Abstract
Supplementary oxygen is commonly administered in current medical practice. Recently it has been suggested that hyperoxia causes acute oxidative stress and produces prompt and substantial changes in coronary resistance in patients with ischemic heart disease. In this report, we examined whether the effects of hyperoxia on coronary blood velocity (CBV) would be associated with a reduction in myocardial function. We were also interested in determining if the postulated changes in left ventricular (LV) function seen with Tissue Doppler Imaging (TDI) could be reversed with intravenous vitamin C, a potent, acute anti-oxidant. LV function was determined in eight healthy subjects with transthoracic echocardiography and TDI before and after hyperoxia and with and without infusing vitamin C. Hyperoxia compared to room air promptly reduced CBV by 28 ± 3% (from 23.50 ± 2.31 cm/s down to 17.00 ± 1.79 cm/s) and increased relative coronary resistance by 34 ± 5% (from 5.63 ± 0.88 up to 7.32 ± 0.94). Meanwhile, LV myocardial systolic velocity decreased by 11 ± 6% (TDI). These effects on flow and function were eliminated by the infusion of vitamin C. This suggests that these changes are mediated by vitamin C-quenchable substances acting on the coronary microcirculation.
Keywords: oxygen, coronary circulation, oxidative stress, nitric oxide
Introduction
Patients presenting to hospitals with acute shortness of breath often receive empiric supplemental oxygen (Moradkhan and Sinoway 2010). This is potentially problematic since both animal and human studies have found that hyperoxia increases coronary vascular resistance and decreases cardiac output (Daniell and Bagwell 1968; Haque et al. 1996). This effect of high arterial oxygen tension to increase vascular tone may be mediated by the generation of reactive oxygen species (ROS) in the vessel wall (Jamieson et al. 1986; Lee and Choi 2003). ROS rapidly react with nitric oxide (NO) thus reducing vasodilator responses (Landmesser et al. 2006; Lavi et al. 2008). Studies from this lab have shown that 100% oxygen produces a prompt and substantial change in coronary resistance and blood flow (McNulty et al. 2005; Momen et al. 2009). The constrictor effects of hyperoxia can be acutely reversed by the intravenous administration of high dose vitamin C, a potent anti-oxidant (Mak et al. 2002; McNulty et al. 2007).
Feigl and colleagues (Feigl 1987; Huang and Feigl 1988) demonstrated that coronary vasoconstriction due to adrenergic stimulation evoked epicardial constriction, thereby preserving subendocardial blood flow and ventricular performance during exercise. Thus, adrenergic coronary vasoconstriction seen with exercise may be beneficial. In this report, we postulated that hyperoxia would cause a generalized increase in coronary tone and this would lead to a reduction in cardiac function.
The development of new high frequency, high-resolution ultrasound equipment has allowed investigators to non-invasively measure coronary blood velocity (CBV) with transthoracic echocardiography (Gao et al. 2009; Hozumi et al. 1998; Momen et al. 2009). In an analogous fashion, Tissue Doppler Imaging (TDI) can be used to assess left ventricular (LV) regional and global function (Derumeaux et al. 1998). TDI is an echocardiographic technique employing the Doppler principle to measure the velocity of movement of myocardial tissue (as well as other cardiac structures). It is well suited for the measurement of long-axis ventricular function (Nikitin and Witte 2004; Yu et al. 2007). In this study, we hypothesized that hyperoxia would increase coronary resistance; the hyperoxia-induced vasoconstriction would in turn reduce myocardial function by a mechanism sensitive to the antioxidant vitamin C.
Methods
Study population
Eight healthy normotensive volunteers (4 men and 4 women: age 26.5 ± 1 yr, and body mass index, 23.4 ± 0.9 kg/m2) were studied. All subjects were nonsmokers and on no medications including antioxidant vitamins or supplements. Subjects abstained from drinking caffeine for 24 h before performing the studies. All subjects provided informed consent; the experimental protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine. All studies were performed in the General Clinical Research Center at the Penn State College of Medicine, Hershey, PA.
Study protocol
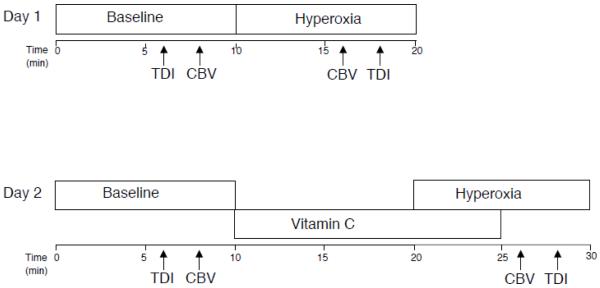
All subjects underwent two hyperoxic challenges (oxygen alone and oxygen with an infusion of vitamin C), which were performed on two separate days. In one hyperoxic challenge, subjects breathed room air for 10 min, and then breathed 100% oxygen via a plastic facemask for 10 min. On the other day, subjects breathed room air for 10 min, then received an intravenous infusion of 3.0 g of vitamin C over 15 min, and then were given 100% oxygen to breath for 10 min. The hyperoxia began 10 min into the vitamin C infusion as described in a previous study (McNulty et al. 2007) (Figure 1). Prior reports have shown that this dose of vitamin C promptly restores coronary flow velocity and prevents coronary constriction during oxidative stress (Kaufmann et al. 2000; Teramoto et al. 2004). Based on body weight, the vitamin C dose in the current study was 94 ± 4 mg/kg.
Figure 1.
Timeline of experimental protocols. The arrows show when TDI and CBV data are recorded.
Echocardiographic examination
All subjects were studied using echocardiographic examination including TDI data at baseline and during hyperoxic protocols, respectively. Standard transthoracic echocardiography was performed with the subjects in the lateral recumbent position using a digital ultrasound system (iE33, Philips Ultrasound, Bothell, WA, USA) and 5.0-1.0 MHz probe (S5-1). Subjects were encouraged to breath normally throughout the protocol. Echocardiographic measurements included LV end-diastolic and end-systolic dimensions (LVEDD, LVESD) using standard M-mode (Sahn et al. 1978), LV end-diastolic and end-systolic volumes (LVEDV, LVESV), stroke volume (SV) and ejection fraction (EF) using Simpson’s biplane method (Lang et al. 2005). Cardiac output (CO) was calculated as SV X HR. LVEDV was used as an index of preload while LVEF and CO were used as indices of systolic function (Giakoumis et al. 2007; Luecke et al. 2004). All measurements were averaged over three to five cardiac cycles.
Assessment of coronary blood velocity (CBV) and coronary vascular resistance (CVR)
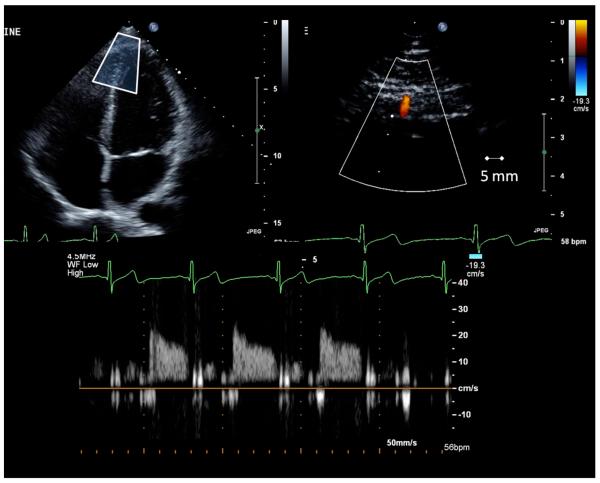
CBV and CVR measurements were performed as described before (Momen et al. 2007; Momen et al. 2009). A variable frequency phased-array transducer (S8-1) was employed at the same ultrasound session. For the coronary blood flow signal acquisition, the left ventricle was first imaged in the standard four-chamber long axis view; then the transducer was adjusted to better explore the LV apex. The imaging depth was set at 5 cm and the focal zones were set at ~2-3 cm. Color flow mapping was used and the 2D gain was adjusted to obtain the best blood flow signal of the LAD. For color Doppler flow mapping, the velocity range was set at ± 19 cm/s. Once the distal portion of the LAD was located in the region of the LV apex, care was taken to orient the transducer so as to acquire the long axis view of the LAD. The average depth of explored coronary branches was 2.18 ± 0.17 cm. With a sample volume (2.0 mm) positioned over the color signal in the LAD, we recorded CBV at the end of expiration before and during the hyperoxic protocols (Figure 2). BP and HR were recorded simultaneously. The Doppler tracing of the diastolic portion of each cardiac cycle was analyzed using Pro Solv®3.0 to obtain peak coronary diastolic blood velocity.
Figure 2.
Original transthoracic Doppler echocardiogram (top) and coronary blood flow velocity profile (bottom) demonstrating data acquisition.
Tissue Doppler imaging (TDI)
TDI was used to measure myocardial tissue velocities. These velocities were obtained with the transducer in the apical four-chamber views and the sampling volume at the septal and lateral mitral annular regions. Measurements included the systolic myocardial velocity (Sm), early (Em), and late (Am) diastolic myocardial velocities at the mitral annulus at the end of the respiration; Sm velocities from the two sites were averaged to derive a sensitive indicator of measurement of myocardial systolic function (Derumeaux et al. 1998). Similarly, Em and Am velocities from the two mitral annular regions were averaged to derive sensitive markers of regional diastolic function (Derumeaux et al. 1998). It has been well acknowledged that the TDI technique is relatively insensitive to changes in loading condition and overcomes several limitations associated with the use of routine Doppler inflow velocities (Abali et al. 2005; Derumeaux et al. 1998; Yu et al. 2007).
The timing of the echocardiographic examination and coronary blood flow recordings are depicted in Figure 1. In an effort to measure coronary blood flow from the same portion of the LAD, CBV was obtained at the end of baseline and 6 minutes into hyperoxia (i.e. the transducer was kept in place). TDI images were then acquired for CBV for the last 2 minutes of hyperoxia. The same examiner (ZG) performed all of these studies.
Blood pressure and heart rate
HR was measured by electrocardiogram and BP using a Finometer (FMS, Netherlands). Baseline BP was established by an automated sphygmomanometer (Dinamp Critikon, Tampa, FL) and the Finometer was adjusted to match that pressure. Continuous BP and HR data were collected with a computer data acquisition system (Power Lab, AD Instruments, CO). Rate-pressure product (RPP), an index of myocardial oxygen consumption, was calculated as HR x systolic BP.
An index of CVR was calculated by dividing diastolic BP by CBV (cm/s). CVR was expressed in arbitrary units. CBV and CVR were recorded at baseline and during hyperoxia.
Data analysis and statistics
Beat-by-beat analysis of HR, BP, and CBV were performed for all subjects during each protocol. After normality was confirmed by the Kolmogorov-Smirnov test (i.e. P > 0.05 for all measurements), a 2 treatment (control, vitamin C) by 2 time point (base, hyperoxia) ANOVA was conducted for all measurements. Paired t-tests were used for post hoc analyses. Intraclass correlations were performed on select baseline data to determine test-retest reliability of the measurement (Day 1 vs. Day 2). Data are presented as means ± SE. A P value of < 0.05 was considered statistically significant.
Results
1. The hemodynamic effects of hyperoxia with and without treatment of vitamin C (Table 1)
Table 1.
Systemic Hemodynamic Responses to Hyperoxia with and without Treatment of Vitamin C
| Day 1 | Day 2 | |||
|---|---|---|---|---|
| Room Air | Hyperoxia | Room Air | Vitamin C + Hyperoxia |
|
| HR (bpm) | 64 ± 3 | 59 ± 2* | 63 ± 2 | 62 ± 2 |
| SBP (mmHg) | 114 ± 5 | 118 ± 3 | 116 ± 4 | 118 ± 1 |
| DBP (mmHg) | 61 ± 2 | 64 ± 2 | 66 ± 2 | 66 ± 3 |
| MBP (mmHg) | 80 ± 3 | 81 ± 2 | 81 ± 4 | 83 ± 3 |
|
RPP
(mmHg•beats/min) |
7467 ± 457 | 7179 ± 359 | 7635 ± 281 | 7340 ± 243 |
Values are means ± S.E.M. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; RPP, rate-pressure product.
P < 0.05, versus respective baseline.
For HR, there was no main effects for treatment [F(1,7) = 0.873, P = 0.381, ηp2 = 0.111] and no main effect for time [F(1,7) = 2.644, P = 0.148, ηp2 = 0.274] but a significant treatment by time interaction was found [F(1,7) = 36.78, P = 0.001, ηp2 = 0.840]. Subsequent paired t-tests revealed that HR was significantly reduced by hyperoxia (P = 0.014). ANOVA did not reveal any main or interaction effects for SBP, DBP, MBP, and RPP (Table 1).
2. The cardiac effects of hyperoxia with and without the vitamin C infusion (Table 2)
Table 2.
Echocardiographic Measurement Changes Due to Hyperoxia.
| Day 1 | Day 2 | |||
|---|---|---|---|---|
| Room Air | Hyperoxia | Room Air | Vitamin C + Hyperoxia |
|
| LVEDD (cm) | 4.53 ± 0.17 | 4.53 ± 0.18 | 4.53 ± 0.17 | 4.44 ± 0.19 |
| LVESD (cm) | 2.89 ± 0.10 | 2.94 ± 0.12 | 2.96 ± 0.14 | 3.04 ± 0.15 |
| LVEDV (ml) | 114.50 ± 5.78 | 113.75 ± 6.79 | 111.63 ± 7.17 | 113.13 ± 7.56 |
| LVESV(ml) | 45.25 ± 3.32 | 48.75 ± 3.41 | 47.38 ± 4.52 | 48.38 ± 3.51 |
| SV (ml) | 71.00 ± 3.87 | 65.00 ± 4.18 | 65.00 ± 3.92 | 65.00 ± 4.89 |
| CO (L) | 4.75 ± 0.34 | 3.77 ± 0.29* | 4.20 ± 0.16 | 4.16 ± 0.24 |
| EF (%) | 60.38 ± 1.46 | 57.13 ± 1.63 | 58.63 ± 1.84 | 57.13 ± 1.55 |
Values are mean ± S.E.M. LVEDD, diameter of left ventricle at the end of diastole; LVESD, diameter of left ventricle at the end of systole; LVEDV, volume of left ventricle at the end of diastole; LVESV, volume of left ventricle at the end of systole; SV, stroke volume; CO, cardiac output; EF, ejection fraction.
P < 0.05, versus respective baseline.
LVEDD, EDV, SV, and EF did not reveal any main or interaction effects (Table 2). For CO, there was no main effect for treatment [F(1,7) = 1.55, p = 0.254, ηp2= 0.181] and no treatment by time interaction [F(1,7) = 5.15, P = 0.058, ηp2 = 0.424], but there was a significant main effect for time [F(1,7) = 18.07, P = 0.004, ηp2 = 0.721]. Subsequent paired t-tests demonstrated that CO was significantly reduced by hyperoxia (P = 0.015).
3. TDI data and coronary hemodynamic responses to hyperoxia with and without Vitamin C
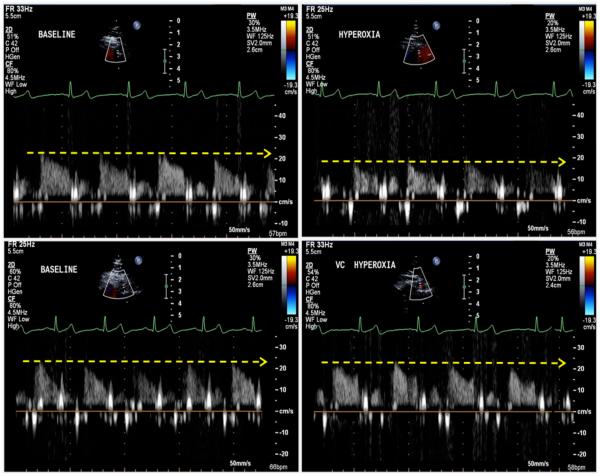
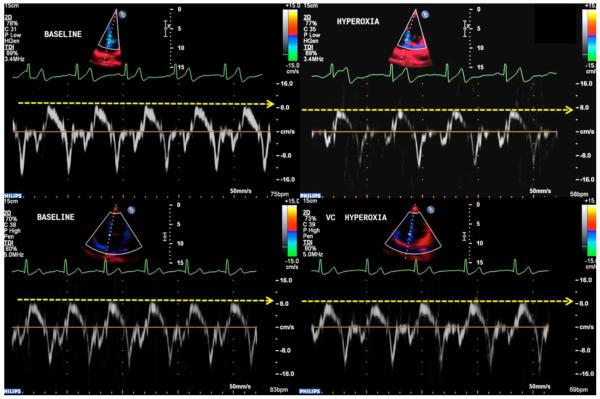
For Sm, there was no main effect for treatment [F(1,7) = 4.19, P = 0.080, ηp2 = 0.374] and no main effect for time [F(1,7) = 4.21, P = 0.079, ηp2 = 0.376], but a significant treatment by time interaction was found [F(1,7) = 79.82, P < 0.001, ηp2 = 0.919]. Paired t-tests showed that Sm was significantly reduced by hyperoxia (P = 0.001) and that treatment with Vitamin C caused a significantly higher Sm during hyperoxia compared to control (P = 0.001). Diastolic function (Em and Am) did not display any main or interaction effects (Figure 3). CBV did not reveal a main effect for treatment [F(1,7) = 4.50, P = 0.072, but did show a main effect for time [F(1,7) = 47.06, P < 0.001, ηp2 = 0.871] and a treatment by time interaction [F(1,7) = 11.04, P = 0.013, ηp2 = 0.612]. Paired samples t-tests demonstrated that hyperoxia significantly reduced CBV (P < 0.001) and Vitamin C caused a significantly higher CBV during hyperoxia compared to control (P = 0.005). CVR did not reveal a main effect for treatment [F(1,6) = 3.27, P = 0.120, ηp2 = 0.353] but did show a main effect for time [F(1,6) = 22.37, P = 0.003, ηp2 = 0.789] and a treatment by time interaction [F(1,6) = 43.51, P = 0.001, ηp2 = 0.879]. Paired samples t-tests demonstrated that hyperoxia significantly reduced CVR (P < 0.001) and Vitamin C caused a significantly higher CVR during hyperoxia compared to control (P = 0.043). Taken together, these data indicate that systolic function (Sm) and CBV are increased and CVR is reduced in the presence of hyperoxia but this effect is abolished in the presence of Vitamin C.
Figure 3.
Effects of hyperoxia on coronary blood velocity with and without vitamin C. Representative recordings of coronary blood velocity at baseline and during hyperoxia. Top, subject without administration of vitamin C, the wave signals are shown in during room air (left) and during hyperoxia (right). Note, that during hyperoxia, the coronary blood velocity is lower than during baseline. Bottom, after administration of vitamin C, the coronary blood velocity is preserved during hyperoxia.
Baseline Sm, and CVR demonstrated moderate test-retest reliability (Cronbach’s alpha = 0.509, and 0.433 respectively). SV and CBV showed strong test-retest reliability (Cronbach’s alpha = 0.815 and 0.747, respectively). Representative recordings of TDI tracings and CBV at baseline and during hyperoxia with and without administration of vitamin C are shown in Figures 3 and 4.
Figure 4.
Effects of hyperoxia on myocardial function with and without vitamin C. Representative recordings of TDI tracings at baseline and during hyperoxia. Top, subject without administration of vitamin C. Bottom, subject with vitamin C. Note, that the administration of vitamin C preserves myocardial function during hyperoxia.
Discussion
Study findings
These studies were performed to examine the effects of hyperoxia on CBV and myocardial function using noninvasive ultrasound techniques in conscious humans. We found that: 1) hyperoxia evoked coronary vasoconstriction and reduced myocardial function; and 2) these effects were abolished by the administration of vitamin C administration.
Previous studies have demonstrated that hyperoxia-mediated vasoconstriction in the forearm (Mak et al. 2002) and the calf (Rousseau et al. 2005) were abolished by vitamin C. Invasive studies from this laboratory demonstrate that hyperoxia causes a reduction in coronary flow and velocity in patients with ischemic heart disease (McNulty et al. 2007).
Hyperoxia and sympathetic nervous system activation
Attempts to analyze the effects of hyperoxia on coronary circulation and cardiac function have been made by others (see review (Feigl 1983)]. The coronary vasoconstrictive effects of high PO have been shown in animal and human studies (Baron et al. 1990; Feigl 1983; McNulty et al. 2007; Momen et al. 2009). Among important determinants of coronary blood flow, hyperoxia has been shown to alter autonomic nervous system activity, raise afterload and alter myocardial oxygen consumption. These responses have been suggested as contributors to hyperoxic constriction (Feigl 1983). However, although adrenergic vasoconstriction has been identified to play an important role in coronary regulation, both animal and human studies suggest that systemic hyperoxia either lowers or does not change sympathetic activity (Daniell and Bagwell 1968; Seals et al. 1991). Moreover, animal experiments demonstrate that even after combined parasympathetic and sympathetic blockade, 100% O2 reduces coronary blood flow by 10% (Lammerant et al. 1969). Similar results have been found in isolated heart preparations (Baron et al. 1990).
Hyperoxia and reactive oxygen species
Hyperoxia leads to an increase in the superoxide anion O2.− (Rubanyi and Vanhoutte 1986). Numerous studies have demonstrated that local O2.− concentration is the main limiting factor for the availability of bioactive NO in healthy and diseased vessels. O2.− interacts rapidly with NO leading to an accelerated reduction in dilator potential (Cai and Harrison 2000). Superoxide dismutase (SOD) plays an important role in combating the effects of the superoxide anion on NO by accelerating the breakdown of O2.− to H2O2. This finding, however, is not universal (Mouren et al. 1997; Sauls and Boegehold 2000).
ROS may affect the redox state of tetrahydrobiopterin (BH4), thus impairing its function as an essential cofactor for endothelial NO synthase (Mayer and Andrew 1998). Hyperoxia may also reduce basal release of NO in porcine conduit coronary arteries (Pasgaard et al. 2007). It is possible that hyperoxia interacts with nitrosylated hemoglobin and/or thiol compounds. Nitrosylated hemoglobin can serve as a NO transport vehicle. Release of NO from these compounds may be inversely related to blood O2 tension (Jia et al. 1996).
Hyperoxia and oxidative stress may also stimulate the production of the endothelium-derived vasoconstrictor endothelin (Dallinger et al. 2000; Messina et al. 1994) and may have an effect on potassium channel conductance and vascular smooth muscle cells resting membrane potential (Mouren et al. 1997).
Vitamin C is known to improve endothelial function acting as a free radical scavenger restoring NO activity (Levine et al. 1996; Taddei et al. 1998). An administration of vitamin C abolished the coronary vasoconstrictor responses during hyperoxia, suggesting that acute coronary vasoconstriction was a consequence of oxidative stress in patients with ischemic heart disease (McNulty et al. 2007).
In this report, vitamin C markedly attenuated the effects of hyperoxia on coronary flow and on the annular longitudinal velocity of LV movement. It has been well known that the longitudinal motion of the ventricle is an important component of LV systolic and diastolic function (Henein and Gibson 1999). Tissue Doppler velocities of mitral annulus velocities have proven to be very sensitive for the assessment of myocardial dysfunction (Alam et al. 2000; Shan et al. 2000). We reasoned that longitudinal ventricular movement would be a valid index of LV function if: 1) regional wall motion was normal; and 2) rotational and translational cardiac motion were minimized (Ommen et al. 2000). To minimize the effects of rotation and translational motion, the transducer was placed at the apex and images were acquired at end expiration as recommended (Lang et al. 2005; Ommen et al. 2000).
Limitations
Several limitations should be noted. First, our study population was small (N = 8). Second, because of the very short half-life of NO, direct measurements of NO within human blood vessels is problematic. Moreover, measurements of oxidative stress in humans (especially as they pertain to acute short lived interventions) are not sensitive nor specific (Bailey et al. 2004). Third, unlike the invasive methods used previously in our catheterization laboratory, we calculated CVR from non-invasive determination of CBV, not from coronary blood flow. Coronary blood flow calculations rely on accurate measurements of vessel diameter. However, the diameter range of the LAD branches in which we measured blood flow velocity signals are only in the range of 0.8-2 mm. This diameter range is too small to be measured accurately and confidently with current commercially available echocardiographic machines. However, previous studies have shown a good correlation between maximal flow velocity and flow even at high flow rates (Marcus et al. 1981; Wilson et al. 1985).
Clinical implications
Oxygen supplementation is widely used in clinical practice and is part of first-line treatment in myocardial infarction (Antman et al. 2004; Van de Werf et al. 2003). We agree with prior important reports that have long posited that hyperoxia in this setting can be cause for concern (Rivas et al. 1980; Rush et al. 1995). Additionally, oxygen is widely and often empirically used in the treatment of a diverse range of acute medical conditions. In one of the few randomized, double-blind, controlled trials of oxygen therapy, patients with uncomplicated myocardial infarction that were randomized to receive supplemental oxygen tended to have a higher mortality and more ventricular tachycardia than those not receiving supplemental O2 (Rawles and Kenmure 1976). The administration of high-concentration supplemental oxygen to the point of hyperoxia can be a frequent occurrence in the hospital setting. Based on the fact that congestive heart failure is so common in the general population, the empiric use of this agent is of potential great concern since previous studies from this lab and others suggests that oxygen therapy can have dose dependent hemodynamic consequences in patients with CHF (Haque et al. 1996; Mak et al. 2001).
Conclusion
In the present study, normal young subjects who acutely received supplemental oxygen were observed to have a reduction in CBV, an effect coincident with significant subendocardial myocardial impairment. Intravenous administration of vitamin C prevented these effects and could stimulate future research in this area.
Table 3.
TDI Data and Coronary Vascular Tone
| Day 1 | Day 2 | |||
|---|---|---|---|---|
| Baseline | Hyperoxia | Baseline | Vitamin C + Hyperoxia | |
|
Sm
(cm/s) |
8.50 ± 0.18 | 7.56 ± 0.26* | 8.31 ± 0.21 | t 8.51 ± 0.17† |
|
Em
(cm/s) |
12.87 ± 0.71 | 12.33 ± 0.65 | 13.15 ± 0.42 | 13.52 ± 0.66 |
|
Am
(cm/s) |
5.96 ± 0.41 | 6.53 ± 0.72 | 6.99 ± 0.33 | 7.08 ± 0.41 |
| E (cm/s) | 81.38 ± 5.08 | 78.50 ± 4.57 | 80.38 ± 5.24 | 84.86 ± 5.92 |
| A (cm/s) | 40.63 ± 3.63 | 39.50 ± 2.07 | 39.00 ± 2.44 | 39.43 ± 3.2 |
| E/Em | 6.39 ± 0.36 | 6.43 ± 0.34 | 6.13 ± 0.37 | 6.38 ± 0.20 |
|
CBV
(cm/s) |
23.50 ± 2.31 | 17.00 ± 1.79* | 23.88 ± 1.36 | t 22.25 ± 1.45† |
| CVR | 5.63 ± 0.88 | 7.32 ±0.94* | 4.83 ± 0.32 | t 5.29 ± 0.37† |
Values are mean ± S.E.M. Sm, peak systolic velocity in ejection period measured at mitral annulus; Em, early diastolic velocity at mitral annulus; Am, late diastolic velocity at mitral annulus; CBV, peak velocity of coronary blood velocity; CVR, coronary vascular resistance.
P< 0.05, vs. respective baseline.
P < 0.05, vs. day 1 hyperoxia
Acknowledgements
We are thankful to Cheryl Blaha and Jessica Mast for their expert study coordination and invaluable technical assistance during the studies. The authors also express gratitude to Jennifer Stoner for her outstanding secretarial skills. Grant R01 HL070222 from the National Institutes of Health (LS) and this project is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions (LS).
Footnotes
Conflict of Interest
None
References
- Abali G, Tokgozoglu L, Ozcebe OI, Aytemir K, Nazli N. Which Doppler parameters are load independent? A study in normal volunteers after blood donation. J Am Soc Echocardiogr. 2005;18:1260–1265. doi: 10.1016/j.echo.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Effects of first myocardial infarction on left ventricular systolic and diastolic function with the use of mitral annular velocity determined by pulsed wave doppler tissue imaging. J Am Soc Echocardiogr. 2000;13:343–352. doi: 10.1016/s0894-7317(00)70003-4. [DOI] [PubMed] [Google Scholar]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC., Jr. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol. 2004;287:H1689–H1699. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- Baron JF, Vicaut E, Hou X, Duvelleroy M. Independent role of arterial O2 tension in local control of coronary blood flow. Am J Physiol Heart Circ Physiol. 1990;258:H1388–H1394. doi: 10.1152/ajpheart.1990.258.5.H1388. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Dallinger S, Dorner GT, Wenzel R, Graselli U, Findl O, Eichler HG, Wolzt M, Schmetterer L. Endothelin-1 contributes to hyperoxia-induced vasoconstriction in the human retina. Invest Ophthalmol Vis Sci. 2000;41:864–869. [PubMed] [Google Scholar]
- Daniell HB, Bagwell EE. Effects of high oxygen on coronary flow and heart force. Am J Physiol. 1968;214:1454–1459. doi: 10.1152/ajplegacy.1968.214.6.1454. [DOI] [PubMed] [Google Scholar]
- Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, Letac B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation. 1998;97:1970–1977. doi: 10.1161/01.cir.97.19.1970. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Feigl EO. The paradox of adrenergic coronary vasoconstriction. Circulation. 1987;76:737–745. doi: 10.1161/01.cir.76.4.737. [DOI] [PubMed] [Google Scholar]
- Gao Z, Momen A, Novick M, Williams R, Cyran S, Blaha C, Mast J, Spilk S, Leuenberger U, Sinoway L. Weight loss attenuates oxidative stress related-coronary vasoconstriction in obese adolescents. FASEB J. 2009;23:1032–1033. doi: 10.1007/s00421-012-2459-9. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoumis A, Berdoukas V, Gotsis E, Aessopos A. Comparison of echocardiographic (US) volumetry with cardiac magnetic resonance (CMR) imaging in transfusion dependent thalassemia major (TM) Cardiovasc Ultrasound. 2007;5:24. doi: 10.1186/1476-7120-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol. 1996;27:353–357. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- Henein MY, Gibson DG. Long axis function in disease. Heart. 1999;81:229–231. doi: 10.1136/hrt.81.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32:1251–1259. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res. 1988;62:286–298. doi: 10.1161/01.res.62.2.286. [DOI] [PubMed] [Google Scholar]
- Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schafers KP, Luscher TF, Camici PG. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–1238. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- Lammerant J, Schryver CD, Becsei I, Camphyn M, Mertens-Strijthagen J. Coronary circulation response to hyperoxia after vagotomy and combined alpha and beta adrenergic receptors blockade in the anesthetized intact dog. Pflügers Arch. 1969;308:185–196. doi: 10.1007/BF00586552. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Harrison D, Drexler H. Oxidant stress—a major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur J Clin Pharmacol. 2006;62:13–19. [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension. 2008;51:127–133. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35:341–350. doi: 10.1016/s0891-5849(03)00279-x. [DOI] [PubMed] [Google Scholar]
- Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr., Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- Luecke T, Roth H, Herrmann P, Joachim A, Weisser G, Pelosi P, Quintel M. Assessment of cardiac preload and left ventricular function under increasing levels of positive end-expiratory pressure. Intensive Care Med. 2004;30:119–126. doi: 10.1007/s00134-003-1993-7. [DOI] [PubMed] [Google Scholar]
- Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–473. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002;282:H2414–H2421. doi: 10.1152/ajpheart.00947.2001. [DOI] [PubMed] [Google Scholar]
- Marcus M, Wright C, Doty D, Eastham C, Laughlin D, Krumm P, Fastenow C, Brody M. Measurements of coronary velocity and reactive hyperemia in the coronary circulation of humans. Circ Res. 1981;49:877–891. doi: 10.1161/01.res.49.4.877. [DOI] [PubMed] [Google Scholar]
- Mayer B, Andrew P. Nitric oxide synthases: catalytic function and progress towards selective inhibition. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:127–133. doi: 10.1007/pl00005233. [DOI] [PubMed] [Google Scholar]
- McNulty PH, King N, Scott S, Hartman G, McCann J, Kozak M, Chambers CE, Demers LM, Sinoway LI. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–H1062. doi: 10.1152/ajpheart.00625.2004. [DOI] [PubMed] [Google Scholar]
- McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, Sinoway LI. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 2007;102:2040–2045. doi: 10.1152/japplphysiol.00595.2006. [DOI] [PubMed] [Google Scholar]
- Messina EJ, Sun D, Koller A, Wolin MS, Kaley G. Increases in oxygen tension evoke arteriolar constriction by inhibiting endothelial prostaglandin synthesis. Microvasc Res. 1994;48:151–160. doi: 10.1006/mvre.1994.1046. [DOI] [PubMed] [Google Scholar]
- Momen A, Gahremanpour A, Mansoor A, Kunselman A, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol. 2007;102:735–739. doi: 10.1152/japplphysiol.00618.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer J, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol. 2009;296:H854–H861. doi: 10.1152/ajpheart.01075.2007. PMCID: PMC2660236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradkhan R, Sinoway LI. Revisiting the role of oxygen therapy in cardiac patients. J Am Coll Cardiol. 2010;56:1013–1016. doi: 10.1016/j.jacc.2010.04.052. NIHMSID: NIHMS211824, PMCID: PMC2941910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouren S, Souktani R, Beaussier M, Abdenour L, Arthaud M, Duvelleroy M, Vicaut E. Mechanisms of coronary vasoconstriction induced by high arterial oxygen tension. Am J Physiol Heart Circ Physiol. 1997;272:H67–H75. doi: 10.1152/ajpheart.1997.272.1.H67. [DOI] [PubMed] [Google Scholar]
- Nikitin NP, Witte KK. Application of tissue Doppler imaging in cardiology. Cardiology. 2004;101:170–184. doi: 10.1159/000076694. [DOI] [PubMed] [Google Scholar]
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- Pasgaard T, Stankevicius E, Jorgensen MM, Ostergaard L, Simonsen U, Frobert O. Hyperoxia reduces basal release of nitric oxide and contracts porcine coronary arteries. Acta Physiol (Oxf) 2007;191:285–296. doi: 10.1111/j.1748-1716.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1:1121–1123. doi: 10.1136/bmj.1.6018.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas F, Rembert JC, Bache RJ, Cobb FR, Greenfield JC., Jr. Effect of hyperoxia on regional blood flow after coronary occlusion in awake dogs. Am J Physiol Heart Circ Physiol. 1980;238:H244–H248. doi: 10.1152/ajpheart.1980.238.2.H244. [DOI] [PubMed] [Google Scholar]
- Rousseau A, Bak Z, Janerot-Sjoberg B, Sjoberg F. Acute hyperoxaemia-induced effects on regional blood flow, oxygen consumption and central circulation in man. Acta Physiol Scand. 2005;183:231–240. doi: 10.1111/j.1365-201X.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Rush JW, MacLean DA, Hultman E, Graham TE. Exercise causes branched-chain oxoacid dehydrogenase dephosphorylation but not AMP deaminase binding. J Appl Physiol. 1995;78:2193–2200. doi: 10.1152/jappl.1995.78.6.2193. [DOI] [PubMed] [Google Scholar]
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Sauls BA, Boegehold MA. Arteriolar wall PO(2) and nitric oxide release during sympathetic vasoconstriction in the rat intestine. Am J Physiol Heart Circ Physiol. 2000;279:H484–H491. doi: 10.1152/ajpheart.2000.279.2.H484. [DOI] [PubMed] [Google Scholar]
- Seals DR, Johnson DG, Fregosi RF. Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol Regul Integr Comp Physiol. 1991;260:R873–R878. doi: 10.1152/ajpregu.1991.260.5.R873. [DOI] [PubMed] [Google Scholar]
- Shan K, Bick RJ, Poindexter BJ, Shimoni S, Letsou GV, Reardon MJ, Howell JF, Zoghbi WA, Nagueh SF. Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta-adrenergic receptor density in humans. J Am Coll Cardiol. 2000;36:891–896. doi: 10.1016/s0735-1097(00)00786-5. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Teramoto K, Daimon M, Hasegawa R, Toyoda T, Sekine T, Kawata T, Yoshida K, Komuro I. Acute effect of oral vitamin C on coronary circulation in young healthy smokers. Am Heart J. 2004;148:300–305. doi: 10.1016/j.ahj.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, Thygesen C, Underwood SR, Vahanian A, Verheugt FW, Wijns W. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. doi: 10.1016/s0195-668x(02)00618-8. [DOI] [PubMed] [Google Scholar]
- Wilson RF, Laughlin DE, Ackell PH, Chilian WM, Holida MD, Hartley CJ, Armstrong ML, Marcus ML, White CW. Transluminal, subselective measurement of coronary artery blood flow velocity and vasodilator reserve in man. Circulation. 1985;72:82–92. doi: 10.1161/01.cir.72.1.82. [DOI] [PubMed] [Google Scholar]
- Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]