Abstract
Peripheral arterial disease (PAD) now affects approximately 20% of adults older than 55 years to an estimated total of 27 million people in the Western World. The aim of this paper is to describe the medical management of PAD for the non-vascular specialist, particularly general practitioners, where PAD has now been included in the Northern Ireland Department of Health's Primary Care Service Framework (Directed Enhanced Service).
Keywords: Peripheral Arterial Disease, Epidemiology, Investigation, Treatment
DEFINITION
Peripheral arterial disease (PAD) is defined by the presence of significant narrowing of arteries distal to the arch of the aorta, most often due to atherosclerosis1, 2. Clinically, PAD most commonly affects the lower limbs as a consequence of arterial narrowing distal to the aortic bifurcation1, 2.
CLINICAL PRESENTATION
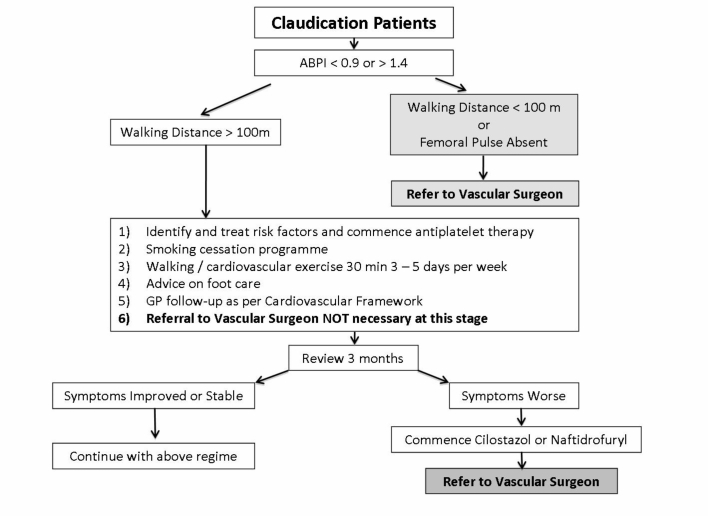
PAD is initially asymptomatic. Intermittent Claudication (IC) is defined as reproducible lower extremity muscular pain induced by exercise and relieved by short periods of rest. It is the symptomatic expression of the inability of the lower limb vasculature to maintain adequate tissue perfusion and oxygenation during exertional activity2, 3. Although an ankle-brachial pressure index (ABPI) less than 0.9 suggests arterial disease, only 10% to 30% of such patients will have classic symptoms of IC4. The presence of slight abnormalities in peripheral blood flow does not necessarily result in symptomatic PAD. Table 1 describes the differential diagnoses of lower limb exertional discomfort.
Table 1.
Differential diagnoses for lower limb exertional symptomatology
| Condition | Prevalence | Anatomical Distribution | Character of Pain | Effect of Exercise | Effect of Rest | Effect of Position | Additional Factors |
|---|---|---|---|---|---|---|---|
| Thigh and Buttock Claudication | Rare | Buttock, hip and thigh | Crampy, aching discomfort | Reproducible onset (same distance for each episode) | Quickly relieved | None | Proximal pulses may be reduced combined with normal distal pulses |
| Calf Claudication | 3% - 5% of the adult population | Calf muscles | Crampy, aching discomfort | Reproducible onset | Quickly relieved | None | May have atypical symptoms on exercise |
| Foot Claudication | Rare | Foot arch | Severe pain on exercise | Reproducible onset | Quickly relieved | None | Numbness can also be associated with pain |
| Venous Claudication | Rare | Entire lower limb affected. Worse in calf. | Tight, bursting pain | Occurs after walking | Slow to settle | Elevation enhances recovery | Signs of deep venous congestion and oedema present. May have history of iliofemoral thrombosis |
| Chronic Compartment Syndrome | Rare | Calf muscles | Tight, bursting pain | Occurs after walking | Very slow to settle | Elevation enhances recovery | Tends to affect heavily muscled athletes |
| Spinal Stenosis | Common | Buttocks and posterior aspects of lower limb. Often bilateral | Pain and weakness | Occurs with exercise and can mimic claudication | Varies but can have a prolonged recovery time | Lumbar spine flexion eases discomfort | Exacerbated by standing and extending spine |
| Nerve Root Compression | Common | Radiation of pain down lower limb | Sharp | May be induced by sitting, standing or walking | Often present at rest | Positional change can improve symptoms | History of back pain |
| Bakers Cyst | Rare | Behind knee down calf | Tenderness and associated swelling | Occurs with exercise | Present at rest | None | Usually a constant discomfort |
| Hip Arthritis | Common | Lateral aspect hip and thigh | Dull to severe ache | Can occur following a period of exercise | Not relieved quickly | Rest and minimal weight bearing helps | Symptoms can vary. Increased in patients with high BMI |
| Foot or Ankle Arthritis | Common | Ankle and foot arch | Aching pain | Variable onset following exercise | Not quickly relieved | Rest and minimal weight bearing helps | Variable. Can relate to exercise but present at rest |
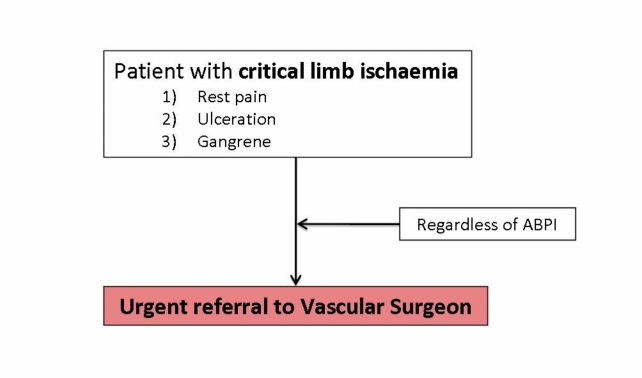
Critical limb ischaemia is lower extremity arterial occlusive disease of a magnitude that potentially threatens the viability of the limb. Critical ischaemia (CI) is persistent rest pain for a period greater than two weeks duration requiring regular analgesia, with an ankle systolic pressure of less than 50mmHg or toe pressure of less than 30mmHg, with or without associated tissue ulceration or gangrene5.
EPIDEMIOLOGY
PAD now affects approximately 20% of adults older than 55 years with an estimated total prevalence of 27 million people in North America and Europe6. The peak incidence is 60 new cases per 10,000 persons per year with a prevalence of 8 million people in the United States7. In Europe, Hooi et al (2001) documented an overall incidence of 1.0 per 1000 people for symptomatic PAD in the Netherlands8.
The Framingham study, and others, documented an increasing prevalence of intermittent claudication (IC) with age and a two-fold male predominance (1.8% for women and 3.6% for men). However, the Edinburgh Artery Study failed to demonstrate any significant difference in PAD between men and women9. The risk of PAD increases two- to three-fold for every 10 year increase in age after 40 years10. More recently, IC was reported to be more common in men, whereas asymptomatic PAD and severe ischaemia were more frequent in women1.
NATURAL HISTORY OF PERIPHERAL ARTERIAL DISEASE
Between 5% and 10% of individuals with asymptomatic PAD develop IC over 5 years and 75% of such patients will experience symptom stabilisation or improvement over their lifetime without intervention11. This trend occurs despite arteriographic evidence of disease progression in the majority of patients. Symptoms may then deteriorate in the remaining 25%, most commonly in the first year (7-9%), and subsequently at rates of 2% to 3% per year, resulting in an incidence of CI between 0.25 to 0.45 per 1000 people per year3. In the first 5 years following diagnosis, 5% of IC patients will require a therapeutic intervention, while only 2% to 4% will ever require a major amputation12. The Edinburgh Artery Study demonstrated that 28.8% of IC patients still had pain after 5 years, 8.2% had undergone a vascular surgical revascularization or amputation and that 1.4% developed leg ulceration9. Although PAD progression is usually slow and the risk of limb loss is low, approximately 20-25% of symptomatic PAD patients will die from coronary or cerebrovascular events within 5 years9, 13. For this reason, risk factor modification and secondary prevention treatment in PAD patients is as important as in patients with coronary and cerebrovascular disease.
MODIFIABLE RISK FACTORS
Atherosclerosis is a multifactorial disease process associated with the interaction of numerous risk factors. The National Health and Nutrition Examination Survey (1999-2000) analysed 2174 participants over the age of 40 and identified a 4.3% prevalence of PAD based on ABPI < 0.90 in either lower limb14. Using age and gender adjusted logistic regression analyses, they reported odds ratios for risk factors significantly associated with PAD including current smoking (4.46), black race (2.83), diabetes (2.71), poor kidney function (2.00), hypertension (1.75) and hypercholesterolaemia (1.68).
Smoking: Smokers have a 3 times greater relative risk of developing IC and experience symptoms 10 years earlier than their non-smoking counterparts3. The Framingham study also showed that smokers are twice as likely to develop PAD as coronary artery disease15. There is also a dose-dependant relationship between smoking and severity of PAD16.
Diabetes: Diabetes mellitus is strongly linked to early onset vascular disease, leading to premature cardiovascular events and mortality15. The Framingham study showed that 20% of IC patients have diabetes and that diabetes increases two- to three-fold the risk of IC. Although most reported studies do not stratify patients according to Type 1 and Type 2 diabetes, patients with both diabetes and IC have a two- to four-fold increased risk of a further cardiovascular event15. PAD prevalence increases with the duration of diabetes with 15% of diabetic patients affected by PAD at 10 years following diagnosis, rising to 45% at 20 years17. Of major concern, Elhadd et al (1999) reported a 25% prevalence of undetected PAD in Scottish diabetic patients attending hospital clinics18. There is a more rapid progression in diabetic patients with an 11 times higher rate of major lower limb amputation compared to non-diabetics and a doubling of the five-year mortality18. Diabetic ulcers also heal more slowly and are the main cause of non-traumatic lower limb amputation in developed countries accounting for more hospital bed occupancy than any other diabetic complication19.
Hypertension: Hypertension contributes to atherosclerosis development, particularly in the coronary and cerebral circulations as well as a two- to three-fold increased risk of developing IC15.
Hypercholesterolaemia: Elevated serum cholesterol, triglyceride and low density lipoproteins (LDL) with reduced high density lipoprotein levels are all implicated as important factors for the progression of atherosclerosis.
Hyperhomocysteinaemia: Elevated homocysteine level may initiate atherosclerosis by modulating cholesterol biosynthesis, thus inducing other cardiovascular risk factors. It is considered an independently graded risk factor for arteriosclerotic vascular disease20.
INVESTIGATION OF PERIPHERAL ARTERIAL DISEASE
Risk Factors: All patients should have a risk factor analysis on their first consultation, particularly smoking and blood pressure measurement. Assessment of haemoglobin, urea and electrolytes to determine baseline renal function as well as fasting serum glucose and lipid profiles should also be performed. Liver function testing should be considered at this stage prior to commencing pharmacological therapy, particularly statins.
Vascular Flow Investigation: An assessment of peripheral blood flow ranges from non-invasive ankle-brachial pressure index, arterial duplex, computerised tomography and magnetic resonance imaging to conventional transfemoral angiography.
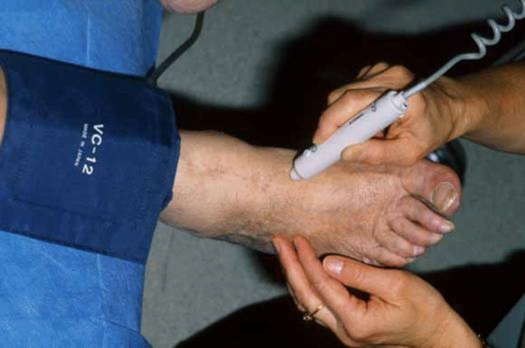
Ankle-Brachial Pressure Index (ABPI) is derived from the brachial, posterior tibial and dorsalis pedis artery systolic pressures which are measured using cuff occlusion by a sphygmomanometer and Doppler ultrasound (Figure 1). A resting ABPI of <0.90 indicates a haemodynamically significant arterial stenosis and is most often used as a haemodynamic definition of PAD. An ABPI <0.5 would suggest moderate to severe claudication while an ABPI <0.3 is suggestive of impending critical ischaemia. An ABPI < 0.90 is also 95% sensitive in detecting arteriogram-positive lesions in symptomatic individuals2. Calcification and inability to compress the arteries secondary to diabetes or renal insufficiency may result in a false elevation of ABPI > 1.4 in some cases2. In these patients, arterial duplex at multiple sites in the lower limb may identify a haemodynamically significant lesion2. Significant disease may also be demonstrated by the characteristics of the actual Doppler waveforms which range from normal triphasic waveforms to atherosclerotic biphasic and monophasic patterns which occur distal to 50% and >70% stenoses respectively.
Fig 1.
Ankle-brachial index – Doppler assessment of dorsalis pedis which is measured using cuff occlusion by a sphygmomanometer and Doppler ultrasound (Super Dopplex® II Huntleigh Healthcare, UK).
The Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC) II guidelines recommend that ABPI measurements should be performed in all patients between the age of 50-69 with a cardiovascular risk factor, who have exertional leg symptoms, who are over the age of 70 regardless of their risk-factor status and in all patients with a Framingham risk score of 10-20% (Framingham risk score is based on patient gender, age, blood pressure, total cholesterol and HDL levels and presence of smoking and diabetes)2.
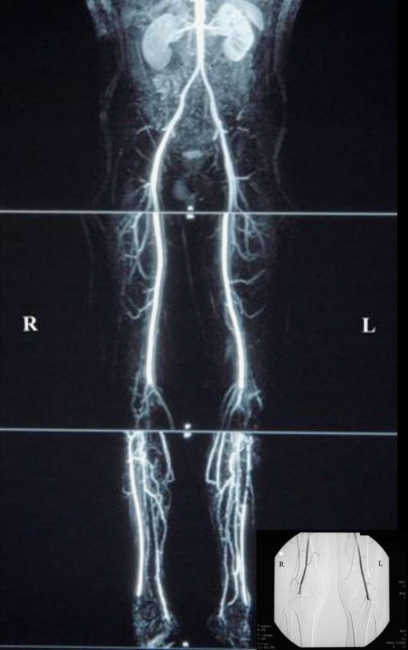
Magnetic resonance angiography (MRA) is a relatively safe modality offering three-dimensional images of the abdomen, pelvis and lower extremities (Figure 2). The use of techniques to minimise venous contamination have improved the accuracy compared to invasive angiography21. Although gadolinium-based contrast agents administered during MRA scanning may be associated with a lower risk of renal dysfunction when compared to iodinated contrast media, nephrogenic systemic fibrosis has now been reported which can lead to severe disability and even death through the induction of skin, muscle and organ fibrosis in patients with background renal impairment.
Fig 2.
Magnetic Resonance Angiogram (MRA) of the peripheral arterial system extending from the abdominal aorta to the pedal vessels which demonstrated bilateral popliteal artery occlusion with surrounding collateralization (Inset figure is patients pre-operative transfemoral angiogram demonstrating bilateral popliteal artery occlusion).
Multidetector Computerised Tomography Angiography yields rapid high quality imaging (Figure 3)22. Major limitations include iodinated contrast and radiation exposure combined with the requirement for careful interpretation during automated digital subtraction due to the presence of arterial wall calcification which has a Hounsfield unit value close to arterially opacified blood.
Fig 3.
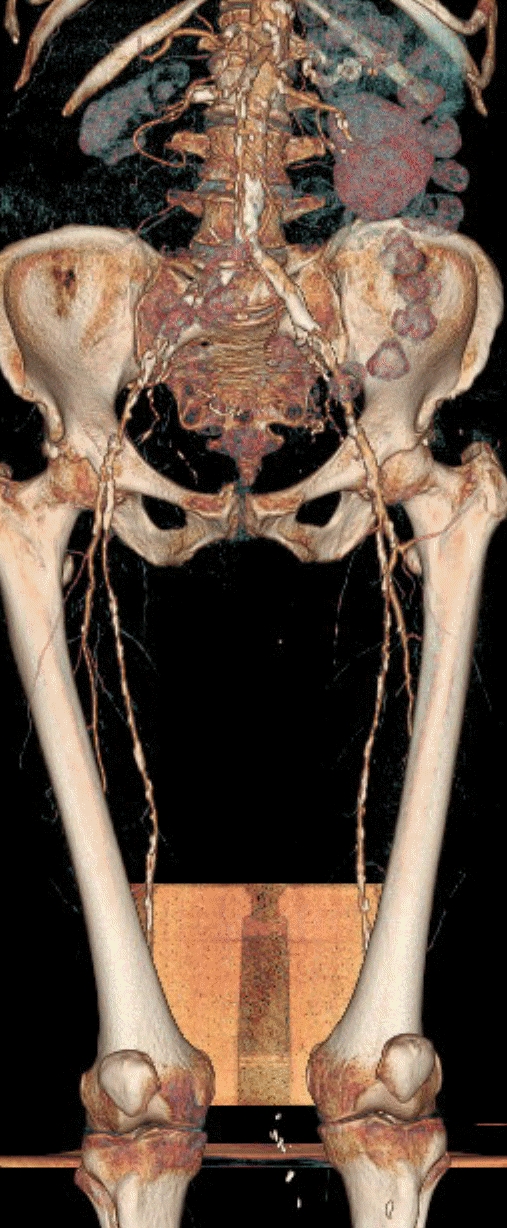
Computerised tomography angiogram of the peripheral arterial system demonstrating proximal disease of both lower limbs extending from the external iliac to the superficial femoral arteries.
Transarterial Angiography assessing the infrarenal vasculature remains the “gold standard” arterial imaging test, with concurrent therapeutic intervention potential (Figure 2). However, complications can arise as a consequence of the arterial puncture or from administration of the contrast material2.
Vascular Function Assessment: includes both a subjective assessment of quality of life combined with quantitative walking distances on a treadmill.
Quality of Life is an important outcome measure for interventions based on global and specific measures23. Generic health questionnaires such as the Short Form-36 (SF-36) evaluate QoL through measurement of physical, social and emotional dimensions of health. However, they are less sensitive in detecting subtle but clinically important treatment differences24. Disease-specific QoL instruments for patients with PAD include the Walking Impairment Questionnaire (WIQ) and the VascuQoL Questionnaire23, 25.
Treadmill Testing provides an objective measure of walking capacity, performed on calibrated treadmill machines at a constant speed of 2mph (3.2 kph) and gradient of 10%2, 26. The time of onset of claudication pain is called the initial claudication distance (ICD) or claudication onset time (COT). The maximal walking performance before the patient stops due to pain is called the absolute claudication or maximal walking distance (ACD or MWD)27. Claudication patients have reduced walking capacities, with a 50% to 60% reduction in peak treadmill performance, compared to age-matched healthy controls. A therapeutic intervention which results in a greater than 25% increase in both ICD and ACD is considered significant28.
TREATMENT OF PERIPHERAL ARTERIAL DISEASE
Treatment of PAD aims to improve longer-term cardiovascular outcomes through risk factor modification combined with antiplatelet and lipid lowering primary medical therapy whilst attenuation of lower limb symptomatology is mediated through walking specific pharmacological agents, endovascular or surgical interventions. The Vascular and Endovascular Units from the Belfast Health and Social Care Trust have issued PAD referral pathways to assist non-vascular practitioners in their management of such patients (Figures 4-7).
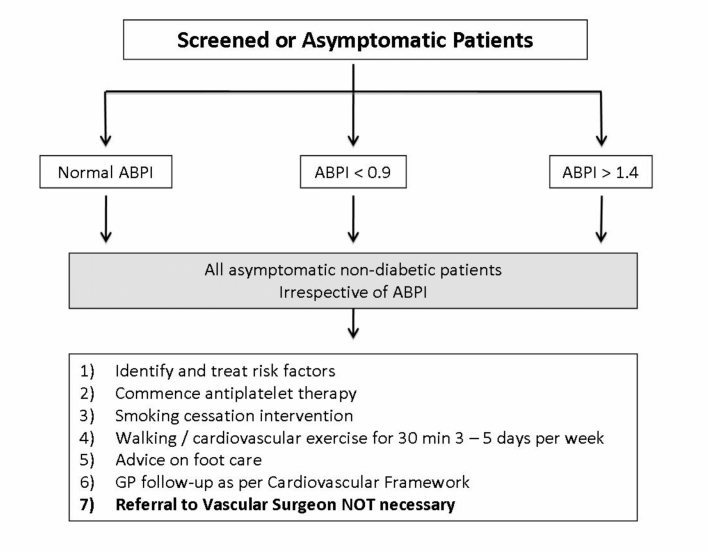
Fig 4.
Belfast Health and Social Care Trust Referral Pathway for screened or asymptomatic patients.
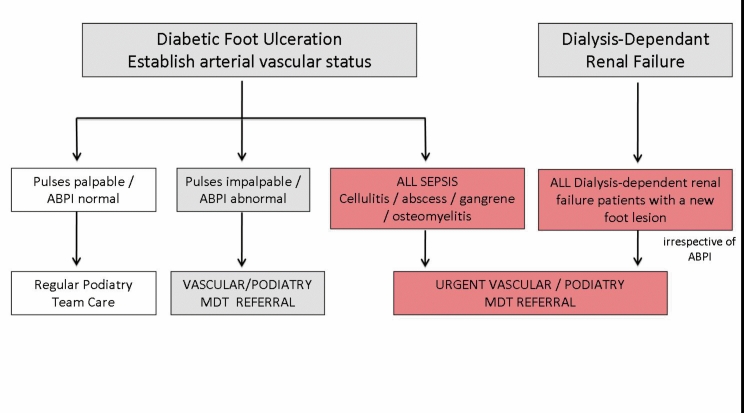
Figure 7.
Belfast Health and Social Care Trust Referral Pathway for patients with diabetes and renal failure.
Fig 5.
Belfast Health and Social Care Trust Referral Pathway for patients with intermittent claudication.
Fig 6.
Belfast Health and Social Care Trust Referral Pathway for patients with critical ischaemia.
CONSERVATIVE
Dietary therapy has beneficial effects on the occurrence and progression of atherosclerosis29. The National Cholesterol Education Program III (NCEP) report recommends a tiered dietary approach to weight reduction, with the further addition of cholesterol lowering agents if required29.
Smokers should be strongly advised to stop through physician advice, group counselling sessions and nicotine replacement2. Smoking cessation reduces the severity of claudication and the risk of developing rest pain30.
Exercise significantly improves maximum walking time and overall walking ability in stable IC patients. The absolute or peak walking distance can be improved by more than 100% with a greater effect identified in longer-term programmes31. Exercise has been reported as more effective than angioplasty or antiplatelet therapy for improving walking time and quality of life, but remains similar to surgical treatment. Exercise also improves gait patterns in non-surgical patients32. Exercise programmes deliver better outcomes when supervised by hospital based-departments compared to home based-programmes and if the mean duration is greater than 2 months31. Badger et al (2007) also commented on the efficacy of a supervised exercise programme, which led to further improvements in walking distance after lower limb arterial bypass surgery.
MEDICAL
PAD medical therapies include systemic cardiovascular or walking specific therapies.
Systemic Cardiovascular Therapies
Antiplatelet Therapy: Antiplatelets delay the rate of PAD progression, reduce the need for intervention and the rate of graft failure following revascularisation procedures33. More importantly a meta-analysis of 195 placebo-controlled trials of antiplatelet therapy demonstrated a reduced cardiovascular death risk of approximately 25% in high risk patients including those with symptomatic PAD33. The Clopidogrel vs. Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial reported a 8.7% relative risk reduction for MI, stroke or cardiovascular death when clopidogrel (75mg/day) was compared to aspirin (325mg/day) in high-risk patients34. Aspirin (75mg) is therefore indicated in all patients with PAD while Clopidogrel (75mg) should be considered for higher risk patients particularly those with severe cardiac disease and diabetes. Other antiplatelet therapies including picotamide, triflusal and ketaserin have not been reported to be superior to aspirin for preventing systematic complications in PAD patients33.
Lipid Lowering Therapy: Statins are clearly efficacious in cholesterol homeostasis with additional cardiovascular benefits which include a 20% relative risk reduction of stroke, MI, vascular death and revascularisation procedures in PAD patients with increased baseline cholesterol levels35. Statins have been reported to reduce the incidence of new onset intermittent claudication whilst improving pain-free walking distances as well as reducing intimal-media thickness in patients with carotid artery stenosis36, 37. Current guidelines for lipid control state that symptomatic PAD patients should have their LDL lowered to <2.59 mmol/ with a target for further reduction down to <1.81 mmol/l in patients with concomitant vascular disease elsewhere2. In conjunction with dietary modification, statins are the primary pharmacological agent with fibrates and/or niacin reserved for patients with abnormalities in triglyceride and HDL profiles.
Antihypertensives: Control of hypertension in PAD patients reduces cardiovascular co-morbidities over the medium to longer-term period38. All PAD patients with hypertension should have blood pressure controlled to <140/90 mmHg or <130/80 mmHg if they also have diabetes or renal insufficiency. Thiazides and angiotensin converting enzyme inhibitors are the initial antihypertensive agents to reduce the risk of cardiovascular disease2. Angiotensin-converting enzyme inhibitors, in hypertensive patients with PAD, may also improve walking distances without a change in ABPI measurements39. Although ß-blockers do not affect IC distance and are not actually contraindicated in PAD, a cautious approach is advocated for more severely affected PAD patients40.
WALKING SPECIFIC THERAPIES
Traditional PAD vasodilators such as naftidrofuryl and pentoxifylline have largely been superseded by cilostazol whereas other arterial vasodilators such as α-blockers, papaverine, nylidrin and nifedipine are not clinically efficacious in PAD41, 42.
Cilostazol: Cilostazol (Pletal® - 100mg twice a day orally) has antiplatelet, antithrombotic, vasodilatory, antimitogenic and cardiogenic properties43. A meta-analysis showed a significant increase of 67% vs. 40% in ICD and 50% vs. 21% for ACD for oral cilostazol 100mg and placebo respectively27. A further meta-analysis from the Cochrane Peripheral Vascular Diseases Group confirmed cilostazol's efficacy on ICD and ACD in patients with stable, moderate to severe IC44. Thompson et al (2002) and Regensteiner et al (2002) have also reported improvements in quality of life with cilostazol therapy as assessed by the SF-36 and Walking Impairment Questionnaires25, 27.
Cilostazol is contraindicated in congestive heart failure, haemostatic disorders or active bleeding. Although cilostazol does not alter coagulation parameters, caution is advised with initial clinical monitoring of patients on antiplatelet and warfarin therapy as well as its use in patients with moderate renal or hepatic dysfunction. Cilostazol is associated with a high frequency of side effects, particularly headache, diarrhoea and palpitations due to vasodilatory properties, occurring in up to 32% of patients27, 45. However, most of these effects are reported to settle within 6 weeks45, 46.
Naftidrofuryl: Naftidrofuryl (Praxilene®) has vasodilatory effects, improving tissue oxygenation and muscle metabolism2. In a meta-analysis of 888 patients with IC, naftidrofuryl increased pain-free walking distance by 26% compared to placebo47. Patients with IC who have a poor quality of life may be considered for treatment with naftidrofuryl2, 48.
Pentoxifylline: Pentoxifylline (Trental®) produces dose-related haemorrheologic effects, lowers blood viscosity and improves erythrocyte flexibility. Although pentoxifylline is associated with modest increases in walking distance over placebo, the overall clinical efficacy is unclear. It is no longer recommended for use in PAD patients2, 48.
Inositol and Cinnarizine: Inositol nicotinate causes vasodilation, lysis of fibrin, a mild hypolipidaemic effect and inhibition of oxidative metabolism in hypoxic tissues. Cinnarizine acts via antagonism of vasoconstrictive substances such as noradrenaline, serotonin and angiotensin49. Although trials for these agents suggested efficacy by improving walking distances, their overall methodology was poor and have not shown clear evidence of benefit over placebo48.
Alternative Peripheral Vasodilators and Treatments: Ticlodipine and ginkgo biloba special extract (Egb 761) significantly increase pain-free walking distance50, 51. Assessment of angiogenic stimulation of new collateral channels by growth factors, autologous bone marrow cells and transcription factor gene therapy modalities are currently being evaluated52-54. Although dietary supplementation with B-vitamins and / or folate has been shown to lower homocysteine levels, high-level evidence for beneficial effects in PAD patients in terms of preventing cardiovascular events is lacking and such therapy is not recommended55.
THERAPEUTIC INTERVENTION
Revascularisation should be considered in patients with critical limb ischaemia or severely debilitating claudication symptoms that persist despite maximal medical treatment. Although operative arterial bypass appears to have a better long term patency, the risks of surgery are significantly greater when compared to endovascular interventions in terms of mortality, major morbidity and return to the normal activities of daily living.
Endovascular
The technical success of percutaneous transluminal angioplasty (PTA) and/or stenting for proximal lesions in the iliac artery exceeds 90% with reported variations between 80% and 100% depending on the presence of either long or focal stenotic iliac lesions respectively2. Five year primary patency rates range between 64% and 75% for all cases with a higher rates of 79% reported for claudicants2, 56, 57.
Endovascular treatment of infrainguinal disease is also associated with a low morbidity and mortality with a technical and clinical success rate of PTA for femoropopliteal stenoses exceeding 95%2. However, longer term outcomes are lower compared to proximal iliac interventions with 5 year patency rates of 55% (range 52-62%) and 42% (range 33-51%) for femoropopliteal stenoses and occlusions respectively2.
Although PTA improves symptomatology and median ACD at 6-months in patients with mild to moderate claudication, longer term benefits are limited with no significant difference in walking distances or quality of life at 2 or 6 years post-PTA58. Independent risk factors for re-stenosis include the disease severity (claudication vs. critical ischaemia), the actual length of the stenosed / occluded segment and the quality of the distal outflow (run-off) vessels.
Surgical
Surgical intervention is indicated for critical limb ischaemia where the primary goal is to relieve pain, encourage ulcer healing, prevent limb loss and improve patient function, quality of life and survival2. Surgical intervention may also be considered in intermittent claudication in patients with proximal lesions or deteriorating symptomatology despite best medical therapy and endovascular intervention. Although surgical procedures improve medium to long-term outcome rates compared to endovascular intervention, they carry a higher early post-procedural complication rate2, 59, 60.
An aortobifemoral arterial bypass is usually recommended for diffuse aortoiliac disease with limb-based patency rates for patients with claudication of 91.0% and 86.8% at 5 and 10 years, respectively, as compared with 87.5% and 81.8% for patients with critical limb ischemia59. In higher risk patients with medical co-morbidities, extra-anatomical bypass modalities include femoro-femoral or axillo-femoral arterial conduits. However, these routes have a lower success rate with 5 year patency rates between 51% and 75%2. Infrainguinal arterial bypass requires uncompromised inflow at the proximal anastomosis, preferably to a native artery rather than graft. A satisfactory distal run-off vessel is the most important determinant of longer-term patency which ranges between 35% for prosthetic material to 60% for vein grafts over 5 years2.
IMPLICATIONS FOR NORTHERN IRELAND
General Practitioners in Northern Ireland participate in the Quality and Outcomes Framework which incentivises a number of activities to enhance patient outcomes. The Department of Health is currently drafting a Cardiovascular Service Framework including a care pathway from prevention, promotion, protection of health and wellbeing, to appropriate assessment, diagnosis, treatment and care provision, rehabilitation and end of life care based on evidence from established sources such as the National Institute of Clinical Excellence. The Northern Ireland Department of Health's Primary Care Service Framework (Directed Enhanced Service) is now developing standards which will cover risk assessment for those patients who have a high risk of or who have PAD61.
Provisional service descriptions as described in Table 2 suggest the initial training of staff members in the assessment of PAD and the development of two disease registers to account for all patients with PAD followed by a second phase to incorporate patients greater than 50 years of age at risk of peripheral and cardiovascular disease particularly those who smoke. Finally, treatment protocols should then be implemented for patients on both registries61.
Table 2.
Provisional service descriptions of the Northern Ireland Department of Health's Primary Care Service Framework (Directed Enhanced Service) for the treatment of PAD
| Year | Target Goals |
|---|---|
| 1 | • Purchase of equipment for ABPI assessment (doppler, sphygmomanometer). |
| • Training of staff to conduct ABPI assessment. | |
| • Completion of a symptomatic PAD registry. | |
| 2 | • Completion of appropriate treatment for all symptomatic PAD patients on registry. |
| • Commencement of PAD assessment registry for at-risk patients over the age of 50 years who smoke. | |
| 3 | • Completion of appropriate treatment for all at-risk PAD patients on registry. |
CONCLUSION
Peripheral arterial disease (PAD) now affects approximately 20% of adults older than 55 years. However, less than 30% of patients diagnosed with PAD based on the ankle-brachial index will have classic symptoms of IC. Asymptomatic and patients able to walk greater than 100 yards should be initially managed in the community with risk factor modification, peripheral arterial examination combined with the calculation of ankle-brachial indices. All patients should be considered for antiplatelet and lipid lowering therapies. Diabetic patients should also receive foot care advice. Symptomatic patients able to walk further than 100 yards require walking-specific medication such as cilostazol for a period of three months. Tertiary referral should be reserved for refractory symptomatology despite maximal conservative management in the community and short distance claudication (<100 yards). Critical limb ischaemia warrants urgent referral.
Acknowledgments
The authors have no conflict of interest.
References
- 1.Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45(6):1185–91. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Schmieder FA, Comerota AJ. Intermittent claudication: magnitude of the problem, patient evaluation, and therapeutic strategies. Amer J Cardiol. 2001;87(12A):3D–13D. doi: 10.1016/s0002-9149(01)01671-x. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med. 2006;73(Suppl 4):S2–7. doi: 10.3949/ccjm.73.suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- 5.Second European Consensus Document on chronic critical leg ischaemia, editor. Eur J Vasc Surg. 1992;6(Supp A):1–32. [PubMed] [Google Scholar]
- 6.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006;295(5):547–53. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 7.Hiatt WR, Nehler MR. Peripheral arterial disease. Adv Int Med. 2001;47:89–110. [PubMed] [Google Scholar]
- 8.Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153(7):666–72. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 9.Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. In J Epidemiol. 1996;25(6):1172–81. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 10.Hiatt WR. Pharmacologic therapy for peripheral arterial disease and claudication. J Vasc Surg, 2002;36(6):1283–91. doi: 10.1067/mva.2002.129654. [DOI] [PubMed] [Google Scholar]
- 11.Hooi JD, Stoffers HE, Knottnerus JA, van Ree JW. The prognosis of non-critical limb ischaemia: a systematic review of population-based evidence. Br J Gen Pract, 1999;49(438):49–55. [PMC free article] [PubMed] [Google Scholar]
- 12.Dormandy J, Heeck L, Vig S. Predictors of early disease in the lower limbs. Semin Vasc Surg. 1999;12(2):109–17. [PubMed] [Google Scholar]
- 13.Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiol. 2004;57(3):294–300. doi: 10.1016/j.jclinepi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 15.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96(1):44–9. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 16.Willigendael EM, Teijink JA, Bartelink ML, Kuiken BW, Boiten J, Moll FL, et al. Influence of smoking on incidence and prevalence of peripheral arterial disease. J Vasc Surg. 2004;40(6):1158–65. doi: 10.1016/j.jvs.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 17.Kreines K, Johnson E, Albrink M, Knatterud GL, Levin ME, Lewitan A, et al. The course of peripheral vascular disease in non-insulin-dependent diabetes. Diabetes Care. 1985;8(3):235–43. doi: 10.2337/diacare.8.3.235. [DOI] [PubMed] [Google Scholar]
- 18.Elhadd T, Robb R, Jung R, Stonebridge P, Belch J. Pilot study of prevalence of asymptomatic peripheral arterial occlusive disease in patients with diabetes attending a hospital clinic. Pract Diabetes Int. 1999;16(6):163–6. [Google Scholar]
- 19.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. New Eng J Med. 1994;331(13):854–60. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- 20.Sharma M, Rai SK, Tiwari M, Chandra R. Effect of hyperhomocysteinemia on cardiovascular risk factors and initiation of atherosclerosis in Wistar rats. Eur J Pharmacol. 2007;574(1):49–60. doi: 10.1016/j.ejphar.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Koelemay MJ, Legemate DA, Reekers JA, Koedam NA, Balm R, Jacobs MJ. Interobserver variation in interpretation of arteriography and management of severe lower leg arterial disease. Eur J Vasc Endovasc Surg. 2001;21(5):417–22. doi: 10.1053/ejvs.2001.1328. [DOI] [PubMed] [Google Scholar]
- 22.Ota H, Takase K, Igarashi K, Chiba Y, Haga K, Saito H, et al. MDCT compared with digital subtraction angiography for assessment of lower extremity arterial occlusive disease: importance of reviewing cross-sectional images. AJR Am J Roentgenol. 2004;182(1):201–209. doi: 10.2214/ajr.182.1.1820201. [DOI] [PubMed] [Google Scholar]
- 23.Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33(4):679–87. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 24.Mehta T, Venkata Subramaniam A, Chetter I, McCollum P. Disease-specific quality of life assessment in intermittent claudication: review. Eur J Vasc Endovasc Surg. 2003;25(3):202–8. doi: 10.1053/ejvs.2002.1837. [DOI] [PubMed] [Google Scholar]
- 25.Regensteiner JG, Ware JE, Jr., et al. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002;50(12):1939–46. doi: 10.1046/j.1532-5415.2002.50604.x. [DOI] [PubMed] [Google Scholar]
- 26.Badger SA, Soong CV, O'Donnell ME, Boreham CA, McGuigan KE. Benefits of a supervised exercise program after lower limb bypass surgery. Vasc Endovascul Surg. 2007;41(1):27–32. doi: 10.1177/1538574406296209. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol. 2002;90(12):1314–9. doi: 10.1016/s0002-9149(02)02869-2. [DOI] [PubMed] [Google Scholar]
- 28.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92(3):614–21. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, editor. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews (Online) 2008;(2) doi: 10.1002/14651858.CD000165.pub3. CD000165. [DOI] [PubMed] [Google Scholar]
- 31.Ernst E, Fialka V. A review of the clinical effectiveness of exercise therapy for intermittent claudication. Arch Int Med. 1993;153(20):2357–60. [PubMed] [Google Scholar]
- 32.Lord SR, Lloyd DG, Nirui M, Raymond J, Williams P, Stewart RA. The effect of exercise on gait patterns in older women: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 1996;51(2):M64–70. doi: 10.1093/gerona/51a.2.m64. [DOI] [PubMed] [Google Scholar]
- 33.Antithrombotic Trialist's Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for the prevention of death, myocardial infarction, and stroke in high risk patients, editor. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CAPRIE Steering Committee, editor. A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348(9038):1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 35.Heart Protection Study Collaborative Group, editor. MRC/BHF Heart Protection Study of Cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 36.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108(12):1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 37.Reid JA, Wolsley C, Lau LL, Hannon RJ, Lee B, Young IS, et al. The effect of pravastatin on intima media thickness of the carotid artery in patients with normal cholesterol. Eur J Vasc Endovasc Surg. 2005;30(5):464–8. doi: 10.1016/j.ejvs.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Yusef S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study (HOPES) Investigators. N Eng J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 39.Lip GY, Makin AJ. Treatment of hypertension in peripheral arterial disease. Cochrane Database Systematic Reviews (Online) 2009;(4) doi: 10.1002/14651858.CD003075. CD003075. [DOI] [PubMed] [Google Scholar]
- 40.Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease. A meta-analysis of randomized controlled trials. Arch Int Med. 1991;151(9):1769–76. [PubMed] [Google Scholar]
- 41.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19(4):369–86. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 42.Dean SM. Pharmacologic treatment for intermittent claudication. Vasc Med. 2002;7(4):301–9. doi: 10.1191/1358863x02vm447ra. [DOI] [PubMed] [Google Scholar]
- 43.Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs. 2003;3(2):117–38. doi: 10.2165/00129784-200303020-00006. [DOI] [PubMed] [Google Scholar]
- 44.Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database of Systematic Reviews (Online) 2008;(1) doi: 10.1002/14651858.CD003748.pub3. CD003748. [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell ME, Badger SA, Sharif MA, Young IS, Lee B, Soong CV. The vascular and biochemical effects of cilostazol in patients with peripheral arterial disease. J Vasc Surg. 2009;49(5):1226–34. doi: 10.1016/j.jvs.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 46.O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The vascular and biochemical effects of cilostazol in diabetic patients with peripheral arterial disease. Vasc Endovascular Surg. 2009;43(2):132–43. doi: 10.1177/1538574408328586. [DOI] [PubMed] [Google Scholar]
- 47.Lehert P, Comte S, Gamand S, Brown TM. Naftidrofuryl in intermittent claudication: a retrospective analysis. J Cardiovasc Pharmacol. 1994;23(Suppl 3):S48–52. [PubMed] [Google Scholar]
- 48.Scottish Collegiate Guidelines Network (SIGN) Diagnosis and Management of Peripheral Arterial Disease - A National Clinical Guideline, editor. Edinburgh: SIGN; 2006. Available online from: http://www.sign.ac.uk/pdf/sign89.pdf. Last accessed November 2010.
- 49.Van Neuten JM, Janssen PA. Effect of cinnarizine on peripheral circulation in dogs. Eur J Pharmacol. 1972;17(1):103–6. doi: 10.1016/0014-2999(72)90275-0. [DOI] [PubMed] [Google Scholar]
- 50.Balsano F, Coccheri S, Libretti A, Nenci GG, Catalano M, Fortunato G, et al. Ticlopidine in the treatment of intermittent claudication: a 21-month double-blind trial. J Lab Clin Med. 1989;114(1):84–91. [PubMed] [Google Scholar]
- 51.Horsch S, Walther C. Ginkgo biloba special extract EGb 761 in the treatment of peripheral arterial occlusive disease (PAOD)–a review based on randomized, controlled studies. Int J Clin Pharmacol Ther. 2004;42(2):63–72. doi: 10.5414/cpp42063. [DOI] [PubMed] [Google Scholar]
- 52.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359(9323):2053–8. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 53.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 54.Rajagopalan S, Olin J, Deitcher S, Pieczek A, Laird J, Grossman PM, et al. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115(10):1234–43. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 55.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. New England J Med. 2006;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 56.Becker GJ, Katzen BT, Dake MD. Noncoronary angioplasty. Radiology. 1989;170(3 Pt 2):921–40. doi: 10.1148/radiology.170.3.2521745. [DOI] [PubMed] [Google Scholar]
- 57.Rutherford R, Durham J. Percutaneous balloon angioplasty for arteriosclerosis obliterans: long term results. In: Yao J, Pearce W, editors. Techniques in Vascular Surgery. Philadelphia: Saunders; 1992. pp. 329–45. [Google Scholar]
- 58.Fowkes G, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database of Systematic Reviews (Online) 1998;(2) doi: 10.1002/14651858.CD000017.pub2. CD000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Vries SO, Hunink MG. Results of aortic bifurcation: a meta-analysis. J Vasc Surg. 1997;26(4):558–69. doi: 10.1016/s0741-5214(97)70053-3. [DOI] [PubMed] [Google Scholar]
- 60.Bachoo P. Peripheral arterial disease. Clin Evid. 2004;(12):144–58. [PubMed] [Google Scholar]
- 61.Department of Health, Social Services and Public Safety, Northern Ireland, editor. The primary medical services (direct enhanced services) directions (Northern Ireland) 2008. Belfast: DHSS; 2008. Available online from: http://www.dhsspsni.gov.uk/specification_on_the_primary_medical_services_des_directions_ni_2008.pdf.