Abstract
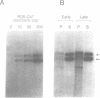
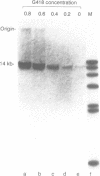
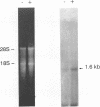
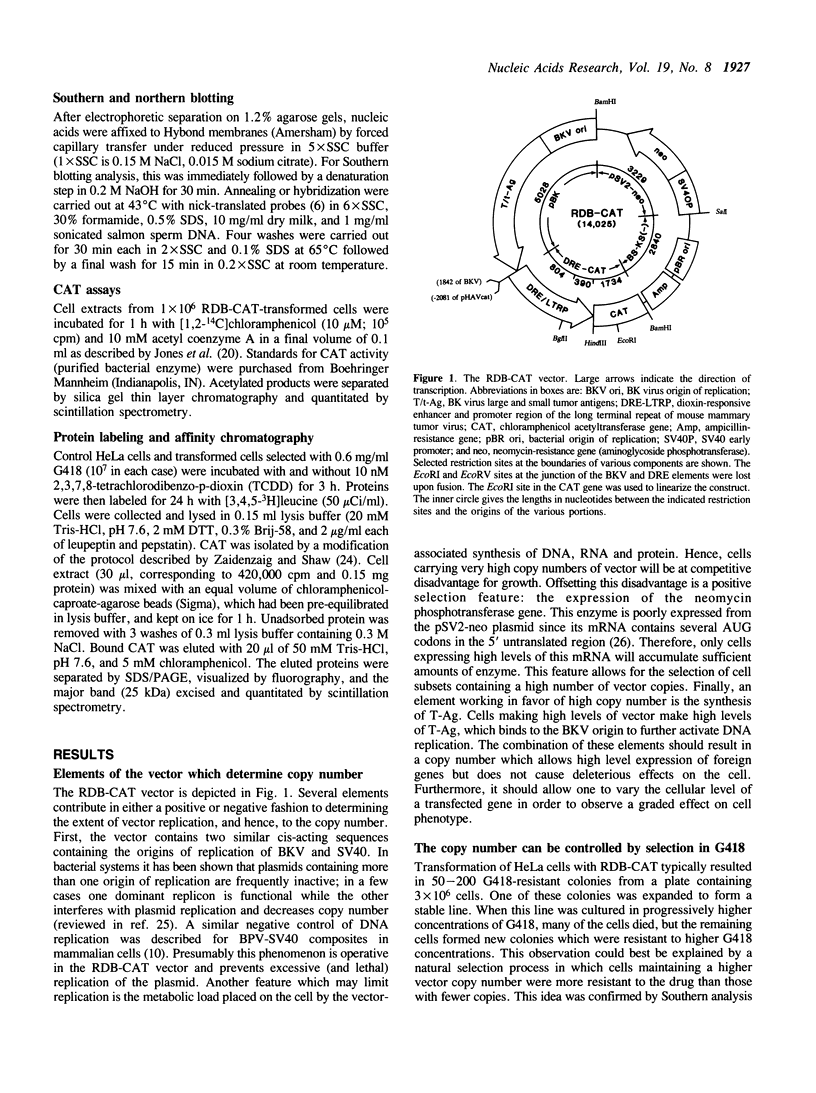
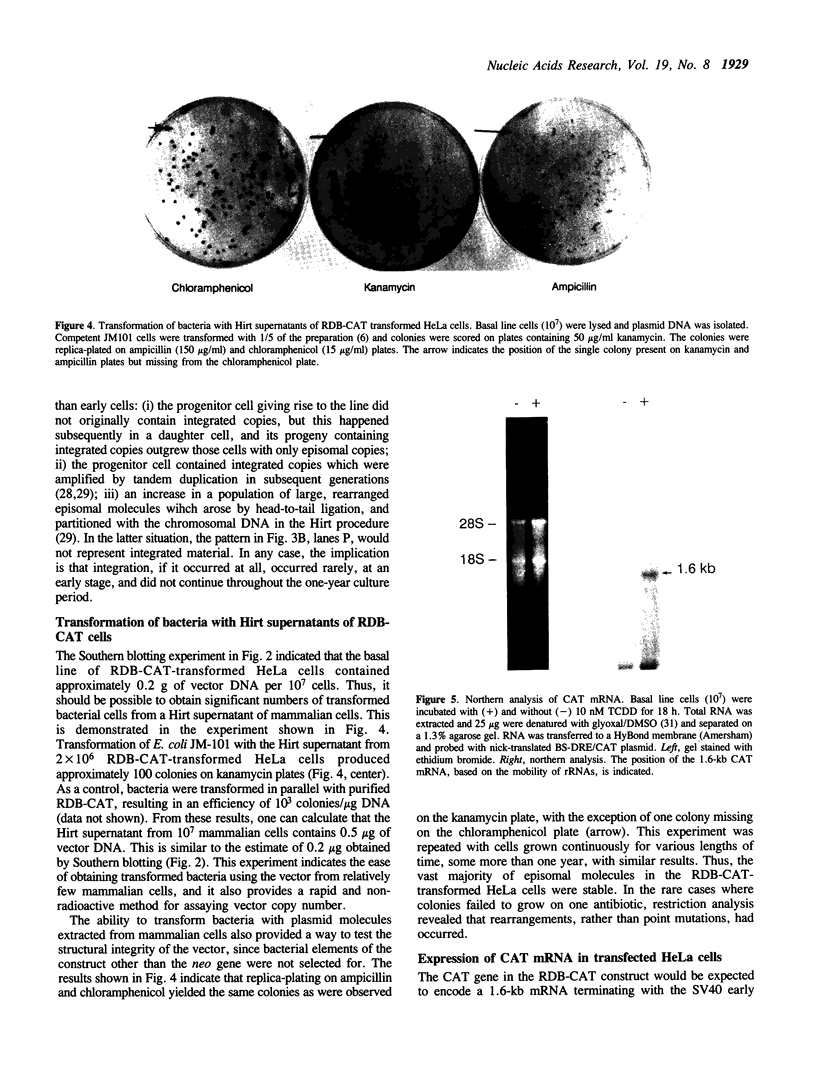
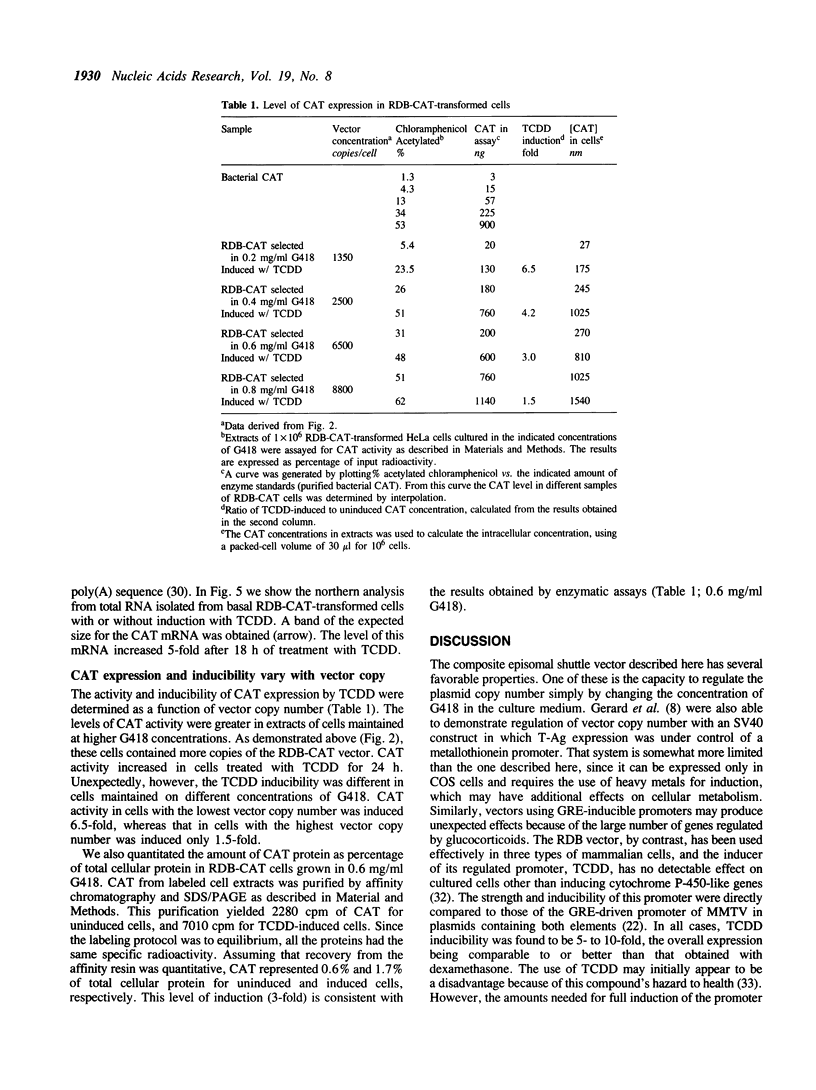
A composite mammalian cell-E. coli shuttle vector was developed based on the human papova virus BK and pSV-neo. The vector contains a dioxin-responsive enhancer (DRE) controlling a mouse mammary tumor virus (MMTV) promoter for the inducible expression of inserted genes. In human cells the vector replicates episomally, presumably utilizing the BKV rather than the SV40 origin, and expresses the BK T/t antigens. A deletion in the late BK region precludes the expression of the core/capsid proteins VP1, VP2, and VP3, thereby preventing the infectious lytic cycle. HeLa cells which were transfected with this vector and selected for resistance to the antibiotic G418 maintained the construct primarily in episomal form during more than one year of continuous culture, with little or no integration into the host genome. Transformed cells cultured in higher concentrations of G418 contained higher copy numbers of the vector. This permits one to vary the dosage of an inserted gene easily and reversibly without the need of conventional amplification techniques and clonal analysis. Using a chloramphenicol acetyl transferase (CAT) reporter gene inserted downstream of the MMTV promoter, we found that CAT expression was greater in clones with higher vector copy number. CAT expression was inducible with 2,3,7,8-tetrachlorodibenzo-p-dioxin, but inducibility was found to be inversely proportional to the copy number. Transformation of bacteria with plasmid molecules retrieved from the mammalian host was efficient, making this vector well adapted for the screening of cDNA libraries for the ability to express a phenotype in mammalian cells. Moreover, DNA sequences were stable during long-term passage in mammalian cells; vector passaged continuously for more than one year retained fully functional bacterial genes for resistance to chloramphenicol and ampicillin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S., Nordström K. Partition-mediated incompatibility of bacterial plasmids. Cell. 1990 Feb 9;60(3):351–354. doi: 10.1016/0092-8674(90)90584-2. [DOI] [PubMed] [Google Scholar]
- Berghard A., Gradin K., Toftgård R. Serum and extracellular calcium modulate induction of cytochrome P-450IA1 in human keratinocytes. J Biol Chem. 1990 Dec 5;265(34):21086–21090. [PubMed] [Google Scholar]
- Carswell S., Alwine J. C. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol. 1989 Oct;9(10):4248–4258. doi: 10.1128/mcb.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Williams G. J., Baglioni C. Inhibition of binding to initiation complexes of nascent reovirus mRNA by double-stranded RNA-dependent protein kinase. J Virol. 1985 May;54(2):408–413. doi: 10.1128/jvi.54.2.408-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Valle G., Fenton R. G., Basilico C. Polyoma large T antigen regulates the integration of viral DNA sequences into the genome of transformed cells. Cell. 1981 Feb;23(2):347–355. doi: 10.1016/0092-8674(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Wu L., Denison M. S., Whitlock J. P., Jr Organization and function of a dioxin-responsive enhancer. J Biol Chem. 1990 Jun 15;265(17):9676–9681. [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Construction, propagation and expression of simian virus 40 recombinant genomes containing the Escherichia coli gene for thymidine kinase and a Saccharomyces cerevisae gene for tyrosine transfer RNA. J Mol Biol. 1979 Sep 25;133(3):359–383. doi: 10.1016/0022-2836(79)90398-x. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Smith K. D., Boyer S. H., Leder P. SV40 recombinants carrying rabbit beta-globin gene coding sequences. Cell. 1979 Jul;17(3):725–735. doi: 10.1016/0092-8674(79)90279-4. [DOI] [PubMed] [Google Scholar]
- Heinzel S. S., Krysan P. J., Calos M. P., DuBridge R. B. Use of simian virus 40 replication to amplify Epstein-Barr virus shuttle vectors in human cells. J Virol. 1988 Oct;62(10):3738–3746. doi: 10.1128/jvi.62.10.3738-3746.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R. A., Song K., Villaret D., Margolskee R., Dunne J., Hayakawa H., Ringold G. M. Amplified expression of tumor necrosis factor receptor in cells transfected with Epstein-Barr virus shuttle vector cDNA libraries. J Biol Chem. 1990 Apr 5;265(10):5708–5717. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Durrin L. K., Galeazzi D. R., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression: analysis of a dioxin-responsive enhancer system. Proc Natl Acad Sci U S A. 1986 May;83(9):2802–2806. doi: 10.1073/pnas.83.9.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough R. D. Human health effects of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annu Rev Pharmacol Toxicol. 1987;27:87–111. doi: 10.1146/annurev.pa.27.040187.000511. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., Poland A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: failure to demonstrate toxicity in twenty-three cultured cell types. Toxicol Appl Pharmacol. 1980 Jul;54(3):377–383. doi: 10.1016/0041-008x(80)90163-5. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Margolskee R. F., Kavathas P., Berg P. Epstein-Barr virus shuttle vector for stable episomal replication of cDNA expression libraries in human cells. Mol Cell Biol. 1988 Jul;8(7):2837–2847. doi: 10.1128/mcb.8.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi G., Barbanti-Brodano G., Negrini M., Lee D., Corallini A., Caputo A., Grossi M. P., Ricciardi R. P. BK virus-plasmid expression vector that persists episomally in human cells and shuttles into Escherichia coli. Mol Cell Biol. 1984 Aug;4(8):1551–1560. doi: 10.1128/mcb.4.8.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W. Cloning vectors derived from animal viruses. J Gen Virol. 1983 Feb;64(Pt 2):255–266. doi: 10.1099/0022-1317-64-2-255. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Negative control of DNA replication in composite SV40-bovine papilloma virus plasmids. Cell. 1986 Aug 29;46(5):741–752. doi: 10.1016/0092-8674(86)90350-8. [DOI] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steinberg V. I., Norkin L. C. SV40 DNA in a carrier system of human glioblastoma cells. Virology. 1988 Apr;163(2):420–428. doi: 10.1016/0042-6822(88)90283-8. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasavada H. A., Ganguly S., Chorney M., Mathur R., Shukla H., Swaroop A., Weissman S. M. pSH4: a mammalian cDNA expression vector. Nucleic Acids Res. 1990 Jun 25;18(12):3668–3668. doi: 10.1093/nar/18.12.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida A., Sogawa K., Yasumoto K. I., Fujii-Kuriyama Y. A novel cis-acting DNA element required for a high level of inducible expression of the rat P-450c gene. Mol Cell Biol. 1990 Apr;10(4):1470–1475. doi: 10.1128/mcb.10.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. Affinity and hydrophobic chromatography of three variants of chloramphenicol acetyltransferases specified by R factors in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):266–271. doi: 10.1016/0014-5793(76)80072-5. [DOI] [PubMed] [Google Scholar]