Abstract
Introduction:
Current smoking cessation guidelines recommend setting a quit date prior to starting pharmacotherapy. However, providing flexibility in the date of quitting may be more acceptable to some smokers. The objective of this study was to compare varenicline 1 mg twice daily (b.i.d.) with placebo in subjects using a flexible quit date paradigm after starting medication.
Methods:
In this double-blind, randomized, placebo-controlled international study, smokers of ≥10 cigarettes/day, aged 18–75 years, and who were motivated to quit were randomized (3:1) to receive varenicline 1 mg b.i.d. or placebo for 12 weeks. Subjects were followed up through Week 24. Subjects were instructed to quit between Days 8 and 35 after starting medication. The primary endpoint was carbon monoxide–confirmed continuous abstinence during Weeks 9–12, and a key secondary endpoint was continuous abstinence during Weeks 9–24.
Results:
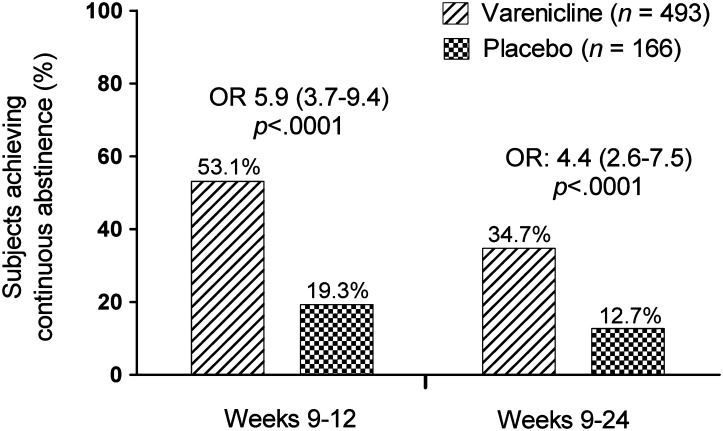
Overall, 493 subjects were randomized to varenicline and 166 to placebo. Continuous abstinence was higher for varenicline than for placebo subjects at the end of treatment (Weeks 9–12: 53.1% vs. 19.3%; odds ratio [OR] 5.9; 95% CI, 3.7–9.4; p < .0001) and through 24 weeks follow-up (Weeks 9–24: 34.7% vs. 12.7%; OR 4.4; 95% CI, 2.6–7.5; p < .0001). Serious adverse events occurred in 1.2% varenicline (none were psychiatric) and 0.6% placebo subjects. Fewer varenicline than placebo subjects reported depression-related adverse events (2.3% vs. 6.7%, respectively).
Conclusions:
Varenicline 1 mg b.i.d. using a flexible quit date paradigm had similar efficacy and safety compared with previous fixed quit date studies.
Introduction
Cigarette smoking is the single most important cause of preventable morbidity and mortality in the developed world (World Health Organization, 2008). The majority of smokers express a desire to quit, but annually less than 5%–6% quit and maintain abstinence after 1 year (Hughes, Keely, & Naud, 2004). Pharmacotherapy can at least double quit rates over counseling alone (Fiore et al., 2008; Molyneux et al., 2003) but remains underutilized (Shiffman, Brockwell, Pillitteri, & Gitchell, 2008). In part, this may be due to medication management guidelines, which recommend pharmacotherapy be initiated in relation to a fixed quit date (Chambers, 2009; Fiore et al., 2008), with the expectation that cessation will occur at treatment initiation or shortly thereafter.
Although setting a quit date is thought to increase the probability of successful quitting (Fiore et al., 2008), we are unaware of empirical tests demonstrating the benefit of this approach. Gradual cessation studies allow smokers to delay setting a quit date until later in the treatment (Hughes, Solomon, Livingston, Callas, & Peters, 2010; Shiffman, Ferguson, & Strahs, 2009), but we are unaware of abrupt cessation studies that have allowed smokers flexible quit dates. The flexibility to quit at some point during a period of several weeks after treatment initiation may make treatment more acceptable for some smokers. The current study was designed to evaluate whether the most recently approved smoking cessation pharmacotherapy—varenicline—could be used successfully with a protocol that allowed smokers to start medication without fixing the quit date as Day 8 of treatment.
In clinical trials, varenicline increases the odds of quitting threefold compared with placebo when used with a fixed quit date (Cahill, Stead, & Lancaster, 2008; Gonzales et al., 2006; Jorenby et al., 2006). As varenicline is a partial agonist active at the α4β2 nicotinic acetylcholine receptor and is believed to block the reward associated with nicotine and reduce nicotine craving (Coe et al., 2005), it may be particularly suitable for a flexible quit date strategy. After starting varenicline treatment while still smoking, smokers find smoking less rewarding (West, Baker, Cappelleri, & Bushmakin, 2008). Experiencing the beneficial effects of varenicline while still smoking should increase the smoker’s confidence that varenicline will be helpful during a quit attempt. Such use also promotes a reduction in cigarettes smoked, increases self-efficacy, and decreases nicotine dependence (Hughes et al., 2010). Similarly, “pretreatment” prior to the quit date increases abstinence with the nicotine patch (Rose, Herskovic, Behm, & Westman, 2009).
Allowing smokers to choose their own quit date within a specified time period provides a means for accommodating smokers who are unable or unwilling to set a fixed quit date, although we are not aware of any empirical evidence that fixed quit dates may be a barrier to treatment uptake. The aim of the current study was to examine whether varenicline would be effective and have the same safety profile when used within a flexible quit date approach to treatment.
Methods
Design Overview
In this randomized, double-blind, placebo-controlled multinational study, smokers interested in quitting were randomized to the standard dose of varenicline or placebo. They were free to choose to quit at any time between Days 8 and 35 (Weeks 2–5) after starting treatment (i.e., following drug titration that took place within the first week). The aim was to assess the efficacy and safety of 12 weeks of varenicline 1 mg twice daily (b.i.d.) versus placebo in aiding cessation within this flexible quit date setting and included a 12-week follow-up period without medication.
Clinic visits occurred weekly until Week 12 then at Weeks 13, 16, 20, and 24. Telephone contact was made at Weeks 14, 18, and 22. At each visit, subjects were queried about their potential quit date, the number of cigarettes smoked per day, and their quit attempts since the last visit. A quit attempt was defined as a self-reported attempt that lasted for at least a few hours with the conviction to quit permanently and included both spontaneous and planned attempts. Cessation was monitored by self-report and was confirmed by ≤10 ppm expired carbon monoxide (CO). At each clinic or telephone contact, subjects received brief (up to 10 min) smoking cessation counseling, consistent with Agency for Healthcare Research and Quality guidelines (Fiore et al., 2008). At baseline, subjects received the Clearing the Air: Quit Smoking Today (National Cancer Institute [U.S.], 2003) self-help booklet. As in previous varenicline studies, subjects were encouraged to continue in the study even if they discontinued medication or failed to stop smoking.
To facilitate cross-study comparisons, the same major outcomes were used as in previous varenicline studies, i.e., continuous abstinence from Weeks 9–12 and Weeks 9–24.
Randomization and Interventions
A predefined, central, computer-generated randomization sequence assigned subjects in a 3:1 ratio to receive either varenicline or placebo (block size: 4, stratified by center). A 3:1 randomization was chosen to promote rapid enrollment because the efficacy of varenicline has been established and it is commercially available. Varenicline subjects were titrated to the full dose during the first week: 0.5 mg once daily for 3 days then 0.5 mg b.i.d. for 4 days. Those randomized to placebo received matched placebo dosing with identical appearance to varenicline.
In order to preserve the blind of the investigative centers, subjects, and sponsor, no unblinded data listings and tables were produced, other than for the Data Monitoring Committee, until data from the non-treatment follow-up period had been entered into a database and cleaned.
Written informed consent was obtained from all subjects. Consent forms and procedures were approved by institutional boards at each site. The study was conducted in compliance with the ethical principles of the Declaration of Helsinki (World Medical Association, 2008) and International Conference on Harmonisation Good Clinical Practice guidelines (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 1996) and applicable local regulatory requirements and laws. An independent Data Monitoring Committee assured the safety of the subjects.
Setting and Subjects
The study was conducted at 33 centers across 14 countries (Argentina, Brazil, Canada, China, Czech Republic, France, Germany, Hungary, Italy, Mexico, Republic of Korea, Taiwan, United Kingdom, and United States), between September 22, 2008 and December 10, 2009. Sites included research centers, private practice offices, and research clinics. Many centers in multiple countries were used in order to increase the external validity of the trial and to facilitate expeditious recruitment. Enrollment per center varied from 9 (1.4%) to 43 (6.5%) subjects with 17 centers enrolling between 16 and 20 subjects.
Inclusion and exclusion criteria were similar to the varenicline registration studies (Gonzales et al., 2006; Jorenby et al., 2006). Male and female smokers were eligible for the study if they were aged between 18 and 75 years, had smoked ≥10 cigarettes/day during the previous year with no longer than 3 months abstinence during that time, and were motivated to stop smoking.
Subjects were excluded from the study if they had used a nicotine replacement product, bupropion, clonidine, or nortriptyline within the past 3 months or had taken varenicline previously. Subjects were also excluded for serious or unstable psychiatric disorders in the past 6 months or on the basis of medical history. This encompassed current depression or depression diagnosed or treated within the past 12 months; any history of suicidal ideation or suicidal behavior in the past 5 years; past history of—or present—psychosis, panic attacks, or anxiety disorders; or bipolar disorder. It also excluded those with a history of drug (except nicotine) or alcohol abuse/dependence within the past 12 months and those with a positive urine drug screen for drugs of abuse/potential abuse not prescribed for the treatment of a medical condition.
Outcomes and Follow-up
The primary efficacy objective was to compare 12 weeks of varenicline 1 mg b.i.d. with placebo for smoking cessation in the setting of subject self-selected quit date. The primary efficacy endpoint was 4-week continuous abstinence for Weeks 9–12. The key secondary efficacy endpoint was continuous abstinence from Weeks 9 to 24. Additional secondary endpoints included the 7-day point prevalence of abstinence at Weeks 12 and 24 and time to first quit attempt.
Adverse events were recorded and subsequently systematized using the Medical Dictionary for Regulatory Activities (MedDRA). Additionally, at each contact, two neuropsychiatric assessments were conducted: interviewer–administration of the Columbia Suicide-Severity Rating Scale (C-SSRS; Posner, Oquendo, Gould, Stanley, & Davies, 2007) to assess suicidal ideation and behavior and self-administration of the Patient Health Questionnaire (PHQ-9; Kroenke, Spitzer, & Williams, 2001) to assess depression/depressed mood.
Statistical Analyses
Logistic regression models were used to assess primary and key secondary endpoints. The model included treatment and center as independent variables. The type 1 family-wise error rate of .05, for the analyses of the primary and key secondary endpoints, was preserved using a step-down procedure. The hierarchy of comparisons was (1) 4-week continuous abstinence for Weeks 9–12 and (2) continuous abstinence for Weeks 9–24. For these endpoints, expanded logistic regression models were used to assess the center by treatment interaction. The primary efficacy analysis population was all subjects randomized to treatment. Subjects who discontinued the study were assumed to be smokers from the point of discontinuation to end of study. In the case of missed visits during the evaluation periods for continuous abstinence (Weeks 9–12 and 9–24), a subject was considered abstinent if they reported they had not smoked or used nicotine products “since the last visit” at the first visit after the missing visit(s). Missing CO measurements did not disqualify the subject for abstinence endpoints. All statistical testing was two sided and used a .05 level of significance (no adjustment for multiplicity was used other than for the primary and key secondary endpoints).
The Kaplan–Meier method was used to analyze the time to first quit attempt, defined as the number of days from the first dose of study medication to the date of the first quit attempt based on subjects who took at least one dose of study medication. Subjects who did not report quit attempts within the quit window, or who had missing data, were classified as censored, and their time to first quit attempt was set to Day 35.
Expanded logistic regression models were used to assess the impact of country and baseline characteristics on the primary and key secondary outcomes. The sample size calculation was based on a two-group continuity-corrected Pearson chi-square two-sided test with a .05 significance level and a 3:1 randomization ratio of varenicline to placebo. Assuming true continuous abstinence rates of 0.24 for Weeks 9–12 and 0.18 for Weeks 9–24 for placebo and continuous abstinence rates of 0.46 for Weeks 9–12 and 0.31 for Weeks 9–24 for varenicline, a sample size of 652 subjects was calculated. This provided at least 90% power to detect a difference between the varenicline and placebo groups for the primary and key secondary endpoints.
Results
Subjects
Of 831 screened subjects, 172 did not fulfill the inclusion criteria, the most frequent reason being a positive urine drug test result (26.7%). In total, 493 subjects were randomized to varenicline and 166 to placebo and were included in the efficacy analysis. Of these, 486 in the varenicline group and 165 in the placebo group received at least one dose of medication and were included in the safety analysis. There were 61 (12.4%) subjects who discontinued treatment in the varenicline arm (24 [4.9%] due to adverse events [AEs]) and 34 (20.5%) in the placebo arm (13 [7.8%] due to AEs). Some of the subjects who discontinued treatment remained in the study. Overall, 425 (86.2%) subjects completed the 24 weeks of the study in the varenicline arm and 141 (84.9%) in placebo. The flow of subjects through the study is shown in full in Supplementary Figure 1.
The two treatment groups were similar with respect to baseline demographics, smoking history, and level of nicotine dependence (Table 1).
Table 1.
Subject Baseline Characteristics
| Varenicline (n = 493) | Placebo (n = 166) | |
| Overall subjects per site, median (range) | 20 (9–43) | |
| Demographic characteristics | ||
| Age, years, mean (SD) | 43.9 (12.5) | 43.2 (12.2) |
| Men, n (%) | 296 (60.0) | 99 (59.6) |
| Race, n (%) | ||
| White | 335 (68.0) | 113 (68.1) |
| Black | 33 (6.7) | 8 (4.8) |
| Asian | 103 (20.9) | 36 (21.7) |
| Other | 22 (4.5) | 9 (5.4) |
| Body mass index, kg/m2, mean (SD) | 26.2 (4.3) | 26.7 (4.7) |
| Smoking history | ||
| Years smoked cigarettes, mean (range) | 26.0 (2–57) | 24.6 (2–59) |
| Cigarettes/day (past month), mean (range) | 21.3 (10–70) | 21.5 (10–65) |
| ≥1 previous quit attempt, n (%) | 334 (67.7) | 106 (63.9) |
| Longest previous abstinent period in past year, days, mean (range) | 4.4 (0–90) | 4.7 (0–90) |
| Fagerström Test for Nicotine Dependence Score, mean (SD)a | 5.6 (2.2) | 5.4 (2.1) |
Note. aScores range from 0 to 10, with higher scores indicating greater dependence.
Study completion rates were similar for both study groups: 86.2% (n = 425) for varenicline and 84.9% (n = 141) for placebo. The median duration of treatment (study drug exposure) was 83 days for each of the treatment groups, and the majority of subjects were exposed for 61–90 days across the two treatment groups.
Efficacy Outcomes
Among all randomized subjects, CO-confirmed continuous abstinence was significantly higher for those randomized to varenicline than for those randomized to placebo at the end of treatment (Weeks 9–12: 53.1% vs. 19.3%; odds ratio [OR] 5.9; 95% CI, 3.7–9.4; p < .0001; Figure 1) and at Week 24 (Weeks 9–24: 34.7% vs. 12.7%; OR 4.4; 95% CI, 2.6–7.5; p < .0001). For both endpoints, the result of the expanded models showed no statistically significant interaction (p > .5) of center and treatment.
Figure 1.
Continuous abstinence at Weeks 9–12 and 9–24. OR = odds ratio (shown with 95% CI).
Seven-day point prevalence of abstinence rates were significantly higher for varenicline compared with placebo at the end of treatment at Week 12 (varenicline 58.6% vs. placebo 24.1%; OR 5.6; 95% CI, 3.6–8.6; p < .0001) and at the end of follow-up at Week 24 (varenicline 42.4% vs. placebo 17.5%; OR 4.1; 95% CI, 2.6–6.5; p < .0001). Seven-day point prevalence of abstinence rates is shown in Supplementary Figure 2.
Moderators of Study Results
The results of the models exploring the effect of replacing investigative centers by country showed a significant effect on continuous abstinence at Weeks 9–12 (p < .0001) but no significant interaction with the treatment (p > .4). A series of models adjusting separately for gender, age, race, Fagerström Test for Nicotine Dependence score, and average number of cigarettes per day over the last month showed that adjusted ORs for varenicline versus placebo ranged from 5.92 to 6.42, favoring active medication. None of the treatment by baseline characteristic interactions was significant (all p > .1226), i.e., the efficacy of varenicline was stable across variations in baseline characteristics.
Time to First Quit Attempt
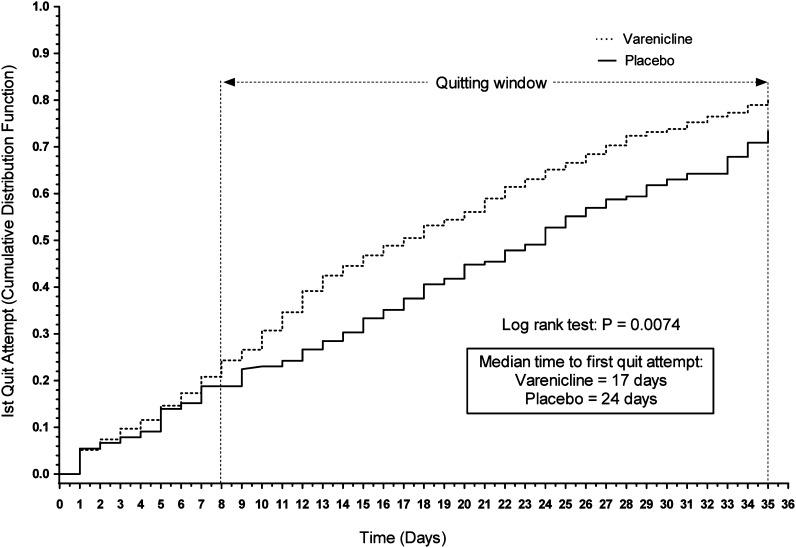
By the end of the quit window (Day 35), 391 (80.5%) varenicline subjects and 121 (73.3%) placebo subjects reported making a quit attempt (p = .062). Varenicline-treated subjects made their first quit attempt significantly earlier than placebo-treated subjects (p = .0074), with a median of 17 versus 24 days, respectively (Figure 2).
Figure 2.
Time to first quit attempt (Kaplan–Meier).
Safety Outcomes
Varenicline was generally well tolerated and had a safety profile similar to previous clinical trials (Gonzales et al., 2006; Jorenby et al., 2006). The most frequent adverse events (occurring in ≥5% of either treatment group) were nausea, headache, insomnia, nasopharyngitis, and abnormal dreams (Table 2). Serious adverse events occurred in six (1.2%) varenicline subjects (intervertebral disc protrusion [two cases], carotid artery stenosis, syncope, peripheral arterial occlusive disease, and ureteric calculus with obstruction) and one (0.6%) placebo subject (suicidal ideation).
Table 2.
Adverse Events, Shown As n (%)
| Varenicline (n = 486) | Placebo (n = 165) | |
| Any adverse events | 348 (71.6) | 89 (53.9) |
| Severe adverse events | 43 (8.8) | 18 (10.9) |
| Serious adverse events | 6 (1.2) | 1 (0.6) |
| Deaths (all causes) | 0 | 0 |
| Study drug discontinuations due to adverse events | ||
| All causes | 24 (4.9) | 13 (7.9) |
| Nausea | 5 (1.0) | 0 |
| Dose reductions or temporary discontinuations due to adverse events | 53 (10.9) | 5 (3.0) |
| Most frequent adverse events (≥5% in either group; except psychiatric adverse events) | ||
| GI disorders | ||
| Nausea | 142 (29.2) | 15 (9.1) |
| Infections and infestations | ||
| Nasopharyngitis | 34 (7.0) | 14 (8.5) |
| Nervous system disorders | ||
| Headache | 55 (11.3) | 20 (12.1) |
| Psychiatric adverse events (≥1% in any group) | ||
| Anxiety disorders and symptoms | 13 (2.7) | 8 (4.8) |
| Anxiety | 4 (0.8) | 5 (3.0) |
| Depressed mood disorders and disturbances | 11 (2.3) | 11 (6.7) |
| Depressed mood | 5 (1.0) | 5 (3.0) |
| Depression | 4 (0.8) | 5 (3.0) |
| Mood disorders and disturbances (not elsewhere) classified | 8 (1.6) | 2 (1.2) |
| Psychiatric disorders (not elsewhere classified) | 2 (0.4) | 2 (1.2) |
| Sleep disorders and disturbances | 116 (23.9) | 17 (10.3) |
| Abnormal dreams | 61 (12.6) | 5 (3.0) |
| Insomnia | 43 (8.8) | 6 (3.6) |
| Nightmare | 8 (1.6) | 2 (1.2) |
| Sleep disorder | 20 (4.1) | 6 (3.6) |
| Suicidal and self-injurious behaviors | 1 (0.2) | 2 (1.2) |
| Suicidal ideation | 1 (0.2) | 2 (1.2) |
Note. Adverse events were defined as those that began or increased in severity during study drug treatment or up to 30 days after the last dose, irrespective of the causal relationship to study drug according to the investigator’s opinion.
Other than sleep disorders, psychiatric adverse events were uncommon (<5%) in both groups (Table 2). Depressed mood was experienced by 1% and 3% and depression by 0.8% and 3% of varenicline and placebo subjects, respectively. A similar number of subjects had an increase in the PHQ-9 depression severity categorization in both varenicline (13.4%) and placebo (17.6%) treatment groups. Most often, these shifts were from “None” at screening and baseline to a postbaseline assessment of “Mild.” One varenicline subject (0.2%) and two (1.2%) placebo subjects had positive postbaseline C-SSRS answers for suicidal ideation and reported these adverse events during the study. There were no instances of suicide attempts.
Discussion
In this randomized placebo-controlled trial, varenicline was found to be efficacious for smoking cessation in a flexible quit date setting. For continuous abstinence at the end of treatment, the OR of 5.9 in favor of varenicline is toward the mid-to-upper end of ORs observed in previously published smoking cessation randomized, placebo-controlled clinical trials of varenicline with a similar dose regimen and treatment duration (OR range: 2.3–8.4; Gonzales et al., 2006; Jorenby et al., 2006; Nakamura et al., 2007; Oncken et al., 2006; Rigotti et al., 2010; Tashkin et al., 2010; Tsai et al., 2007; Wang et al., 2009). The difference was maintained through 24 weeks, with the OR of 4.4 being close to the upper value of the range for ORs of previously published clinical trials of varenicline (OR range: 1.9–4.9; Gonzales et al., 2006; Jorenby et al., 2006; Nakamura et al., 2007; Oncken et al., 2006; Rigotti et al., 2010; Tashkin et al., 2010; Tsai et al., 2007; Wang et al., 2009). Continuous abstinence for varenicline of 53.1% for Weeks 9–12 is in the mid range of continuous abstinence observed in previous studies of varenicline (range: 42.3%–65.4%), as is the continuous abstinence of 34.7% for Weeks 9–24 (range: 25.8%–46.8%; Gonzales et al., 2006; Jorenby et al., 2006; Nakamura et al., 2007; Oncken et al., 2006; Rigotti et al., 2010; Tashkin et al., 2010; Tsai et al., 2007; Wang et al., 2009).
One potential drawback of allowing smokers to delay setting a quit date until after starting pharmacotherapy might be an increased probability of the smoker postponing the quit attempt indefinitely. However, in this study, 80.6% of varenicline and 73.3% of placebo subjects reported making a quit attempt during the quitting window. The number of subjects who made a quit attempt during the treatment phase is actually higher because there are some subjects (44 [9.1%] receiving varenicline and 7 [4.2%] receiving placebo) who were abstinent during Weeks 9–12 but did not report an attempt in the quit window. These subjects either made their first quit attempt outside the quit window (during Weeks 6–9) or failed to report it during the quit window. Thus, the vast majority of subjects made a quit attempt during the first 9 weeks of the study. The median time to quit attempt in the varenicline group in this flexible protocol was longer than in the traditional fixed quit date protocol (17 vs. 8 days), assuming that everybody in the previous studies made a quit attempt on Day 8, as required by the protocol. However, this extended time to quit date did not appear to result in lower efficacy than that observed in previous studies with a similar population (Gonzales et al., 2006; Jorenby et al., 2006).
Prolongation of the precessation treatment period may have allowed smokers to experience the reward-blocking and craving-relieving benefits of varenicline prior to the quit date and thus increased their confidence and motivation to make a quit attempt and remain abstinent after the quit attempt. Although we observed improved abstinence with varenicline in this study, relative to placebo, we did not observe more quit attempts in the active drug group. This is probably due to the fact that smokers entering a clinical trial are typically highly motivated to quit smoking; therefore, the vast majority of subjects in both treatment arms made at least one attempt. In addition, unlike in prior studies (Gonzales et al., 2006; Jorenby et al., 2006), the time between the target quit date and follow-up varied among individuals, resulting in an increase in the days smokers in the varenicline group were at risk for relapse, since on average they quit earlier than the placebo group. However, this did not unfavorably influence the abstinence rates in the active treatment group.
The overall safety profile observed in this trial was similar to previous community trials with varenicline (Gonzales et al., 2006; Jorenby et al., 2006). Gastrointestinal and sleep-related adverse events were higher in the varenicline group compared with placebo, but few subjects discontinued treatment due to adverse events. In fact more placebo than varenicline subjects discontinued due to adverse events (7.9% vs. 4.9%, respectively). Psychiatric adverse events appeared to be less prevalent in the varenicline than in the placebo group. Exclusion of subjects with psychiatric illness was a limitation of this study.
As discussed above, the odds of quitting with this protocol were within the range observed in studies in which subjects were required to set a quit date a priori, which possibly suggests no particular disadvantage to using a fixed quit date approach in terms of overall treatment efficacy. However, such cross-study comparisons can be misleading; thus only a randomized trial would answer whether a fixed quit date or a flexible quit date protocol is superior. The present study was not designed to determine which cessation paradigm would be superior. By demonstrating similar effect size, however, the current study demonstrates that a flexible quit strategy is an effective option and that a study comparing flexible with fixed quit dates in a general population would need to be very large. Alternatively, if the two approaches to quitting are more suitable for different smoker populations, a direct comparison may not be relevant. Our results are consistent with two prior studies examining the effect of precessation nicotine replacement therapy in conjunction with a flexible quit date (Hughes et al., 2010; Shiffman et al., 2009); however, these studies used a gradual cessation approach and were conducted among smokers who were less motivated to quit.
Allowing smokers to start treatment without setting a fixed quit date may make quitting more appealing to some smokers. Current guidelines recommend that all smokers should be counseled at every clinical encounter to quit (Fiore et al., 2008). Those willing to make a quit attempt should be supported to the fullest extent possible. For the clinician with a smoker willing to quit but unable to commit to a fixed quit date, the current study endorses an alternative approach: quitting within a flexible quit window.
Supplementary Material
Supplementary Figures 1 and 2 can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was supported by Pfizer Inc., which was the sponsor and funding source for the clinical trial reported here (ClinicalTrials.gov identification number: NCT00691483).
Declaration of Interests
SR, JH, PC, EK, and TR did not receive any financial support with respect to the writing or development of this manuscript. Funding from Pfizer Inc. has been received by the authors or their institutions for the following in the 36 months prior to submission: research grants (JH, PC, EK, and TR); advisory board membership (SR and JH); reimbursement for travel and accommodation expenses (SR, PC, EK, and TR); lectures/speaker bureau (PC); consultancy (EK); and development of educational materials (EK and TR). CA, LSA, and CR are employees of Pfizer Inc. JH has received consulting fees from several non-profit and for-profit entities that develop or promote smoking cessation products or that advocate for tobacco control measures.
Supplementary Material
Acknowledgments
Editorial assistance in the form of proofreading, collation of review comments, formatting the manuscript for submission, preparation of figures, and formatting of references was provided by Tina Morley and Abegale Templar, PhD, of UBC Scientific Solutions and was funded by Pfizer Inc. The authors would like to thank all study site personnel involved in the study. The Principle Investigators were: Argentina: Marta Angueira (Instituto Medico Especializado); Brazil: Elie Fiss (Fundação do ABC e Faculdade de Medicina do ABC); Canada: Claude Gagne (Lipid Research Center); Douglas S. Helmersen (Peter Lougheed Center); China: Ping Chen (The General Hospital of Shenyang Military Command); Rong Chang Chen (Guangzhou Institute of Respiratory Disease); Chen Wang (Beijing Chaoyang Hospital); Czech Republic: Ondrej Sochor (Fakultni nemocnice u sv. Anny v Brne); France: Beatrice Le Maitre (CHU Côte de Nacre, Unité de Coordination de Tabacologie); Germany: Isabelle Schenkenberger (Klinische Forschung Berlin); Hungary: Iren Herjavecz (Orszagos Koranyi TBC es Pulmonologiai Intezet); Maria Szilasi (Debreceni Egyetem Orvos—es Egeszsegtudomanyi Centrum); Italy: Laura Carrozzi (Dipartimento Cardiotoracico—Azienda Ospedaliero Universitaria Pisana); Mexico: Rodolfo Posadas (Hospital Universitario Dr. Jose E. Gonzalez); Republic of Korea: Ho Joong Kim (Samsung Medical Center); Young Whan Kim (Division of Pulmonary and Critical Care Medicine); Taiwan: Huey-Shinn Cheng (Chang Gung Memorial Hospital-Linkou); Kuang-Chieh Hsueh (Kaohsiung Veterans General Hospital); United Kingdom: Alex Bobak (Wandsworth Medical Centre); United States: Corey G. Anderson (Dedicated Clinical Research, Inc.); James L. Borders (Central Kentucky Research Association, Inc.); Victor Alan Elinoff (Regional Clinical Research, Inc.); Gary E. Johnson (Paramount Clinical Research); Nabil Charle Morcos (Apex Research Institute); Anthony D. Puopolo (Milford Emergency Associates, Inc.); Jon Andrew Shapiro (Philadelphia Health Associates—Adult Medicine); Stephan C. Sharp (Clinical Research Associates, Inc.); Mark Edward Shirley (Omaha Clinical Research, P.C.); Donald P. Tashkin (UCLA Medical Center); Bradley D. Vince (Vince and Associates Clinical Research).
References
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systemic Reviews. 2008;(3) CD006103. doi:10.1002/14651858.CD006103.pub3. [Google Scholar]
- Chambers M. NHS stop smoking services: Service and monitoring guidance 2010/11. 2009. Retrieved. from http://www.scsrn.org/policy_guidance/2010_11_NHS_Stop_Smoking_Services_service_and_monitoring_guidance.pdf. [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: An α4β2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48:3474–3477. doi: 10.1021/jm050069n. doi:10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. 2008. Retrieved from http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi:10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. doi:10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Solomon LJ, Livingston AE, Callas PW, Peters EN. A randomized, controlled trial of NRT-aided gradual vs. abrupt cessation in smokers actively trying to quit. Drug and Alcohol Dependence. 2010;111:105–113. doi: 10.1016/j.drugalcdep.2010.04.007. doi:10.1016/j.drugalcdep.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: Guideline for good clinical practice E6(R1) 1996. Retrieved from http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf. [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. doi:10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax. 2003;58:484–488. doi: 10.1136/thorax.58.6.484. doi:10.1136/thorax.58.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clinical Therapeutics. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. doi:10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (U.S.) Clearing the air: Quit smoking today. 2003. NIH publication 03-1647. Retrieved from http://scc.uchicago.edu/pdf/library/stop_smoking_nci.pdf. [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. doi:10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. doi:10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: A randomized trial. Circulation. 2010;121:221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. doi:10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine & Tobacco Research. 2009;11:1067–1075. doi: 10.1093/ntr/ntp103. doi:10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. doi:10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: A randomized controlled trial. American Journal of Preventive Medicine. 2009;36:96–104. doi: 10.1016/j.amepre.2008.09.039. doi:10.1016/j.amepre.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in mild-to-moderate COPD: A randomized controlled trial. Chest. 2010;139:591–599. doi: 10.1378/chest.10-0865. doi:10.1378/chest.10-0865. [DOI] [PubMed] [Google Scholar]
- Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB, Jr., et al. A randomized, placebo-controlled trial of varenicline, a selective α4β2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clinical Therapeutics. 2007;29:1027–1039. doi: 10.1016/j.clinthera.2007.06.011. doi:10.1016/j.clinthera.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: A placebo-controlled, randomized study. Respirology. 2009;14:384–392. doi: 10.1111/j.1440-1843.2008.01476.x. doi:10.1111/j.1440-1843.2008.01476.x. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. doi:10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2008. The MPower package. 2008. Retrieved from http://www.who.int/tobacco/mpower/gtcr_download/en/index.html. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki—ethical principles for medical research involving human subjects. 2008. Retrieved from http://www.wma.net/en/30publications/10policies/b3/index.html. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.