Abstract
AIM: To investigate prognostic factors of survival following curative, non-palliative surgical removal of lung metastases secondary to colorectal cancer (CRC).
METHODS: Between 1999 and 2009, a radical metastasectomy with curative intent was performed on lung metastases in 21 patients with CRC (15 male and 6 female; mean age: 57.4 ± 11.8 years; age range: 29-74 years) who had already undergone primary tumour resection.
RESULTS: The mean number of lung metastases ranged from one to five. The mean overall survival was 71 ± 35 mo (median: 25 mo). After adjusting for potential confounders, multivariable Cox regression analyses predicted only the number of lung metastases (1 vs ≥ 2; hazard ratio: 7.60, 95% confidence interval: 1.18-17.2, P = 0.03) as an independent predictor of poor survival following lung resection for metastatic CRC.
CONCLUSION: Resection of lung metastases is a safe and effective treatment in selected CRC patients with single lung metastases.
Keywords: Lung metastases, Colorectal cancer, Metastasectomy, Prognostic factors, Survival
INTRODUCTION
The lung is one of the most frequently affected metastatic sites in patients with colorectal cancer (CRC)[1]. Indeed, lung metastases may be detected sequentially or simultaneously in approximately 10% of patients with this malignancy[2,3]. Since Blalock first described pulmonary resection for metastases of colorectal carcinoma[4], several studies have demonstrated the efficacy of lung metastasectomy in CRC patients[5-14]. As the safety of the operation has improved over time, more patients may be able to undergo this surgery. In a recent single-center retrospective study, Maeda et al[15] reported an analysis of patients with pulmonary metastases from colorectal carcinoma who underwent surgical resection. The overall 5-year survival rate was 74%. Importantly, the number of pulmonary metastases and prethoracotomy carcinoembryonic antigen (CEA) levels were significant independent predictors of survival after the first pulmonary metastasectomy[15]. Earlier studies reported wide ranges of survival percentages from 27% to 40.5% and tried to identify independent factors associated with clinical outcomes[5-14]. This knowledge is clinically helpful for defining a subset of patients who are most likely to benefit from surgical resection.
Here, we report our experience in lung metastasectomy in patients with primary CRC who were referred to a Turkish tertiary hospital during the past 10 years. A number of prognostic factors were analyzed to identify their impact on survival.
MATERIALS AND METHODS
This study was designed as a single-center retrospective investigation at the Department of Oncology, Uludag University Medical School, Bursa, Turkey. Retrospective analysis of the patient data was approved by the local ethics committee.
Patients
Between 1999 and 2009, a radical metastasectomy with curative purposes was performed in 21 patients with CRC (15 males and 6 females). These patients had already undergone primary tumor resection. The pulmonary lesions were also evaluated with conventional chest computed tomography. In addition, all patients underwent bronchoscopy, pulmonary function tests, and endoscopic evaluations before the operation. Indications for pulmonary resection of metastatic CRC were as follows: (1) completely resectable lung lesions diagnosed by preoperative imaging; (2) ability of the patient to tolerate the required surgical procedure and to carry out a normal life with the remaining respiratory function; and (3) surgically controllable extrapulmonary disease, including the primary lesion. All thoracotomy specimens were processed according to standard procedures and histologically confirmed to contain cancer consistent with CRC origin. All resected lung metastasis specimens had pathological tumor-free margins.
Clinical data and follow-up
The medical charts of all patients were reviewed and examined for sex, age, tumor differentiation, history of previous liver metastasis, prethoracotomy CEA levels, disease-free interval between the colorectal resection and the first pulmonary resection, number of metastases, location of metastases, type of resection, history of hilar or mediastinal node resection, size of lung metastases, and administration of postoperative chemotherapy after pulmonary resection. Clinical data and follow-up information were obtained from the medical records and were further complemented using telephone contacts with patients, family members, and physicians.
Statistical analysis
The primary endpoint was the time from lung resection to the time of death. Univariate and multivariable Cox proportional hazards models were used to identify predictors of survival. The appropriateness of the proportional hazards assumption was verified using graphical methods and tested as described by Grambsch and Therneau[16]. The assumption of linearity for the Cox models was examined by visual inspection, and no violation was found. All tests were two-sided, and P < 0.05 was considered statistically significant. All calculations were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, United States).
RESULTS
Characteristics of CRC patients
The mean age was 57.4 ± 11.8 years, with a range of 29 years to 74 years. All patients had adenocarcinoma. Regarding localization at the time of primary tumor diagnosis, 11 of 21 patients had rectal cancer, and the other 10 had colon cancer. Tumor differentiation grades were as follows: G1, one patient; G2, 18 patients; and G3, two patients. Seven patients had a history of previous liver metastases. There were no operative or hospital deaths. The median preoperative CEA level was 3.6 ng/mL (range: 1.2-77.0 ng/mL).
Characteristics of pulmonary metastatic lesions
The mean disease-free interval (interval between initial treatment and onset of pulmonary metastasis) was 28 ± 21 mo (range: 3-75 mo). A solitary metastatic lesion was found in 11 patients. Multiple metastases were found in the other 10 patients, that is, two metastatic lesions in five patients, three metastatic lesions in one patient, four metastatic lesions in two patients, and five metastatic lesions in two patients. In patients with a solitary metastasis, left lung metastasis was found in four patients, and right lung metastasis in seven. In patients with multiple metastases, left lung metastases were found in two patients, right lung metastases were found in five, and metastases were found in both lungs in three patients. Wedge resection was performed in eight patients (five with a solitary lesion, one with two lesions, one with four lesions, and one with five lesions); segmentectomy in eight patients (three with a solitary lesion, two with two lesions, one with three lesions, one with four lesions, and one with five lesions); lobectomy in four patients (two with a solitary lesion and two with two lesions); and pneumectomy in one patient with a solitary lesion. Four patients underwent either hilar or mediastinal lymph node dissection. The pulmonary metastatic tumor size was obtained for all patients and ranged from 5 mm to 41 mm (mean size: 18 ± 10 mm). Nineteen patients received postoperative chemotherapy after pulmonary resection.
Survival data
Survival was calculated from the time of lung resection for metastasis, and the primary end point was death. The mean overall survival was 71 ± 35 mo (median: 25 mo), ranging from 21 mo to 139 mo. The actuarial overall survival following lung metastasectomy for CRC was 71.4% at 3 years and 47.6% at 5 years.
Prognostic factors
Several prognostic factors were analyzed to identify their impact on survival. The factors that were associated with poor survival outcome following univariate analysis were the number of lung metastases (P = 0.01), history of previous liver metastasis (P = 0.04), and preoperative CEA levels (P = 0.04) (Table 1). No other factors had an influence on survival (Table 1). After adjusting for potential confounders, multivariable Cox regression analyses showed that only the number of lung metastases (1 vs ≥ 2; hazard ratio: 7.60, 95% confidence interval: 1.18-17.2, P = 0.03) was an independent predictor of poor survival following lung resection for CRC (Figure 1).
Table 1.
Univariate analysis of prognostic factors
| Risk factors | P value1 |
| Sex (male vs female) | 0.52 |
| Age (yr) | 0.39 |
| Tumor differentiation (G1 and 2 vs G3) | 0.71 |
| History of previous liver metastasis (yes vs no) | 0.04 |
| Preoperative CEA levels (below vs above median) | 0.04 |
| Disease-free interval (mo) | 0.42 |
| Number of metastases (1 vs ≥ 2) | 0.01 |
| Location of metastasis (left vs others) | 0.82 |
| Type of resection (wedge vs others) | 0.74 |
| Hilar or mediastinal node resection (yes vs no) | 0.51 |
| Lung metastatic tumour size (mm) | 0.43 |
| Postoperative chemotherapy (yes vs no) | 0.89 |
Calculated with univariate Cox regression. CEA: Carcinoembryonic antigen.
Figure 1.
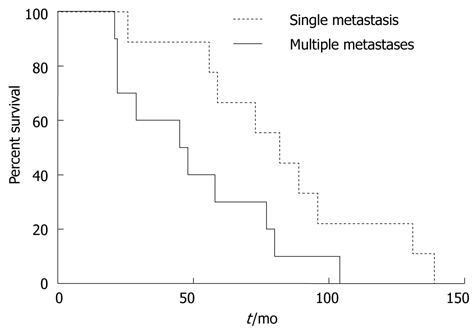
Kaplan-Meier plot for patients with single and multiple lung metastases from colorectal cancer.
DISCUSSION
The results from this study indicate that the presence of a single pulmonary metastasis is the main indication for a metastasectomy in Turkish patients with CRC. The lung is a key site of metastases from CRC, and several previous studies have attempted to identify significant prognostic factors for pulmonary metastasectomy. Previously identified prognostic factors include the number of pulmonary metastases, hilar and/or mediastinal lymph node metastasis, prethoracotomy CEA levels, time of appearance of metastasis, liver metastasis before thoracotomy, mode of operation, and location of pulmonary metastases[5-14]. In our study, there were no prognostic factors except for the number of pulmonary metastases. The overall 5-year survival rate in our series was largely in agreement with previous reports[5-14], and we suggest that surgery should remain the mainstay for treating patients with single pulmonary metastases from CRC. In agreement with our current findings, Pfannschmidt et al[9] have reported solitary metastases as a significant prognostic factor; the post-resection outcome of patients with two or more lesions was significantly worse than that of patients with a single metastasis. Prethoracotomy CEA levels may serve as a biochemical marker for post-resection outcome[14]. CEA participates in intracellular recognition and metastasis by functioning as an attachment factor[14]. CEA levels may therefore reflect the highly malignant nature of cancer cells that undergo systemic dissemination. In our current study, an abnormal CEA level was identified as a prognostic factor in univariate but not multivariate analysis. The observation that CEA levels did not serve as a significant independent prognostic factor suggests that concentrations of this molecule simply reflect the number of lung metastases observed in our patients. A history of previous liver metastases had prognostic significance in univariate but not multivariate analysis. Our results are in agreement with numerous previous reports[17,18]. In this regard, it has been suggested that selected patients with simultaneous presentation with technically resectable liver and lung metastases may benefit from an aggressive surgical approach[19]. We cannot, however, exclude the possibility that these favorable results may be found only in selected patients.
The limitations of this study are the small patient population and the retrospective nature of the study, although the data were collected prospectively. The other drawback is that the patients included in the study may be a selected group and may not represent the general population of patients with lung metastases from CRC. These limitations notwithstanding, our results suggest a favorable outcome in Turkish CRC patients with solitary lung metastases.
In summary, the results from this study indicate that lung resection for metastatic CRC appears to be valuable in selected patients showing isolated lesions, with acceptable long-term survival. This study demonstrated that the presence of multiple lung metastases is associated with poor survival. Surgery should not be considered as the first modality of treatment for patients with multiple lung metastases, because it is associated with a relatively poor outcome. The presence of previous liver metastases should not be considered a contraindication for lung metastasectomy. Large multicenter studies are, however, required to confirm these results.
COMMENTS
Background
The lung is one of the most frequently affected metastatic sites in patients with colorectal cancer (CRC). Several studies have demonstrated the efficacy of lung metastasectomy in CRC patients.
Research frontiers
Earlier studies have reported outcomes that varied widely and have tried to identify independent factors associated with clinical outcomes. This knowledge is clinically helpful for defining the subset of patients who are most likely to benefit from surgical resection.
Innovations and breakthroughs
Here, we report our experience concerning lung metastasectomy in patients with primary CRC referred during the past 10 years. A number of prognostic factors were analyzed to identify their impact on survival.
Applications
The results from this study indicate that lung resection for metastatic CRC appears to be valuable in selected patients with single lesions, providing acceptable long-term survival. The presence of multiple lung metastases is associated with poor survival.
Peer review
The paper is a nice addition to a growing body of literature illustrating the potential benefits of lung metastasectomy for CRC patients. This was a small series of 21 patients, yet multivariate analysis correctly identified the number of lung metastases (1 vs ≥ 2) as a significant predictor of survival. The message is therefore clear, and the small number of patients is not a problem. The paper is concise and well written, the results are clearly presented, and the survival data (71% and 47% at 3 years and 5 years, respectively) are in accordance with previously published series. These results also suggest that we deal with a selected population of CRC patients (mostly young individuals with resectable metastatic disease), but this is not a surprise.
Footnotes
Peer reviewers: Pascal Gervaz, PD, Department of Surgery, University Hospital Geneva, 4, Rue Gabrielle Perret Gentile, Geneva 1211, Switzerland; Jean S Wang, MD, PhD, Assistant Professor of Medicine, Division of Gastroenterology, Washington University School of Medicine, Saint Louis, Missouri, Campus Box 8124, 660 South Euclid Ave, Saint Louis, MO 63110-1093, United States
S- Editor Lv S L- Editor Kerr C E- Editor Zhang DN
References
- 1.August DA, Ottow RT, Sugerbaker PH. Clinical prospective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3:303–324. doi: 10.1007/BF00051457. [DOI] [PubMed] [Google Scholar]
- 2.McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg. 1992;127:1403–1406. doi: 10.1001/archsurg.1992.01420120037006. [DOI] [PubMed] [Google Scholar]
- 3.Goya T, Miyazawa N, Kondo H, Tsuchiya R, Naruke T, Suemasu K. Surgical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer. 1989;64:1418–1421. doi: 10.1002/1097-0142(19891001)64:7<1418::aid-cncr2820640709>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Blalock A. Recent advances in surgery. N Engl J Med. 1994;231:261–267. [Google Scholar]
- 5.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Präuer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066–1071. doi: 10.1002/bjs.4602. [DOI] [PubMed] [Google Scholar]
- 6.Yedibela S, Klein P, Feuchter K, Hoffmann M, Meyer T, Papadopoulos T, Göhl J, Hohenberger W. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol. 2006;13:1538–1544. doi: 10.1245/s10434-006-9100-2. [DOI] [PubMed] [Google Scholar]
- 7.Iizasa T, Suzuki M, Yoshida S, Motohashi S, Yasufuku K, Iyoda A, Shibuya K, Hiroshima K, Nakatani Y, Fujisawa T. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg. 2006;82:254–260. doi: 10.1016/j.athoracsur.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Pfannschmidt J, Muley T, Hoffmann H, Dienemann H. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg. 2003;126:732–739. doi: 10.1016/s0022-5223(03)00587-7. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Omiya H, Kohno K, Kobayashi T, Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H, Nakade M. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg. 2002;124:1007–1013. doi: 10.1067/mtc.2002.125165. [DOI] [PubMed] [Google Scholar]
- 11.Rena O, Casadio C, Viano F, Cristofori R, Ruffini E, Filosso PL, Maggi G. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg. 2002;21:906–912. doi: 10.1016/s1010-7940(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 12.Zink S, Kayser G, Gabius HJ, Kayser K. Survival, disease-free interval, and associated tumor features in patients with colonyrectal carcinomas and their resected intra-pulmonary metastases. Eur J Cardiothorac Surg. 2001;19:908–913. doi: 10.1016/s1010-7940(01)00724-2. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto T, Tsubota N, Iwanaga K, Yuki T, Matsuoka H, Yoshimura M. Pulmonary resection for metastases from colorectal cancer. Chest. 2001;119:1069–1072. doi: 10.1378/chest.119.4.1069. [DOI] [PubMed] [Google Scholar]
- 14.Koga R, Yamamoto J, Saiura A, Yamaguchi T, Hata E, Sakamoto M. Surgical resection of pulmonary metastases from colorectal cancer: Four favourable prognostic factors. Jpn J Clin Oncol. 2006;36:643–648. doi: 10.1093/jjco/hyl076. [DOI] [PubMed] [Google Scholar]
- 15.Maeda R, Isowa N, Onuma H, Miura H, Harada T, Touge H, Tokuyasu H, Kawasaki Y. Pulmonary resection for metastases from colorectal carcinoma. Interact Cardiovasc Thorac Surg. 2009;9:640–644. doi: 10.1510/icvts.2009.202598. [DOI] [PubMed] [Google Scholar]
- 16.Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, Geroldi D. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J. 2006;27:802–807. doi: 10.1093/eurheartj/ehi730. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Kawamura M, Ishihara T. Surgical treatment for both pulmonary and hepatic metastases from colorectal cancer. J Thorac Cardiovasc Surg. 1999;118:1090–1096. doi: 10.1016/S0022-5223(99)70106-6. [DOI] [PubMed] [Google Scholar]
- 18.Lehnert T, Knaebel HP, Dück M, Bülzebruck H, Herfarth C. Sequential hepatic and pulmonary resections for metastatic colorectal cancer. Br J Surg. 1999;86:241–243. doi: 10.1046/j.1365-2168.1999.01010.x. [DOI] [PubMed] [Google Scholar]
- 19.Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, Taylor BR, Langer B, Gallinger S, Wei AC. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–475. doi: 10.1016/j.jamcollsurg.2005.11.008. [DOI] [PubMed] [Google Scholar]


