Abstract
Objective
The purpose of this article is to describe the effects of the pediatric antidepressant controversy on the Treatment of Serotonin-Selective Reuptake Inhibitor (SSRI) Resistant Depression in Adolescents (TORDIA) trial.
Method
Adolescents, ages 12–18 years, with SSRI resistant depression were randomized to one of four treatments for a 12 week trial: Switch to different SSRI, switch to an alternate antidepressant (venlafaxine), switch to an alternate SSRI plus cognitive behavior therapy (CBT), or switch to venlafaxine plus CBT.
Results
The health advisories and “black box” warnings regarding suicidality and antidepressants in adolescents occurred during the course of the TORDIA trial. Revisions to the protocol, multiple-consent form changes, and re-consenting of patients were necessary. Recruitment of participants was adversely affected.
Conclusion
Despite a cascade of unforeseen events that delayed the completion of the study, the TORDIA trial resulted in clinically important information about treatment-resistant depression in adolescents.
Introduction
There is a continued need to identify effective treatments for psychiatric disorders in youth. Unfortunately, conducting clinical trials in the pediatric population has challenges. Since children are a vulnerable population, ethical issues are foremost in the conduct of treatment studies (Field and Behrman 2004; Tan and Koelch 2008). Parents should make an informed decision to consent for their children to participate in a treatment study. Parents and their children need to be informed about the specific purpose of the trial and procedures during the study (Koelch et al. 2009). The potential benefits of treatment need to be weighed against the risk of participation in the study (McIntosh et al. 2000; Field and Behrman 2004). New information that becomes available about the treatments in a trial should be explained to parents, as it may alter the risk-benefit ratio.
Recruitment and retention of participants in clinical trials is key to the successful completion of the trial (Furimsky et al. 2008). Recruiting and retaining adolescents is especially challenging (McClure et al. 2004). To ensure adequate sample size, pediatric clinical trials are often conducted at multiple sites. Although multisite trials enhance recruitment, these trials pose more organizational, management, and administrative demands than single-site studies (Fuhrer 2005). Importantly, each site's Institutional Review Board (IRB) acts independently of one another. There can be marked variability in the ethics review at each of these sites and due to lack of standardized forms, differences in the experience of board members, institutional culture, and regional thinking (Gold and Dewa 2005). Given the sensitivity of conducting research with children, each IRB's threshold for risk-benefit ratio of the proposed treatment or protocol revisions may vary.
Pretrial planning has been emphasized as an additional way to ensure the success of clinical studies (Curtis et al. 2009). Parents of child subjects anticipate that clinical studies will proceed as expected (Wagner et al. 2006). However, despite adequate preplanning, a well-designed study, and organized administrative oversight, there can be unforeseen events that affect a treatment study. The purpose of this article is to describe the impact of the antidepressant suicide controversy, which emerged in 2003, on the conduct of the multisite Treatment of serotonin-selective reuptake inhibitor (SSRI)-Resistant Depression in Adolescents (TORDIA) trial.
Description of TORDIA Trial
The TORDIA trial was a six-site, National Institute of Mental Health-Funded study that enrolled participants from February 2001 to August 2006. Adolescents, aged 12–18 years, who had been in treatment for major depressive disorder and had clinically significant depression (Children's Depression Rating Scale-Revised total score of at least 40 and a Clinical Global Impressions-Severity subscale of at least 4 [moderate severity]) who had not responded to an adequate 8 week course of an SSRI were eligible to participate in the study. Participants were randomized to one of four treatments: Switch to a different SSRI, switch to an alternate antidepressant (venlafaxine), switch to an alternate SSRI plus cognitive behavior therapy (CBT), or switch to venlafaxine plus CBT. As initially designed, in the SSRI switch treatment arm, participants were randomized to either paroxetine or fluoxetine. The acute phase study was 12 weeks; responders were eligible to continue in their treatment arm, and nonresponders received open treatment for an additional 12 weeks.
The study was approved by each site's IRB. All parents or guardians gave written informed consent, and adolescents gave written informed assent for participation in this study. A National Institute of Mental Health–Constituted Data and Safety Monitoring Board (DSMB) monitored recruitment, outcomes, and adverse events on a quarterly basis. The University of Pittsburgh was the coordinating site.
Unforeseen Events
June 2003
Paroxetine
The Food and Drug Administration (FDA) issued a statement about a possible increased risk of suicidal thinking and suicide attempts in children and adolescents under the age of 18 years treated with paroxetine (Paxil) for major depressive disorder (U.S. Food and Drug Administration 2003b). The FDA recommended that paroxetine not be used to treat depression in children and adolescents. For patients who were currently being treated with paroxetine, the FDA recommended that the medication not be abruptly discontinued and that medication changes should occur under medical supervision. Similar warnings were issued by the United Kingdom's Chairman of the Committee on Safety of Medicines (CSM) during this same time period (Medicines and Health Care Products Regulatory Agency 2003a).
At that time, TORDIA participants randomized to the different SSRI study condition had a 50% chance of receiving paroxetine. The FDA recommendation regarding paroxetine necessitated a significant revision to the TORDIA protocol. No further randomization to paroxetine was allowed at any site. Additionally, there was no further recruitment of potential participants who were being prescribed paroxetine. All sites were required to submit and seek approval of these changes from their respective IRBs.
Since some earlier participants had received paroxetine and some participants were currently receiving paroxetine, it was necessary to inform them of this new information regarding paroxetine and suicidality. Letters were immediately sent to previous participants and their parents informing them of both the FDA and CSM recommendations that paroxetine not be used for the treatment of depression in children and adolescents. The parents were also informed as to whether their child had or had not taken paroxetine as a part of the TORDIA trial. For those participants who had previously taken paroxetine during the study, an in-person appointment was scheduled to discuss the issue of suicidality. For those three participants currently receiving paroxetine in this study, an appointment was scheduled in which the medication was tapered and discontinued. All participants who were tapered from paroxetine were offered an alternate medication based on the clinical judgment of the study physician. These participants were followed for 12 weeks of open treatment consisting of medication, therapy, or both.
A decision by the TORDIA investigators was made that citalopram would replace paroxetine as a randomization option in the study. All sites were required to inform and seek approval of their IRBs for this protocol change.
Venlafaxine
The FDA released a clinical report of two unpublished efficacy studies indicating that venlafaxine (Effexor) was not more effective than placebo in the treatment of pediatric depression.
Since half of the participants in the TORDIA study would be randomized to venlafaxine, all sites IRBs were informed about these venlafaxine findings. The consent form for participants was modified to include information about the lack of efficacy of venlafaxine in the treatment of pediatric depression. The DSMB was also provided this information.
August 2003
The Wyeth Corporation sent a letter to all the U.S. health care professionals about the use of Effexor for children and adolescents with major depressive disorder (Wyeth Pharmaceuticals 2003). In their pediatric depression clinical trials, there were increased reports of hostility and suicide-related adverse events such as suicidal ideation and self-harm. Rates of hostility were 2% for venlafaxine XR treated patients compared with <1% for placebo, and rates of suicidal ideation were 2% for venlafaxine XR treated patients compared with 0% for placebo. All sites were required to inform and obtain approval from their IRBs of this new information regarding venlafaxine. Consent forms were modified to include this information. In addition, letters were sent to patients and their parents to inform them of the findings regarding hostility and suicidality with venlafaxine.
September 2003
The United Kingdom's Chairman of the CSM released a report recommending that venlafaxine XR not be used for the treatment of depression in youth under the age of 18 years (Medicines and Health Care Products Regulatory Agency 2003b). All sites informed their IRBs about this new information. The coordinating site was informed by their IRB that enrollment should be immediately suspended pending further review by the IRB and DSMB. All sites were informed and immediately suspended enrollment pending approval by the University of Pittsburgh IRB, local site IRBs, and the DSMB.
The DSMB reviewed an analysis by the treatment arm of the relevant safety data, including serious adverse events and treatment emergent suicidality. This analysis was not suggestive of increased human subject risk with the study medications. However, all sites were requested to increase clinical monitoring for participants. Safety assessments were increased to weekly assessments. The 12-item Clinician Weekly Monitoring Scale was administered weekly to assess adverse events that may be associated with antidepressants. Items assessed included problems such as sleeping, irritability, hostility, aggression, restlessness/agitated/anxious/nervous, tremors, overly excited, emotional lability, disinhibition, and altered thinking, and nonsuicidal self injurious behavior. Suicidal ideation and behavior were monitored weekly using the Suicide Severity Rating Scale (SSRS) (Posner et al. 2007). This is a two-item scale that rates suicidal ideation on a 0 to 5 scale (no ideation to suicidal ideation with intent and a clear plan) and suicidal behavior on a 0 to 5 scale (no behavior to multiple attempts). A suicidal adverse event was defined as a two-point change on either the suicidal ideation or suicidal behavior scale on the SSRS.
Participants and parents were provided with an information sheet that described warning signs for suicidality and actions to be taken should this occur. All sites submitted this new information to their IRBs for review and approval.
October 2003
The FDA issued an alert regarding the occurrence of suicidality (both suicidal ideation and suicidal attempts) in clinical trials of antidepressant medications for pediatric patients with major depressive disorder (U.S. Food and Drug Administration 2003a). Results of a preliminary review of eight antidepressants drugs including citalopram, fluoxetine, and venlafaxine were reported. Although the FDA noted that an association between the use of these drugs and increased suicidal thoughts or actions by pediatric patients has not been established, the agency stated that it is not possible to rule out an increased risk of suicidal thoughts or actions for any of these drugs and that additional data and analysis and public discussion of the available data are needed. This FDA warning statement was submitted to all sites IRBs, and the consent form and protocol were revised to reflect this new information.
March 2004
The FDA asked manufacturers of antidepressants to include in their label a warning statement that recommends close observation of patients treated with antidepressants for worsening depression or the emergence of suicidality (U.S. Food and Drug Administration 2004b). The antidepressants in the warning included fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, escitalopram, bupropion, venlafaxine, nefazadone, and mirtazapine. All sites submitted this FDA warning to their IRBs for review, and consent forms were modified if requested by the IRB to reflect this FDA statement.
June 2004
The FDA and Wyeth Pharmaceuticals notified health care professionals that neonates exposed to venlafaxine or SSRIs in the third trimester of pregnancy have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding (Wyeth Pharmaceuticals 2004). All sites were required to submit this information to their IRBs for review and to modify their consent forms.
September 2004
The FDA completed its review of antidepressants and suicidality. An increased risk (2%) of suicidal thoughts or behavior was found among patients taking antidepressants compared with those taking placebo. An FDA joint advisory committee recommended that a “black box” warning be added to all antidepressants regarding the increased risk of suicidality in pediatric patients. All sites submitted this new information to their IRBs for review.
October 2004
The FDA released a Public Health Advisory announcing a multipronged strategy to warn the public about the increased risk of suicidality in children and adolescents treated with antidepressant medications (U.S. Food and Drug Administration 2004a). Manufacturers were directed to add a “black box” warning for all antidepressants to describe the risk of suicidality and to emphasize the need for close monitoring of patients. The FDA also decided that a Patient Medication Guide needed to be developed to explain the risks of suicidality and appropriate course of action. This Public Health Advisory was submitted by all sites to their IRBs for review, and consent forms were revised to reflect this new information.
July 2006
The FDA released two Public Health Advisories regarding antidepressants (U.S. Food and Drug Administration 2006a, 2006b). One advisory included information from two studies about benefits (decreased relapse rates for maternal depression) and risks (persistent pulmonary hypertension in neonates) of antidepressants in pregnancy. The other advisory discussed the risk of serotonin syndrome when taking triptans and certain types of antidepressant medications. All sites were requested to submit this new information to their IRBs for review, and consent forms were modified to reflect this new information.
October 2006
Wyeth Corporation released a Dear Healthcare Provider letter about the risk of overdose of venlafaxine SR primarily in combination with alcohol and other drugs (Wyeth Pharmaceuticals 2006). All sites submitted the Wyeth letter to their IRBs for review, and consent forms were modified as needed.
Discussion
Effect on study
The FDA warnings and subsequent events beginning in 2003 raised the question of whether the TORDIA trial could continue after 2 years of enrollment. Every time a new warning or health advisory was sent from the FDA or pharmaceutical companies, each of the sites was required to submit this new information to their IRBs for review. Since this was a multisite study, each IRB acted independently so that individual investigators were uncertain as to whether their IRB would allow the study to continue at their site. Moreover, it was essential that the coordinating site receive IRB approval to continue this study; otherwise, it would not be possible for the individual sites to continue with study recruitment. In addition, the DSMB had to approve continuation of the study.
New findings regarding antidepressants necessitated multiple consent form changes. Participants and their parents needed to be contacted about safety issues related to antidepressants and be re-consented to participate in the trial.
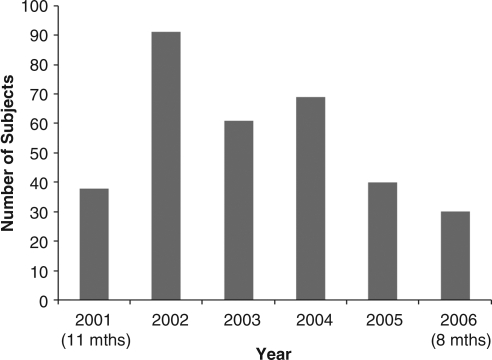
After the June 2003 FDA warning about Paxil, there was a substantial negative impact on recruitment into the study. During September 2003 to December 2003, enrollment into the study was suspended. Enrollment by year is shown in Figure 1. From 2003 to the completion of the study, enrollment numbers never achieved those of 2002, the year before the FDA warnings regarding antidepressant use in children and adolescents (Fig. 1).
FIG. 1.
Enrollment in Treatment of Resistant Depression in Adolescents (TORDIA) Study.
The study design needed to be altered to address these FDA warnings. After 181 of 334 participants had been enrolled, one of the treatment options in the SSRI switch was changed from paroxetine to citalopram. Participants in the first half of the study had self-harm adverse events monitored by spontaneous report. The remaining 153 participants in the latter half of the study were monitored by systematic weekly assessment of suicidal ideation and behavior as well as adverse events associated with antidepressants such as irritability, lability, and hostility using the SSRS scale and the Clinician Weekly Monitoring Scale.
This was one of the first clinical trials to use the SSRS in real time. During this trial, we were able to establish reliablility of the measure (Brent et al. 2009). The Clinical Weekly Monitoring Scale was developed to try to identify specific side effects that might be precursors or correlates of suicidal behavior. The measure was reliable (intraclass correlation coefficient (ICC) of individual items 0.6 to 1.0, p's<.001) and showed good internal consistency (Cronbach's α=0.69, nine items). Factor Analysis identified four factors explaining 67% of the variance: (1) Irritability, Restless/agitated/anxious/nervous, Emotional lability; (2) Hostility, Aggression; (3) Tremors, Altered Thinking; and (4) Problems sleeping, Non-suicidal self-injury behavior. Participants who experienced suicidal events had higher scores on these factors; however, the association was not statistically significant (p's>0.05).
Findings from the TORDIA trial
Despite the cascade of unforeseen events that affected the investigators, participants, and the trial protocol, the results from the TORDIA study have provided valuable clinical information about SSRI treatment resistant depression in adolescents.
In adolescents with SSRI treatment resistant depression,
Approximately 50% adolescents will respond to an alternate antidepressant (Brent et al. 2008).
Response rates are similar for a switch to another SSRI (47%) or to an alternative class of medication such as venlafaxine (48%) (Brent et al. 2008).
Venlafaxine is associated with more side effects during acute treatment, and higher suicidality and lower self-reported depression over long-term follow-up (Brent et al. 2008; Vitiello et al. 2011)
The combination of CBT and a switch to another antidepressant yields a higher response rate (55%) than a medication switch alone (41%) (Brent et al. 2008).
Adolescents who have more than nine CBT sessions are 2.5 times more likely to have adequate treatment response than those who had 9 or few sessions (Kennard et al. 2009).
Substance use is common (28%) among adolescents with treatment resistant depression and is associated with poorer treatment response (Goldstein et al. 2009).
Systematic monitoring of suicidal and nonsuicidal self-injury detects higher rates of these events than spontaneous report (Brent et al. 2009).
Suicide events are predicted by high baseline suicidal ideation, family conflict, and drug and alcohol use (Brent et al. 2009).
Less severe depression, less family conflict, and lack of nonsuicidal self-injurious behavior predicts better treatment response (Asarnow et al. 2009).
A better response to combination treatment versus medication alone was predicted by a greater number of comorbid conditions, no abuse history, and lower hopelessness (Asarnow et al. 2009).
FKBP5 polymorphisms are associated with suicidal events (Brent et al. 2010).
Approximately one-third of adolescents will achieve remission by 24 weeks of treatment, and there is a greater likelihood of remission for those adolescents who had a clinical response by 12 weeks (Emslie et al. 2010).
One-fourth of remitted adolescents experience a relapse,, but around 60% will achieve remission through 72 weeks of treatment (Vitiello et al. 2011).
Treatment response to combination treatment, compared with medication alone, was lower in those adolescents who had a history of physical abuse (Shamseddeen et al. 2011).
Clinical response to citalopram and fluoxetine may be related to antidepressant plasma concentration (Sakolsky et al. 2011).
Higher adherence to the prescribed medication regimen predicted treatment response (Woldu et al. 2011).
Combined CBT plus medication decreases the number of days with depression but is more costly than medication alone (Lynch et al. 2011).
Conclusion
Despite the numerous difficulties, NIMH, our DSMB, and each of the sites and investigators remained committed to the study, which allowed us to see it to fruition. We are especially grateful to the participants and families who had to read and sign multiple versions of consent forms and were still willing to commit to completing the protocol.
To recruit a treatment-resistant sample, we needed to allow participants who were actively suicidal. Nearly 60% had self-rated suicidal ideation at intake that was above the established clinical cut-offs. In collaboration with our IRBs and the DSMB, we developed protocols for monitoring and managing that have been adapted by other investigators for other treatment protocols. We were able to demonstrate that suicidal patients could be safely managed in the context of a clinical trial and did not need to be systematically excluded from study, as they had been in the past.
Clinical Significance
As investigators, we have learned that during the course of a trial, new publically available information about the efficacy and safety of the study medications may result in numerous protocol and consent form modifications. A single IRB that has authority to approve these modifications, rather than use of multiple independent site IRBs, would expedite this process. We have also learned that patience, persistence, equanimity, and collegiality are keys to successful study completion.
Disclosures
Dr. Karen Wagner (for the period 4/2008–4/2011) has received honoraria from American Psychiatric Association, American Institute of Biological Sciences, Physicians Postgraduate Press, CMP Medica, American Academy of Child and Adolescent Psychiatry, Madison Institute of Medicine, NIH, Mexican Psychiatric Association, Contemporary Forums, Doctors Hospital at Renaissance, UBM Medica, Quantia Communications, CME LLC, Nevada Psychiatric Association, Letters and Sciences, and American Society of Clinical Psychopharmacology. In the past, Dr. Wagner received research support and/or was a consultant to Forest, GlaxoSmithKline, Lilly, Wyeth-Ayerst, and Pfizer.
Dr. Martin Keller is or has been a consultant or received honoraria from Abbott, CENEREX, Cephalon, Cypress Bioscience, Cyberonics, Forest Laboratories, Medtronic, Organon, Novartis, Pfizer, Solvay, Wyeth, and Glaxo-Smith-Kline. He has received grants or research funding from Pfizer and Wyeth. He is or has been on an advisory board for Abbott Laboratories, CENEREX, Cyberonics, Cypress Bioscience, Forest Laboratories, Neuronetics, Novartis, Organon, Pfizer, and Glaxo-Smith-Kline.
Dr. David Brent receives research support from the National Institute of Mental Health. He receives royalties from Guilford Press and is UpToDate Psychiatry Editor.
Dr. Graham Emslie has received research support from the National Institute of Mental Health, Biobehavioral Diagnostics, Inc., Eli Lilly, Forest Laboratories, GlaxoSmithKline, and Somerset; he has been a consultant for Biobehavioral Diagnostics, Inc., Eli Lilly, Forest Laboratories, GlaxoSmithKline, INC Research Inc., Lundbeck Pfizer, Seaside, Shire Pharmaceuticals, Validus Pharmaceuticals, and Wyeth Pharmaceuticals; and has served on the Speakers Bureau for Forest Laboratories.
Authors' Vitiello, Clarke, Porta, Ryan, Ritz, Iyengar, Zelanzny, and Onorato have no disclosures to report.
References
- Asarnow JR. Emslie G. Clarke G. Wagner KD. Spirito A. Vitiello B. Iyengar S. Shamseddeen W. Ritz L. Mccracken J. Strober M. Suddath R. Leonard H. Porta G. Keller M. Brent D. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: Predictors and moderators of treatment response. J Am Acad Child Adolesc Psychiatry. 2009;48:330–339. doi: 10.1097/CHI.0b013e3181977476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D. Emslie G. Clarke G. Wagner KD. Asarnow JR. Keller M. Vitiello B. Ritz L. Iyengar S. Abebe K. Birmaher B. Ryan N. Kennard B. Hughes C. DeBar L. McCracken J. Strober M. Suddath R. Spirito A. Leonard H. Melhem N. Porta G. Onorato M. Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D. Melhem N. Ferrell R. Emslie G. Wagner KD. Ryan N. Vitiello B. Birmaher B. Mayes T. Zelazny J. Onorato M. Devlin B. Clarke G. DeBar L. Keller M. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Am J Psychiatry. 2010;167:190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA. Emslie GJ. Clarke GN. Asarnow J. Spirito A. Ritz L. Vitiello B. Iyengar S. Birmaher B. Ryan ND. Zelazny J. Onorato M. Kennard B. Mayes TL. Debar LL. McCracken JT. Strober M. Suddath R. Leonard H. Porta G. Keller MB. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166:418–426. doi: 10.1176/appi.ajp.2008.08070976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BM. Barrett BJ. Parfrey PS. The design of randomized controlled trials. Methods Mol Biol. 2009;473:95–111. doi: 10.1007/978-1-59745-385-1_5. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Mayes T. Porta G. Vitiello B. Clarke G. Wagner KD. Asarnow JR. Spirito A. Birmaher B. Ryan N. Kennard B. DeBar L. McCracken J. Strober M. Onorato M. Zelazny J. Keller M. Iyengar S. Brent D. Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. Am J Psychiatry. 2010;167:782–791. doi: 10.1176/appi.ajp.2010.09040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ. Behrman RE. Ethical Conduct of Clinical Research Involving Children. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- Fuhrer MJ. Conducting multiple-site clinical trials in medical rehabilitation research. Am J Phys Med Rehabil. 2005;84:823–831. doi: 10.1097/01.phm.0000184103.57599.01. [DOI] [PubMed] [Google Scholar]
- Furimsky I. Cheung AH. Dewa CS. Zipursky RB. Strategies to enhance patient recruitment and retention in research involving patients with a first episode of mental illness. Contemp Clin Trials. 2008;29:862–866. doi: 10.1016/j.cct.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Gold JL. Dewa CS. Institutional review boards and multisite studies in health services research: Is there a better way? Health Serv Res. 2005;40:291–307. doi: 10.1111/j.1475-6773.2005.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI. Shamseddeen W. Spirito A. Emslie G. Clarke G. Wagner KD. Asarnow JR. Vitiello B. Ryan N. Birmaher B. Mayes T. Onorato M. Zelazny J. Brent DA. Substance use and the treatment of resistant depression in adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:1182–1192. doi: 10.1097/CHI.0b013e3181bef6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard BD. Clarke GN. Weersing VR. Asarnow JR. Shamseddeen W. Porta G. Berk M. Hughes JL. Spirito A. Emslie GJ. Keller MB. Wagner KD. Brent DA. Effective components of TORDIA cognitive-behavioral therapy for adolescent depression: Preliminary findings. J Consult Clin Psychol. 2009;77:1033–1041. doi: 10.1037/a0017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelch M. Singer H. Prestel A. Burkert J. Schulze U. Fegert JM. “… because I am something special” or “I think I will be something like a guinea pig”: Information, assent of legal minors in clinical trials—assessment of understanding, appreciation, reasoning. Child Adolesc Psychiatry Ment Health. 2009;3:2. doi: 10.1186/1753-2000-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch FL. Dickerson JF. Clarke G. Vitiello B. Porta G. Wagner KD. Emslie G. Asarnow JR., Jr. Keller MB. Birmaher B. Ryan ND. Kennard B. Mayes T. Debar L. McCracken JT. Strober M. Suddath RL. Spirito A. Onorato M. Zelazny J. Iyengar S. Brent D. Incremental cost-effectiveness of combined therapy vs medication only for youth with selective serotonin reuptake inhibitor-resistant depression: Treatment of SSRI-resistant depression in adolescents trial findings. Arch Gen Psychiatry. 2011;68:253–262. doi: 10.1001/archgenpsychiatry.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure CA. Gray G. Rybczyk GK. Wright PF. Challenges to conducting HIV preventative vaccine trials with adolescents. J Acquir Immune Defic Syndr. 2004;36:726–733. doi: 10.1097/00126334-200406010-00010. [DOI] [PubMed] [Google Scholar]
- McIntosh N. Bates P. Brykczynska G. Dunstan G. Goldman A. Harvey D. Larcher V. McCrae D. McKinnon A. Patton M. Saunders J. Shelley P. Guidelines for the ethical conduct of medical research involving children. Royal College of Paediatrics, Child Health: Ethics Advisory Committee. Arch Dis Child. 2000;82:177–182. doi: 10.1136/adc.82.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicines and Health Care Products Regulatory Agency. Safety of seroxat (paroxetine) in children and adolescents under 18 years-contraindication in the treatment of depressive illness. Message from Professor G Duff, Chairman of the committee on safety of medicines. Jun 10, 2003a. www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con019507.pdf. [Mar 17;2011 ]. www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con019507.pdf
- Medicines and Health Care Products Regulatory Agency. Safety of velafaxine in children and adolescents under 18 years in the treatment of depressive illness. Message from Professor G Duff, Chairman of the committee on safety of medicines. Sep 19, 2003b. www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con019501.pdf. [Mar 17;2011 ]. www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con019501.pdf
- Posner K. Oquendo MA. Stanley B. Davies M. Gould M. Columbia Classification Algorithm of Suicide Assessment (C-CACA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakolsky DJ. Perel JM. Emslie GJ. Clarke GN. Wagner KD. Vitiello B. Keller MB. Birmaher B. Asarnow JR. Ryan ND. McCracken JT. Strober MJ. Iyengar S. Porta G. Brent DA. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31:92–97. doi: 10.1097/JCP.0b013e318204b117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddeen W. Asarnow JR. Clarke G. Vitiello B. Wagner KD. Birmaher B. Keller MB. Emslie G. Iyengar S. Ryan ND. McCracken JT. Porta G. Mayes T. Brent DA. Impact of physical and sexual abuse on treatment response in the treatment of resistant depression in adolescent study (TORDIA) J Am Acad Child Adolesc Psychiatry. 2011;50:293–301. doi: 10.1016/j.jaac.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JO. Koelch M. The ethics of psychopharmacological research in legal minors. Child Adolesc Psychiatry Ment Health. 2008;2:39. doi: 10.1186/1753-2000-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. FDA Public Health Advisory, reports of suicidality in pediatric patients being treated with antidepressant medications for major depressive disorder (MDD) Oct 27, 2003a. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm168828.htm. [Mar 17;2011 ]. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm168828.htm
- U.S. Food and Drug Administration. FDA Statement regarding the anti-depressant paxil for pediatric population. FDA Talk Paper. Jun 19, 2003b. www.ahrp.org/infomail/0603/19a.php. [Mar 17;2011 ]. www.ahrp.org/infomail/0603/19a.php
- U.S. Food and Drug Administration. FDA Public Health Advisory: FDA launches a multi-pronged strategy to strengthen safeguards for children treated with antidepressant medications. Oct 15, 2004a. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108363.htm. [Mar 17;2011 ]. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108363.htm
- U.S. Food and Drug Administration. FDA Public Health Advisory, worsening depression and suicidality in patients being treated with antidepressant medications. Mar 22, 2004b. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm161696.htm. [Mar 17;2011 ]. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm161696.htm
- U.S. Food and Drug Administration. Public Health Advisory—Combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. Jul 19, 2006a. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm124349.htm. [Mar 17;2011 ]. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm124349.htm
- U.S. Food and Drug Administration. Public Health Advisory: Treatment challenges of depression in pregnancy and the possibility of persistent pulmonary hypertension in newborns. Jul 19, 2006b. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm124348.htm. [Mar 17;2011 ]. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm124348.htm
- Vitiello B. Emslie G. Clarke G. Wagner KD. Asarnow JR. Keller MB. Birmaher B. Ryan ND. Kennard B. Mayes TL. Debar L. Lynch F. Dickerson J. Strober M. Suddath R. McCracken JT. Spirito A. Onorato M. Zelazny J. Porta G. Iyengar S. Brent DA. Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: A follow-up study of the TORDIA sample. J Clin Psychiatry. 2011;72:388–396. doi: 10.4088/JCP.09m05885blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KD. Martinez M. Joiner T. Youths' and their parents' attitudes and experiences about participation in psychopharmacology treatment research. J Child Adolesc Psychopharmacol. 2006;16:298–307. doi: 10.1089/cap.2006.16.298. [DOI] [PubMed] [Google Scholar]
- Woldu H. Porta G. Goldstein T. Sakolsky D. Perel J. Emslie G. Mayes T. Clarke G. Ryan ND. Birmaher B. Wagner KD. Asarnow JR. Keller MB. Brent D. Pharmacokinetically and clinician-determined adherence to an antidepressant regimen and clinical outcome in the TORDIA trial. J Am Acad Child Adolesc Psychiatry. 2011;50:490–498. doi: 10.1016/j.jaac.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth Pharmaceuticals. Letter to health professionals. Aug 22, 2003. www.antidepressantsfacts.com/2003-08-22-Wyeth-Effexor-kids.pdf. [Mar 17;2011 ]. www.antidepressantsfacts.com/2003-08-22-Wyeth-Effexor-kids.pdf
- Wyeth Pharmaceuticals. Letter to health professionals. Jun 3, 2004. www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM166425.pdf. [Mar 17;2011 ]. www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM166425.pdf
- Wyeth Pharmaceuticals. Letter to health professionals. Oct 17, 2006. www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM153177.pdf. [Mar 17;2011 ]. www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM153177.pdf