Abstract
Somatic mutation of ten-eleven translocation 2 (TET2) gene is frequently found in human myeloid malignancies. Recent reports showed that loss of Tet2 led to pleiotropic hematopoietic abnormalities including increased competitive repopulating capacity of bone marrow (BM) HSCs and myeloid transformation. However, precise impact of Tet2 loss on the function of fetal liver (FL) HSCs has not been examined. Here we show that disruption of Tet2 results in the expansion of Lin−Sca-1+c-Kit+ (LSK) cells in FL. Furthermore, Tet2 loss led to enhanced self-renewal and long-term repopulating capacity of FL-HSCs in in vivo serial transplantation assay. Disruption of Tet2 in FL also led to altered differentiation of mature blood cells, expansion of common myeloid progenitors and increased resistance for hematopoietic progenitor cells (HPCs) to differentiation stimuli in vitro. These results demonstrate that Tet2 plays a critical role in homeostasis of HSCs and HPCs not only in the BM, but also in FL.
Myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN) are two major hematopoietic malignancies, thought to arise from hematopoietic stem or progenitor cells. MDS is characterized by dysregulated hematopoietic differentiation with propensity to develop acute myeloid leukemia (AML)1. MPN is a spectrum of disease characterized by the expansion of maturing hematopoietic elements with minimal dysplasia. Some of the patients with MPN also progress to myeloid leukemia by acquiring additional mutations altering differentiation. Recent advances in high throughput genome-wide sequencing have made it possible to identify a number of recurrent somatic mutations in human MDS and MPN. One of the most frequently found mutations is a loss-of-function mutation of ten-eleven translocation 2 (TET2), which is located at chromosome 4q24 where uniparental disomy was frequently observed in human myeloid malignancies. Recurrent TET2 mutation was identified in 10–20% of MDS and MPN2,3, and subsequent studies reported high incidence of TET2 mutations in secondary AML (20–40%) and chronic myelomonocytic leukemia (CMML) (40–50%)4,5. These results underscore the importance of TET2 in maintaining homeostasis and malignant transformation of hematopoietic system.
DNA methylation is one of the most important epigenetic modifications, and aberrant DNA methylations are hallmark of cancers including AML6. It was recently reported that TET family proteins could convert 5-methylcytosine (5 mC) to 5-hydroxymethylcytosine (5 hmC)7,8,9 and 5 hmC production by Tet1 is critical for ES cell self-renewal and inner cell mass specification8,10. Moreover, TET2 mutations associated with myeloid malignancies disrupt its catalytic activity, and BM cells from patients with TET2 mutations contained lower levels of 5 hmC compared to normal controls9. On the other hand, mutations of isocitrate dehydrogenase (IDH) 1 and IDH2, enzymes involved in citrate metabolism, are seen in AML and brain tumors in mutually exclusive manner with TET211,12,13,14. These mutations inhibit catalytic activity of TET2 by 2-hydroxyglutarate (2-HG), an oncometabolite generated by mutant IDH1/214. TET2 is dependent on alpha-keto-glutarate (α-KG) for its catalytic activity, and 2-HG has been shown to inhibit TET2 by competing with α-KG15. These results strongly suggest that impaired 5 hmC generation by mutant TET2 or by mutant IDH1/2 is one of the critical steps in myeloid transformation.
FL-HSCs and adult HSCs differ in several aspects of their phenotypes and functions. For example, FL-HSCs are different from adult HSCs in the expression of CD11b/ Mac-1 and CD34, while some markers such as CD150 and endomucin are commonly expressed in both populations16,17. FL-HSCs have higher, more robust capacity to reconstitute hematopoietic compartment in irradiated recipients as compared to adult HSCs18,19. Moreover, regulation of self-renewal, one of the most critical features of HSCs, differs between FL and adult HSCs as marked by distinct dependence on polycomb group proteins such as Bmi-1, Rae28, and Mel-1820,21,22. These observations clearly indicate that FL-HSCs and adult HSCs are phenotypically and functionally distinct, and suggest that they may be regulated by distinct molecular machinery.
It was recently reported that inactivation of Tet2 in mouse genome results in increased long-term repopulating capacity and competitive advantage of HSCs from adult BM, and eventually leads to myeloid transformation23,24,25. However, self-renewal capacity of HSCs was not precisely assessed by serial transplantation assay and effects of Tet2 disruption on FL-HSCs have not been examined. Here we show that disruption of Tet2 leads to the expansion of lineage negative (Lin−), Sca-1+, c-Kit+ (LSK) multipotent progenitor (MPP) fraction and common myeloid progenitors (CMPs) in FL. In addition, self-renewal and long-term repopulating capacities were enhanced by Tet2 disruption as evidenced by serial transplantation assay. These results clearly indicate critical roles of Tet2 in homeostasis of HSCs and HPCs in FL.
Results
Characterization of Tet2 gene-trap mice
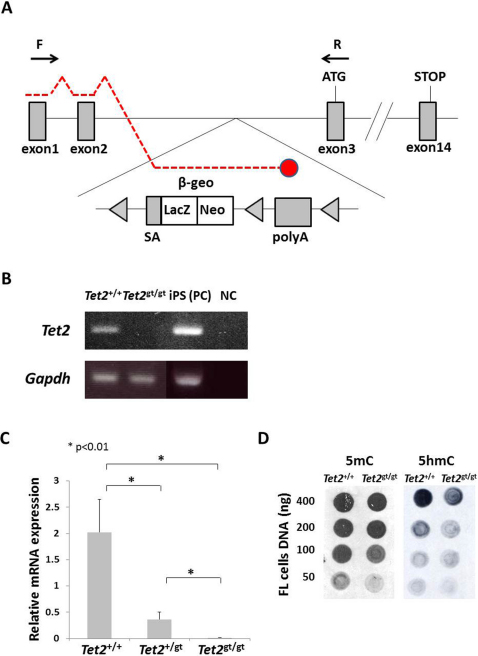
Tet2 gene was disrupted by inserting LacZ/ neomycin resistance (β-geo) cassette in intron 2, just before exon 3 (Figure 1A). mRNAs transcribed from the endogenous promoter are expected to terminate by being spliced to the trap cassette, which carries poly A signal at the end. Since exon 3 is the first coding exon, trapping the Tet2 message before exon 3 should lead to complete ablation of Tet2. We first tested whether mRNA was efficiently terminated by the trap-cassette. Semi-quantitative and real-time quantitative RT-PCR using primers amplifying exon 1–3 revealed that mRNA reading through exon 3 in Tet2gt/gt mice was far less than 1% of that of Tet2+/+ mice, indicating that almost all mRNAs were trapped by the cassette (Figure 1B and C). Of note, the level of mRNA in Tet2+/gt mice was approximately 20% of WT. We have also examined the catalytic activity of TET2 in gene-trap mice by dot blot assay. Quantification of the 5 mC and 5 hmC levels in DNA from FL cells confirmed a marked reduction of 5 hmC signals in Tet2gt/gt mice as compared to WT (Figure 1D). These data indicate that transcription of Tet2 gene was efficiently disrupted in Tet2gt/gt mice, and therefore, Tet2gt allele can be regarded as a null allele.
Figure 1. Schematic illustration of Tet2 gene-trap allele and validation of Tet2 ablation.
(A) Schematic illustration of Tet2 gene-trap allele. SA; mouse En2 splicing acceptor site, β-geo; β-galactosidase/ neomycin-resistance fusion gene, LacZ; β-galactosidase, Neo; neomycin phosphotransferase, polyA; polyadenylation signal. Detailed structure and feature of gene-trap vector was described previously26,31. Arrows indicate the primers used for RT-PCR. Red broken lines are mRNA transcribed from the endogenous promoter. Red circle shows that mRNA is terminated by poly A signal in the trap cassette. (B) Efficiency of Tet2 mRNA knockdown by RT-PCR. RT-PCR was performed as described in Methods using cDNAs derived from Tet2+/+ and Tet2gt/gt fetal liver cells. PCR was run for 35 cycles. The positions of forward and reverse primers are shown in Figure 1A. cDNA from iPS cells was used for positive control. The electrophoretic gels for Gapdh are cropped. Uncropped images of the full-length gels are presented in Supplementary Figure 8. PC; positive control, NC; negative control. (C) Efficiency of Tet2 mRNA knockdown by quantitative RT-PCR. qRT-PCR was performed as described in Methods using cDNAs derived from Tet2+/+, Tet2+/gt and Tet2gt/gt fetal liver cells. The positions of forward and reverse primers are shown in figure 1A. Expression was normalized to the expression level of Gapdh in each fetal liver cells. The data represents the mean ± standard deviation (S.D.) (n = 3 for each genotype). (D) Quantification of 5 mC and 5 hmC levels in DNA from FL cells by dot blot assay. Genomic DNA was spotted onto the membrane at the amount indicated on the left. DNA extraction and immunoblot were performed as described in Methods.
As reported previously, intercross of heterozygous mice resulted in perinatal lethality of homozygous mice, and very few Tet2gt/gt mice survived to 1 week after birth26. Therefore, we used fetal liver (FL) cells for analyzing hematopoiesis for the following analysis.
Tet2 disruption leads to the expansion of multipotent progenitor cells and myeloid progenitors in fetal liver
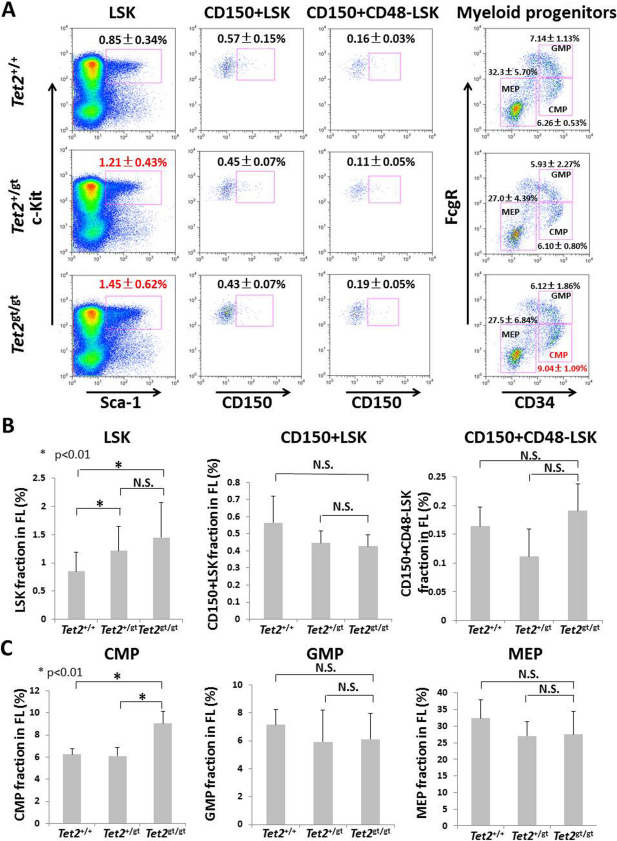
Initial analysis revealed that the numbers of whole FL cells or various hematopoietic progenitors in FLs were not significantly different between WT and Tet2-mutant embryos (Supplemental Figure S1 A and B). In addition, apoptotic status of FL cells as shown by the staining with annexin V and propidium iodide (PI) was indistinguishable between WT and Tet2-mutants (Supplemental Figure S2). Next we analyzed the frequency and numbers of hematopoietic stem and progenitor cells in Tet2-mutant FLs. Interestingly, percentage of lineage negative (Lin−), Sca-1+, c-Kit+ (LSK) fraction that mainly consists of MPPs increased in Tet2gt/gt (1.45 +/− 0.62%) and Tet2+/gt FLs (1.21 +/− 0.43%) as compared to wild-type (WT) (0.85 +/−0.34%) FLs (Figure 2A and B). Absolute number of LSK cells was also increased in Tet2-mutant FLs (Suppl. Figure S3A). However, percentages and absolute numbers of CD150+LSK, CD150+CD48−LSK and CD34+LSK cells, highly enriched fractions of FL-HSCs, were not statistically different between WT and Tet2 mutants (Figure 2A and B, Suppl. Figure S3A). Analysis of myeloid-committed progenitor cells revealed that the frequency of common myeloid progenitors (CMPs) significantly increased in Tet2gt/gt FLs, whereas those of granulocyte-monocyte progenitors (GMPs) and megakaryocyte-erythroid progenitors (MEPs) did not (Figure 2A and C). The average cell number of Tet2gt/gt CMPs was higher than that of WT or Tet2+/gt CMPs, although the difference was not statistically significant (Suppl. Figure S3B). Taken together, disruption of Tet2 leads to the expansion of LSK cells and CMPs, but not highly purified HSCs in FLs.
Figure 2. Characterization of HSC, HPC and myeloid progenitor fractions in fetal livers of Tet2 gene-trap mice.
(A) Flow cytometric analysis of HSC/ HPC fractions (LSK, CD150+LSK, CD150+CD48−LSK) and myeloid progenitor fractions (CMP, GMP, MEP) in FLs was performed as described in Methods. Representative figures for each genotype are shown. The data represents the mean ± S.D. for each fraction (LSK; Tet2+/+: n = 14, Tet2+/gt: n = 23, Tet2gt/gt: n = 11, CD150+LSK, CD150+CD48−LSK, and myeloid progenitors; n = 3 for each genotype). (B and C) Percentages of each HSC/HPC fractions (B) or each myeloid progenitor fractions (C) within whole FL cells. The data represents the mean ± S.D. for each fraction (LSK; Tet2+/+: n = 14, Tet2+/gt: n = 23, Tet2gt/gt: n = 11, CD150+LSK, CD150+CD48−LSK, and myeloid progenitors; n = 3 for each genotype).
Self-renewal capacity of HSCs was dramatically enhanced by ablation of Tet2
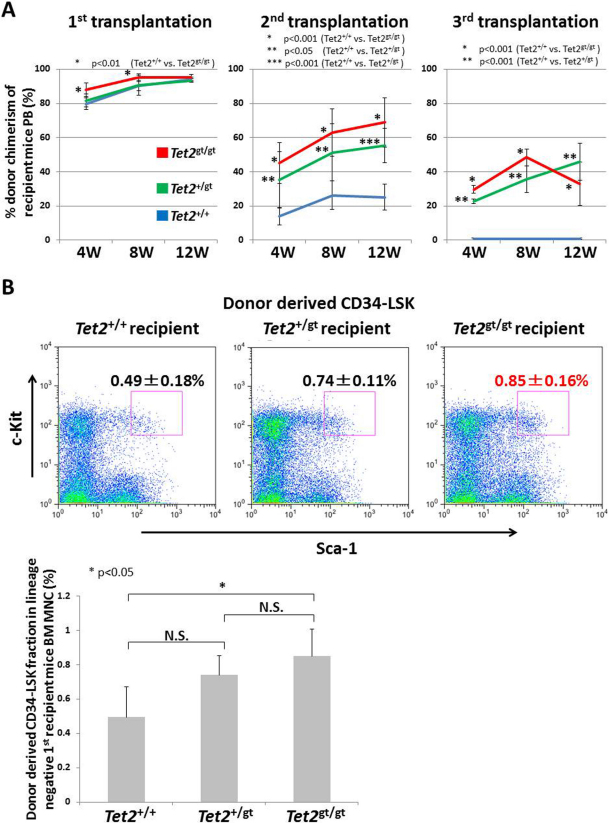
Increased LSK fraction in Tet2gt/gt FLs with expansion of the MPP population could be due to enhanced self-renewal capacity of FL-HSCs upon disruption of Tet2. To address this issue, we performed serial transplantation assay using FL cells from WT and Tet2-mutant embryos (Figure 3A). Transplantation of 1 x 106 FL cells with 2 x 105 competitor cells into lethally irradiated recipients resulted in over 80% peripheral engraftment in all genotypes. Strikingly however, secondary and tertiary transplant led to dramatically increased peripheral blood (PB) chimerism of Tet2gt/gt cells compared to WT cells. Interestingly, chimerism of Tet2+/gt cells was intermediate between WT and Tet2gt/gt which was statistically higher compared to WT in the secondary and the tertiary transplants, while the difference to Tet2gt/gt cells was not statistically significant. We speculated that FL-HSCs might expand in the engrafted microenvironment, and therefore went on to examine the fraction of donor-derived HSCs in the recipients' bone marrow (BM). As expected, percentage of donor-derived HSCs (CD34−LSK cells) in the recipient's marrow was significantly higher in Tet2gt/gt group (0.85 +/− 0.16%) compared to WT (0.49 +/− 0.18%) (Figure 3B). Tet2+/gt CD34−LSK cells again showed intermediate expansion (0.74 +/− 0.11%), the difference of which was not statistically significant against WT or Tet2gt/gt cells. To investigate whether enhanced engraftment of Tet2-mutant FL cells was due to the increased number or enhanced long-term repopulating (LTR) and self-renewal capacity of FL-HSCs, we transplanted equal number of highly purified FL-HSCs (CD34+LSK cells) from WT or Tet2gt/gt embryos with competitor cells and examined their engraftment in the recipients' PB (Suppl. Figure S4). Interestingly, Tet2gt/gt CD34+LSK cells showed higher engraftment as compared to WT cells, indicating that LTR/ self-renewal capacity of FL-HSCs was enhanced by Tet2-loss.
Figure 3. Serial transplantation of Tet2-mutant fetal liver cells.
(A) WT and Tet2-mutant FL cells (Ly5.2) were transplanted into lethally irradiated recipients (Ly5.1), and percentages of donor chimerism in recipient's PB were analyzed by flow cytometry at the indicated time points after transplantation. Serial transplantations were performed as described in Methods. The data represents the mean ± S.D. (1st transplantation; Tet2+/+: n = 4, Tet2+/gt and Tet2gt/gt: n = 5, 2nd and 3rd transplantation; Tet2+/gt: n = 4, Tet2+/+ and Tet2gt/gt: n = 5). (B) (Upper panel) Flow cytometric analysis of donor derived HSC fraction (CD34−LSK cells) within Lin− fraction of BM mononuclear cells in mice receiving the first transplants. Representative FACS pictures are shown. (Lower panel) Percentages of donor derived CD34−LSK cells within Lin− fraction of BM mononuclear cells in mice receiving the first transplants. The data represents mean ± S.D. (n = 3 for each recipient).
Taken together, these results clearly indicate that LTR and self-renewal capacity of FL-HSCs is enhanced by disruption of Tet2, and they can expand in the BM microenvironment of transplanted recipients.
Disruption of Tet2 impairs myeloid differentiation
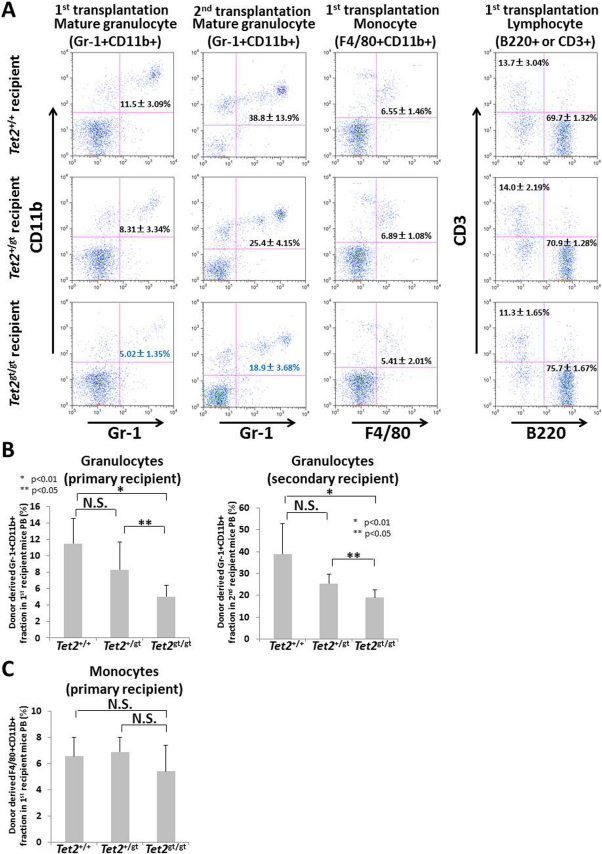
In serial transplantation experiment, multilineage differentiation potential of Tet2-mutant FL cells was grossly maintained throughout transplants. However, close examination of peripheral blood (PB) by flow cytometry revealed some alteration of myeloid differentiation in Tet2-mutant cells. Interestingly, significant decrease of Gr-1+CD11b+ mature granulocytes was observed in both primary and secondary recipients of Tet2gt/gt cells (Figure 4A and B), while F4/80+CD11b+ monocytes were not affected (Figure 4A and C). Of note, we observed slight increase of mature B cells accompanied by slight decrease of mature T cells in Tet2gt/gt cells, whose significance must be substantiated by further investigation (Figure 4A). Taken together, these data suggest that disruption of Tet2 not only affects HSC function, but also impose a significant defect on myeloid differentiation.
Figure 4. Differentiation of Tet2-mutant FL cells in transplanted recipient mice.
Fractions of mature granulocytes (Gr-1+CD11b+), monocytes (F4/80+CD11b+) and lymphocytes (B220+ or CD3+) in mice transplanted with WT or Tet2-mutant FL cells were analyzed at 12 weeks after the 1st or 2nd transplantation by flow cytometry. The data represents the mean ± S.D. (1st transplantation; Tet2+/+ recipient: n = 4, Tet2+/gt and Tet2gt/gt recipient : n = 5, 2nd transplantation; Tet2+/gt recipient : n = 4, Tet2+/+ and Tet2gt/gt recipient : n = 5). (A) Representative FACS pictures are shown. (B, C, D) Percentages of granulocytes (B), monocytes (C), and B or T cells (D) are shown on the graphs. The data are mean ± S.D.
Tet2 loss confers hematopoietic progenitor cells with resistance to differentiative stress
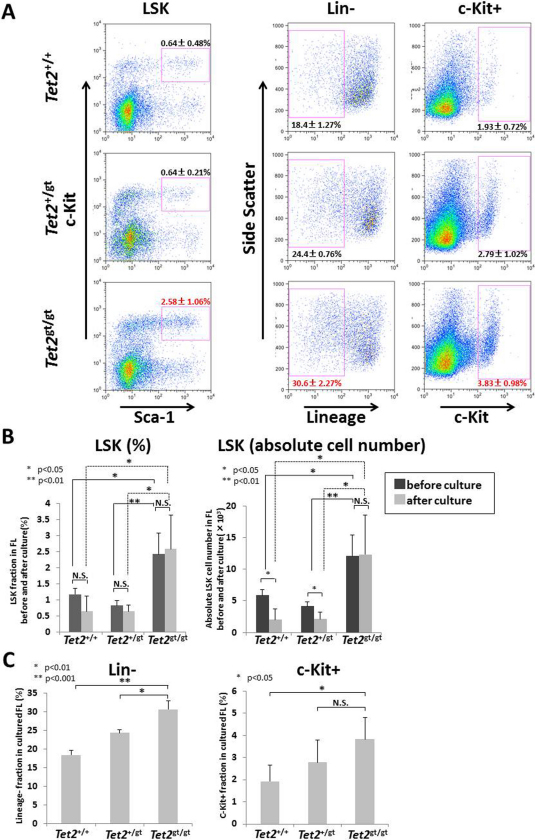
Enhanced self-renewal capacity is often accompanied by resistance to differentiating stimuli. We tested this hypothesis by culturing FL cells from WT or Tet2-mutant embryos under a differentiative condition and examined their surface phenotype by flow cytometry (Suppl. Figure S5A). The rate of proliferation was comparable between each genotypes (Suppl. Figure S5B). As shown in Figure 5, Tet2gt/gt FL cells contained higher fraction of immature cells such as LSK (Figure 5A and B), Lin− and c-Kit+ cells (Figure 5A and C) as compared to WT after a liquid culture for 7-days with cocktails of cytokines. Surprisingly, percentage and absolute number of LSK fraction of Tet2gt/gt cells did not drop after 7-days of culture, while the latter of Tet2+/+ and Tet2+/gt cells decreased approximately by half (Figure 5B). These results strongly suggest that Tet2 loss confers immature hematopoietic cells in FL with resistance to differentiation in in vitro culture condition.
Figure 5. Tet2-mutant FL cells are resistant to differentiative stress in liquid culture.
WT or Tet2-mutant FL cells were cultured for 7-days with various cytokines (rmSCF, rmIL-6, rhFLT3L, rhTPO, and rmIL-3), and LSK, Lin− and c-Kit+ cells were analyzed by flow cytometry as described in the Methods. (A) Representative FACS pictures of the cells after the cultures are shown. The data are mean ± S.D. (n = 3 for each genotype). (B) Percentages and absolute cell numbers of LSK cells before and after liquid culture for 7-days with various cytokines (rmSCF, rmIL-6, rhFLT3L, rhTPO, and rmIL-3). The data are mean ± S.D. (n = 3 for each genotype). (C) Percentages of Lin− or c-Kit+ cells after liquid culture for 7-days. The data are mean ± S.D. (n = 3 for each genotype).
Leukocytosis and HSC/ HPC expansion in adult heterozygous gene-trap mice
It was reported that myeloproliferation and extramedullary hematopoiesis occurred with age in Tet2−/− and Tet2+/− mice23,24. Since Tet2gt/gt mice did not survive to adulthood, we examined Tet2+/gt mice for evidence of myeloproliferation. Consistent with the previous reports, white blood cell (WBC) count in PB was significantly increased in Tet2+/gt mice at the age of 38-weeks (Suppl. Figure S6A). In addition, percentage of LSK fraction in Tet2+/gt BM was significantly higher compared to that of WT, while CD150+LSK cells and myeloid progenitors were not statistically different between Tet2+/gt and WT (Suppl. Figure S6B and C). In contrast however, signs of extramedullary hematopoiesis such as splenomegaly or expansion of HPCs in spleen were not evident in Tet2+/gt mice, and they did not develop fatal myeloproliferative disorder during an observation over 40-weeks. It is also interesting to note that there was no sign of myeloproliferation such as increased WBC count or expansion of mature myeloid cells in the Tet2gt/gt FL cell recipients with more than 80% donor chimerism at least until 12-weeks after transplantation (Suppl. Figure S7). Taken together, these data indicate that extensive myeloproliferation is not a frequent phenomenon in Tet2+/gt mice or mice transplanted with Tet2gt/gt FL cells, and suggest that additional factors must cooperate with Tet2 to develop myeloid transformation.
Discussion
Accumulating evidence suggests that altered regulation of cytosine hydroxymethylation is a critical pathogenic event in myeloid malignancies, such as MDS, MPN and AML. TET family proteins are reported to convert 5 mC to 5 hmC, and TET2 mutations found in myeloid malignancies disrupt this enzymatic functions. Moreover, it was recently reported that TET2 catalytic activity was inhibited by 2-hydroxyglutarate, an abnormal catalytic product generated by mutant IDH1 or IDH2 proteins that are frequently found in myeloid malignancies. These findings strongly suggest that dysregulation of 5 mC to 5 hmC conversion can be a critical step in myeloid transformation.
We showed that disruption of Tet2 in FL led to increased self-renewal and LTR capacity of FL-HSCs. Furthermore, LSK fraction that mainly consists of MPPs clearly increased in Tet2gt/gt FL, whereas both percentages and numbers of highly enriched FL-HSC fractions (CD150+LSK, CD150+CD48−LSK and CD34+LSK cells) were not significantly different between WT and Tet2gt/gt mice. These findings are consistent with the ones reported recently for BM cells, showing increased in vitro serial replating capacity and competitive advantage of Tet2−/− HSCs over WT cells23,24,25. Taken together with our data, it is suggested that Tet2 is critical for HSC/ HPC homeostasis in both FL and adult BM. It should be noted, however, that the previous studies have only examined competitive repopulating capacity of Tet2−/− HSCs in a single round of transplantation, and did not precisely addressed self-renewal capacity of HSCs by ‘serial' transplantation. In contrast, we performed serial transplantation assays and showed that Tet2gt/gt FL-HSCs presented dramatically increased PB chimerism over WT cells in secondary and tertiary recipients. Furthermore, CD34−LSK HSC fraction derived from Tet2gt/gt FL was significantly increased in the recipient's BM as compared to the one from WT, indicating that expansion of Tet2gt/gt HSCs is cell autonomous phenomenon and can occur in the BM microenvironment. Importantly, Tet2gt/gt cells showed only a mild impairment in myeloid differentiation. Taken together, these data clearly indicate that self-renewal capacity of FL-HSC is enhanced by inactivation of Tet2 without major defects on multilineage differentiation capacity.
Enhanced self-renewal capacity of HSCs by Tet2 inactivation is compatible with high incidence of TET2 mutation in MDS. MDS is characterized by an expansion of self-renewing malignant clone, which ultimately overrides normal hematopoiesis in the BM. Loss-of–function mutation of TET2 clearly endows HSCs with such fundamental feature of MDS, setting a molecular basis for acquiring additional mutations and disease progression. Since Tet2 mutation causes only a mild impairment in myeloid differentiation, it seems that clonal evolution of MDS to overt leukemia must include a step acquiring mutation that blocks differentiation.
We have also shown that CMP fraction, but not GMP and MEP, was significantly increased to 9.04 +/− 1.09% in Tet2gt/gt FL, as compared to 6.26 +/− 0.53% in WT. Two recent studies have described increased percentage of CMP and GMP, or increased absolute number (but not percentage) of CMP and MEP in the Tet2−/− BM23,24,25. Despite some differences in amplifying cell types, CMP amplification is commonly observed either in FL or in the BM. This is in fact consistent with high incidence of TET2 mutation in human myeloid tumors such as chronic myelomonocytic leukemia (CMML), which is characterized by extensive myeloproliferation and myelodysplasia. Recent studies actually reported extramedullary hematopoiesis and the following myeloid transformation in Tet2−/− mice that was reminiscent of human CMML23,24,25. They showed peripheral leukocytosis and splenomegaly with proliferation of myeloid elements occurring in aged mice. Although we could not examine adult Tet2gt/gt mice due to their perinatal lethality, analysis of Tet2+/gt mice over 30-weeks of age showed a significant increase of WBC counts in PB. However, in contrast to their results, expansion of HSCs/ HPCs and myeloid cells in spleen could not be documented in Tet2+/gt mice. Phenotypic discrepancies between the studies also exist in differentiation of myeloid cells in PB. We observed impaired differentiation of Tet2gt/gt FL cells to Gr-1+CD11b+ mature granulocytes in the transplanted recipients (Figure 4), whereas previous studies have shown the increase of mature granulocytes in PB of Tet2−/− mice23,24,25. On the other hand, shRNA-mediated knockdown of Tet2 or introduction of mutant IDH2 into murine BM cells resulted in decreased differentiation to granulocytes14, which is consistent with our data. These data suggest that the effect of Tet2 loss on myeloid differentiation can be affected by various experimental factors including the strategies for Tet2 targeting, the cell source (BM vs. FL) and levels of Tet2 expression. It is clear that disruption of Tet2 critically affects early and late stages of myeloid differentiation, however, revealing the precise molecular mechanism of myeloid regulation by Tet2 awaits future investigations.
Epigenetic modification is a fundamental process for stem cells to maintain pluripotency and capacity to self-renew. Although TET family protein is a major player in this process, the way in which they regulate self-renewal seems different between cell types or among family members. Tet1 is essential for self-renewal and maintenance of ES cells (ESCs) as shown by shRNA-mediated knockdown studies, and therefore it has a ‘positive' regulatory role in this process8. Interestingly however, Tet2 is clearly a ‘negative' regulator for self-renewal of HSCs as revealed by the present study and others. Therefore, while both Tet1 and Tet2 are critical for stemness, they work in opposite manner in regulating self-renewal. This may be the reflection of different cellular environment (such as epigenetic status) between ESCs and HSCs, or due to the different inherent function of Tet1 and Tet2. Revealing the molecular targets of these genes is absolutely essential for answering these questions.
In summary, we showed that Tet2 inactivation in FL resulted in enhanced LTR and self-renewal capacity of FL-HSCs and altered differentiation in myeloid lineage. Current data indicate that conversion of 5 mC to 5 hmC as well as 5-formylcytosine and 5-carboxylcytosine27 is a key enzymatic function of Tet2 in HSC regulation. However, there still might be unknown features of Tet2 that are essential for these processes. Moreover, recent study suggests that the role of TET proteins is not a mere transcriptional de-repressor, but they fine-tunes transcription either positively or negatively, acting as global regulators of transcription28. Further studies are definitely required to elucidate precise molecular mechanism by which TET2 regulates HSC stemness and hematopoietic differentiation.
Methods
Mice
C57BL/6 (B6) mice were from Japan CLEA Inc. (Tokyo, Japan), and B6-Ly5.1 mice were from Sankyo Lab Service Co. (Tsukuba, Japan). Tet2 gene trap mice were described previously26. All mice were housed and maintained under specific pathogen-free (SPF) condition. E14.5 embryos were used in all FL experiments. All animal experiments were reviewed and approved by the Internal Review Board of Keio University School of Medicine.
RT-PCR
Total RNA was extracted from FL cells using a TRIZOL Reagent (Invitrogen) according to the manufacturer's protocol. RNA was treated with RNase-free DNase I (Invitrogen) to remove contaminating genomic DNA. cDNA was reverse-transcribed using SuperScript II reverse transcriptase (Invitrogen). The quantity of cDNA was normalized according to the expression of GAPDH measured by real-time RT-PCR using a THUNDERBIRD SYBRqPCR Mix kit (TOYOBO) and StepOnePlusTM real-time PCR system (Applied Biosystems). Real-time PCR was peformed according to the manufacturer's protocol. Semi-quantitative RT-PCR was performed using Ex Taq–HS polymerase (TaKaRa Bio) as described previously29.
Dot blot assay
Genomic DNA was extracted from FL cells using a DNeasy Blood & Tissue Kit (QIAGEN) according to the manufacturer's protocol. DNA amount was measured using SmartSpec 3000 (BIO-RAD). DNA was manually spotted onto PROTRAN BA85 nitrocellulose membranes (Schleicher & Schuell). Membranes were first probed with anti-5-methylcytidine antibody (Eurogentec) or anti-5-hydroxymethylcytidine antibody (Active Motif), which were then probed with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin (Ig) polyclonal antibody. Bound antibodies were visualized by enhanced chemiluminescence (ECL; Amersham).
Analysis of fetal liver cells by flow cytometry
For the analysis of hematopoietic stem/progenitor cells and myeloid progenitor cells in FLs, FLs were dissected from E14.5 embryos and single cell suspension was made in phosphate-buffered saline (PBS). After lysing red blood cells in ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) at 37°C for 5 minutes, cells were spun and suspended in PBS supplemented with 5% fetal bovine serum (FBS). For HSC/ HPC (LSK, CD150+LSK, CD150+CD48−LSK) analysis, cells were stained with biotin anti-B220 (RAE-6B2, e-Bioscience), biotin anti-CD19 (6D5, BioLegend), biotin anti-Ter119 (TER-119, e-Bioscience), biotin anti-Gr-1 (RB6-8C5, BioLegend), phycoerythrin (PE)-Sca-1 (D7, e-Bioscience), allophycocyanin (APC)-c-Kit (2B8, e-Bioscience), Alexa488-CD150 (TC15-12F12.2, BioLegend), and biotin anti-CD48 (HM48-1, BioLegend), followed by staining with streptavidin-PE-Cy7 (BioLegend). For the analysis of myeloid progenitor cells (CMP, GMP, MEP), cells were stained with fluorescein isothyocyanate (FITC)-CD34 (RAM34, BD Pharmingen), PE-FcγRII/III (2.4G2, BD Pharmingen), APC-c-Kit, biotin anti-B220, biotin anti-CD19, biotin anti-Ter119, biotin anti-Gr-1, biotin anti-IL7Rα (A7R34, e-Bioscience) and biotin anti-Sca-1 (D7, BioLegend), followed by staining with streptavidin-PE-Cy7. Stained cells were analyzed by MoFlo (Beckman Coulter) or FACS Calibur (BD Bioscience).
Analysis of donor-derived mature blood cells and BM HSCs after transplantation
Percentages of donor chimerism together with differentiation to multiple lineages in recipient's PB were analyzed by flow cytometry at 4, 8, and 12 weeks after transplantation. After lysis of red blood cells, total white blood cells were stained with peridinin-chlorophyll proteins-cyanin 5.5 (PerCp-Cy5.5)-CD45.2 (104, BioLegend), or combination of the following monoclonal antibodies: FITC-Gr-1 (RB6-8C5, BD Pharmingen), PE-CD11b (M1/70, BD Pharmingen), FITC-F4/80 (BM8, BioLegend), FITC-B220 (RA3-6B2, BioLegend), and PE-CD3 (145-2C11, e-Bioscience). For analyzing donor-derived HSCs in the BM, cells were collected from bilateral femurs and tibias of the recipient mice 20 weeks after transplantation. Mononuclear cells were separated by density-gradient centrifugation using Lymphoprep (Axis-Shield Poc AS), and lineage-positive cells were depleted using Lineage Cell Depletion Kit (Miltenyi Biotec) according to the manufacturer's protocol. CD34−LSK cells were analyzed as described previously30. Briefly, lineage-negative cells were stained with FITC-CD34 (RAM34, BD Pharmingen), APC-Cy7-CD45.1 (A20, BioLegend), PE-Sca-1 (D7, e-Bioscience), APC-c-Kit (2B8, e-Bioscience), and an anti-lineage antibody cocktail in the Lineage Cell Depletion Kit, followed by staining with streptavidin-PE-Cy7. Cells were analyzed by FACS Calibur and MoFlow cytometer.
Serial transplantation assay
FL cells were separated from E14.5 embryos (Ly5.2) and suspended in IMDM supplemented with 10% FBS, 100 U/ml penicillin G, 100 μg/ml streptomycin, 50 μg/ml gentamicin and 2 mM L-glutamine. Competitor cells were collected from the BM of 7-week-old Ly5.1 mice. 1×106 whole fetal liver cells (Ly5.2) with 2×105 competitor BM cells (Ly5.1) were intravenously injected into lethally irradiated recipient mice (Ly5.1) through tail veins. For secondary or tertiary transplantation, 2×106 whole BM cells taken from the first or secondary recipient mice 12 weeks after transplantation were transplanted into lethally irradiated recipient mice (Ly5.1).
In vitro liquid culture assay
Whole FL cells were collected from E14.5 embryos and suspended in IMDM supplemented with 15% FBS, 100 U/ml penicillin G, 100 μg/ml streptomycin, 50 μg/ml gentamicin and 2 mM L-glutamine and cytokines (rmSCF 50 ng/mL, rmIL-6 50 ng/mL, rhFLT3L 50 ng/mL, rhTPO 50 ng/mL, rmIL-3 20 ng/mL). Cells were cultured in humidified atmosphere with 5% CO2 at 37°C, and split on day 2, 4, and 6 to keep cell density between 5 x105/ ml and 1 x106/ ml. Cell numbers were enumerated on day 2, 4, and 6. Cells were collected on day 7 for the analysis of LSK, lineage negative, and c-Kit positive cells.
Statistical analysis
All statistical analyses were performed using unpaired Student's t-test. P values<0.05 were considered statistically significant.
Author Contributions
HK performed research, analyzed the data, and wrote a part of the paper. YF, MS and KS performed research. YI and SO supervised the study. HN designed research, analyzed the data, provided financial and administrative support, and wrote the paper.
Supplementary Material
Supporting online material
Acknowledgments
We thank excellent technical assistance by Akiko Ito and Junko Kawakita. We also thank S. Suzuki (FACS Core Laboratory, Keio University School of Medicine) for FACS sorting and K. Takubo (Department of Cell Differentiation, The Sakaguchi Laboratory of Developmental Biology, Keio University School of Medicine) for FACS sorting and valuable advice. This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects.
Footnotes
Yasuo Ikeda is a member of Board of Directors of Chugai Pharmaceutical Co., Ltd.
References
- Corey S. J. et al. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer 7, 118–129 (2007). [DOI] [PubMed] [Google Scholar]
- Delhommeau F. et al. Mutation in TET2 in myeloid cancers. N Engl J Med 360, 2289–2301 (2009). [DOI] [PubMed] [Google Scholar]
- Langemeijer S. M. et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet 41, 838–842 (2009). [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab O. et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 114, 144–147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska A. M. et al. Loss of heterozygosity 4q24 and TET2 mutations associated with myelodysplastic/myeloproliferative neoplasms. Blood 113, 6403–6410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M. E. et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 17, 13–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. et al. Role of Tet proteins in 5 mC to 5 hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M. et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468, 839–843 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K. P. et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8, 200–213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis E. R. et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361, 1058–1066 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., He S., Yilmaz O. H., Kiel M. J. & Morrison S. J. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood 108, 737–744 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A. et al. Endomucin, a CD34-like sialomucin, marks hematopoietic stem cells throughout development. J Exp Med 202, 1483–1492 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Hemmati H. D., Wandycz A. M. & Weissman I. L. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A 92, 10302–10306 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Zhong R. K., Jordan C. T., Lemischka I. R. & Astle C. M. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp Hematol 25, 293–297 (1997). [PubMed] [Google Scholar]
- Park I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003). [DOI] [PubMed] [Google Scholar]
- Kajiume T., Ninomiya Y., Ishihara H., Kanno R. & Kanno M. Polycomb group gene mel-18 modulates the self-renewal activity and cell cycle status of hematopoietic stem cells. Exp Hematol 32, 571–578 (2004). [DOI] [PubMed] [Google Scholar]
- Ohta H. et al. Polycomb group gene rae28 is required for sustaining activity of hematopoietic stem cells. J Exp Med 195, 759–770 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K. et al. Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self-Renewal and Myeloid Transformation. Cancer Cell 20, 11–24 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivoron C. et al. TET2 Inactivation Results in Pleiotropic Hematopoietic Abnormalities in Mouse and Is a Recurrent Event during Human Lymphomagenesis. Cancer Cell 20, 25–38 (2011). [DOI] [PubMed] [Google Scholar]
- Li Z. et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Araki K., Li Z. & Yamamura K. Characterization of Ayu17-449 gene expression and resultant kidney pathology in a knockout mouse model. Transgenic Res 17, 599–608 (2008). [DOI] [PubMed] [Google Scholar]
- Ito S. et al. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science 333, 1300–1303 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi Y. et al. Comprehensive analysis of myeloid lineage conversion using mice expressing an inducible form of C/EBP alpha. EMBO J 25, 3398–3410 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata F. et al. Roundabout 4 is expressed on hematopoietic stem cells and potentially involved in the niche-mediated regulation of the side population phenotype. Stem Cells 27, 183–190 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T. et al. Characterization of an exchangeable gene trap using pU-17 carrying a stop codon-beta geo cassette. Dev Growth Differ 47, 163–172 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting online material