Abstract
AIM: To investigate the angiogenesis-related protein expression profile characterizing metastatic colorectal cancer (mCRC) with the aim of identifying prognostic markers.
METHODS: The expression of 44 angiogenesis-secreted factors was measured by a novel cytokine antibody array methodology. The study evaluated vascular endothelial growth factor (VEGF) and its soluble vascular endothelial growth factor receptor (sVEGFR)-1 protein levels by enzyme immunoassay (EIA) in a panel of 16 CRC cell lines. mRNA VEGF and VEGF-A isoforms were quantified by quantitative reverse-transcription polymerase chain reaction (Q-RT-PCR) and vascular endothelial growth factor receptor (VEGFR)-2 expression was analyzed by flow cytometry.
RESULTS: Metastasis-derived CRC cell lines expressed a distinctive molecular profile as compared with those isolated from a primary tumor site. Metastatic CRC cell lines were characterized by higher expression of angiopoietin-2 (Ang-2), macrophage chemoattractant proteins-3/4 (MCP-3/4), matrix metalloproteinase-1 (MMP-1), and the chemokines interferon γ inducible T cell α chemoattractant protein (I-TAC), monocyte chemoattractant protein I-309, and interleukins interleukin (IL)-2 and IL-1α, as compared to primary tumor cell lines. In contrast, primary CRC cell lines expressed higher levels of interferon γ (IFN-γ), insulin-like growth factor-1 (IGF-1), IL-6, leptin, epidermal growth factor (EGF), placental growth factor (PlGF), thrombopoietin, transforming growth factor β1 (TGF-β1) and VEGF-D, as compared with the metastatic cell lines. VEGF expression does not significantly differ according to the CRC cellular origin in normoxia. Severe hypoxia induced VEGF expression up-regulation but contrary to expectations, metastatic CRC cell lines did not respond as much as primary cell lines to the hypoxic stimulus. In CRC primary-derived cell lines, we observed a two-fold increase in VEGF expression between normoxia and hypoxia as compared to metastatic cell lines. CRC cell lines express a similar pattern of VEGF isoforms (VEGF121, VEGF165 and VEGF189) despite variability in VEGF expression, where the major transcript was VEGF121. No relevant expression of VEGFR-2 was found in CRC cell lines, as compared to that of human umbilical vein endothelial cells and sVEGFR-1 expression did not depend on the CRC cellular origin.
CONCLUSION: A distinct angiogenesis-related expression pattern characterizes metastatic CRC cell lines. Factors other than VEGF appear as prognostic markers and intervention targets in the metastatic CRC setting.
Keywords: Colorectal cancer metastasis, Cytokine-antibody array, Angiogenesis, Vascular endothelial growth factor, Biomarkers
INTRODUCTION
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths. The prognosis of CRC is dependent upon the extent of disease and approximately 60% of patients develop metastases after surgical resection. With a 5-year survival rate of less than 10% in patients with distant metastatic disease, targeting the metastatic process and sites should provide an effective treatment[1]. The progressive growth of colon cancer and subsequent metastatic process is dependent on an angiogenic network[2,3]. Thus, anti-angiogenic strategies have emerged as effective therapies in patients with colon cancer, especially in the metastatic setting of the disease[4-6]. Yet, differences in the magnitude of survival benefit point to alternative pathways in the tumor microenvironment as responsible for inconsistent outcomes[7].
Angiogenesis is a complex process dependent on the angiogenic factors secreted by the tumor and stroma cells[8]. Vascular endothelial growth factor is considered the major pro-angiogenic factor[9]. The vascular endothelial growth factor (VEGF) gene encodes for six alternatively spliced isoforms[10] with differential diffusion potential and binding to receptors[11]. The question currently consists of understanding the significance of VEGF/vascular endothelial growth factor receptor (VEGFR) signaling in cancer cells[12,13]. The VEGF isoforms and VEGF receptor expression pattern would drive the activity and functionality of the VEGF/VEGFR pathway in both tumor and endothelial cells. The multistep process of angiogenesis accompanies the multistage development of a tumor[14]. The switch into the metastatic phenotype brings a number of changes within the tumor microenvironment, including acquisition of hypoxia-tolerance mechanisms[15]. While up-regulation of VEGF expression is activated mainly under hypoxia[9], recent reports reflect on the question of whether metastatic tumors rely as much on angiogenesis and VEGF as primary tumors[15].
Other studies report that tumors in more advanced stages do not rely on a unique angiogenesis driver[2]. A network of multiple cytokines and growth factors create a crosstalk within the tumor microenvironment which ultimately drives tumor angiogenesis[2,16]. The mediators of vessel wall remodeling matrix metalloproteinases, macrophage chemoattractant proteins and angiopoietin, involved in invasion and metastasis processes, exert pro-angiogenic signals[8,17]. Chemokines such as interleukin (IL)-1α and IL-8 play an important role in colon cancer progression and angiogenesis[18], and IL-8 up-regulates MMPs[19]. VEGF expression actually determines the activity of Ang-1/Ang-2 and the expression of MCPs[20,21].
Great efforts have been made to characterize biomarkers in CRC[22]. However, the question of biomarkers of CRC metastasis remains currently unresolved. On this basis, the aim of this study was to characterize the protein factors behind the angiogenic potential of CRC cell lines of metastatic origin.
MATERIALS AND METHODS
Cell cultures and conditioned media
We used 16 CRC cell lines: HT29, WiDr, HCT116, RKO, SW480, Colo320, Caco2, SW1116, LS174T, SW1417, DLD-1, LS513, HCT15, SW620, LoVo and T84 (all from American Type Culture Collection, Manassas, VA) (Table 1). The cell lines were maintained in the recommended growth media supplemented with 10% fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (GIBCO). For harvesting conditioned media, CRC lines cells were grown approximately to 70% confluence in serum free media. The conditioned media were collected after 24 h of incubation, centrifuged and kept frozen.
Table 1.
Colorectal cancer cell lines origin
| Cell line | Type/origin |
| SW620 | Colon adenocarcinoma. Derived from: metastasis to lymph node |
| T84 | Colon carcinoma. Derived from metastasis to lung |
| LoVo | Derived from metastatic site: left supraclavicular region |
| SW480 | Colon adenocarcinoma |
| WiDr | Colon adenocarcinoma |
| RKO | Colon carcinoma |
| HT29 | Colon adenocarcinoma |
| HCT15 | Colon adenocarcinoma |
| HCT116 | Colon carcinoma |
| SW1116 | Colon adenocarcinoma |
| SW1417 | Colon adenocarcinoma |
| LS174T | Colon adenocarcinoma |
| LS513 | Colon carcinoma |
| Caco2 | Colon adenocarcinoma |
| DLD-1 | Colon adenocarcinoma |
| LS411N | Colon adenocarcinoma |
| Colo320 | Colon adenocarcinoma |
VEGF and sVEGFR1 protein detection by quantitative immunoassay
VEGF-A in supernatant was determined using the Human VEGF Quantikine® EIA kit (R and D Systems) and soluble vascular endothelial growth factor receptor (sVEGFR)-1 was quantified by EIA (Human sVEGF R1/Flt-1 Quantikine®, R and D Systems), according to the manufacturer’s instructions. We normalized VEGF and sVEGFR-1 protein levels per number of cells. Results are the average of replicates.
Total VEGF and isoforms mRNA determination by quantitative reverse-transcription polymerase chain reaction
Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA). Single strand DNA was synthesized from 1 μg total RNA using the cDNA Archive kit (Applied Biosystems). Quantitative reverse-transcription polymerase chain reaction (Q-RT-PCR) for total VEGF was performed using primers and probes purchased from Applied Biosystems (Hs00900054_m1). RNA18s (Hs99999901_s1) was used as an endogenous control and data obtained was represented as 2-ΔCt.
VEGF isoforms were determined by Q-RT-PCR using primers designed specifically for VEGF121, VEGF165, and VEGF189, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control (Table 2). The relative quantification of samples was performed using a standard curve by dilution of a specific plasmid for each isoform (ranging from 10 pg to 1 fg). Human VEGF cDNA for each isoform and GAPDH were cloned from total RNA isolated from lung cancer resection as follows. PCR products were run through a 1% agar gel and bands of the size expected for VEGF121, VEGF165 and VEGF189 were isolated and purified. Each VEGF isoform was cloned into the pCRII vector (Invitrogen) and sequenced (ABI PRISM Big Dye Terminator Cycle Sequencing reaction kit; ABI Protocol, Gene Amp 9600, Applied Biosystems) to verify its identity.
Table 2.
Primer and probe sequences for vascular endothelial growth factor isoforms quantitative reverse-transcription polymerase chain reaction
| Sense primer | Antisense primer | Taqman probe | Amplicon size (bp) | |
| VEGF end-point and cloning | ACTGCCATCCAATCGAGACC | GATGGCTTGAAGATGTACTCGATCT | ||
| GAPDH end-point and cloning | TGGTATCGTGGAAGGACTCATGAC | ATGCCAGTGAGCTTCCCGTTCAGC | 189 | |
| VEGF121 mRNA | CAAGGCCAGCACATAGGAGA | CTCGGCTTGTCACATTTTTC | CTTCCTACAGCACAACAAATGTGAATGCAGA | 101 |
| VEGF165 mRNA | TGTGAATGCAGACCAAAGAAAGA | TGCTTTCTCCGCTCTGAGC | AGAGCAAGACAAGAAAATCCCTGTGGGC | 74 |
| VEGF189 mRNA | CGCAAGAAATCCCGGTATAAGT | TGCTTTCTCCGCTCTGAGC | AGGCCCACAGGGAACGCTCCAG | 65 |
| GAPDH | TGGTATCGTGGAAGGACTCATGAC | ATGCCAGTGAGCTTCCCGTTCAGC | CCCAGAGACTGTGGATGGCCCC | 189 |
VEGF: Vascular endothelial growth factor; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Time course hypoxia-normoxia
The cell lines were maintained in the recommended growth media supplemented with 10% fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (GIBCO). After washing with phosphate buffered saline (PBS), serum-free medium was added and the cells exposed to normoxic or hypoxic conditions for 6 h, 12 h, 24 h, 36 h, 48 h and 72 h. Hypoxic conditions were achieved by culturing cells in a modulator incubation chamber (Sanyo MCO-18 M) gassed with 1% O2, 50 mL/L CO2, and 94% N2. VEGF protein secretion was measured in the supernatant by enzyme immune-assay (EIA) and VEGF mRNA levels by Q-RT-PCR. Cell proliferation was evaluated by the Trypan Blue exclusion method.
VEGFR-2 expression in colorectal cancer cell lines by flow cytometry
The expression of VEGFR-2 (KDR) in CRC cell lines was determined by flow cytometry (FacScan, Becton-Dickinson). After trypsinization, cells were incubated in medium for 12 h on a rocker platform to enable regeneration of the receptors. Cells were Fc-blocked by treatment with 100 μL of AB human serum for 15 min at room temperature prior to staining with 10 μL of PE-conjugated anti-VEGFR-2 antibody (Becton Dickinson Biosystems) for 30 min at 4 °C. Following the incubation, unbounded anti-VEGFR-2 antibody was removed by washing the cells twice in 4 mL PBS buffer. The human umbilical vein endothelial cells (HUVEC) cell line was used as a positive control.
Secreted angiogenic profile by cytokine antibody-array
The secretion of angiogenic factors by CRC cell lines was evaluated in duplicate using a protein array method (RayBio® Human Angiogenesis Antibody Array, RayBiotech C Series 1000, RayBiotech, Inc Norgross, GA). This assay is capable of simultaneously detecting 44 different angiogenic factors (spotted in sub-arrays I and II) with high specificity. The sensitivity of the antibodies present in the arrays ranged from 1-2000 pg/mL. Conditioned media was obtained after the incubation of 2 × 105 cells in serum-free medium for 20 h at 37 °C and 5% CO2. Each array was incubated with 1.2 mL of medium at 4 °C overnight, and bound antigens were detected according to the manufacturer’s instructions. To determine the relative concentrations of angiogenic factors in the media, the densities of individual spots were measured using Imagene 4.1 software (Biodiscovery Inc., Marina Del Rey, United States) for image capture and analysis.
Statistical analysis
Statistical analysis was carried out with SPSS 13.0 software (SPSS Inc.). Associations between VEGF expression and VEGF isoforms pattern were determined with the Spearman correlation. Differences between groups were determined by the Mann-Whitney U test. The level of two-tailed statistical significance was 0.05.
CRC cell line angiogenesis cytokine antibody-arrays raw data were normalized to the global median [BRB Array Tools 3.6.0 (NCI)] of signals detected as per manufacturer’s instructions. GENESIS software (Institute for biomedical engineering, Graz University of Technology, Graz, Austria) was used for the analyses of clustering of samples and genes and K-means and hierarchical unsupervised clustering analyses were performed to determine cytokine profiles.
RESULTS
Distinct angiogenesis-related expression pattern in primary and metastatic colorectal cancer cell lines
To identify the angiogenesis-related “secretome” of CRC cell lines in normoxia, we analyzed 44 angiogenesis-related cytokines and growth factors by an antibody-array in primary (Caco2, SW1417, DLD1, HT29 and SW480) and metastatic (SW620 and T84) CRC cell lines. K-means analysis classified CRC cell line angiogenesis-related secreted factors according to their level of secretion (Figure 1A). Cluster I showed a homogeneous high expression of the pro-angiogenic IL-8, MMP-1, MCP-1, growth related oncogene (GRO)-α, regulated upon activation, normal T-cell expressed, and secreted protein (RANTES), urokinase-type plasminogen activator-receptor (uPAR) and VEGF; and the anti-angiogenic tissue inhibitor of metalloproteinases tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 (Figure 1A, cluster I). Cluster II integrated angiogenic factors not secreted by CRC cell lines in normoxia, including VEGF family proteins placental growth factor (PlGF) and sVEGFR-2 and inflammatory cytokines with pro-angiogenic properties granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), IFN-γ, tumor necrosis factor-α (TNF-α) (Figure 1A, cluster II). Primary tumor- and metastasis-derived CRC cell lines were characterized by a distinct angiogenesis-related molecular pattern in normoxia (Figure 1A, cluster III and IV). Figure 1B shows the unsupervised hierarchical clustering of the antibody-array proteins significantly differing in expression according to their cellular origin. One-way ANOVA (P < 0.05) grouped primary and metastatic cell lines according to their differential molecular expression pattern. Metastasis-derived cell lines were characterized by higher expression of Ang-2, MCP-3, MCP-4, MMP-1 and the chemokines I-TAC, I-309, IL-2 and IL-1α (P < 0.05), and a trend was found for MMP-9, as compared to primary tumor cell lines (Figure 1B). On the other hand, CRC cell lines isolated from a primary tumor site were clustered together according to the higher expression of IFN-γ, IGF-1, IL-6, leptin, EGF, PlGF, thrombopoietin, TGF-β1 and VEGF-D (P < 0.05), as compared with the metastatic ones (Figure 1B). Interestingly, VEGF-A (VEGF) was not found among the proteins differentially expressed according to the cellular source of isolation. Figure 1C illustrates processed antibody-arrays and the images captured of Caco2 (primary CRC cell line) and T84 (metastatic CRC cell line).
Figure 1.
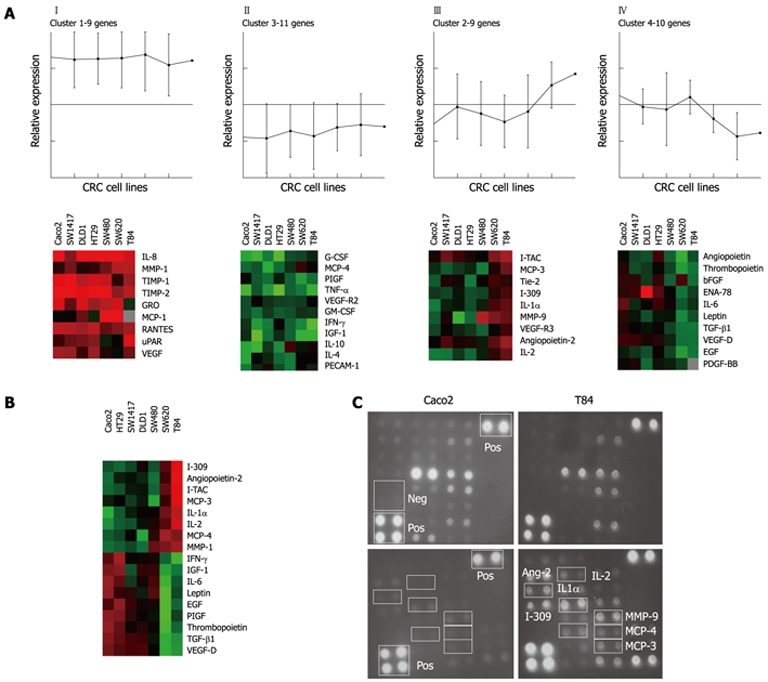
Angiogenesis-related factors expression profile in colorectal cancer cell lines as determined by cytokine antibody-array. A: K-means (n = 4) clustering grouped the angiogenesis-related proteins according to level of expression; B: Unsupervised-hierarchical clustering of the factors with a significantly different expression in primary and metastatic colorectal cancer (CRC) cell lines; C: Images of subarrays I and II of the primary Caco2 and the metastatic T84 CRC cell lines after detection and processing. IL: Interleukin; MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of metalloproteinases; GRO: Growth related oncogene; MCP: Macrophage chemoattractant proteins; RANTES: Regulated upon activation normally T-expressed and secreted; uPAR: Urokinase-type plasminogen activator-receptor; G-CSF: Granulocyte colony-stimulating factor; PIGF: Phosphatidylinositol glycan, class F; TNF-α: Tumor necrosis factor-α; GM-CSF: Granulocyte macrophage colony-stimulating factor; IFN-γ: Interferon γ; IGF: Insulin-like growth factor; PECAM: Platelet-endothelial cell adhesion molecule; I-TAC: Inducible T cell α chemoattractant protein; ENA: Epithelial neutrophil activating protein; EGF: Epidermal growth factor; PDGF-BB: Platelet-derived growth factor, β polypeptide; TGF-β1: Transforming growth factor β1; Neg: Negative control; Pos: Positive control.
VEGF expression in primary and metastatic colorectal cancer cell lines
The antibody array data showed no significant changes in VEGF secretion between primary and metastasis-derived CRC cell lines (Figure 1B). To validate the antibody array results, we analyzed VEGF levels by EIA. The results were confirmed by a statistically significant positive correlation between VEGF protein as determined by the antibody-array and by EIA (r Spearman = 0.7, P < 0.05) (Figure 2A).
Figure 2.
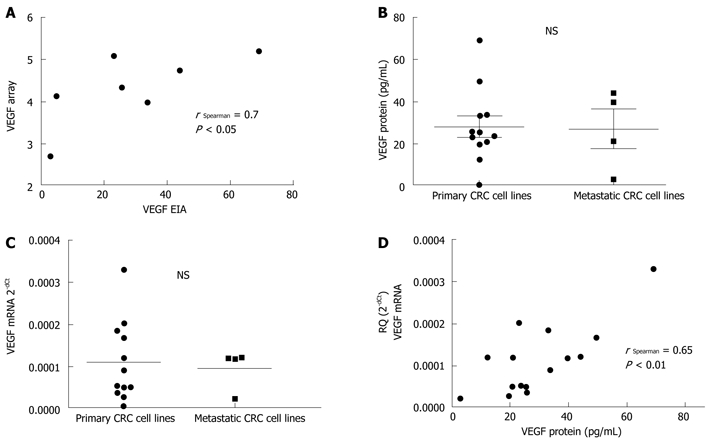
Vascular endothelial growth factor expression in colorectal cancer cell lines. A: A statistically significant positive correlation is found between vascular endothelial growth factor (VEGF) protein as determined by antibody-array and by enzyme immunoassay (EIA), validating the array method; B and C: Colorectal cancer (CRC) cell lines exhibit variability in VEGF protein (B) and mRNA (C) expression according to their primary or metastatic origin (not statistically significant); D: A statistically significant positive correlation is found between VEGF protein by EIA and VEGF mRNA, suggesting the major role of transcriptional mechanisms in the regulation of VEGF expression. NS: Not significant.
In a second step, VEGF secretion by EIA and VEGF mRNA expression was analyzed in a larger panel of 16 CRC cell lines. As shown in Figure 2B and C, we did not detect any significant difference in VEGF expression according to the primary or metastatic CRC cell lines (mean of 28.9 pg/mL and 22.7 pg/mL VEGF protein; 0.011 and 0.009 (relative quantification) VEGF mRNA, respectively). Further, a strong correlation (r = 0.65, P < 0.01) was detected between VEGF protein (by EIA) and VEGF mRNA expression (Figure 2D) in CRC cell lines, indicative of the major role of transcriptional mechanisms in the regulation of VEGF expression[23]. A similar correlation was observed in hypoxia between VEGF protein (by EIA) and VEGF mRNA expression (Figure 3A). Severe hypoxia induced different levels of VEGF expression up-regulation depending on the CRC cellular origin. Surprisingly, the fold change normoxia-hypoxia in VEGF expression of metastatic CRC cell lines was ≤ 1.5 in the majority of time points tested, as compared with the > 1.5-4.0 fold change in primary cell lines for both protein and mRNA VEGF (Figure 3A).
Figure 3.
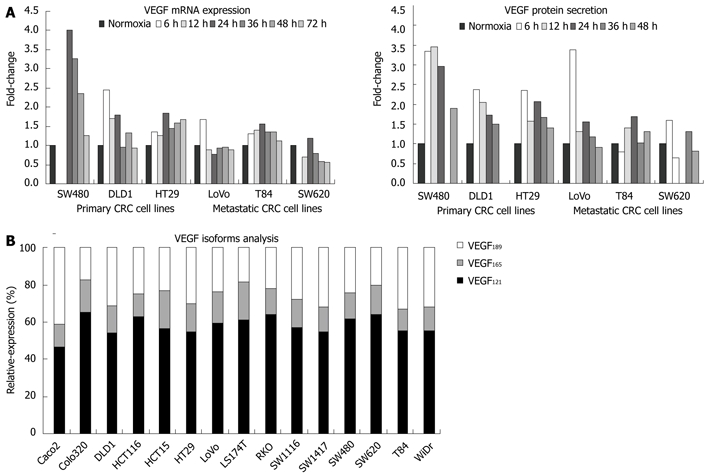
Vascular endothelial growth factor expression regulation. A: Modulation of vascular endothelial growth factor (VEGF) expression (mRNA and protein) in response to severe hypoxia in primary and metastatic colorectal cancer (CRC) cell lines; B: Expression of VEGF isoforms 121, 189 and 165 by CRC cells in normoxia.
VEGF isoforms have differential angiogenic and tumorigenic activities and their expression pattern may also define the CRC cell angiogenic capacity[24]. Primary and metastatic CRC cell lines had a similar expression pattern of the three major isoforms VEGF121, VEGF169 and VEGF185, despite variability in VEGF expression (Figure 3B), implying a similar mechanism of regulation. VEGF121 was the predominant isoform expressed by CRC cell lines (58.23% ± 5.05% of total VEGF mRNA), as compared toVEGF165 and VEGF189 (15.13% ± 2.71% and 26.6% ± 6.5% of VEGF transcripts, respectively). In line with a previous study on tumor tissue[25], the expression of the three isoforms was significantly associated with total VEGF protein; r = 0.55, P < 0.05 for VEGF121 and furthermore, VEGF165 and VEGF189 showed higher correlation (r = 0.67 and r = 0.69, P < 0.01, respectively) (Table 3).
Table 3.
Association between vascular endothelial growth factor mRNA isoforms and vascular endothelial growth factor protein secretion
| VEGF protein | VEGF121 mRNA | VEGF165 mRNA | |
| VEGF121 mRNA | r = 0.55 | ||
| P = 0.034 | |||
| VEGF165 mRNA | r = 0.67 | r = 0.93 | |
| P = 0.007 | P = 0.000 | ||
| VEGF189 mRNA | r = 0.69 | r = 0.95 | r = 0.92 |
| P = 0.005 | P = 0.000 | P = 0.000 |
VEGF: Vascular endothelial growth factor.
VEGFR expression in colorectal cancer cell lines
While the role of the VEGF/VEGFR pathway in endothelial cells is well characterized, its functionality and expression by tumor cells is still controversial[13]. Soluble VEGFR-1 was quantified in CRC cell line supernatants at a lower range than VEGF (mean 8.3 and 27.8 pg/mL respectively) and no differences were found according to the cellular origin (7.57 ± 2.12 and 10.67 ± 3.1, in primary and metastatic CRC cell lines, respectively) (Figure 4A). In agreement with other studies[26], a trend was observed for an inverse correlation between sVEGFR-1 and VEGF expression (data not shown), indicative of the angiogenesis inhibiting role of sVEGFR-1[13].
Figure 4.

Vascular endothelial growth factor receptors expression in colorectal cancer cell lines. A: Soluble vascular endothelial growth factor receptor (sVEGFR)-1 expression measured by EIA is not significantly different between primary and metastatic colorectal cancer (CRC) cell lines; B: Flow cytometry of the surface expression of vascular endothelial growth factor receptor (VEGFR)-2 in human umbilical vein endothelial cells (HUVEC) and the primary CRC cell lines HCT116, Caco2 and RKO under normoxic conditions reveals a general lack of VEGFR-2 expression on the surface of CRC cells as compared to HUVEC. NS: Not significant.
In our CRC cell lines panel, the antibody array data showed a lack of expression of sVEGFR-2 (Figure 1A). Given the hypothesis that earlier tumor stages are more dependent on the VEGF/VEGFR signaling pathway[15], we analyzed surface VEGFR-2 expression in CRC cells of primary origin. Flow cytometry revealed a general lack of surface VEGFR-2 expression in CRC cells of medium to high VEGF expression, as compared to HUVEC cell line (Figure 4B). These findings add to the stock of controversial results to date[27,28].
DISCUSSION
Identifying the proteins responsible for the different behavior of more advanced CRC tumors seems warranted in order to more effectively use current treatment options. Furthermore, there is a need to characterize definite biomarkers of CRC metastasis to serve as prognostic indicators and novel interventional targets. As derived from our findings in vitro, the tumor microenvironment of CRC metastases would be different to that of primary tumors, because of the effect of the CRC cells secreted factors. Metastatic CRC cell lines are characterized by a greater expression of cytokines majorly involved in metastasis, migration and invasion, while being proven pro-angiogenic effectors. MMP-1 plays an important role in CRC tumor invasion and metastasis[29] and MMP-9 has proved to be of prognostic value in stage II colon cancer patients, where tumors with higher protein expression had a higher recurrence rate[30]. The monocyte attractant chemokine I-309 has been shown to stimulate chemotaxis and invasion of endothelial cells and the roles of IL-1α in colon cancer angiogenesis and of IL-2 in inflammation and apoptosis, seem also consistent with the metastatic phenotype[18,31,32].
Hypoxia is widely recognized as the major transcription effector for VEGF expression[9]. However, the greater (two-fold increase) induction of VEGF expression in hypoxia observed in primary CRC as compared to metastatic cell lines is an interesting finding which agrees with recent hypotheses. Tolerance to hypoxia is frequently acquired by tumor cells progressing towards more advanced phenotypes[15]. Our finding suggests the metastatic CRC molecular phenotype provides some intrinsic resistance to the hypoxic induction of VEGF expression. Some authors have shown that hypoxia would select more malignant metastatic cells, less sensitive to anti-angiogenic treatment[33], to yield poorer patients outcomes[34,35]. The community still agrees that angiogenesis is a hallmark of cancer in metastatic stages[36]. However, given the broad angiogenic network in the tumor microenvironment, research should move in the direction of investigating the mechanisms by which metastatic tumors depend on VEGF, since they seem to be different to those exploited by primary tumors[15]. Furthermore, with the objective of individualized care in mCRC, the distinct metastatic “secretome” proteins emerge as alternative targets to consider in the management of advanced disease.
Further to the VEGF expression profile, the pattern of VEGF isoforms represents the next step to identifying intrinsic differences to guide treatment choice. However, the similar expression of VEGF isoforms across cell lines does not offer clarification. Further to this finding, it would be of interest to explore how VEGF transcription factors modulate the ratio of VEGF isoforms as disease progresses, given the changes on VEGF dependence. Interestingly, a novel class of VEGF isoforms, VEGFxxxb, generated through alternative splicing of exon 8, has been recently described[37]. Studies suggest anti-angiogenic or weak angiogenic properties for these isoforms[38,39]. Not exempt from controversy, this discovery will help in further defining the role of VEGF/VEGFR signaling in CRC, yet still the testing techniques need refinement in specificity between the two classes.
Emerging data suggest VEGF to be a growth factor also for tumor cells and VEGF/VEGFR signaling to regulate their expression. However, this hypothesis remains unproven until consolidated results on VEGF receptor expression on tumor cells become available[12,28]. Extensive work has been done on the activity of VEGF/VEGFR-1 signaling in CRC cells showing that it mediates cell motility and invasiveness but not cell proliferation[13]. While this would involve VEGF/VEGFR-1 in CRC progression and metastatic processes, sVEGFR-1 secretion was not found of significant relevance in metastasis-derived CRC cells. In contrast, not so much is known about the activity of VEGF/VEGFR-2 in cancer cells. Reports suggest an involvement in the sensitivity of CRC cells to inhibition of VEGF-related survival pathways[40]. However, controversial results on the VEGFR-2 expression on tumor cells to date[27,28], to which our results add, do not help to resolve this question. Definite confirmation of the expression and functionality of this pathway is necessary in order to shed more light on the mechanism of action of anti-VEGF therapies[40].
Consistent with the key role of VEGF in the “angiogenic switch” and the hypoxia-resistance mechanisms in metastatic stages, CRC cell dependence on VEGF in more advanced settings seems attenuated in favor of other cytokines in the progression of metastasis. Further investigation of these findings and testing the significance of the distinct “secretome” of CRC metastases at the clinic side seems warranted given the implications for patient outcomes.
ACKNOWLEDGMENTS
We thank Dr. Paul Miller for English editorial work.
COMMENTS
Background
Identifying the proteins responsible for the different behavior of more advanced colorectal cancer is necessary in order to more effectively use current treatment options. The progressive growth of colon cancer depends on the blood vessels (angiogenesis) network within the tumor. Therapies targeting angiogenesis have emerged in the field; however, variances in the magnitude benefit lead to great amount of research to explain inter-individual differences. It is thought that different proteins or biomarkers in the tumor microenvironment are responsible for these facts.
Research frontiers
The lack in understanding of biomarkers of colorectal cancer metastasis led the authors to set up this work. Using a novel cytokine antibody array technique, this work identifies the differences in angiogenesis-related protein expression of colorectal cancer cell lines of primary and metastatic origin. This is the first step prior to translation into a clinic setting, where these differences are to be corroborated in patients with colorectal cancer.
Innovations and breakthroughs
The distinct profile of metastatic cell lines comprises eight proteins with different cellular properties, including favoring the growth of those tumor blood vessels. Interestingly, the classical angiogenesis marker vascular endothelial growth factor is not in such a profile, indicating that tumors in more advanced phases tend to rely on different mechanisms for their growth.
Applications
The findings of this work show that a number of markers might be of value when determining the course of disease in colorectal cancer. Furthermore, these proteins arise as novel intervention targets in the metastatic colorectal cancer setting.
Peer review
The researchers intent was to investigate the angiogenesis-related protein expression profile characterizing metastatic colorectal cancer with the aim of identifying prognostic markers. The subject of biomarkers of colorectal cancer (CRC) metastasis is not well understood up to this time. Because of that, efforts of authors to characterize the protein factors behind the angiogenic potential of CRC cell lines of metastatic origin is of great importance. This work is a next step forward to identify the proteins responsible for the different behavior of metastatic colorectal cancers and for developing new treatment options.
Footnotes
Supported by A grant of “Department of Health, Government of Navarra, Spain (23/2009)”
Peer reviewers: Marek Bebenek, MD, PhD, Department of Surgical Oncology, Regional Comprehensive Cancer Center, pl. Hirszfelda 12, 53-413 Wroclaw, Poland; Lucia Ricci Vitiani, Dr., Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, Viale Regina Elena 299, Rome 00161, Italy
S- Editor Lv S L- Editor O’Neill M E- Editor Zhang DN
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Emmanouilides C, Pegram M, Robinson R, Hecht R, Kabbinavar F, Isacoff W. Anti-VEGF antibody bevacizumab (Avastin) with 5FU/LV as third line treatment for colorectal cancer. Tech Coloproctol. 2004;8 Suppl 1:s50–s52. doi: 10.1007/s10151-004-0110-4. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69 Suppl 3:11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 10.Akiri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein O, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998;17:227–236. doi: 10.1038/sj.onc.1202019. [DOI] [PubMed] [Google Scholar]
- 11.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Slavin RE, Gallagher JA, Zhu G, Biehl TR, Swanstrom LL, Hansen PD. Expression of vascular endothelial growth factor and receptor flk-1 in colon cancer liver metastases. J Hepatobiliary Pancreat Surg. 2004;11:164–170. doi: 10.1007/s00534-003-0883-2. [DOI] [PubMed] [Google Scholar]
- 13.Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, Somcio RJ, Wu Y, Hooper A, Hicklin DJ, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 14.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 15.Brahimi-Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med ( Berl) 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 16.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JE, Jung SA, Kim SE, Joo YH, Shim KN, Kim TH, Yoo K, Moon IH. [Expression of MMP-2, HIF-1alpha and VEGF in colon adenoma and colon cancer] Korean J Gastroenterol. 2007;50:9–18. [PubMed] [Google Scholar]
- 18.Matsuo Y, Sawai H, Ma J, Xu D, Ochi N, Yasuda A, Takahashi H, Funahashi H, Takeyama H. IL-1alpha secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1alpha release and tumor cells’ potential for liver metastasis. J Surg Oncol. 2009;99:361–367. doi: 10.1002/jso.21245. [DOI] [PubMed] [Google Scholar]
- 19.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 20.Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48:1131–1137. doi: 10.2337/diabetes.48.5.1131. [DOI] [PubMed] [Google Scholar]
- 21.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, Batchelor TT, Sorensen AG. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loureiro RM, D’Amore PA. Transcriptional regulation of vascular endothelial growth factor in cancer. Cytokine Growth Factor Rev. 2005;16:77–89. doi: 10.1016/j.cytogfr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, Harris AL, Ziche M, Bicknell R. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br J Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cressey R, Wattananupong O, Lertprasertsuke N, Vinitketkumnuen U. Alteration of protein expression pattern of vascular endothelial growth factor (VEGF) from soluble to cell-associated isoform during tumourigenesis. BMC Cancer. 2005;5:128. doi: 10.1186/1471-2407-5-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 27.Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, Sproat G, Swann R, Gray N, Ryan A, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010;16:3548–3561. doi: 10.1158/1078-0432.CCR-09-2797. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Seo JH, Lee YJ, Yoon JH, Choi CW, Kim BS, Shin SW, Kim YH, Kim JS. Autocrine vascular endothelial growth factor/vascular endothelial growth factor receptor-2 growth pathway represents a cyclooxygenase-2-independent target for the cyclooxygenase-2 inhibitor NS-398 in colon cancer cells. Oncology. 2005;68:204–211. doi: 10.1159/000086775. [DOI] [PubMed] [Google Scholar]
- 29.Bendardaf R, Buhmeida A, Ristamäki R, Syrjänen K, Pyrhönen S. MMP-1 (collagenase-1) expression in primary colorectal cancer and its metastases. Scand J Gastroenterol. 2007;42:1473–1478. doi: 10.1080/00365520701485449. [DOI] [PubMed] [Google Scholar]
- 30.Buhmeida A, Bendardaf R, Hilska M, Collan Y, Laato M, Syrjänen S, Syrjänen K, Pyrhönen S. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in stage II colorectal carcinoma. J Gastrointest Cancer. 2009;40:91–97. doi: 10.1007/s12029-009-9091-x. [DOI] [PubMed] [Google Scholar]
- 31.Bernardini G, Spinetti G, Ribatti D, Camarda G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood. 2000;96:4039–4045. [PubMed] [Google Scholar]
- 32.Bae J, Park D, Lee YS, Jeoung D. Interleukin-2 promotes angiogenesis by activation of Akt and increase of ROS. J Microbiol Biotechnol. 2008;18:377–382. [PubMed] [Google Scholar]
- 33.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 34.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 35.Lièvre A, Samalin E, Mitry E, Assenat E, Boyer-Gestin C, Lepère C, Bachet JB, Portales F, Vaillant JN, Ychou M, et al. Bevacizumab plus FOLFIRI or FOLFOX in chemotherapy-refractory patients with metastatic colorectal cancer: a retrospective study. BMC Cancer. 2009;9:347. doi: 10.1186/1471-2407-9-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verheul HM, Hammers H, van Erp K, Wei Y, Sanni T, Salumbides B, Qian DZ, Yancopoulos GD, Pili R. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 37.Ladomery MR, Harper SJ, Bates DO. Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett. 2007;249:133–142. doi: 10.1016/j.canlet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Catena R, Larzabal L, Larrayoz M, Molina E, Hermida J, Agorreta J, Montes R, Pio R, Montuenga LM, Calvo A. VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Mol Cancer. 2010;9:320. doi: 10.1186/1476-4598-9-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 40.Calvani M, Trisciuoglio D, Bergamaschi C, Shoemaker RH, Melillo G. Differential involvement of vascular endothelial growth factor in the survival of hypoxic colon cancer cells. Cancer Res. 2008;68:285–291. doi: 10.1158/0008-5472.CAN-07-5564. [DOI] [PubMed] [Google Scholar]


