-like syndrome in children and adults. Pediatr. Neurol. 2008;39:223–235. doi: 10.1016/j.pediatrneurol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Treatment of mitochondrial disorders. Eur. J. Paediatr. Neurol. 2010;14:29–44. doi: 10.1016/j.ejpn.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Fryer R.M., Eells J.T., Hsu A.K., Henry M.M., Gross G.J. Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- Garcia-Rivas Gde J., Carvajal K., Correa F., Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br. J. Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid K.D., Paucek P., Yarov-Yarovoy V., Murray H.N., Darbenzio R.B., D'Alonzo A.J., Lodge N.J., Smith M.A., Grover G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Romagnoli A., Pinton P., Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- Giorgi C., De Stefani D., Bononi A., Rizzuto R., Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P.G. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Goh S.S., Woodman O.L., Pepe S., Cao A.H., Qin C., Ritchie R.H. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia–reperfusion via multiple sites and mechanisms. Antioxid. Redox Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- Goldberg S.R., Bursey C.R. Prevalence of the nematode Spauligodon giganticus (Oxyurida: Pharyngodonidae) in neonatal Yarrow's spiny lizards, Sceloporus jarrovii (Sauria: Iguanidae) J. Parasitol. 1992;78:539–541. [PubMed] [Google Scholar]
- Gomez L., Thibault H., Gharib A., Dumont J.M., Vuagniaux G., Scalfaro P., Derumeaux G., Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- Griffiths E.J., Halestrap A.P. Protection by cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J. Mol. Cell. Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- Griffiths E.J., Ocampo C.J., Savage J.S., Stern M.D., Silverman H.S. Protective effects of low and high doses of cyclosporin A against reoxygenation injury in isolated rat cardiomyocytes are associated with differential effects on mitochondrial calcium levels. Cell Calcium. 2000;27:87–95. doi: 10.1054/ceca.1999.0094. [DOI] [PubMed] [Google Scholar]
- Grover G.J., D'Alonzo A.J., Garlid K.D., Bajgar R., Lodge N.J., Sleph P.G., Darbenzio R.B., Hess T.A., Smith M.A., Paucek P., Atwal K.S. Pharmacologic characterization of BMS-191095, a mitochondrial K(ATP) opener with no peripheral vasodilator or cardiac action potential shortening activity. J. Pharmacol. Exp. Ther. 2001;297:1184–1192. [PubMed] [Google Scholar]
- Gustafsson A.B., Gottlieb R.A. Bcl-2 family members and apoptosis, taken to heart. Am. J. Physiol. Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- Gustafsson A.B., Gottlieb R.A. Heart mitochondria: gates of life and death. Cardiovasc. Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- Guy J., Qi X., Pallotti F., Schon E.A., Manfredi G., Carelli V., Martinuzzi A., Hauswirth W.W., Lewin A.S. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann. Neurol. 2002;52:534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Csordas G. Calcium signalling: fishing out molecules of mitochondrial calcium transport. Curr. Biol. 2010;20:R888–R891. doi: 10.1016/j.cub.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A.P., Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Duchen M.R., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia–reperfusion injury. Cardiovasc. Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Hausser M., Raman I.M., Otis T., Smith S.L., Nelson A., du Lac S., Loewenstein Y., Mahon S., Pennartz C., Cohen I., Yarom Y. The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J. Neurosci. 2004;24:9215–9219. doi: 10.1523/JNEUROSCI.3375-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C., Vitte P.A., Bombrun A., Rostovtseva T.K., Montessuit S., Hiver A., Schwarz M.K., Church D.J., Korsmeyer S.J., Martinou J.C., Antonsson B. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- Ide T., Tsutsui H., Hayashidani S., Kang D., Suematsu N., Nakamura K., Utsumi H., Hamasaki N., Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- Ishii H., Ichimiya S., Kanashiro M., Amano T., Imai K., Murohara T., Matsubara T. Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation. 2005;112:1284–1288. doi: 10.1161/CIRCULATIONAHA.104.530329. [DOI] [PubMed] [Google Scholar]
- Iwai T., Tanonaka K., Koshimizu M., Takeo S. Preservation of mitochondrial function by diazoxide during sustained ischaemia in the rat heart. Br. J. Pharmacol. 2000;129:1219–1227. doi: 10.1038/sj.bjp.0703148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai T., Tanonaka K., Motegi K., Inoue R., Kasahara S., Takeo S. Nicorandil preserves mitochondrial function during ischemia in perfused rat heart. Eur. J. Pharmacol. 2002;446:119–127. doi: 10.1016/s0014-2999(02)01645-x. [DOI] [PubMed] [Google Scholar]
- Javadov S., Karmazyn M., Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J. Pharmacol. Exp. Ther. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- Jiang D., Zhao L., Clapham D.E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung F., Weiland U., Johns R.A., Ihling C., Dimmeler S. Chronic hypoxia induces apoptosis in cardiac myocytes: a possible role for Bcl-2-like proteins. Biochem. Biophys. Res. Commun. 2001;286:419–425. doi: 10.1006/bbrc.2001.5406. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B., Austad S.N. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Asakura M., Kim J., Shintani Y., Asanuma H., Hamasaki T., Seguchi O., Myoishi M., Minamino T., Ohara T., Nagai Y., Nanto S., Watanabe K., Fukuzawa S., Hirayama A., Nakamura N., Kimura K., Fujii K., Ishihara M., Saito Y., Tomoike H., Kitamura S. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- Lee B., Miles P.D., Vargas L., Luan P., Glasco S., Kushnareva Y., Kornbrust E.S., Grako K.A., Wollheim C.B., Maechler P., Olefsky J.M., Anderson C.M. Inhibition of mitochondrial Na + − Ca2+ exchanger increases mitochondrial metabolism and potentiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes. 2003;52:965–973. doi: 10.2337/diabetes.52.4.965. [DOI] [PubMed] [Google Scholar]
- Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sato T., O'Rourke B., Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Maechler P., Wollheim C.B. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- Marriage B., Clandinin M.T., Glerum D.M. Nutritional cofactor treatment in mitochondrial disorders. J. Am. Diet. Assoc. 2003;103:1029–1038. doi: 10.1016/s0002-8223(03)00476-0. [DOI] [PubMed] [Google Scholar]
- Mattson M.P. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Merlini L., Angelin A., Tiepolo T., Braghetta P., Sabatelli P., Zamparelli A., Ferlini A., Maraldi N.M., Bonaldo P., Bernardi P. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc. Natl Acad. Sci. USA. 2008;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P.P., Lanfrancone L., Pelicci P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Migliaccio E., Giorgio M., Pelicci P.G. Apoptosis and aging: role of p66Shc redox protein. Antioxid. Redox Signal. 2006;8:600–608. doi: 10.1089/ars.2006.8.600. [DOI] [PubMed] [Google Scholar]
- Miyamae M., Camacho S.A., Weiner M.W., Figueredo V.M. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am. J. Physiol. 1996;271:H2145–H2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nagarkatti N., Deshpande L.S., DeLorenzo R.J. Development of the calcium plateau following status epilepticus: role of calcium in epileptogenesis. Expert. Rev. Neurother. 2009;9:813–824. doi: 10.1586/ern.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth W., Yafei N., Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J. Mol. Cell. Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- Nemoto S., Combs C.A., French S., Ahn B.H., Fergusson M.M., Balaban R.S., Finkel T. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J. Biol. Chem. 2006;281:10555–10560. doi: 10.1074/jbc.M511626200. [DOI] [PubMed] [Google Scholar]
- Nowikovsky K., Froschauer E.M., Zsurka G., Samaj J., Reipert S., Kolisek M., Wiesenberger G., Schweyen R.J. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf–Hirschhorn syndrome. J. Biol. Chem. 2004;279:30307–30315. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- Orsini F., Migliaccio E., Moroni M., Contursi C., Raker V.A., Piccini D., Martin-Padura I., Pelliccia G., Trinei M., Bono M., Puri C., Tacchetti C., Ferrini M., Mannucci R., Nicoletti I., Lanfrancone L., Giorgio M., Pelicci P.G. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J. Biol. Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- Ow Y.P., Green D.R., Hao Z., Mak T.W. Cytochrome c: functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Bowles D.K., Kehrer J.P. Protection against hypoxic injury in isolated-perfused rat heart by ruthenium red. J. Pharmacol. Exp. Ther. 1990;253:628–635. [PubMed] [Google Scholar]
- Patti M.E., Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M. Calcineurin and the heartbeat, an evolving story. J. Mol. Cell. Cardiol. 2002;34:259–262. doi: 10.1006/jmcc.2001.1520. [DOI] [PubMed] [Google Scholar]
- Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Rimessi A., Marchi S., Orsini F., Migliaccio E., Giorgio M., Contursi C., Minucci S., Mantovani F., Wieckowski M.R., Del Sal G., Pelicci P.G., Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- Raha S., Robinson B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Ravagnan L., Roumier T., Kroemer G. Mitochondria, the killer organelles and their weapons. J. Cell. Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- Ray P.S., Maulik G., Cordis G.A., Bertelli A.A., Bertelli A., Das D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- Reed J.C. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol. Med. 2001;7:314–319. doi: 10.1016/s1471-4914(01)02026-3. [DOI] [PubMed] [Google Scholar]
- Reeve J.L., Duffy A.M., O'Brien T., Samali A. Don't lose heart—therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J. Cell. Mol. Med. 2005;9:609–622. doi: 10.1111/j.1582-4934.2005.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke F., Smeitink J.A., van der Westhuizen F.H. OXPHOS gene expression and control in mitochondrial disorders. Biochim. Biophys. Acta. 2009;1792:1113–1121. doi: 10.1016/j.bbadis.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Rimessi A., Giorgi C., Pinton P., Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta. 2008;1777:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bernardi P., Pozzan T. Mitochondria as all-round players of the calcium game. J. Physiol. 2000;529(Pt 1):37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Rutter G.A., Tsuboi T., Ravier M.A. Ca2+ microdomains and the control of insulin secretion. Cell Calcium. 2006;40:539–551. doi: 10.1016/j.ceca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Schaller S., Paradis S., Ngoh G.A., Assaly R., Buisson B., Drouot C., Ostuni M.A., Lacapere J.J., Bassissi F., Bordet T., Berdeaux A., Jones S.P., Morin D., Pruss R.M. TRO40303, a new cardioprotective compound, inhibits mitochondrial permeability transition. J. Pharmacol. Exp. Ther. 2010;333:696–706. doi: 10.1124/jpet.110.167486. [DOI] [PubMed] [Google Scholar]
- Schreiber S.L., Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Seif-El-Nasr M., El-Fattah A.A. Lipid peroxide, phospholipids, glutathione levels and superoxide dismutase activity in rat brain after ischaemia: effect of ginkgo biloba extract. Pharmacol. Res. 1995;32:273–278. doi: 10.1016/s1043-6618(05)80014-3. [DOI] [PubMed] [Google Scholar]
- Sethi R., Takeda N., Nagano M., Dhalla N.S. Beneficial effects of vitamin E treatment in acute myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 2000;5:51–58. doi: 10.1177/107424840000500107. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V., De Pinto V., Zweckstetter M., Raviv Z., Keinan N., Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Simamura E., Shimada H., Hatta T., Hirai K. Mitochondrial voltage-dependent anion channels (VDACs) as novel pharmacological targets for anti-cancer agents. J. Bioenerg. Biomembr. 2008;40:213–217. doi: 10.1007/s10863-008-9158-6. [DOI] [PubMed] [Google Scholar]
- Smeitink J., van den Heuvel L., DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2001;2:342–352. doi: 10.1038/35072063. [DOI] [PubMed] [Google Scholar]
- Smeitink J.A., Zeviani M., Turnbull D.M., Jacobs H.T. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006;3:9–13. doi: 10.1016/j.cmet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Surmeier D.J., Guzman J.N., Sanchez-Padilla J. Calcium, cellular aging, and selective neuronal vulnerability in Parkinson's disease. Cell Calcium. 2010;47:175–182. doi: 10.1016/j.ceca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O., Gant J.C., Landfield P.W. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B., Beal M.F. Mitochondrial therapies for Parkinson's disease. Mov. Disord. 2010;25(Suppl 1):S155–S160. doi: 10.1002/mds.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenker M., Malli R., Fertschai I., Levak-Frank S., Graier W.F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita K., Shimomura H., Kaikita K., Kawano H., Hokamaki J., Nagayoshi Y., Yamashita T., Fukuda M., Nakamura Y., Sakamoto T., Yoshimura M., Ogawa H. Long-term efficacy of edaravone in patients with acute myocardial infarction. Circ. J. 2006;70:832–837. doi: 10.1253/circj.70.832. [DOI] [PubMed] [Google Scholar]
- Tuppen H.A., Blakely E.L., Turnbull D.M., Taylor R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Veeramani S., Yuan T.C., Lin F.F., Lin M.F. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene. 2008;27:5057–5068. doi: 10.1038/onc.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Fernyhough P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium. 2008;44:112–122. doi: 10.1016/j.ceca.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Victor V.M., Rocha M. Targeting antioxidants to mitochondria: a potential new therapeutic strategy for cardiovascular diseases. Curr. Pharm. Des. 2007;13:845–863. doi: 10.2174/138161207780363077. [DOI] [PubMed] [Google Scholar]
- Visch H.J., Rutter G.A., Koopman W.J., Koenderink J.B., Verkaart S., de Groot T., Varadi A., Mitchell K.J., van den Heuvel L.P., Smeitink J.A., Willems P.H. Inhibition of mitochondrial Na+–Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J. Biol. Chem. 2004;279:40328–40336. doi: 10.1074/jbc.M408068200. [DOI] [PubMed] [Google Scholar]
- Visch H.J., Koopman W.J., Zeegers D., van Emst-de Vries S.E., van Kuppeveld F.J., van den Heuvel L.W., Smeitink J.A., Willems P.H. Ca2+-mobilizing agonists increase mitochondrial ATP production to accelerate cytosolic Ca2+ removal: aberrations in human complex I deficiency. Am. J. Physiol. Cell Physiol. 2006;291:C308–C316. doi: 10.1152/ajpcell.00561.2005. [DOI] [PubMed] [Google Scholar]
- Walter D.H., Haendeler J., Galle J., Zeiher A.M., Dimmeler S. Cyclosporin A inhibits apoptosis of human endothelial cells by preventing release of cytochrome C from mitochondria. Circulation. 1998;98:1153–1157. doi: 10.1161/01.cir.98.12.1153. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A., Wollheim C.B. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Wulf G., Finn G., Suizu F., Lu K.P. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nat. Cell Biol. 2005;7:435–441. doi: 10.1038/ncb0505-435. [DOI] [PubMed] [Google Scholar]
- Xiao D., Singh S.V. p66Shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Cancer Res. 2010;70:3150–3158. doi: 10.1158/0008-5472.CAN-09-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yu T. Prolonged donor heart preservation with pinacidil: the role of mitochondria and the mitochondrial adenosine triphosphate-sensitive potassium channel. J. Thorac. Cardiovasc. Surg. 2010;139:1057–1063. doi: 10.1016/j.jtcvs.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Zaffanello M., Zamboni G. Therapeutic approach in a case of Pearson's syndrome. Minerva Pediatr. 2005;57:143–146. [PubMed] [Google Scholar]