Abstract
Irritable bowel syndrome (IBS) is a functional gut disorder with high prevalence. Because of various factors involved in its pathophysiology and disappointing results from conventional IBS medications, the treatment of IBS is challenging and use of complementary and alternative medicines especially herbal therapies is increasing. In this paper, electronic databases including PubMed, Scopus, and Cochrane library were searched to obtain any in vitro, in vivo or human studies evaluating single or compound herbal preparations in the management of IBS. One in vitro, 3 in vivo and 23 human studies were included and systematically reviewed. The majority of studies are about essential oil of Menta piperita as a single preparation and STW 5 as a compound preparation. Some evaluated herbs such as Curcuma xanthorriza and Fumaria officinalis did not demonstrate any benefits in IBS. However, it seems there are many other herbal preparations such as those proposed in traditional medicine of different countries that could be studied and investigated for their efficacy in management of IBS.
Keywords: Herbal medicines, Irritable bowel syndrome, Systematic review
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gut disorder characterized by abdominal pain or discomfort, bloating, and bowel disturbances[1] with a higher prevalence ratio of women to men (ratio of 2:1)[2]. Studies from Asia suggest higher prevalence of IBS in more developed countries such as Singapore (8.6%) and Japan (9.8%) compared with India with the lowest prevalence (4.2%)[3]. The pathophysiology of IBS is most likely multifactorial involving visceral hypersensitivity, abnormal gut motility, intestinal microbiota, inflammation and immune disturbance, genetic factors, abnormal gas handling, psychosocial factors, intestinal infections, central nervous system, and serotonin[1,4,5]. Pharmacological treatment of IBS varies from antidepressants including tricyclic antidepressants[6] and selective serotonin reuptake inhibitors[7], to antispasmodics[8,9], 5-hydroxytryptamine-3 receptor (5-HT3) antagonists[10], 5-HT4 agonists[11], antibiotics[12], probiotics[13], and melatonin[14]. But involvement of numerous factors in pathophysiology and a very significant placebo effect[15] cause therapy of this disease to be more complex. Due to disappointing results with conventional IBS treatments, complementary and alternative medicines are becoming attractive options for many patients[16].
In the present paper, management of IBS by herbal medicines and their modes of action have been evaluated in detail. For this purpose, electronic databases including PubMed, Scopus, and Cochrane library were searched to obtain studies giving any in vitro, in vivo, or human evidence of the efficacy of herbs in the treatment of IBS. Data were collected for the years 1966 to 2011 (up to February). The search terms were: “complementary and alternative medicine”, “plant”, or “herb” and “irritable bowel syndrome”. Reference lists of the retrieved articles were also reviewed for additional applicable studies. The title and abstract of each article were examined to eliminate duplicates, reviews, studies examining functional bowel diseases other than IBS, and studies assessing complementary and alternative medicines other than herbs. Figure 1 shows a flow diagram of the study selection process.
Figure 1.
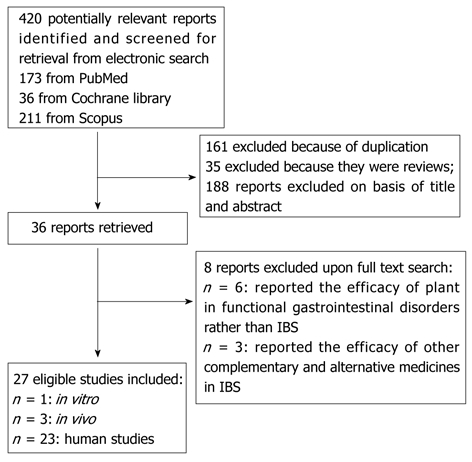
Flow diagram of the study selection process. IBS: Irritable bowel syndrome.
INVESTIGATED HERBAL PREPARATIONS IN IBS
Single preparations
Preparations containing only one herb and investigated in IBS are discussed below and also in Table 1.
Table 1.
Single herbs used for treatment of irritable bowel syndrome
| Scientific name | Part | Type of study | Model | Concomitant drugs | Duration of study | Results | Ref. |
| Aloe vera | Gel | Placebo-controlled double-blind trial | IBS patients | - | 1 mo | No difference between treatment and placebo groups in response to treatment at 1 mo diarrhea-predominant patients showed a trend towards a response to treatment at 1 mo | [17] |
| Curcuma longa | Rhizome | Partially blinded, randomized, two-dose, pilot study | IBS patients | - | 8 wk | ↓Abdominal pain/discomfort score Significant improvements in IBS QOL scales Approximately two thirds of all subjects reported an improvement in symptoms after treatment | [18] |
| Curcuma xanthorrhiza | Rhizome | Randomized, double-blind, placebo-controlled trial | IBS patients | - | 18 wk | ↑IBS-related pain ↓IBS-related distension but more decrease was seen in placebo The global assessment of changes in IBS symptoms and psychological stress due to IBS did not differ significantly among groups | [19] |
| Cynara scolymus | Aqueous-alcohol extract of leaf | Postmarketing surveillance study | IBS patients | - | 6 wk | ↓Severity of symptoms reported by both physicians and patients 96% of patients rated this drug as better than or at least equal to previous therapies Very good tolerability | [24] |
| Aqueous extract of leaf | Dose-ranging, open, postal study | IBS patients | - | 2 mo | ↓IBS incidence by 26.4% A significant shift in self-reported usual bowel pattern away from “alternating constipation/diarrhea” toward “normal” ↓NDI total symptom score by 41% 20% improvement in the NDI total QOL score | [23] | |
| Fumaria officinalis | Whole plant | Randomized, double-blind, placebo-controlled trial | IBS patients | - | 18 wk | ↓IBS-related pain ↑IBS-related distension The global assessment of changes in IBS symptoms and psychological stress due to IBS did not differ significantly among group | [19] |
| Hypericum perforatum | Aerial parts | Randomized, double-blind, placebo-controlled trial | IBS patients | - | 12 wk | ↓Overall BSS in both groups with the placebo arm having significantly lower scores at 12 wk compared with Hypericum group A similar proportion of subjects in each treatment group believed that the study drug they received decreased IBS life interferences | [28] |
| Open-label, uncontrolled trial | IBS women | - | 8 wk | ↓autonomic nervous system to different stressor Improvement of Gastrointestinal symptoms of IBS | [29] | ||
| Iberis amara | Whole plant extract | Double-blind, randomized, placebo-controlled, multi-centre trial | IBS patients | - | 4 wk | Significant improvement in IBS symptom scale and abdominal pain scale in Iberis group compared with placebo | [44] |
| Maranta arundinacea | Root | Uncontrolled | Diarrhea predominant- IBS patients | - | 1 mo | ↓Diarrhoea ↓Abdominal pain | [30] |
| Menthe piperita | Essence | Prospective double blind placebo-controlled randomized trial | IBS patients | - | 4 wk | 75% of the patients in the treatment group showed a > 50% reduction of basal total IBS symptoms score compared with 38% in the placebo group (P < 0.009) a statistically significant reduction of the total IBS symptoms score in treatment group compared with T (0), while no change was found with the placebo | [34] |
| Randomized double-blind placebo-controlled study | IBS patients | - | 8 wk | The number of subjects free from abdominal pain or discomfort changed from 0 at wk 0 to 14 at wk 8 in the treatment group and from 0 to 6 in controls (P < 0.001). ↓Severity of abdominal pain significantly in the drug group as compared to controls Improvement in the QOL in the treatment group There was no significant adverse reaction | [33] | ||
| Randomized, double-blind controlled trial | IBS patients | - | 2 wk | 76% of the patients receiving peppermint oil reported changes in the severity of symptom scale at the end of trial compared with 19% receiving placebo | [35] | ||
| Improvements in the change of symptom scale in 71% of the patients receiving peppermint oil compared with 43% receiving placebo | |||||||
| No significant differences between groups in the Gastrointestinal Symptom Rating Scale | |||||||
| No changes in symptoms such as abdominal rumbling, abdominal distention, belching, gas, and heartburn in treatment group compared with placebo | |||||||
| Mean severity of pain symptoms in the treatment group was significantly lower than that in the placebo | |||||||
| IBS patients | - | 1 mo | Significant reduction in the abdominal pain, abdominal distension, stool frequency, borborygmi, flatulence in the treatment group compared to placebo | [32] | |||
| Symptom improvements after essence therapy were significantly better than after placebo | |||||||
| No significant changes in liver function test results. | |||||||
| Paeonia lactiflora | Paeoniflorin; Active principle of root | In vivo | Neonatal maternal separation-induced visceral hyperalgesia in rats | - | Single dose | A dose-dependent analgesic effect Blockage of analgesic effect of Paeoniflorin by nor-binaltorphimine, dl-α-methyltyrosine, and yohimbine. Analgesic effect may be mediated by kappa-opioid receptors and α(2)-adrenoceptors in the central nervous system | [39] |
| Plantago psyllium | Seed | Randomized placebo controlled trial | IBs patients | - | 12 wk | Significantly greater proportion of responders in the psyllium group than in the placebo group | [40] |
| ↓Symptom severity significantly in the psyllium group compared with the placebo | |||||||
| No differences in QOL |
IBS: Irritable bowel syndrome; QOL: Quality of life; NDI: Nepean dyspepsia index; BSS: Bowel symptom score.
Aloe vera
Fifty-eight patients with IBS were randomized to receive Aloe vera or matching placebo for a month but no significant difference was found between Aloe and placebo groups[17].
Curcuma species
Eight-week treatment of IBS patients with Curcuma longa extract tablet decreased IBS prevalence and abdominal pain/discomfort score significantly between baseline and after treatment. There were significant improvements in the IBS quality of life (QOL) scales. Approximately two thirds of all subjects reported an improvement in symptoms after treatment, and there was a favorable shift in self-reported bowel pattern[18]. In a randomized, double-blind, placebo-controlled trial, IBS patients were randomly assigned to receive Curcuma xanthorriza or placebo. IBS-related pain increased in the Curcuma group and decreased in the placebo group. IBS-related distension showed a greater reduction in the placebo group compared to the curcuma group. Additionally, the global assessment of changes in IBS symptoms and psychological stress due to IBS did not differ significantly among the two treatment groups. Thus, Curcuma xanthorriza did not show any therapeutic benefit over placebo in patients with IBS[19]. Thus, the species of Curcuma used is an important factor in determining its efficacy in IBS. The efficacy of Curcuma in IBS may be due to bactericidal[20], anti-inflammatory[21], and spasmolytic[22] activities.
Cynara scolymus
Cynara scolymus was demonstrated to have both preventive and curative roles in IBS. The leaf extract of Cynara scolymus was evaluated in healthy volunteers suffering concomitant dyspepsia and showed a 26.4% fall in IBS incidence after treatment. A significant shift in self-reported usual bowel pattern away from “alternating constipation/diarrhea” toward “normal” was observed. The nepean dyspepsia index (NDI) total symptom score significantly decreased by 41% after treatment. Similarly, there was 20% improvement in the NDI total QOL score in the subset after treatment[23]. When the leaf extract of Cynara scolymus was administered to patients with IBS for 6 wk, a significant reduction in the severity of symptoms was observed. Ninety-six percent of patients rated this extract better than or at least equal to previous therapies administered for their symptoms. Furthermore, the tolerability of Cynara scolymus extract was very good[24]. It was reported that Cynara scolymus affects intestinal microbiota[25] and has antispasmodic activity[26].
Fumaria officinalis
The efficacy of Fumaria officinalis, because of its antispasmodic activity, has been investigated in IBS patients. In the randomized, double-blind, placebo-controlled trial, IBS-related pain decreased more in the fumitory group compared to the placebo group. IBS-related distension decreased in the placebo group and increased in the fumitory group. Additionally, the global assessment of changes in IBS symptoms and psychological stress due to IBS did not differ significantly among the two treatment groups[19].
Hypericum perforatum
Hypericum perforatum is a popular herbal medicine for the treatment of depression and it may be beneficial in the management of IBS by modulating psychological stress and serotonin[27]. The efficacy of Hypericum perforatum (St John’s wort) was evaluated in IBS patients during a 12-wk randomized, double-blind, placebo-controlled trial. The overall bowel symptom score (BSS) from baseline was decreased both in Hypericum and placebo groups whereas the placebo arm showed significantly lower scores at the end of treatment. Individual BSS for diarrhea (D-BSS), constipation (C-BSS), pain or discomfort, and bloating, adequate relief (AR) of IBS of at least 50% during the last 4 wk of therapy and IBS quality-of-life score showed greater improvement in the placebo group when compared with the Hypericum group. Thus Hypericum perforatum showed lower efficacy for treatment of IBS than placebo[28]. Another study showed that Hypericum perforatum can improve the psychologic symptoms and the ANS reactivity to stress and relieve intestinal symptoms in women with IBS. In patients with IBS, intestinal symptoms of IBS were also relieved significantly[29].
Maranta arundinacea
The efficacy of powdered root of Maranta arundinacea (Arrowroot) was assessed in patients with diarrhea predominant-IBS. It reduced diarrhea with a long-term effect on constipation. It also reduced abdominal pain[30].
Mentha × piperita
The evidence for the efficacy of essential oil of Mentha × piperita (peppermint oil) in IBS seems to be more than other herbal preparations[9,31]. In a prospective, randomized, double-blind, placebo-controlled clinical study, the efficacy of an enteric-coated peppermint oil formulation was evaluated in outpatients with IBS. Seventy-nine percent of patients on Mentha capsule experienced an alleviation of the severity of abdominal pain, 83% had less abdominal distension, 83% showed a reduced stool frequency, 73% had fewer borborygmi, and 79% less flatulence. Corresponding figures for the placebo group were: 43% with reduced pain, 29% with reduced distension, 32% with reduced stool frequency, 31% with fewer borborygmi, and 22% with less flatulence. Symptom improvements after Mentha capsule were significantly better than after placebo. No significant side effect was seen in the Mentha group and peppermint oil was well tolerated[32]. In another randomized, double-blind, placebo-controlled study conducted on outpatients with IBS, the number of subjects free from abdominal pain or discomfort changed from 0 at week 0 to 14 at week 8 in the peppermint oil group and from 0 to 6 in the placebo group. The severity of abdominal pain was reduced significantly in the peppermint group as compared to the placebo group. Furthermore, peppermint oil capsule significantly improved the QOL. No significant adverse reaction was reported from peppermint oil capsule[33]. In another study, the effectiveness of an enteric-coated peppermint oil capsule was evaluated in patients with IBS in whom small intestinal bacterial overgrowth, lactose intolerance and celiac disease were excluded. The symptoms evaluated were: abdominal bloating, abdominal pain or discomfort, diarrhea, constipation, feeling of incomplete evacuation, pain at defecation, passage of gas or mucus and urgency at defecation. The number of patients who showed reduction of the basal total IBS symptoms score in the peppermint oil group was greater than that in the placebo group[34]. In a randomized, double-blind controlled trial of children with IBS, 75% of those receiving peppermint oil showed a reduced severity of pain associated with IBS. At the end of the trial, the peppermint oil group reported a more significant improvement in the change of symptom scale than the placebo group. Symptoms such as changes in abdominal rumbling, abdominal distention, belching, gas, and heartburn exhibited no changes when peppermint oil was compared with placebo. The most predominant effect of peppermint oil was reduction in the severity of abdominal pain. No side effects were reported by either the investigator or patients during the 2-week study period[35]. Mentha was shown to have antimicrobial[36] and antispasmodic[37] activity and cause reduction in gastric motility[38].
Paeonia lactiflora
The root of Paeonia lactiflora is used in many herbal preparations for IBS. Paeoniflorin (PF) is one of the principle active ingredients of the root of Paeonia lactiflora. A dose-dependent analgesic effect was produced by both intraperitoneal and central administration of PF on visceral pain in rats with neonatal maternal separation. Further investigation showed that this effect may be mediated by kappa-opioid receptors and α (2)-adrenoceptors in the central nervous system[39].
Plantago psyllium
The efficacy of seed from Plantago psyllium in IBS was determined during a randomized controlled trial. The proportion of responders was significantly greater in the Plantago group than in the placebo group during the first month and the second month of treatment. After three months of treatment, symptom severity in the Plantago group was reduced by 90 points, compared with 49 points in the placebo group. No differences were found with respect to QOL[40].
COMPOUND PREPARATIONS
Preparations containing more than one herb are discussed below and summarized in Table 2.
Table 2.
Combination herbal therapies used for irritable bowel syndrome
| Name of preparation | Composition (part) | Type of study | Model | Concomitant drugs | Duration of study | Results | Ref. |
| Carmint | Mentha piperita (leaf) | Double-blind, randomized, placebo-controlled, multicenter clinical trial | IBS patients | Loperamide or psyllium (based on their predominant bowel function) | 8 wk | Severity and frequency of abdominal pain/discomfort were significantly lower in the Carmint group than the placebo group | [41] |
| Melissa officinalis (leaf) | |||||||
| Coriandrum sativum (fruit) | |||||||
| CHM | Codonopsis pilosulae (root) | Randomized, double-blind, placebo-controlled trial | IBS patients | - | 16 wk | Significant improvement in bowel symptom scores as rated by patients and by gastroenterologists | [42] |
| Agastaches seu pogostemi (whole plant) | Significant global improvement as rated by patients and by gastroenterologists | ||||||
| Ledebouriella sesloidis (root) | Patients reported that treatment significantly ↓ the degree of interference with life caused by IBS symptoms | ||||||
| Coicis lachryma-jobi (seed) | |||||||
| Bupleurum chinensis (whole plant) | |||||||
| Artemisia capillaries (whole plant) | |||||||
| Atractylodis macrocephalae (rhizome) | |||||||
| Magnolia officinalis (bark) | |||||||
| Citrus reticulata (pericarp) | |||||||
| Zingiber officinale (rhizome) | |||||||
| Fraxinus spp. (bark) | |||||||
| Poria cocos (sclerotium) | |||||||
| Angelica dahurica (root) | |||||||
| Plantago spp. (seed) | |||||||
| Phellodendron spp. (bark) | |||||||
| Glycyrrhiza uralensis (root) | |||||||
| Paeonia lactiflora (root) | |||||||
| Saussurea lappa (root) | |||||||
| Coptidis spp. (rhizome) | |||||||
| Schisandra spp. (fruit) | |||||||
| C-IBS formula | Lactulose Ulmus fulva (bark) Glycyrrhiza glabra (root) Avena sativa (bran) | A two arm, open-label, uncontrolled pilot study | Constipation-predominant IBS | - | 2 wk | A 20% increase in bowel movement frequency ↓ in straining, abdominal pain, bloating, and global IBS symptom severity improvements in stool consistency well-tolerated | [54] |
| DA-IBS formula | Vaccinium myrtillus (fruit) | A two arm, open-label, uncontrolled pilot study | Diarrhea-predominant and alternating bowel | - | 2 wk | a small, but significant increase in bowel movement frequency | [54] |
| Ulmus fulva (bark) Cinnamomum zeylanicum (bark) | habit IBS patients | ↓ in straining, abdominal pain, bloating, flatulence, and global IBS symptoms well-tolerated | |||||
| Agrimonia eupatoria (aerial part) | - | ||||||
| Iberogast (STW 5) | Iberis amara (whole plant) | In vitro | Human intestinal mucosa/submucosa preparations | A dose-dependent increase in ion secretion in human tissue and T84 cells evoke an increased spike discharge in 51% of human submucous neurons | [45] | ||
| Chelidonium majus (root) | human epithelial cell line T84 human enteric neurons | ||||||
| Silybum marianum (fruit) | |||||||
| Melissa officinalis (leaf) | |||||||
| Carum carvi (fruit) | |||||||
| Glycyrrhiza glabra (root) | |||||||
| Angelica sinensis (root) | In vivo | Wistar rats | - | Single dose | ↑Afferent discharge to 5-HT and bradykinin dose-dependently | [47] | |
| Matricaria recutita (flower) | |||||||
| Mentha piperita (leaf) | Double-blind, randomized, placebo-controlled, multi-centre trial | IBS patients | - | 4 wk | Significant improvement in IBS symptom scale and abdominal pain scale in STW 5 group compared with placebo | [44] | |
| STW 5-II | Iberis amara (whole plant) Melissa officinalis (leaf) Carum carvi (fruit) | Double-blind, randomized, placebo-controlled, multi-centre trial | IBS patients | - | 4 wk | Significant improvement in IBS symptom scale and abdominal pain scale in STW 5-II group compared with placebo | [44] |
| Glycyrrhiza glabra (root) | |||||||
| Matricaria recutita (flower) | |||||||
| Mentha piperita (leaf) | |||||||
| Padma Lax | Aloe barbadensis A. ferox (extract) | Randomized, double-blind, placebo-controlled trial | Constipation predominant-IBS patients | - | 3 mo | Significant improvement compared to placebo in constipation, severity of abdominal pain, incomplete evacuation, abdominal distension and flatus/flatulence | [43] |
| Jateorhiza palmata (root) | Significantly more Padma Lax patients compared to placebo rated the current treatment superior to previous therapies tried for IBS | ||||||
| Marsdenia condurango (bark), Rhamnus frangula (bark) | Laboratory parameters displayed no clinically significant changes | ||||||
| Gentiana lutea (root) | |||||||
| Inula helenium (rhizome) | |||||||
| Terminalia chebula (fruit) | |||||||
| Piper longum (fruit) Rhamnus purshiana. (bark) | |||||||
| Rheum palmatum (root) | |||||||
| Strychnos nux-vomica (seed) | |||||||
| Zingiber officinale (root) | |||||||
| TXNG | Paeonia lactiflora (root) | Prospective, randomized, double-blind, placebo-controlled trial | Diarrhea predominant-IBS patients | - | 3 wk | ↓IBS-related pain in the TXNG group compared with the placebo | [52] |
| Atractylodes macrocephala (rhizome) | ↓Frequency and the duration of abdominal pain between the TXNG group and the placebo | ||||||
| Citrus reticulate (green unripe exocarp) | Improvement of IBS-related stool in form or appearance in the TXNG group in comparison with the placebo | ||||||
| Allium macrostemon (bulb) | ↓Stool frequency in the TXNG group compared with the placebo | ||||||
| Improvement of stool passage (urgency or | |||||||
| Feeling of incomplete rectal emptying) in the TXNG group compared with the placebo. | |||||||
| Improvement in IBS-related diarrhea in the TXNG group compared to placebo | |||||||
| No statistical difference in either the effective time of IBS-related pain or the effective time of IBS-related diarrhea between the two groups | |||||||
| ↓IBS-related pain alleviation time and the IBS-related diarrhea alleviation time in the TXNG group compared to those in the placebo group | |||||||
| TXYF | Atractylodes macrocephala (rhizome) | In vivo | Maternal separation-induced visceral hypersensitivity rats | - | 2 wk | ↓Pain threshold pressure and abdominal withdrawal reflex scores in a dose-dependent manner | [54] |
| Paeonia lactiflora (root) | ↓ 5-HT levels in serum | ||||||
| Citrus sinensis (dried old peel) | ↓Corticotrophin releasing factor concentrations in the brain | ||||||
| Ledebouriella divaricata (root) | Visceral hypersensitivity alleviation was dependent on the substance P expression in the colon mucosa | ||||||
| Randomized placebo-controlled trial | Diarrhea predominant-IBS patients | Miyarisan | 4 wk | No significant difference between two groups in terms of the total efficacy or the scores of symptoms before and after treatment | [53] | ||
| ↓The number of activated mast cells in the intervention | |||||||
| TCM | Atractylodes macrocephala (rhizome) | Randomized placebo-controlled trial | Diarrhea predominant-IBS patients | - | 16 wk (8 wk drug administration +8 wk follow up) | No significant difference in the proportion of patients with global symptom improvement between the TCM and placebo groups at week 8 and at week 16 | [51] |
| Astragalus membranaceus (root) | No difference in individual symptom scores and the quality-of-life assessment between the two groups at all time points | ||||||
| Paeonia lactiflora (peeled root, fried) | |||||||
| Atractylodes chinensis (rhizome) | |||||||
| Bupleurum chinense (root) | |||||||
| Citrus reticulata (peel) | |||||||
| Saposhnikovia divaricata (root) | |||||||
| Paniculata (twigs) | |||||||
| Punica granatum (rind) | |||||||
| Portulaca oleracea (above-ground parts) | |||||||
| Coptis chinensis (rhizome) |
CHM: Chinese herbal medicine; TXNG: Tong-xie-ning; TXYF: Tong-Xie-Yao-Fang; TCM: Traditional Chinese Medicine; 5-HT: Serotonin; spp.: Schisandra chinensis.
Carmint
Carmint is an Iranian herbal medicine containing total extracts of Melissa officinalis, Mentha spicata, and Coriandrum sativum. Thirty-two IBS patients randomly received either carmint or placebo, plus loperamide or psyllium (based on their predominant bowel function), for 8 wk. The severity and frequency of abdominal pain/discomfort were significantly lower in the carmint group than the placebo group at the end of the treatment according to severity and frequency of bloating[41].
A Chinese herbal medicine
A randomized, double-blind, placebo-controlled trial was conducted to determine whether a Chinese herbal medicine (CHM) is of any benefit in the treatment of IBS. This formulation composed of 20 different herbs (Table 2). Compared with patients in the placebo group, patients in the CHM groups showed a significant improvement in bowel symptom and global improvement scores as rated by patients and by gastroenterologists. Patients reported that treatment significantly reduced the degree of interference with life caused by IBS symptoms[42].
Padma Lax
Padma Lax, a complex Tibetan herbal formula (Table 2), was evaluated for safety and effectiveness in treating constipation-predominant IBS in a 3-mo double-blind randomized pilot study. Significant improvement was demonstrated after 3 mo in the Padma Lax group compared to placebo in constipation, severity of abdominal pain, daily activities, incomplete evacuation, abdominal distension and flatus/flatulence. Significantly more Padma Lax patients than placebo patients rated the current treatment superior to previous therapies tried for IBS. Laboratory parameters displayed no clinically significant changes. The primary side effect of Padma Lax was loose stools which improved by lowering dose[43].
STW 5
STW 5 (Iberogast), a formula composed of hydroethanolic extracts of nine herbs, has been prepared for functional gastrointestinal disorders like IBS and its efficacy and mechanisms of action were investigated in several studies. It was significantly better than placebo in reducing the total abdominal pain and the IBS symptom scores. In a double-blind, placebo-controlled, multicentre trial, STW 5-II, another formula composed of 6 of 9 herbal extracts used in STW 5, and a preparation containing only one of 9 herbal extracts (extract of Iberis amara) were compared with placebo. STW 5-II like STW 5 showed more significant reduction in the total abdominal pain and the IBS symptom scores compared with placebo. There were no statistically significant differences between the mono-extract group and the placebo group[44]. Different mechanisms have been proposed for the efficacy of STW 5 in IBS. A study evaluating STW 5 effects on mucosal secretion in human intestinal mucosa/submucosa preparations and in the human epithelial cell line T84 suggested that this herbal preparation is a secretogogue in the human intestine by direct epithelial actions and through activation of enteric neurons. The prosecretory effect is due to increased epithelial chloride fluxes via cystic fibrosis transmembrane conductance regulator and calcium dependent chloride channels[45]. STW 5 was studied in vitro for binding affinities to serotonin (5-HT3 and 5-HT4) and muscarinic (M3) receptors of the intestine that play central roles in the etiology of IBS. STW 5 showed binding affinity to 5-HT4, M3 and to a lesser degree 5-HT3[46]. STW 5 has also controlled visceral hypersensitivity by reducing intestinal afferent sensitivity to mechanical and chemical stimuli in the upper gastrointestinal tract in male Wistar rats. Following the different doses of serotonin and bradykinin, the peak in afferent nerve discharge was always reduced after pretreatment with STW 5 compared to controls. The ramp distension of the intestinal loop stimulated a rise in intestinal afferent nerve discharge that was lower in the STW 5 pretreated group compared to controls[47]. STW 5 decreased acetylcholine- and histamine-induced contraction of guinea pig ileum. This was also true for extracts of some constituents of this compound formula including Mentha piperita leaf, Matricaria recutita flower and Glycyrrhiza uralensis root. Extract from Iberis amara, however, showed no spasmolytic action; on the contrary, it increased the basal resting tone and contraction of atonic ileal segments. These data may explain, at least in part, the clinically observed therapeutic efficacy of STW 5 in both hypotonic and spastic dysmotility symptoms of IBS[48]. Another study on STW 5 and its components showed that STW 5 evoked a relaxation of the proximal stomach but increased antral motility whereas both effects are myogenic. The extracts of Angelica sinensis root, Matricaria recutita flower and Glycyrrhiza uralensis root mimicked the inhibitory effects in the proximal stomach whereas the extracts of Chelidonium majus, Melissa officinalis leaf, Carum carvi fruit and Iberis amara increased motility of the proximal stomach. All extracts increased motility in the antrum comparable to the effects of STW 5[49]. These data justify the differential effect of STW 5 which is a result of the combined actions of its individual components explaining the clinically observed therapeutic efficacy of STW 5 in both hypotonic and spastic dysmotility symptoms of IBS. Moreover, STW 5 reduced inflammation-induced alterations in ileum/jejunum segments. The effects were associated with a restoration of the disturbed acetylcholine-induced contraction, pathohistological protection and inhibition of tumor necrosis factor (TNF)-α[50].
A traditional Chinese medicine
Therapeutic efficacy of a traditional Chinese medicine (TCM) (Table 2) was investigated in patients with diarrhea-predominant IBS. There was no significant difference in the proportion of patients with global symptom improvement between the TCM and placebo groups. Moreover, there was no difference in individual symptom scores and the quality-of-life assessment between the two groups[51].
Tong-xie-ning
Tong-xie-ning (TXNG) is a traditional Chinese medicine composed of four different herbs. The efficacy of this preparation was evaluated in diarrhea predominant-IBS patients by a prospective, randomized, double-blind, placebo-controlled trial. IBS-related pain measured by the numeric pain intensity scale in the TXNG group, significantly decreased as compared with the placebo group. A total of 82.7% of the patients reported a reduction in IBS-related pain in the TXNG group compared with 39.3% in the placebo group. Furthermore, there was a significant reduction in the frequency and the duration of abdominal pain between the TXNG group and the placebo group. In addition, IBS-related stools improved in form or appearance in the TXNG group in comparison to the placebo group. The stool frequency was significantly decreased in the TXNG group compared with the placebo group. Moreover, stool passage (urgency or feeling of incomplete rectal emptying) in the TXNG group was significantly improved when compared with the placebo group. There was a 20.7% and 42.9% loss of appetite in the TXNG and placebo groups, respectively. An observable improvement in IBS-related diarrhea was seen in 86.2% of subjects in the TXNG group and 42.9% of subjects in the placebo group. There was no statistical difference in either the effective time of IBS-related pain or the effective time of IBS-related diarrhea between the two groups. However, the IBS-related pain alleviation time and the IBS-related diarrhea alleviation time in the TXNG group were markedly shorter than those in the placebo group[52].
Tong-Xie-Yao-Fang
Tong-Xie-Yao-Fang (TXYF) is a prescription in TCM, prepared from four herbs (Table 2). A study was done to compare the efficacy of this formulation with Myarisan, a probiotic formulation, in treating diarrhea-predominant IBS. No significant difference between the two groups in terms of the total efficacy or the scores of symptoms before and after treatment was found. In this study, the number of activated mast cells was decreased in the TXYF group after treatment, showing a significant difference as compared with that before treatment as well as with that in the Myarisan group after treatment. This result suggested that the mechanism of action of TXYF might be through adjustment of mast cells activation to decrease visceral hypersensitivity[53]. This product has been investigated in experimental visceral hypersensitivity models that showed a dose-dependent analgesic effect. It significantly decreased serotonin levels in serum and CRF concentrations in the brain. Moreover, it was found that visceral hypersensitivity alleviation by TXYF was dependent on substance P (SP) expression in the colon mucosa[54].
C-IBS and DA-IBS formulations
The efficacy and tolerability of C-IBS (Table 2), a formula designed to treat constipation-predominant IBS, and DA-IBS, a formula designed to treat diarrhea-predominant and alternating bowel habit IBS, were evaluated in an uncontrolled study. Ingestion of the DA-IBS formula was associated with a small, but significant, increase in bowel movement frequency. Reductions in straining, abdominal pain, bloating, flatulence, and global IBS symptoms were also demonstrated in patients using this formula. Subjects in the C-IBS group experienced a 20% increase in bowel movement frequency and significant reductions in straining, abdominal pain, bloating, and global IBS symptom severity, as well as improvements in stool consistency. Both formulas were well-tolerated[55].
CONCLUSION
In this paper, different herbal preparations investigated for the management of IBS and their possible mechanisms of action were reviewed. Among the single preparations, the most evidence for efficacy in IBS patients was found for essential oil of Mentha piperita. Some single preparations including Aloe vera, Curcuma xanthorriza, Fumaria officinalis showed no benefit in IBS. There are conflicting results for the efficacy of Hypericum perforatum in IBS. Among compound preparations, most studies had been performed with STW 5, a formula containing hydroethanolic extract of 9 herbs. Hopeful results come from the efficacy of this herbal preparation in the management of IBS with different mechanisms of action such as anti-inflammatory, prosecretory activity, and affecting gastrointestinal motility. Because of multifactorial nature of the pathophysiology of IBS, it seems that compound preparations can be more efficacious than single ones. Despite the wide prevalence of IBS, there are few studies on the use of herbal medicine in IBS and most studies in this area have focused on the use of peppermint oil and STW 5; while it seems that more effective herbal preparations can be found. For example, in traditional Iranian medicine (TIM) many single and compound herbal preparations have been introduced for the management of various gut disorders such as IBS[56]. Among the herbal contents of this preparations, oleogumresin from Boswellia carterii, fruit of Trachyspermum amum, flower of Eugenia caryophyllata, gall of Quercus infectoria, seed of Nigella sativa, fruit of Cuminum cyminum, tabasheer (a hard, whitish, translucent substance extracted from the joints of different Bambusa species), and fruit of Cucurbita pepo can be stated[57-59]. There is evidence in modern phytotherapy for the beneficial effects of these plants in IBS. Boswellia carterii has shown anti-inflammatory[60,61] and immunomodulatory activity[62]. An in vitro study demonstrated that Trachyspermum copticum has a beneficial effect on intestinal microbiota by inhibiting the growth of potential pathogens[63]. Eugenia caryophyllata has anti-inflammatory[64], immunomodulatory[65], and antimicrobial[66] properties. Gall of Quercus infectoria possesses anti-inflammatory[67] and antibacterial[68] activity. Nigella sativa showed anti-inflammatory, antimicrobial, and immunomodulatory activity[69]. Antimicrobial properties were reported for Cuminum cyminum[70] and Cucurbita pepo[71]. However, more studies are required to get more conclusive results about the efficacy of these herbs in IBS.
Footnotes
Peer reviewer: Mauro Bortolotti, MD, Professor, Department of Internal Medicine and Gastroenterology, University of Bologna, Via Massarenti 48, Bologna 40138, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Zhang DN
References
- 1.Chang JY, Talley NJ. Current and emerging therapies in irritable bowel syndrome: from pathophysiology to treatment. Trends Pharmacol Sci. 2010;31:326–334. doi: 10.1016/j.tips.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691–699. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607. doi: 10.1111/j.1440-1746.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Salvioli B, Corinaldesi R. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20 Suppl 2:1–9. doi: 10.1111/j.1365-2036.2004.02036.x. [DOI] [PubMed] [Google Scholar]
- 5.Karantanos T, Markoutsaki T, Gazouli M, Anagnou NP, Karamanolis DG. Current insights in to the pathophysiology of Irritable Bowel Syndrome. Gut Pathog. 2010;2:3. doi: 10.1186/1757-4749-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi R, Nikfar S, Abdollahi M. Selective serotonin reuptake inhibitors for the management of irritable bowel syndrome: A meta-analysis of randomized controlled trials. Arch Med Sci. 2008;4:71–76. [Google Scholar]
- 8.Darvish-Damavandi M, Nikfar S, Abdollahi M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J Gastroenterol. 2010;16:547–553. doi: 10.3748/wjg.v16.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–1843; quiz 1844. doi: 10.1038/ajg.2009.223. [DOI] [PubMed] [Google Scholar]
- 12.Rezaie A, Nikfar S, Abdollahi M. The place of antibiotics in management of irritable bowel syndrome: a systematic review and meta-analysis. Arch Med Sci. 2010;6:49–55. doi: 10.5114/aoms.2010.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–1780. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16:3646–3655. doi: 10.2174/138161210794079254. [DOI] [PubMed] [Google Scholar]
- 15.Duracinsky M, Chassany O. [How can an effective drug to treat irritable bowel syndrome be successfully developed?] Gastroenterol Clin Biol. 2009;33 Suppl 1:S26–S34. doi: 10.1016/S0399-8320(09)71522-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu JC. Complementary and alternative medicine modalities for the treatment of irritable bowel syndrome: facts or myths? Gastroenterol Hepatol ( NY) 2010;6:705–711. [PMC free article] [PubMed] [Google Scholar]
- 17.Davis K, Philpott S, Kumar D, Mendall M. Randomised double-blind placebo-controlled trial of aloe vera for irritable bowel syndrome. Int J Clin Pract. 2006;60:1080–1086. doi: 10.1111/j.1742-1241.2006.00980.x. [DOI] [PubMed] [Google Scholar]
- 18.Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004;10:1015–1018. doi: 10.1089/acm.2004.10.1015. [DOI] [PubMed] [Google Scholar]
- 19.Brinkhaus B, Hentschel C, Von Keudell C, Schindler G, Lindner M, Stützer H, Kohnen R, Willich SN, Lehmacher W, Hahn EG. Herbal medicine with curcuma and fumitory in the treatment of irritable bowel syndrome: a randomized, placebo-controlled, double-blind clinical trial. Scand J Gastroenterol. 2005;40:936–943. doi: 10.1080/00365520510023134. [DOI] [PubMed] [Google Scholar]
- 20.Zaidi SF, Yamada K, Kadowaki M, Usmanghani K, Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J Ethnopharmacol. 2009;121:286–291. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 22.Gilani AH, Shah AJ, Ghayur MN, Majeed K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76:3089–3105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Bundy R, Walker AF, Middleton RW, Marakis G, Booth JC. Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: a subset analysis. J Altern Complement Med. 2004;10:667–669. doi: 10.1089/acm.2004.10.667. [DOI] [PubMed] [Google Scholar]
- 24.Walker AF, Middleton RW, Petrowicz O. Artichoke leaf extract reduces symptoms of irritable bowel syndrome in a post-marketing surveillance study. Phytother Res. 2001;15:58–61. doi: 10.1002/1099-1573(200102)15:1<58::aid-ptr805>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 25.Costabile A, Kolida S, Klinder A, Gietl E, Bäuerlein M, Frohberg C, Landschütze V, Gibson GR. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br J Nutr. 2010;104:1007–1017. doi: 10.1017/S0007114510001571. [DOI] [PubMed] [Google Scholar]
- 26.Emendörfer F, Emendörfer F, Bellato F, Noldin VF, Cechinel-Filho V, Yunes RA, Delle Monache F, Cardozo AM. Antispasmodic activity of fractions and cynaropicrin from Cynara scolymus on guinea-pig ileum. Biol Pharm Bull. 2005;28:902–904. doi: 10.1248/bpb.28.902. [DOI] [PubMed] [Google Scholar]
- 27.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:118–127. doi: 10.1016/j.pnpbp.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Saito YA, Rey E, Almazar-Elder AE, Harmsen WS, Zinsmeister AR, Locke GR, Talley NJ. A randomized, double-blind, placebo-controlled trial of St John’s wort for treating irritable bowel syndrome. Am J Gastroenterol. 2010;105:170–177. doi: 10.1038/ajg.2009.577. [DOI] [PubMed] [Google Scholar]
- 29.Wan H, Chen Y. Effects of antidepressive treatment of Saint John’s wort extract related to autonomic nervous function in women with irritable bowel syndrome. Int J Psychiatry Med. 2010;40:45–56. doi: 10.2190/PM.40.1.d. [DOI] [PubMed] [Google Scholar]
- 30.Cooke C, Carr I, Abrams K, Mayberry J. Arrowroot as a treatment for diarrhoea in irritable bowel syndrome patients: a pilot study. Arq Gastroenterol. 2000;37:20–24. doi: 10.1590/s0004-28032000000100005. [DOI] [PubMed] [Google Scholar]
- 31.Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: a critical review and metaanalysis. Am J Gastroenterol. 1998;93:1131–1135. doi: 10.1111/j.1572-0241.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32:765–768. doi: 10.1007/BF02936952. [DOI] [PubMed] [Google Scholar]
- 33.Merat S, Khalili S, Mostajabi P, Ghorbani A, Ansari R, Malekzadeh R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig Dis Sci. 2010;55:1385–1390. doi: 10.1007/s10620-009-0854-9. [DOI] [PubMed] [Google Scholar]
- 34.Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530–536. doi: 10.1016/j.dld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Kline RM, Kline JJ, Di Palma J GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138:125–128. doi: 10.1067/mpd.2001.109606. [DOI] [PubMed] [Google Scholar]
- 36.Logan AC, Beaulne TM. The treatment of small intestinal bacterial overgrowth with enteric-coated peppermint oil: a case report. Altern Med Rev. 2002;7:410–417. [PubMed] [Google Scholar]
- 37.Grigoleit HG, Grigoleit P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine. 2005;12:612–616. doi: 10.1016/j.phymed.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Hiki N. [Peppermint oil reduces gastric motility during the upper gastrointestinal endoscopy] Nihon Rinsho. 2010;68:2126–2134. [PubMed] [Google Scholar]
- 39.Zhang XJ, Li Z, Leung WM, Liu L, Xu HX, Bian ZX. The analgesic effect of paeoniflorin on neonatal maternal separation-induced visceral hyperalgesia in rats. J Pain. 2008;9:497–505. doi: 10.1016/j.jpain.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Bijkerk CJ, de Wit NJ, Muris JW, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ. 2009;339:b3154. doi: 10.1136/bmj.b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vejdani R, Shalmani HR, Mir-Fattahi M, Sajed-Nia F, Abdollahi M, Zali MR, Mohammad Alizadeh AH, Bahari A, Amin G. The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: a pilot study. Dig Dis Sci. 2006;51:1501–1507. doi: 10.1007/s10620-006-9079-3. [DOI] [PubMed] [Google Scholar]
- 42.Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280:1585–1589. doi: 10.1001/jama.280.18.1585. [DOI] [PubMed] [Google Scholar]
- 43.Sallon S, Ben-Arye E, Davidson R, Shapiro H, Ginsberg G, Ligumsky M. A novel treatment for constipation-predominant irritable bowel syndrome using Padma Lax, a Tibetan herbal formula. Digestion. 2002;65:161–171. doi: 10.1159/000064936. [DOI] [PubMed] [Google Scholar]
- 44.Madisch A, Holtmann G, Plein K, Hotz J. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment Pharmacol Ther. 2004;19:271–279. doi: 10.1111/j.1365-2036.2004.01859.x. [DOI] [PubMed] [Google Scholar]
- 45.Krueger D, Gruber L, Buhner S, Zeller F, Langer R, Seidl S, Michel K, Schemann M. The multi-herbal drug STW 5 (Iberogast) has prosecretory action in the human intestine. Neurogastroenterol Motil. 2009;21:1203–e110. doi: 10.1111/j.1365-2982.2008.01242.x. [DOI] [PubMed] [Google Scholar]
- 46.Simmen U, Kelber O, Okpanyi SN, Jaeggi R, Bueter B, Weiser D. Binding of STW 5 (Iberogast) and its components to intestinal 5-HT, muscarinic M3, and opioid receptors. Phytomedicine. 2006;13 Suppl 5:51–55. doi: 10.1016/j.phymed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Müller MH, Liu CY, Glatzle J, Weiser D, Kelber O, Enck P, Grundy D, Kreis ME. STW 5 (Iberogast) reduces afferent sensitivity in the rat small intestine. Phytomedicine. 2006;13 Suppl 5:100–106. doi: 10.1016/j.phymed.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Ammon HP, Kelber O, Okpanyi SN. Spasmolytic and tonic effect of Iberogast (STW 5) in intestinal smooth muscle. Phytomedicine. 2006;13 Suppl 5:67–74. doi: 10.1016/j.phymed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Schemann M, Michel K, Zeller F, Hohenester B, Rühl A. Region-specific effects of STW 5 (Iberogast) and its components in gastric fundus, corpus and antrum. Phytomedicine. 2006;13 Suppl 5:90–99. doi: 10.1016/j.phymed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Michael S, Kelber O, Hauschildt S, Spanel-Borowski K, Nieber K. Inhibition of inflammation-induced alterations in rat small intestine by the herbal preparations STW 5 and STW 6. Phytomedicine. 2009;16:161–171. doi: 10.1016/j.phymed.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Leung WK, Wu JC, Liang SM, Chan LS, Chan FK, Xie H, Fung SS, Hui AJ, Wong VW, Che CT, et al. Treatment of diarrhea-predominant irritable bowel syndrome with traditional Chinese herbal medicine: a randomized placebo-controlled trial. Am J Gastroenterol. 2006;101:1574–1580. doi: 10.1111/j.1572-0241.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Li TQ, Wang L, Xia Q, Chang J, Zhang Y, Wan MH, Guo J, Cheng Y, Huang X, et al. Tong-xie-ning, a Chinese herbal formula, in treatment of diarrhea-predominant irritable bowel syndrome: a prospective, randomized, double-blind, placebo-controlled trial. Zhanghua Yixue Zazhi. 2006;119:2114–2119. [PubMed] [Google Scholar]
- 53.Pan F, Zhang T, Zhang YH, Xu JJ, Chen FM. Effect of Tongxie Yaofang Granule in treating diarrhea-predominate irritable bowel syndrome. Chin J Integr Med. 2009;15:216–219. doi: 10.1007/s11655-009-0216-7. [DOI] [PubMed] [Google Scholar]
- 54.Hu X, Xu D, Zhao Y, Yang X, Meng J, Shen H, Guo J. The Alleviating Pain Effect of Aqueous Extract from Tong-Xie-Yao-Fang, on Experimental Visceral Hypersensitivity and Its Mechanism. Biol Pharm Bull. 2009;32:1075–1079. doi: 10.1248/bpb.32.1075. [DOI] [PubMed] [Google Scholar]
- 55.Hawrelak JA, Myers SP. Effects of two natural medicine formulations on irritable bowel syndrome symptoms: a pilot study. J Altern Complement Med. 2010;16:1065–1071. doi: 10.1089/acm.2009.0090. [DOI] [PubMed] [Google Scholar]
- 56.Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16:4504–4514. doi: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonkaboni A. Resaleh Eshalieh. Rezayizadeh H, Rostambakhsh MR, Tafaghod R, Yazdani M, editors. Tehran: Almaee; 2009. pp. 109–119. Available from: http: //www.adinebook.com/gp/product/9647553315. [Google Scholar]
- 58.Tonkaboni MM. Tohfeh al-Momenin. Rahimi R, Shams Ardekani MR, Farjadmand F, editors. Tehran: Shahid Beheshti University of Medical Sciences; 2007. Available from: http: //www.adinebook.com/gp/product/9642657971. [Google Scholar]
- 59.Aghili MH. Makhzan-al-Advia. Rahimi R, Shams Ardekani MR, Farjadmand F, editors. Tehran: Tehran University of Medical Sciences; 2009. Available from: http: //aqlibrary.org/UserFiles/File/makhzan.pdf. [Google Scholar]
- 60.Fan AY, Lao L, Zhang RX, Wang LB, Lee DY, Ma ZZ, Zhang WY, Berman B. Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on rats with persistent inflammation. J Altern Complement Med. 2005;11:323–331. doi: 10.1089/acm.2005.11.323. [DOI] [PubMed] [Google Scholar]
- 61.Banno N, Akihisa T, Yasukawa K, Tokuda H, Tabata K, Nakamura Y, Nishimura R, Kimura Y, Suzuki T. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J Ethnopharmacol. 2006;107:249–253. doi: 10.1016/j.jep.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Badria FA, Mikhaeil BR, Maatooq GT, Amer MM. Immunomodulatory triterpenoids from the oleogum resin of Boswellia carterii Birdwood. Z Naturforsch C. 2003;58:505–516. doi: 10.1515/znc-2003-7-811. [DOI] [PubMed] [Google Scholar]
- 63.Hawrelak JA, Cattley T, Myers SP. Essential oils in the treatment of intestinal dysbiosis: A preliminary in vitro study. Altern Med Rev. 2009;14:380–384. [PubMed] [Google Scholar]
- 64.Magalhães CB, Riva DR, DePaula LJ, Brando-Lima A, Koatz VL, Leal-Cardoso JH, Zin WA, Faffe DS. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J Appl Physiol. 2010;108:845–851. doi: 10.1152/japplphysiol.00560.2009. [DOI] [PubMed] [Google Scholar]
- 65.Carrasco FR, Schmidt G, Romero AL, Sartoretto JL, Caparroz-Assef SM, Bersani-Amado CA, Cuman RK. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: evidence for humor- and cell-mediated responses. J Pharm Pharmacol. 2009;61:961–967. doi: 10.1211/jpp/61.07.0017. [DOI] [PubMed] [Google Scholar]
- 66.Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65:1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 67.Kaur G, Hamid H, Ali A, Alam MS, Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90:285–292. doi: 10.1016/j.jep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Vermani A. Screening of Quercus infectoria gall extracts as anti-bacterial agents against dental pathogens. Indian J Dent Res. 2009;20:337–339. doi: 10.4103/0970-9290.57380. [DOI] [PubMed] [Google Scholar]
- 69.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 70.Wanner J, Bail S, Jirovetz L, Buchbauer G, Schmidt E, Gochev V, Girova T, Atanasova T, Stoyanova A. Chemical composition and antimicrobial activity of cumin oil (Cuminum cyminum, Apiaceae) Nat Prod Commun. 2010;5:1355–1358. [PubMed] [Google Scholar]
- 71.Badr SE, Shaaban M, Elkholy YM, Helal MH, Hamza AS, Masoud MS, El Safty MM. Chemical composition and biological activity of ripe pumpkin fruits (Cucurbita pepo L.) cultivated in Egyptian habitats. Nat Prod Res. 2011;25:1524–1539. doi: 10.1080/14786410903312991. [DOI] [PubMed] [Google Scholar]


