Abstract
Introduction:
Information on chemical composition of the new oral “spitless” smokeless tobacco products is scarce, and it is not clear whether there is some variability as a function of purchase place or time due to either unintended or intended manufacturing variations or other conditions.
Methods:
We analyzed tobacco-specific N-nitrosamines (TSNA) and nicotine in Marlboro Snus, Camel Snus, and dissolvable Camel products Orbs, Sticks, and Strips that were purchased in various regions of the country during the summer of 2010.
Results:
A total of 117 samples were received from different states representing six regions of the country. Levels of unprotonated nicotine in Marlboro Snus and Camel Snus varied significantly by regions, with the differences between the highest and the lowest average regional levels being relatively small in Marlboro Snus (∼1.3-fold) and large in Camel Snus (∼3-fold). Some regional variations in TSNA levels were also observed. Overall, Camel Snus had significantly higher TSNA levels than Marlboro Snus, and Camel Strips had the lowest TSNA levels among all novel products analyzed here. The amount of unprotonated nicotine in the dissolvable Camel products was comparable to the levels found in Marlboro Snus.
Conclusions:
Our study demonstrates some regional variations in the levels of nicotine and TSNA in Marlboro and Camel novel smokeless tobacco products. Continued monitoring of this category of products is needed as the existing products are being test marketed and modified, and new products are being introduced. This information is particularly important given its relevance to Food and Drug Administration regulation of tobacco products.
Introduction
In recent years, a new generation of oral smokeless tobacco products has been marketed in the United States (Rogers, Biener, & Clark, 2010). In 2006, Philip Morris USA introduced for test-marketing Taboka—small pouches of flavored pasteurized tobacco to be placed between the cheek and gum. Taboka was eventually discontinued, but its successor, Marlboro Snus, has been on the market since 2007. Also, in 2006, Reynolds American launched a similar product—Camel Snus. Currently, both Marlboro Snus and Camel Snus are being marketed nationally. One of the recent developments in this area is a line of products called Camel Dissolvables. This line includes Camel Orbs, Camel Strips, and Camel Sticks—products made from finely ground flavored tobacco that dissolve in the mouth. Overall, this new generation of oral smokeless tobacco products is marketed to smokers as a substitute when smoking is not possible due to bans or as an alternative to smoking. There is evidence of substantial initial interest in the snus products among male smokers (Biener & Bogen, 2009; Biener, McCausland, Curry, & Cullen, 2011).
The potential public health impact of these new smokeless products is unknown. Chronic use of traditional smokeless tobacco, although less harmful than cigarette smoking, can result in nicotine addiction (Hatsukami, Lemmonds, & Tomar, 2004; Hatsukami & Severson, 1999) and cause precancerous oral lesions, oral and pancreatic cancer, and cardiovascular diseases (Advisory Committee to the Surgeon General, 1986; International Agency for Research on Cancer, 2007; Hecht et al., 1986). A number of toxicants and carcinogens present in smokeless tobacco are believed to be responsible for these negative health effects (Hoffmann & Djordjevic, 1997; National Cancer Institute, 1992). Tobacco-specific N-nitrosamines (TSNA) are widely considered to be among the most important carcinogens in smokeless tobacco products (Bartsch & Spiegelhalder, 1996; Hecht, 1998; Hecht & Hoffmann, 1988; Magee, 1996; Preston-Martin & Correa, 1989). Two of these compounds, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N ’-nitrosonornicotine (NNN), are believed to play an important role in development of cancers of the lung, pancreas, oral cavity, and esophagus in users of tobacco products and are classified by the International Agency for Research on Cancer as carcinogenic to humans (Group 1; International Agency for Research on Cancer, 2007). Existing epidemiologic studies indicate that the use of Swedish snus—a smokeless tobacco product low in TSNA—even though associated with an increased risk of pancreatic cancer when compared with never-users of any tobacco is not related to lung cancer and that the risk of oral cancer, if it exists, is very limited (Greer, 2011; Luo et al., 2007). Because of their potential for reducing exposure to TSNA and other carcinogens that are present in cigarette smoke, the use of low-TSNA smokeless products is seen by some as a potential harm-reduction strategy (Bates et al., 2003; Levy et al., 2004). Another critical chemical in smokeless tobacco is nicotine, the main known addictive constituent. Both total nicotine and unprotonated nicotine content—the biologically available form of nicotine that depends on the pH of the product—are critical in consumers’ acceptance of a smokeless tobacco product and potential addiction to it (Fant, Henningfield, Nelson, & Pickworth, 1999; Henningfield, Fant, & Tomar, 1997).
Initial analyses revealed that single pouches of Marlboro Snus, Camel Snus, and similar products contain relatively low levels of TSNA and nicotine as compared with conventional moist snuff, implying lower carcinogenic and addictive potential (Stepanov, Jensen, Hatsukami, & Hecht, 2008). However, information on chemical composition of these products is limited, and the extent of variability of TSNA and nicotine in a particular product because of manufacturing methods, the way the product is stored, or because of the intent of the manufacturer is unknown. This information is essential in view of continuous modifications that this new category of products undergoes as it is being test marketed. Moreover, the potentially important role of TSNA and nicotine levels in labeling and marketing regulations, as well as in the establishment of standards for tobacco products as a part of Food and Drug Administration (FDA) regulation, makes the ongoing evaluation of these toxicants particularly important.
The New Product Watch is a web-based national monitoring network made up mainly of state tobacco program staff and their community partners. It provides tools for monitors to periodically report local observations of new oral tobacco products being sold locally. Results are posted online for use by members around the country. Twice a year, monitors collect product samples for analysis of chemical constituents and product packaging (Rogers, Biener, Nyman, & Crow, 2010).
We present here the results of TSNA and nicotine analyses of Marlboro Snus, Camel Snus, and Camel Dissolvables that were purchased as part of the first phase of the New Product Watch project.
Materials and Methods
Tobacco Samples
Monitors from different states representing six regions of the United States purchased products in retail stores between July and September of 2010. The regions (and the corresponding states) are as follows: Northwest (Alaska), West (Colorado), Midwest (Indiana, Nebraska, Minnesota), Northeast (New Hampshire, New York, Massachusetts), Mid-Atlantic/Appalachian (West Virginia), and South (Arkansas, Georgia). In order to obtain representative averages for constituent levels, we sought to acquire a sample of all flavors of each product from three different locations within each region. The monitors were provided with prepaid envelopes and asked to ship samples to our laboratory the day of purchase via overnight delivery. To provide a reference level of analytes, we also purchased Ariva and Stonewall—lozenges that contain compressed powdered tobacco and have been marketed as an alternative to smokers since 2001. We previously demonstrated that these products contain very low levels of TSNA (Stepanov, Jensen, Hatsukami, & Hecht, 2006). Four flavors of Ariva and two flavors of Stonewall were purchased online. Upon receipt, each sample was labeled with a unique identification number. Detailed information on each sample, including but not limited to the brand, flavor, and date and place of purchase, was recorded. Storage conditions—refrigerated or not upon purchase, between purchase and shipment, and after the sample was received in our laboratory—were also recorded to monitor for potential variations in constituent levels due to different storage conditions. After the information was recorded, the samples were sealed in plastic sleeves and stored at 4 °C until analysis.
Tobacco Analysis
Approach to Sample Analyses
Sample preparation for analysis of TSNA, nicotine, moisture content, and pH started after all the samples were obtained in the laboratory. For the measurement of moisture content and pH, all received samples were treated as one large set, the measurements being performed in a single run. Sample preparation for TSNA and nicotine analysis was carried out in three subsets, and analyses of the prepared samples for each analyte were performed in a single run on the corresponding instrument as described below. During sample preparation, one blank sample (corresponding extraction solvent without addition of tobacco) and one positive control sample (Copenhagen Snuff for which nicotine and TSNA contents were previously established) were included in order to monitor for potential contamination and day-to-day analytical variation.
Tobacco-Specific N-Nitrosamines
Analysis of TSNA in smokeless tobacco was performed essentially as previously described (Stepanov et al., 2006). Briefly, tobacco samples were extracted with citrate–phosphate buffer; the extracts were loaded on ChemElut cartridges (Varian, Harbor City, CA) and extracted with CH2Cl2. This was followed by solid-phase extraction on Sep-Pak Plus silica cartridges (Waters Corp., Milford, MA) and concentration to dryness. The purified samples were redissolved in CH3CN and analyzed by gas chromatography (GC) interfaced with a Thermal Energy Analyzer (Orion Research, Beverly, MA).
Moisture content and pH were measured as previously described (Stepanov et al., 2008). Briefly, moisture content was measured via the difference in weight of a tobacco sample before and after its drying for 3 hr at 99 °C, while pH was measured in aqueous tobacco extracts.
Nicotine and Unprotonated Nicotine
Nicotine was measured as described elsewhere (Stepanov, Hecht, Ramakrishnan, & Gupta, 2005). Tobacco was extracted with MeOH containing KOH, and an aliquot of the extract was mixed with [CD3]nicotine internal standard. The samples were transferred to GC-microinsert vials and analyzed by GC–mass spectrometry–selected ion monitoring using a model 6890 GC equipped with an autosampler and interfaced with a model 5973 mass selective detector (Agilent Technologies, Palo Alto, CA) as previously described (Stepanov et al., 2005). The amount of unprotonated nicotine was calculated using the Henderson–Hasselbalch equation based on the measured nicotine, pH, and pKa of 8.02 (Richter & Spierto, 2003).
Statistical Analysis
The statistical analysis was restricted to products for which three samples were obtained from different vendors in multiple locations around the country. Analysis of variance was conducted to compare the geometric means of the products. When the overall F test was significant, t tests were conducted to make the pairwise comparisons of interest. All p values were adjusted using the method of Bonferroni. The level of significance was set to .05.
Results
A total of 117 samples were received as a part of New Product Watch: 71 samples of Marlboro Snus (Rich, Mild, Spearmint, and Peppermint), 36 samples of Camel Snus (Mellow, Frost, Robust, and Winterchill), 4 samples of Camel Orbs (Mellow and Fresh flavors), 3 samples of Camel Sticks (Mellow flavor), and 3 samples of Camel Strips (Fresh flavor). Marlboro Snus Rich, Mild, Spearmint and Peppermint, as well as Camel Snus Mellow and Frost were obtained from all six regions as planned. Samples from Mid-Atlantic/Appalachia were not included in the statistical analysis because they were procured from the same store. Only one sample of Camel Snus Robust and one sample of Camel Snus Winterchill were obtained from Paris, AR. Camel dissolvables were found only by observers in Indiana. Camel Orbs were purchased in two locations (Spenser and Indianapolis), and Camel Sticks and Strips were purchased in three locations (Angola, Spenser, and Indianapolis). Thus, sample sizes for Camel Snus Robust and Winterchill and for Camel dissolvables were insufficient to make any statistical comparisons.
TSNA and nicotine were not detected in negative control samples. Analysis of NNN and NNK in Copenhagen Snuff produced inter-set relative SDs (RSD) of 6.1% and 5.7%, respectively. The inter-set RSD of the nicotine assay in this positive control was 1.2%. We also determined the RSD for the samples purchased in Mid-Atlantic/Appalachia, where three samples of all flavors of each product were purchased in the same store. The RSD values were 4.4% for total TSNA, 3.9% for nicotine, 0.1% for pH, and 1.8% for moisture.
Tables 1 and 2 summarize constituent levels in all products analyzed here, including those for which regional comparisons were not performed due to inadequate sample size.
Table 1.
Portion Weights, Moisture Content, and Tobacco-Specific N-Nitrosamine Levels in Smokeless Products Analyzed Herea
| μg/g dry weight |
||||||||
| Product | Flavor (n) | Portion weight, g | Moisture, % | NNN | NNK | NAT | NAB | Total TSNA |
| Marlboro Snus | Rich (18) | 0.442 ± 0.02 | 20.7 ± 1.4 | 0.421 ± 0.12 | 0.132 ± 0.03 | 0.400 ± 0.10 | 0.018 ± 0.06 | 0.970 ± 0.26 |
| Mild (18) | 0.396 ± 0.02 | 14.3 ± 0.9 | 0.420 ± 0.08 | 0.160 ± 0.03 | 0.358 ± 0.07 | 0.003 ± 0.01 | 0.941 ± 0.16 | |
| Spearmint (17) | 0.400 ± 0.02 | 15.6 ± 1.1 | 0.431 ± 0.08 | 0.164 ± 0.03 | 0.383 ± 0.07 | 0.004 ± 0.01 | 0.982 ± 0.17 | |
| Peppermint (18) | 0.401 ± 0.02 | 15.6 ± 1.2 | 0.470 ± 0.10 | 0.162 ± 0.02 | 0.389 ± 0.08 | 0.005 ± 0.01 | 1.03 ± 0.18 | |
| Camel Snus | Mellow (17) | 0.516 ± 0.06 | 29.5 ± 2.8 | 0.859 ± 0.22 | 0.404 ± 0.15 | 0.327 ± 0.09 | 0.022 ± 0.03 | 1.61 ± 0.40 |
| Frost (17) | 0.510 ± 0.06 | 29.2 ± 3.4 | 0.896 ± 0.27 | 0.450 ± 0.12 | 0.350 ± 0.08 | 0.026 ± 0.03 | 1.72 ± 0.45 | |
| Robust (1) | 1.04 | 34.5 | 1.28 | 0.595 | 0.482 | 0.027 | 2.39 | |
| Winterchill (1) | 1.10 | 33.4 | 0.909 | 0.609 | 0.343 | 0.021 | 1.93 | |
| Camel Orbs | Mellow (2) | 0.253 ± 0.01 | 13.3 ± 1.7 | 0.245 ± 0.02 | 0.343 ± 0.01 | 0.242 ± 0.07 | 0.009 ± 0.01 | 0.84 ± 0.10 |
| Fresh (2) | 0.248 ± 0.01 | 12.7 ± 1.1 | 0.248 ± 0.05 | 0.300 ± 0.01 | 0.264 ± 0.04 | 0.009 ± 0.01 | 0.82 ± 0.11 | |
| Camel Sticks | Mellow (3) | 0.566 ± 0.01 | 13.1 ± 1.1 | 0.304 ± 0.11 | 0.353 ± 0.07 | 0.300 ± 0.11 | 0.023 ± 0.01 | 0.98 ± 0.30 |
| Camel Strips | Fresh (3) | 0.129 ± 0.01 | 17.7 ± 2.4 | 0.185 ± 0.05 | 0.269 ± 0.01 | 0.192 ± 0.10 | 0.006 ± 0.01 | 0.65 ± 0.15 |
| Ariva | Java (1) | 0.292 | 2.91 | 0.094 | 0.073 | 0.350 | 0.032 | 0.55 |
| Citrus (1) | 0.295 | 3.62 | 0.102 | 0.067 | 0.358 | 0.035 | 0.56 | |
| Cinnamon (1) | 0.290 | 2.11 | 0.096 | 0.070 | 0.311 | 0.040 | 0.52 | |
| Wintergreen (1) | 0.296 | 1.23 | 0.096 | 0.073 | 0.354 | 0.047 | 0.57 | |
| Stonewall | Java (1) | 0.469 | 4.58 | 0.122 | 0.063 | 0.437 | 0.117 | 0.74 |
| Wintergreen (1) | 0.467 | 2.95 | 0.137 | 0.064 | 0.482 | 0.111 | 0.79 | |
Note. aArithmetic means ± SDs are shown for products/flavors for which two or more samples were available. Otherwise, results of single sample analyses are shown.
Table 2.
pH and Nicotine Levels in Smokeless Productsa
| Product | Flavor (n) | pH | Total nicotine, mg/g dry weight | Unprotonated nicotine, % of total | Unprotonated nicotine, mg/g dry weight |
| Marlboro Snus | Rich (18) | 6.72 ± 0.14 | 24.84 ± 2.30 | 4.9 ± 1.6 | 1.23 ± 0.38 |
| Mild (18) | 6.68 ± 0.10 | 18.93 ± 1.89 | 4.5 ± 1.1 | 0.84 ± 0.21 | |
| Spearmint (17) | 6.79 ± 0.07 | 18.82 ± 1.69 | 5.6 ± 1.9 | 1.05 ± 0.16 | |
| Peppermint (18) | 6.81 ± 0.09 | 19.38 ± 2.10 | 5.8 ± 1.2 | 1.13 ± 0.81 | |
| Camel Snus | Mellow (17) | 7.38 ± 0.19 | 16.74 ± 2.56 | 19.6 ± 6.2 | 3.36 ± 1.32 |
| Frost (17) | 7.43 ± 0.23 | 16.46 ± 1.87 | 21.8 ± 7.8 | 3.58 ± 1.32 | |
| Robust (1) | 7.78 | 13.93 | 36.5 | 5.09 | |
| Winterchill (1) | 7.68 | 14.65 | 31.4 | 4.59 | |
| Camel Orbs | Mellow (2) | 8.12 ± 0.06 | 3.83 ± 0.15 | 55.4 ± 3.6 | 2.12 ± 0.06 |
| Fresh (2) | 8.08 ± 0.02 | 3.13 ± 0.09 | 53.6 ± 1.4 | 1.68 ± 0.09 | |
| Camel Sticks | Mellow (3) | 7.76 ± 0.12 | 4.51 ± 1.29 | 35.8 ± 6.6 | 1.67 ± 0.79 |
| Camel Strips | Fresh (3) | 7.88 ± 0.12 | 3.24 ± 0.49 | 41.9 ± 6.5 | 1.35 ± 0.22 |
| Ariva | Java (1) | 6.95 | 6.53 | 7.8 | 0.51 |
| Citrus (1) | 6.97 | 4.56 | 8.2 | 0.37 | |
| Cinnamon (1) | 6.85 | 5.14 | 6.3 | 0.33 | |
| Wintergreen (1) | 6.89 | 4.38 | 6.8 | 0.30 | |
| Stonewall | Java (1) | 7.10 | 7.17 | 10.6 | 0.76 |
| Wintergreen (1) | 7.10 | 7.06 | 10.6 | 0.75 |
Note. aArithmetic means ± SDs are shown for products/flavors for which two or more samples were available. Otherwise, results of single sample analyses are shown.
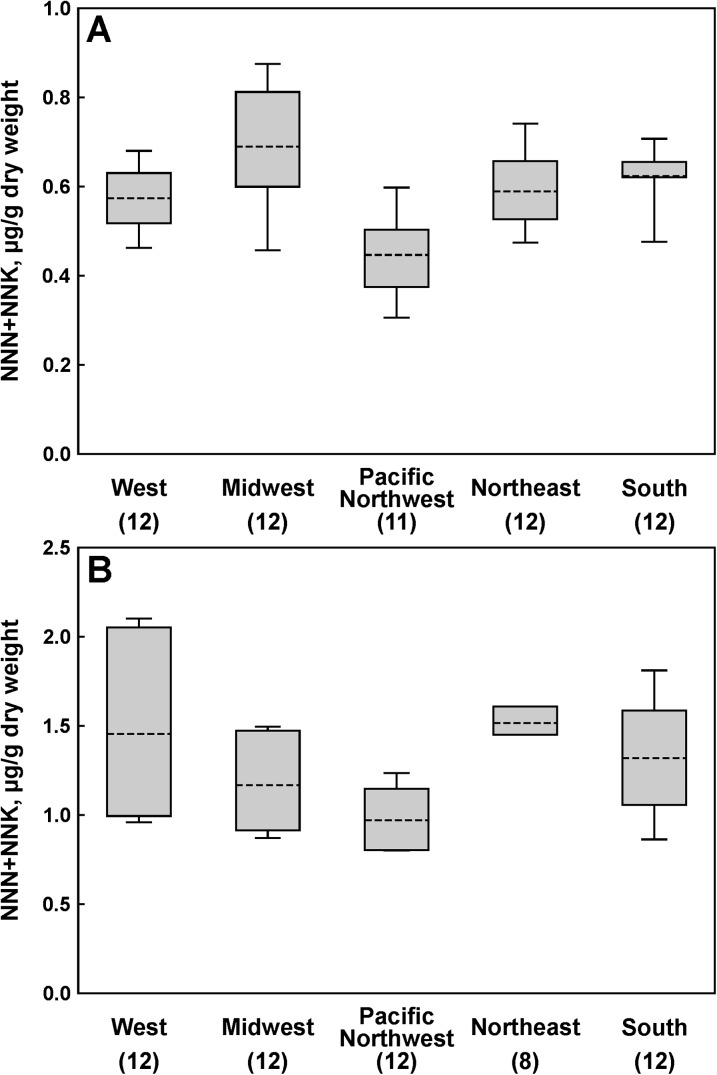
Mean values for single portion weights, moisture content, and TSNA levels per gram dry weight for all products and flavors are provided in Table 1. There were no significant differences in total TSNA levels among various flavors of Marlboro Snus products. When levels of the most carcinogenic TSNA—NNN and NNK—in all flavors of Marlboro Snus combined were compared across all regions (with Mid-Atlantic/Appalachia excluded), some regional variations were observed (Figure 1A). Thus, products purchased in the Midwest had higher NNN + NNK levels than those purchased in the Pacific Northwest (p = .0088) and South (p = .0008). Marlboro Snus purchased in the Pacific Northwest had significantly lower total TSNA levels than that purchased in the Midwest or South (p = .0003 and p = .0001, respectively). There were no detectable differences among individual flavors of Marlboro Snus across the regions. TSNA content was similar for Camel Snus Mellow and Frost, and the slight regional variations of NNN + NNK levels in these two flavors combined were not statistically significant (Figure 1B).
Figure 1.
Regional variations in the sum of NNN and NNK: (A) Marlboro Snus and (B) Camel Snus. Blocks, 95% CI; bars, range; dashed lines, geometric mean; and numbers in parentheses, the number of samples analyzed for a given region.
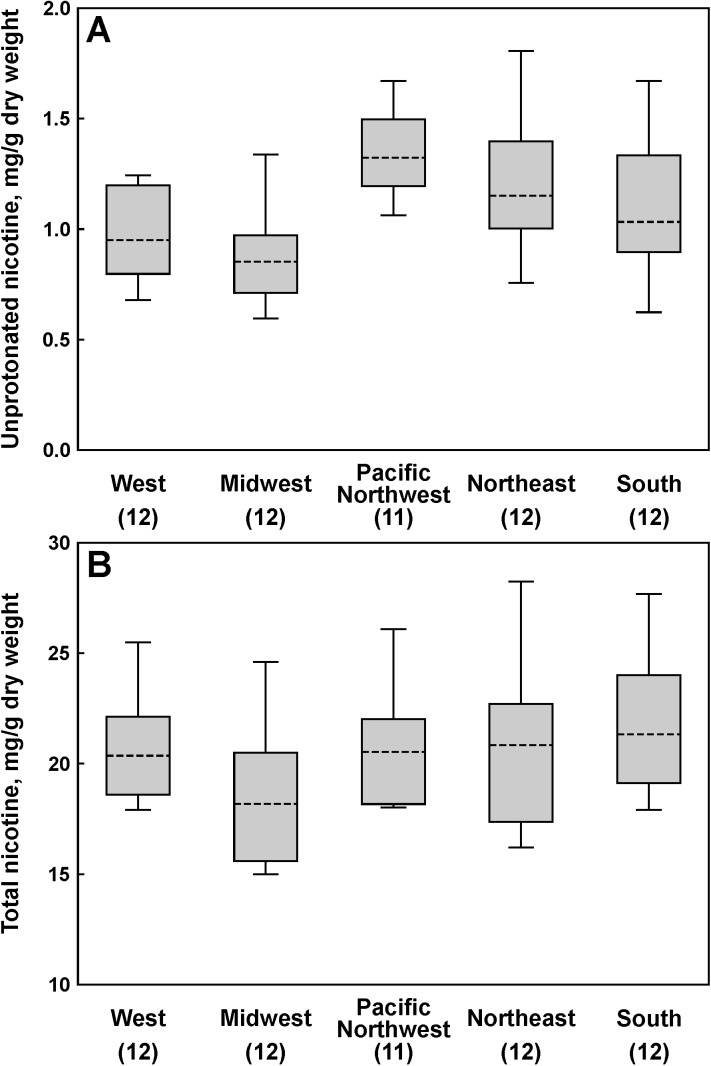
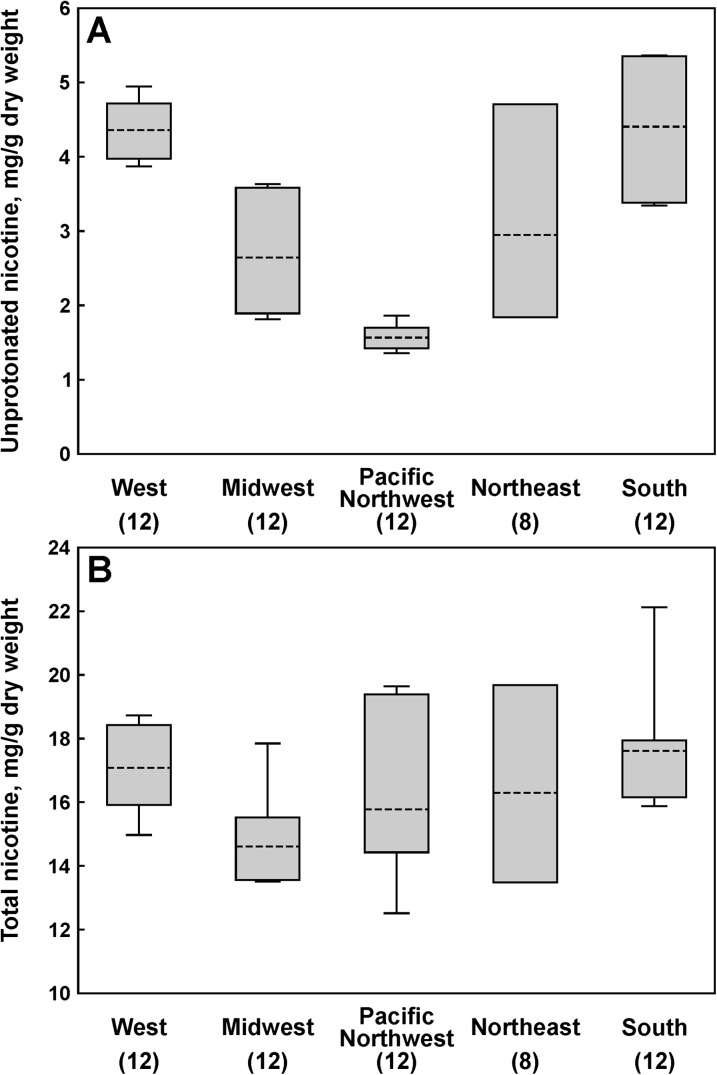
Mean values for pH and the levels of total and unprotonated nicotine per gram dry weight for all products and flavors analyzed here are provided in Table 2. Unprotonated nicotine content in all flavors of Marlboro Snus combined varied by regions (Figure 2A). Thus, products purchased in the Midwest had lower unprotonated nicotine levels than those purchased in the Pacific Northwest (p = .0008) and the Northeast (p = .0435) regions. Products purchased in the West also had lower unprotonated nicotine levels than those purchased in the Pacific Northwest (p = .0231). The remaining means were not detectably different. The levels of total nicotine in Marlboro Snus did not vary significantly by regions (Figure 2B). Analysis of nicotine levels in Camel Snus Mellow and Frost combined showed even larger variation in unprotonated nicotine by regions (Figure 3A), with products purchased in the West and the South having significantly higher unprotonated nicotine levels than in the Midwest (p = .037 and p = .032, respectively) and the Pacific Northwest (p < .0001 for all three comparisons). The products purchased in the Midwest and the Northeast also had significantly higher unprotonated nicotine levels than those acquired in the Pacific Northwest (p = .033 and p = .017, respectively). There was no difference in total or unprotonated nicotine levels between Camel Snus Mild and Frost. No significant regional differences in total nicotine levels in Camel Snus were found (Figure 3B).
Figure 2.
Regional variations in average nicotine levels in Marlboro Snus: (A) unprotonated nicotine and (B) total nicotine. Blocks, 95% CI; bars, range; dashed lines, geometric mean; and numbers in parentheses, the number of samples analyzed for a given region.
Figure 3.
Regional variations in average nicotine levels in Camel Snus: (A) unprotonated nicotine and (B) total nicotine. Blocks, 95% CI; bars, range; dashed lines, geometric mean; and numbers in parentheses, the number of samples analyzed for a given region.
Comparison of analytes across various products showed that Camel Snus products had significantly higher TSNA levels than Marlboro Snus products. Thus, the geometric mean of total TSNA in Camel Snus products was 1.62 μg/g dry weight (95% CI = 1.47, 1.78) and the geometric mean of the sum of carcinogenic NNN and NNK was 1.25 μg/g dry weight (95% CI = 1.13, 1.40); corresponding levels in all flavors of Marlboro Snus were 0.993 ± 0.20 (95% CI = 0.931, 1.06; p < .0001) and 0.601 (95% CI = 0.558, 0.647; p < .0001) μg/g dry weight. TSNA levels in the new flavors of Camel Snus—Robust and Winterchill—were in the range of those measured in the Mellow and Frost flavors (Table 1). Geometric mean of total TSNA levels in Camel Orbs, Strips, and Sticks combined was 0.80 (95% CI = 0.67, 0.95). These values for all flavors of Ariva and Stonewall combined were 0.61 (95% CI = 0.51, 0.74). All flavors of Camel Snus had higher pH (p < .0001), and consequently higher unprotonated nicotine content (p < .0001), than all flavors of Marlboro Snus. Unprotonated nicotine levels in Camel Snus Robust and Winterchill were comparable to those in Camel Snus Mellow and Frost. Camel dissolvables contained higher amounts of unprotonated nicotine than Ariva and Stonewall combined: The geometric mean of unprotonated nicotine in Camel Orbs, Strips, and Sticks combined was 1.60 (95% CI = 1.31, 1.90), and these values for all flavors of Ariva and Stonewall combined were 0.47 (95% CI = 0.30, 0.64; p < .0001).
Discussion
The manufacturing and marketing of recent noncombustible oral “spitless” tobacco products has been and continues to be in a dynamic state since their first introduction by U.S. tobacco manufacturers in 2006. Some modifications, such as changes in marketing and design, can be easily detected. On the other hand, very little is known about potential variations in chemical composition of these products. We report here the results of TSNA and nicotine analyses in Marlboro Snus, Camel Snus, and dissolvable Camel products Orbs, Sticks, and Strips that were purchased in various regions of the country during the summer of 2010.
Regional variation in unprotonated nicotine levels in some products analyzed here is an important finding. Generally, the levels of unprotonated nicotine in Marlboro Snus were relatively low, and the observed variations were not very large—about 1.3-fold difference between the highest and the lowest average regional levels (Figure 2A). In the case of Camel Snus Mellow and Frost, however, this difference was close to threefold, and the levels were ranging from comparable to Marlboro Snus to similar to those observed in some conventional smokeless tobacco products (Figure 3A). Total nicotine levels were similar across the regions for both Marlboro Snus (Figure 2B) and Camel Snus (Figure 3B), and the observed variation in unprotonated nicotine levels was driven mainly by the differences in the pH of products purchased in different regions. Analysis of the data for all Camel Snus samples (including those purchased in the Mid-Atlanic/Appalachia region and Camel Snus Robust and Winterchill) by individual locations, rather than by regions, reveals that unprotonated nicotine levels found in these products sold in 2010 can be roughly divided into three ranges: (a) less than 2 mg/g dry weight, (b) 2–4 mg/g dry weight, and (c) more than 4 mg/g dry weight (Table 3). Most Camel Snus products fall into either the lower (<2 mg/g) or the higher (>4 mg/g) range. Overall, our finding of significant variations in unprotonated nicotine content in Camel Snus samples purchased in different locations may be indicative of an important role of these alterations in the manufacturer’s test-marketing strategy. Having reliable information on regional sales of novel and traditional tobacco products, along with the data on demographics of tobacco users in particular locations, would be a useful adjunct to these data. Regional variations in TSNA levels observed for both Marlboro Snus and Camel Snus, even though statistically significant in some cases, were not extensive: The difference between the highest and the lowest regional average was only 1.5-fold for both products (Figure 1).
Table 3.
Variations in Unprotonated Nicotine Levels in Camel Snus as a Function of Place of Purchase
| Place of purchase (number of samples) | Unprotonated nicotine levelsa, mg/g dry weight |
||
| <2 | 2–4 | >4 | |
| West | |||
| Alamosa, CO (2) | 4.30 ± 0.60 | ||
| Greeley, CO (2) | 4.12 ± 0.21 | ||
| Westcliffe, CO (2) | 4.71 ± 0.33 | ||
| Midwest | |||
| Spenser, IN (2) | 2.79 ± 0.45 | ||
| Indianapolis, IN (2) | 3.61 ± 0.04 | ||
| Lincoln, NE (2) | 1.85 ± 0.06 | ||
| Pacific Northwest | |||
| Anchorage 1, AK (2) | 1.50 ± 0.20 | ||
| Anchorage 2, AK (2) | 1.52 ± 0.13 | ||
| Eagle River, AK (2) | 1.78 ± 0.12 | ||
| Northeast | |||
| Allenstown, NH (2) | 4.65 ± 0.17 | ||
| Nartwick, NY (2) | 1.87 ± 0.09 | ||
| Mid-Atlantic/Appalachia | |||
| Morgantown, WV (6) | 4.28 ± 0.15 | ||
| South | |||
| Suwanee, GA (2) | 5.36 ± 0.01 | ||
| Duluth, GA (2) | 3.36 ± 0.03 | ||
| Paris, AR (4) | 4.79 ± 0.23 | ||
Note. aLevels in all flavors available at a given location were combined to calculate mean and SD.
The results from this study also provided information on the content of TSNA and nicotine across different products. Some findings of this study are consistent with our previously published data on Marlboro Snus and Camel Snus (Stepanov et al., 2008). As in that study, the tobacco of novel smokeless products analyzed here has lower TSNA levels as compared with those found in tobacco of traditional U.S. brands and Swedish Snus. Also, Camel Snus products had larger pouches, higher moisture content, higher pH, and higher unprotonated nicotine content than Marlboro Snus products (Table 2). However, an increase of pouch sizes for all flavors of Marlboro Snus (∼1.8-fold) and for Camel Snus Mellow and Frost (∼1.6-fold) has occurred since our first publication on analysis of these products (Stepanov et al., 2008). Recently introduced flavors Robust and Winterchill contain about 1 g tobacco per pouch (∼3.3-fold increase). Such changes increase consumers’ exposure to various tobacco constituents from single portions of these products. Total TSNA levels in Camel Snus were similar to those measured in products purchased in 2006 (Stepanov et al., 2008). However, total TSNA levels in Marlboro Snus were lower than those previously reported (Stepanov et al., 2008), the difference being driven primarily by the lower NNN and NNK content (Table 1). It is possible that the tobacco blend used for the manufacturing of Marlboro Snus and/or the manufacturing technique itself has changed between 2007 and 2010. TSNA levels in Ariva and Stonewall are somewhat higher than reported previously (Stepanov et al., 2006), which could be also explained by possible changes in tobacco material and/or manufacturing modifications. Nonetheless, similar to previous analysis, TSNA levels in these products are lower than in any other product analyzed here.
This study is the first to report nicotine and TSNA levels in dissolvable Camel products. TSNA analysis of Camel dissolvables showed that Camel Strips had the lowest levels of these carcinogens among all novel products analyzed here; Camel Orbs had the second lowest levels; and Camel Sticks had levels comparable to those in Marlboro Snus (Table 1). Overall, even though these products had very low total nicotine levels as compared with other products analyzed here, the amount of unprotonated nicotine was comparable to the levels found in Marlboro Snus. This is due to the higher pH of Camel dissolvables as compared with all Marlboro Snus products: 7.93 ± 0.17 versus 6.75 ± 0.11, respectively. These results clearly demonstrate how simple it is to adjust unprotonated nicotine levels in a given tobacco product by manipulating its pH. This difference in pH between Camel dissolvables and Marlboro snus drastically affects the portion of total nicotine that is present in unprotonated form in these products: 45.1% ± 9.6% versus 5.2% ± 1.3%, respectively.
The varying levels of unprotonated nicotine across products may have an impact on the acceptability of the product among current or new tobacco users. Smokeless products with higher nicotine content could be more effective in satisfying smokers and completely substituting for cigarettes than those with less nicotine (Hatsukami et al., in press; Kotlyar et al., 2011). This may in part explain the greater popularity of Camel Snus as compared with Marlboro Snus (Rogers et al., 2010). On the other hand, products that are low in unprotonated nicotine could be more easily accepted by young people initiating tobacco use.
In summary, we report here the results of nicotine and TSNA analyses in Marlboro and Camel oral tobacco products that are being marketed to smokers in various regions of the United States and demonstrate some regional variations in the levels of these important tobacco constituents. Furthermore, levels of constituents not only vary across oral tobacco products but also over time within the same brand of product. It is not known whether constituent levels in these products will vary in the future, either by place of purchase or over time. Our findings stress the importance of continued monitoring of this category of products as the existing products are being test marketed and modified, and new products are being introduced. This information is particularly important in view of the U.S. FDA’s regulatory authority over tobacco products. While certain modifications of tobacco products may or may not be subject to FDA restrictions, the disclosure of nicotine, TSNA, and other key constituent levels by the tobacco companies could increase consumer awareness of the variations in the chemical composition of these products and potential health consequences of their use.
Funding
This study was supported by grants R01-CA141631, CA-81301, and R01-CA135884 from the U.S. National Institutes of Health and by National Cancer Institute Contract HHSN261201000544P.
Declaration of Interests
None declared.
Acknowledgments
We would like to thank Bob Carlson for editorial assistance.
References
- Advisory Committee to the Surgeon General. The health consequences of using smokeless tobacco. Bethesda: MD: U.S. Department of Health and Human Sciences; 1986. NIH Publication No. 86-2874. [Google Scholar]
- Bartsch H, Spiegelhalder B. Environmental exposure to N-nitroso compounds (NNOC) and precursors: An overview. European Journal of Cancer Prevention. 1996;5:11–18. doi:10.1097/00008469-199609001-00003. [PubMed] [Google Scholar]
- Bates C, Fagerstrom K, Jarvis MJ, Kunze M, McNeill A, Ramstrom L. European Union policy on smokeless tobacco: A statement in favor of evidence based regulation for public health. Tobacco Control. 2003;12:360–367. doi: 10.1136/tc.12.4.360. doi:10.1136/tc.12.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Bogen K. Receptivity to Taboka and Camel Snus in a U.S. test market. Nicotine & Tobacco Research. 2009;11:1154–1159. doi: 10.1093/ntr/ntp113. doi:10.1093/ntr/ntp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, McCausland K, Curry L, Cullen J. Prevalence of trial of snus products among adult smokers. American Journal of Public Health. 2011 doi: 10.2105/AJPH.2010.200097. E-publication ahead of print (February 17, 2011) Retrieved from http://ajph.aphapublications.org/cgi/doi/10.2105/AJPH.2010.200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tobacco Control. 1999;8:387–392. doi: 10.1136/tc.8.4.387. doi:10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer RO., Jr. Oral manifestations of smokeless tobacco use. Otolaryngologic Clinics of North America. 2011;44:31–56. doi: 10.1016/j.otc.2010.09.002. doi:10.1016/j.otc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Jensen J, Anderson A, Broadbent B, Allen S, Zhang Y, et al. Oral tobacco products: Preference and effects among smokers. Drug and Alcohol Dependence. in press doi: 10.1016/j.drugalcdep.2011.03.026. doi:10.1016/j.drugalcdep.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Lemmonds C, Tomar SL. Smokeless tobacco use: Harm reduction or induction approach? Preventive Medicine. 2004;38:309–317. doi: 10.1016/j.ypmed.2003.10.006. doi:10.1016/j.ypmed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Severson HH. Oral spit tobacco: Addiction, prevention and treatment. Nicotine & Tobacco Research. 1999;1:21–44. doi: 10.1080/14622299050011131. doi:10.1080/14622299050011131. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology. 1998;11:559–603. doi: 10.1021/tx980005y. doi:10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. doi:10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Research. 1986;46:4162–4166. [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Tomar SL. Smokeless tobacco: An addicting drug. Advances in Dental Research. 1997;11:330–335. doi: 10.1177/08959374970110030401. doi:10.1177/08959374970110030401. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Djordjevic MV. Chemical composition and carcinogenicity of smokeless tobacco. Advances in Dental Research. 1997;11:322–329. doi: 10.1177/08959374970110030301. doi:10.1177/08959374970110030301. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 89. Lyon, France: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 548–553. [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Hertsgaard LA, Lindgren BR, Jensen JA, Carmella SG, Stepanov I, et al. Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: A feasibility study. Cancer Epidemiology, Biomarkers & Prevention. 2011;20:91–100. doi: 10.1158/1055-9965.EPI-10-0349. doi:10.1158/1055-9965.EPI-10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Mumford EA, Cummings KM, Gilpin EA, Giovino G, Hyland A, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:2035–2042. [PubMed] [Google Scholar]
- Luo J, Ye W, Zandehdel K, Adami J, Adami HO, Boffetta P, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: A retrospective cohort study. The Lancet. 2007;369:2015–2020. doi: 10.1016/S0140-6736(07)60678-3. doi:10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- Magee PN. Nitrosamines and human cancer: Introduction and overview. European Journal of Cancer Prevention. 1996;5:7–10. doi: 10.1097/00008469-199609001-00002. doi:10.1097/00008469-199609001-00002. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Smoking and tobacco control monographs. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1992. Smokeless tobacco or health, an international perspective; pp. 96–106. [Google Scholar]
- Preston-Martin S, Correa P. Epidemiological evidence for the role of nitroso compounds in human cancer. Cancer Surveys. 1989;8:459–473. [PubMed] [Google Scholar]
- Richter P, Spierto FW. Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine & Tobacco Research. 2003;5:885–889. doi: 10.1080/14622200310001614647. doi:10.1080/14622200310001614647. [DOI] [PubMed] [Google Scholar]
- Rogers JD, Biener L, Clark PI. Test marketing of new smokeless tobacco products in four U.S. cities. Nicotine & Tobacco Research. 2010;12:69–72. doi: 10.1093/ntr/ntp166. doi:10.1093/ntr/ntp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JD, Biener L, Nyman AL, Crow R. Building a national surveillance infrastructure for new smokeless tobacco and nicotine products. February, 2010. Society for Research on Nicotine and Tobacco, Baltimore, MD. [Google Scholar]
- Stepanov I, Hecht SS, Ramakrishnan S, Gupta PC. Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. International Journal of Cancer. 2005;116:16–19. doi: 10.1002/ijc.20966. doi:10.1002/ijc.20966. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8:309–313. doi: 10.1080/14622200500490151. doi:10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research. 2008;10:1773–1782. doi: 10.1080/14622200802443544. doi:10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]