Abstract
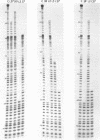
Nucleotide residues in E. coli tRNA(Phe) interacting directly with proteins in pre- and posttranslocated ribosomal complexes have been identified by UV-induced cross-linking. In the tRNA(Phe) molecule located in the Ab-site (pretranslocated complex) residues A9, G18, A26 and U59 are cross-linked with proteins S10, L27, S7 and L2, respectively. In tRNA(Phe) located in the Pt-site (posttranslocated complex) residues C17, G44, C56 and U60 are cross-linked with proteins L2, L5, L27 and S9, respectively. The same cross-links (except for G44-L5) have been found for tRNA in the Pb-site of the pretranslocated ribosomal complex. None of the tRNA(Phe) residues cross-linked with proteins in the complexes examined by us are involved in the stabilization of the secondary structure, but residues A9, G18, A26, G44 and C56 participate in stabilization of tRNA tertiary structure. Since translocation of tRNA(Phe) from Ab- to P-site is accompanied by changes of tRNA contacts with proteins L2 and L27, we postulate that this translocation is coupled with tRNA turn around the axis joining the anticodon loop with the CCA-end of the molecule. This is in agreement with the idea about the presence of a kink in mRNA between codons located in the ribosomal A- and P-sites. In all E. coli tRNAs with known primary structure positions 18 and 56, interacting with L27 protein, when tRNA is located either in A- or P-site, are invariant, whereas positions 17 and 60, interacting with proteins only when tRNA is in the P-site, are strongly conserved. In positions 9, 26 and 59 purines are the preferred residues. In most E. coli tRNAs deviations from the consensus in these three positions is strongly correlated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdurashidova G. G., Baskayeva I. O., Chernyi A. A., Kaminir L. B., Budowsky E. I. Structural characteristics and classification of some tRNA-binding sites of elongating Escherichia coli ribosome. Eur J Biochem. 1986 Aug 15;159(1):103–109. doi: 10.1111/j.1432-1033.1986.tb09838.x. [DOI] [PubMed] [Google Scholar]
- Abdurashidova G. G., Nargisyan M. G., Budowsky E. I. Preparation and properties of monomercurated tRNA. Eur J Biochem. 1983 Oct 17;136(1):147–150. doi: 10.1111/j.1432-1033.1983.tb07718.x. [DOI] [PubMed] [Google Scholar]
- Abdurashidova G. G., Nargizian M. G., Rudenko N. V., Turchinskii M. F., Budovskii E. I. Kontakty ribosomnykh belkov s tRNKPhe i 16S RNK v analogakh 30S initsiatornogo kompleksa. Mol Biol (Mosk) 1985 Mar-Apr;19(2):553–557. [PubMed] [Google Scholar]
- Abdurashidova G. G., Tsvetkova E. A., Budovskii E. I. Izmenenie konformatsii subchastits i ikh vzaimnogo raspolozheniia pri perekhode ot pretranslotsirovannogo sostoianiia k posttranslotsirovannomu. Bioorg Khim. 1985 Mar;11(3):417–419. [PubMed] [Google Scholar]
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Nucleotide residues of tRNA, directly interacting with proteins within the complex of the 30 S subunit of E. coli ribosome with poly(U) and NAcPhe-tRNA(Phe). FEBS Lett. 1989 Jan 30;243(2):299–302. doi: 10.1016/0014-5793(89)80149-8. [DOI] [PubMed] [Google Scholar]
- Abdurashidova G. G., Tsvetkova E. A., Chernyi A. A., Kaminir L. B., Budowsky E. I. Intersubunit RNA-protein contacts in pre- and post-translocated E. coli ribosome. FEBS Lett. 1985 Jun 17;185(2):291–294. doi: 10.1016/0014-5793(85)80925-x. [DOI] [PubMed] [Google Scholar]
- Boni I. V., Isaeva D. M., Budovskii E. I. Ribosomnyi belok S1 v sostave kompleksa 30S ribosomnoi subchastitsy E. coli s RNK faga MS2 vzaimodeistvuet s vnutrennim raionom gena replikazy. Bioorg Khim. 1986 Feb;12(2):293–296. [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Broude N. E., Kussova K. S., Medvedeva N. I., Budowsky E. I. Proteins of the 30-S subunit of Escherichia coli ribosomes which interact directly with natural mRNA. Eur J Biochem. 1983 Apr 15;132(1):139–145. doi: 10.1111/j.1432-1033.1983.tb07338.x. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E. The functional role of ribosomal RNA in protein synthesis. Cell. 1989 May 19;57(4):525–529. doi: 10.1016/0092-8674(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillov S. V., Makhno V. I., Semenkov Y. P. The mechanism of codon-anticodon interaction in ribosomes. Quantitative study of codon-dependent binding of tRNA to the 30-S ribosomal subunits of Escherichia coli. Eur J Biochem. 1978 Aug 15;89(1):297–304. doi: 10.1111/j.1432-1033.1978.tb20927.x. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Voynow P., Hardy S. J., Randall L., Lutter L. Physical and functional heterogeneity of E. coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:17–24. doi: 10.1101/sqb.1969.034.01.006. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989 Nov 9;342(6246):142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Semenkov YuP, Makarov E. M., Makhno V. I., Kirillov S. V. Kinetic aspects of tetracycline action on the acceptor (A) site of Escherichia coli ribosomes. FEBS Lett. 1982 Jul 19;144(1):125–129. doi: 10.1016/0014-5793(82)80584-x. [DOI] [PubMed] [Google Scholar]
- WATSON J. D. THE SYNTHESIS OF PROTEINS UPON RIBOSOMES. Bull Soc Chim Biol (Paris) 1964;46:1399–1425. [PubMed] [Google Scholar]
- Walleczek J., Schüler D., Stöffler-Meilicke M., Brimacombe R., Stöffler G. A model for the spatial arrangement of the proteins in the large subunit of the Escherichia coli ribosome. EMBO J. 1988 Nov;7(11):3571–3576. doi: 10.1002/j.1460-2075.1988.tb03234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]