Abstract
Purpose
STAT3 is constitutively active in human pancreatic cancer cells and can promote cell growth and apoptosis resistance that contribute to tumorigenesis. We determined if sorafenib, a multikinase inhibitor, can induce apoptosis by targeting STAT3 signaling to enhance apoptosis induction by TRAIL.
Experimental Design
Human pancreatic cancer cell lines (PANC-1 and BxPC-3) were pre-incubated with sorafenib (Nexavar®) alone or followed by TRAIL. Apoptosis was determined by Annexin V labeling, caspase cleavage and Bax/Bak activation. Protein expression was analyzed by immunoblotting. Knockdown of STAT3, Mcl-1 and Bim were achieved by lentiviral shRNA. Adenoviral dominant negative (DN) or retroviral constitutively active (CA) STAT3 were also utilized.
Results
Sorafenib inhibited constitutive STAT3 phosphorylation (Tyr705) and suppressed Mcl-1 and Bcl-xL proteins in a dose- and time-dependent manner. CA-STAT3 overexpression was shown to attenuate caspase-3 cleavage and suppression of Mcl-1 by sorafenib. STAT3 knockdown or a DN STAT3 was shown to down-regulate Mcl-1 and Bcl-xL and to sensitize cells to TRAIL-mediated apoptosis. Treatment with sorafenib enhanced TRAIL-induced Annexin V staining and release of mitochondrial cytochrome c and AIF. Since the BH3-only Bim protein is a potent inducer of mitochondrial apoptosis, Bim knockdown was shown to attenuate caspase-3,-9 cleavage and Bax/Bak activation by sorafenib plus TRAIL.
Conclusions
Suppression of STAT3 by genetic means or using sorafenib was shown to down-regulate Mcl-1 and Bcl-xL and to sensitize cells to TRAIL-mediated apoptosis. These data indicate that targeting STAT3 may enhance treatment efficacy against pancreatic cancer.
Keywords: sorafenib, STAT3, Mcl-1, TRAIL, pancreatic cancer
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States (1). The intrinsic resistance of this malignancy to chemotherapy and radiation is due, in large part, to defects in apoptotic signaling pathways (2). Pancreatic cancer and other tumor types show constitutively active Signal Transducer and Activator of Transcription 3 (STAT3) (3, 4). STAT3 is a member of a family of transcription factors that convey signals from the cell surface to the nucleus upon activation by cytokines and growth factors (4, 5). STAT3 can also be activated by nonreceptor tyrosine kinases, including Src in human pancreatic cancer cells (6). Engagement of cell surface receptors by polypeptide ligands, such as interleukin-6 (IL-6), induces tyrosine phosphorylation (Tyr705) of the STAT3 protein that then translocates to the nucleus and regulates expression of genes harboring STAT3-binding sites in their promoters, including genes that govern cell cycle progression, apoptosis, and angiogenesis that contribute to oncogenesis (4, 5). Specifically, STAT3 can transcriptionally regulate pro-survival Bcl-2 proteins such as Bcl-xL, Mcl-1, and survivin, and disruption of STAT3 signaling induces apoptosis and decreases their expression in diverse human tumor cell types (7–10). Together, these data suggest that STAT3 may represent an important therapeutic target in pancreatic cancer.
Sorafenib (Nexavar®, BAY43-9006) is an oral multikinase inhibitor that can block the Ras/Raf/MEK/ERK signaling cascade that is important for the growth of solid tumors (11, 12). The MEK-ERK signaling pathway is a downstream target of oncogenic Ras mutations (12) that occur in ~90% of human pancreatic carcinomas (13). Sorafenib also targets several other receptor tyrosine kinases, including vascular endothelial growth factor receptor 2 (VEGFR2), platelet-derived growth factor receptor (PDGFR), FLT3, Ret, and c-Kit (11). Sorafenib has shown preclinical activity against a variety of tumor types and is a standard treatment for hepatocellular and renal cell carcinomas (14, 15). Sorafenib can induce apoptosis, and has been shown to down-regulate the pro-survival Mcl-1 protein to enhance mitochondrial apoptotic signaling (16, 17). However, Mcl-1 expression has been shown to be regulated by multiple mechanisms (18) including STAT3 (9). Mcl-1 can inhibit apoptosis by sequestering pro-apoptotic BH3-only proteins including Bim and Noxa, as well as the multidomain Bak protein that regulates the permeability of the outer mitochondrial membrane (19). Sorafenib has been shown to induce Bim expression in human leukemia cells (20). Bim can bind to all pro-survival Bcl-2 proteins and is therefore, a potent inducer of apoptosis (19, 21). Sorafenib has been shown to enhance TRAIL (TNF-related apoptosis-inducing ligand)-mediated apoptosis in human leukemia and colon cancer cell lines (22–24). TRAIL is a pro-apoptotic cytokine and promising anti-cancer drug that is currently undergoing phase I/II evaluation in cancer patients (25). TRAIL triggers death receptor (DR)-mediated apoptosis that requires mitochondrial amplification of a membrane DR signal in most human cancer cells (26, 27). We and others have shown that TRAIL-mediated apoptosis can be negatively regulated by Mcl-1 (28, 29) and Bcl-2 (30, 31) proteins.
We hypothesized that sorafenib can induce apoptosis and down-regulate Mcl-1 expression by inhibiting STAT3 activation, and thereby enhance TRAIL-mediated apoptosis. We demonstrate that inhibition of STAT3 activation by sorafenib or alternatively, by STAT3 knockdown or a DN STAT3, can down-regulate Mcl-1 and Bcl-xL proteins to enhance TRAIL-induced apoptosis. Furthermore, overexpression of constitutively active STAT3 was shown to attenuate sorafenib-induced down-regulation of Mcl-1 and to inhibit caspase-3 cleavage by TRAIL and/or sorafenib in human pancreatic cancer cells.
Materials and Methods
Cell culture, drugs and reagents
Human pancreatic cancer cell lines BxPC-3 and PANC-1 cells, previously purchased from the American Type Culture Collection (ATCC, Manassas, VA), were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and with 1% penicillin/streptomycin, 10 mM HEPES, and 1% sodium pyruvate. Human embryonic kidney cell line 293T and 293A cells were maintained in high-glucose DMEM medium (Sigma, St. Louis, MO) with 10% FBS. For lentivirus production, 293T cells were cultured in high-glucose DMEM containing 2% FBS per the manufacturer’s procedure (System Biosciences, Mountain View, CA). Sorafenib (BAY 43-9006) [Bayer Pharmaceutical Corp., West Haven, CT] was utilized from a commercial source and dissolved in DMSO to prepare a stock solution that was aliquoted for storage at −20°C. TRAIL was purchased from Sigma.
Annexin V labeling
After drug treatment, adherent cells were detached from culture dishes by treating with trypsin (Invitrogen) for 3 to 5 min and combined with floating cells. Annexin V labeling was then performed as previously described (32). The extent of apoptosis was quantified as percentage of Annexin V-positive cells, and the extent of drug-specific apoptosis was assessed by this formula: % specific apoptosis = (test − control) × 100 / (100 − control) (32).
DNA fragmentation assay
After treatment, cells were harvested, resuspended in cold PBS and then counted. DNA fragmentation was then quantified by a Cell Death Detection ELISA plus kit (Roche Applied Science, Indianapolis, IN) as per manufacture’s manual. Briefly, an equal number (104) of cells was pelleted and lysed in 200 µl of lysis buffer by incubating for 30 minutes at room temperature (RT). The lysates were centrifuged at 200 × g for 10 min and 20 µl of the supernatant was transferred into the middle of microplate well. Then, 80 µl of immunoreagent was added into each well. The plate was covered and incubated at RT for 2 h under gently shaking. After cells were washed X 3, ABTS solution was added and the color reaction was developed. A stop solution was added and the absorbance at 405 nm was measured (reference wavelength at 490 nm) using a VERSAmax Microplate Reader (Molecular Devices, Inc., Sunnyvale, CA). Samples were run in duplicate and the average values are shown.
Cell Viability assay
Cell viability was determined in the presence or absence of drug treatment using the MTS reduction assay per the manufacturer’s protocol (Promega, Madison, WI) as previously described (32).
Western blotting
Protein samples were prepared in a lysis buffer [5 mmol/L MgCl2, 137 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% CHAPS, 10 mmol/L HEPES (pH 7.5)] containing a protease inhibitor cocktail and/or a phosphotase inhibitor cocktail 2 (both from Sigma), normalized using nanodrop measurement (NanoDrop Technologies), boiled in LDS sample buffer (Invitrogen), and loaded onto 14% SDS-PAGE gels with electrophoretic transfer onto a polyvinylidene difluoride membrane (Bio-Rad). Western blotting was performed, as previously described (32), using anti-mouse antibodies against Mcl-1, caspase-8 (BD Biosciences), Bcl-xL, Bak (EMD Chemicals) and HA (Roche Diagnostics). Anti-rabbit antibodies were utilized against Bim, VEGF (both from Santa Cruz Biotechnology), Bid, Bax, STAT3 and p-STAT3, p-Erk1/2, p-AKT, caspase-9, cleaved caspase-3, anti-FLAG (all from Cell Signaling Technology), and ERK1/2 (EMD). p-Erk1/2 was quantified relative to total Erk1/2 with the ratio arbitrarily set to 1 in vehicle-treated cells. Quantitation was performed using Image J software (National Institutes of Health, Bethesda, MD).
Knockdown of Bim, STAT3 and Mcl-1 using lentiviral small hairpin RNA (shRNA)
Target sequences for Bim and Mcl-1 were selected and the top and bottom strands of the template were synthesized by the Mayo Clinic Molecular Biology Core Facility. The targeting sequences for Mcl-1 was GGCAGTCGCTGGAGATTAT (si1) and GATTGTGACTCTCATTTCT (si8) (33), and the sequence for Bim was GACCGAGAAGGTAGACAATTGC (34). The targeting sequence for STAT3 was CATCTGCCTAGATCGGCTA (35). Cloning of shRNA and generation of lentivirus in the producer cells and transduction of lentivirus into pancreatic cancer cell lines were performed as previously described (32).
Bak and Bax conformational change
After drug treatment, cells were lysed in lysis buffer [5 mM MgCl2, 137 mM KCl, 1mM EDTA, 1 mM EGTA, 1% CHAPS, 10 mM HEPES, pH 7.5] in the presence of protease inhibitor cocktail. Bak and Bax conformational change was then determined by immunoprecipitation followed by immunoblotting using anti-mouse antibodies against Bax 6A7 (Sigma) and Bak (Ab-1, EMD Chemicals), as previously described (32).
Adenovirus construction, production, amplification, purification and transduction
The replication-deficient adenovirus expressing dominant negative (DN) STAT3, where Tyr705 was substituted with a Phe residue (36), was obtained by sequential steps using the Virapower Adenoviral Expression System (Invitrogen). Briefly, DN STAT3 expression cassette was made by PCR-mediated mutagenesis using the wild-type STAT3 cDNA (Origene, Rockville, MD) as the template and cloned into the entry vector pENTR4 (Invitrogen). The DN STAT3 cassette in the vector pENTR4 was then transferred into the adenovirus expression vector pAD/CMV/V5-DEST by recombination using the LR Clonase II enzyme mix (Invitrogen). The DN STAT3 cassette was verified at each step by sequencing. The vector pAD/CMV/V5-DEST containing the DN STAT3 cassette was digested with PacI (NEB) and transfected into 293A cells (Invitrogen) using lipofectamine 2000 according to the protocol as described in Virapower Adenoviral Expression System (Invitrogen). The amplification and purification of adenovirus was performed with an Adeno-X Maxi purification kit (Clontech). The viral titer of the purified adenovirus was estimated based on the absorbance at 260 nm and the titer was calculated and expressed as optical particle unit (opu) per milliliter (ml). Viral titer (opu/ml) =OD260 × viral dilution × 1.1 × 1012. Of note, because opu and plaque forming units (pfu) define different properties, there measurements can’t be directly compared. 1 pfu is estimated as 25 opu (37). Multiplicity of infection (MOI) is expressed as pfu/cell. The transduction of adenovirus into pancreatic cancer cells was performed by directly adding adenovirus into the growth medium of the cells.
Ectopic expression of a constitutively active STAT3 mediated by retrovirus
A retrovirus-based expression vector pBabe-puro containing the constitutively active STAT3 fused with a C-terminal FLAG, or the empty vector (a gift from Dr. J. Bromberg, The Rockefeller University, NY) was mixed with pMD.MLV and pMD.G plasmids and transfected into 293T cells using Lipofectamine and Plus reagent (Invitrogen). Retroviral-containing medium was collected 48 h post-transfection and was concentrated by incubating with 10% (final) PEG-8000 (Sigma) at 4°C overnight, then centrifuged at 1500Xg for 15 min with collection of the pellet. BxPC-3 cells were transduced with concentrated retrovirus in the presence of 8 µg/ml polybrene (Sigma) and non-transduced cells were eliminated with 2 µg/ml puromycin for 48 h post-transduction.
Statistical analysis
The statistical significance of the differences between experimental variables was determined using the Student’s t test. The values shown represent the mean ± SD for triplicate experiments.
Results
Sorafenib inhibits STAT3 phosphorylation(Tyr705) and down-regulates Mcl-1 and Bcl-xL expression
We determined the effect of sorafenib on key mediators of the Ras/Raf/MEK/ERK signaling pathway and its potential downstream effector STAT3 that are frequently activated in pancreatic cancers. STAT3 is activated by phosphorylation at Tyr705, which induces dimerization, nuclear translocation and DNA binding (38). Sorafenib treatment (6, 12, 24 h) was shown to potently inhibit the constitutive phosphorylation at Tyr705 in a dose- and time-dependent manner in PANC-1 (Fig. 1A) and BxPC-3 (Fig. 1B) pancreatic cancer cell lines. Sorafenib inhibited ERK1/2 but not AKT phosphorylation consistent with its ability to inhibit the MAPK pathway (11). Inhibition of STAT3 Tyr705 phosphorylation (p-STAT3) occurred coincident with suppression of pro-survival Mcl-1 or Bcl-xL proteins by sorafenib (Fig.1A, B). Suppression of Mcl-1, but not Bcl-xL by sorafenib was detected after a 6 h incubation (Fig. 1A, B), potentially due to the shorter half-life (t1/2) of Mcl-1 compared to Bcl-xL (39).
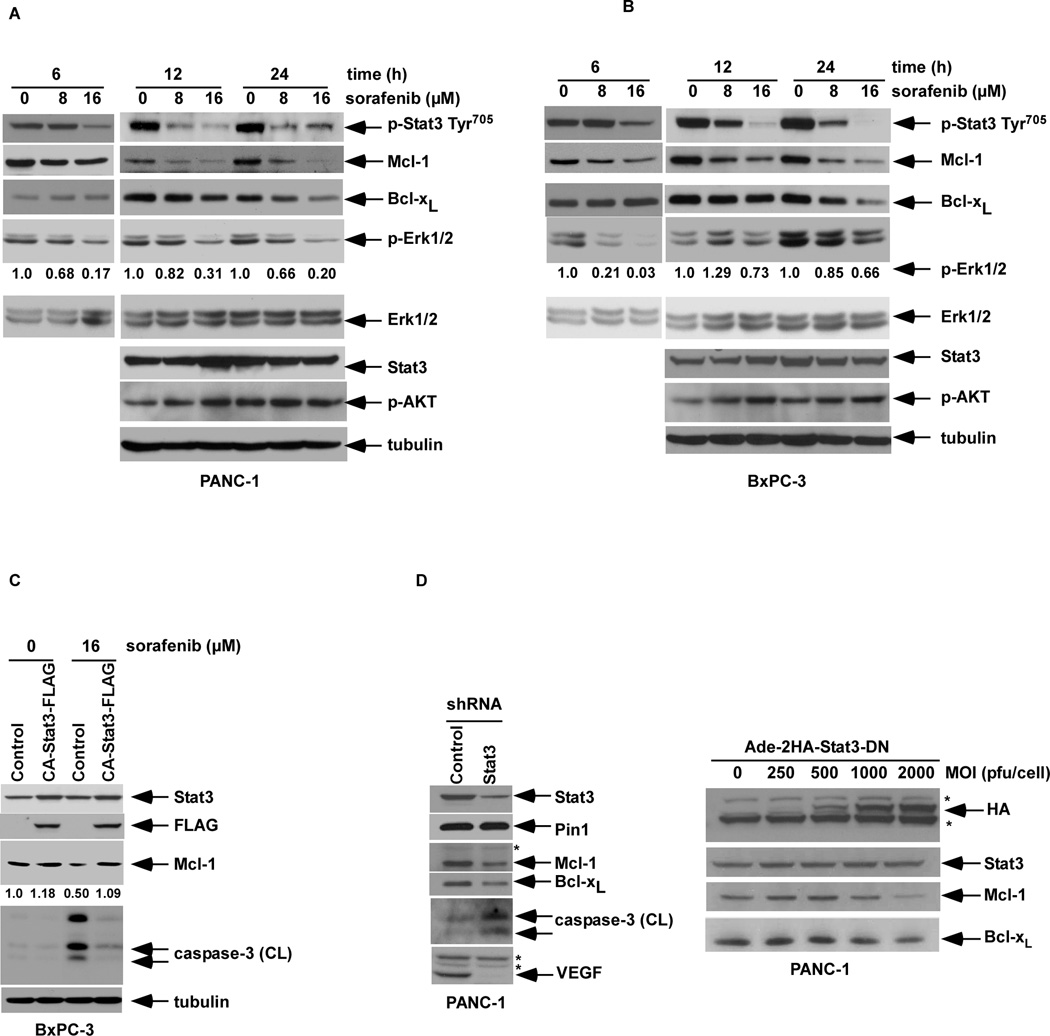
Figure 1.
Sorafenib inhibits constitutive STAT3 phosphorylation at Tyr705 and down-regulates Mcl-1 and Bcl-xL expression in PANC-1 (A) or BxPC-3 (B) cell lines. Cells were incubated with sorafenib at the indicated doses for 6, 12, or 24 h, and the whole cell lysate (WCL) was analyzed by Western blotting using highly specific antibodies. The level of p-ERK1/2 was normalized to total ERK1/2 in the same blots and the relative ratio is shown (see Methods). C, BxPC-3 cells were stably transduced with a retroviral constitutively active STAT3-FLAG or an empty vector. Overexpression of STAT3 was confirmed by detecting STAT3 and the FLAG tag using Western blotting. Cells were treated with sorafenib (0, 16 µM) for 12h and Mcl-1 and cleaved caspase-3 were probed. The relative intensity of the Mcl-1 protein bands was determined by densitometry. D, PANC-1 cells containing a lentiviral STAT3 shRNA or control were probed for Mcl-1, Bcl-xL, VEGF, or cleaved caspase-3 by Western blotting (left panel). Cleaved caspase-3 was detected after a prolonged exposure. Alternatively, PANC-1 cells were transduced with an adenoviral dominant negative STAT3 containing a 2HA tag at the N-terminus at an increasing multiplicity of infection (MOI). The WCL was prepared 48 h post-transduction and subjected to Western blotting for Stat-3, Mcl-1, Bcl-xL or HA tag (* refers to a non-specific band).
Since sorafenib can suppress p-STAT3, we determined whether inhibition of p-STAT3 contributes to the pro-apoptotic effect of this drug. A retroviral vector containing a constitutively active (CA) STAT3 was introduced into BxPC-3 cells that were then incubated with sorafenib. Ectopic CA-STAT3 was shown to potently inhibit caspase-3 cleavage by sorafenib and to attenuate sorafenib-induced Mcl-1 down-regulation consistent with a STAT3-mediated pro-survival effect (Fig. 1C). Furthermore, knockdown of STAT3 using short hairpin RNA (shRNA) was shown to reduce Mcl-1 and Bcl-xL expression associated with an increase of basal caspase-3 cleavage, whereas no change in peptidyl-prolyl cis/trans isomerase 1 (Pin1), included as a control, was found (Fig. 1D). Pin1 interacts with STAT3 upon cytokine/growth factor stimulation and its overexpression promotes STAT3 transcriptional activity and target gene expression (40). In addition. STAT3 shRNA was shown to inhibit downstream VEGF expression (Fig. 1D), a known transcriptional target of STAT3 (6, 41). To further support these data, STAT3 suppression was performed using an adenovirus-expressing construct containing a dominant negative (DN) STAT3 whose phosphorylation site at Tyr705 was substituted with a phosphorylation defective residue Phe (36). In PANC-1 cells transduced with the DN STAT3 construct, expression of Mcl-1 and to a lesser extent Bcl-xL were attenuated (Fig. 1D).
Sorafenib potentiates TRAIL-induced apoptosis that is negatively regulated by STAT3
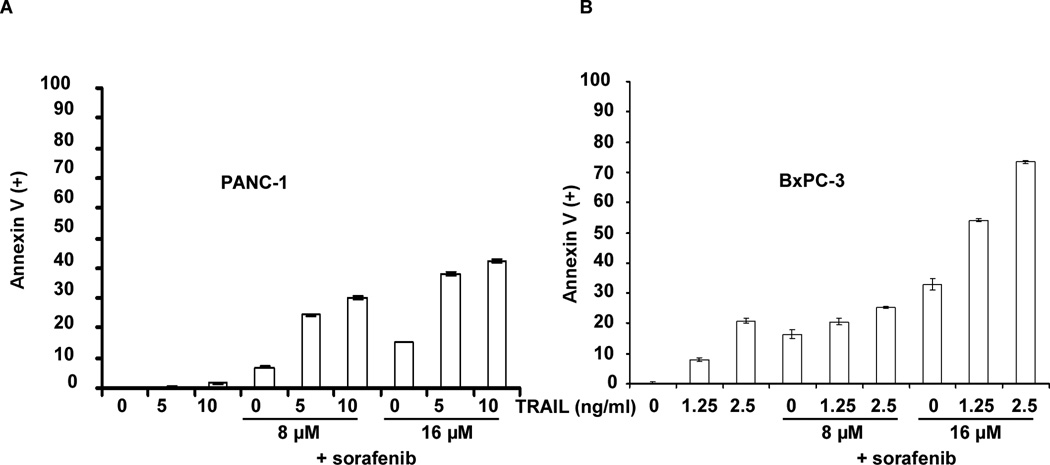
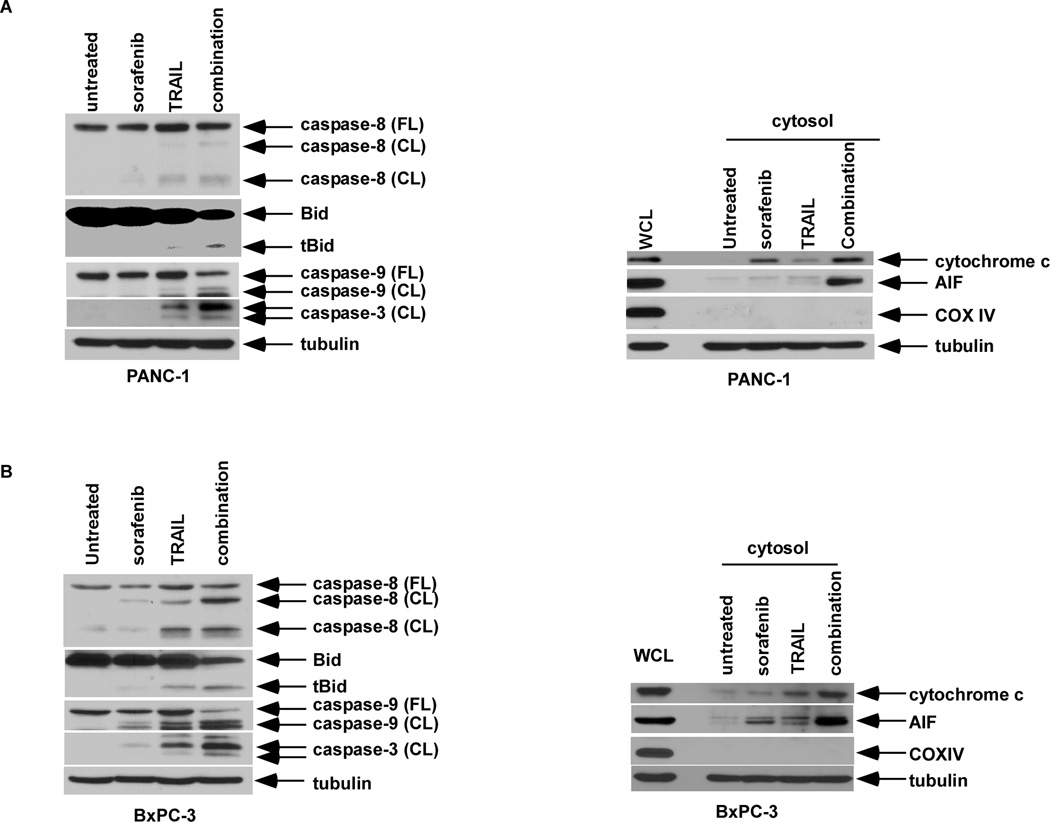
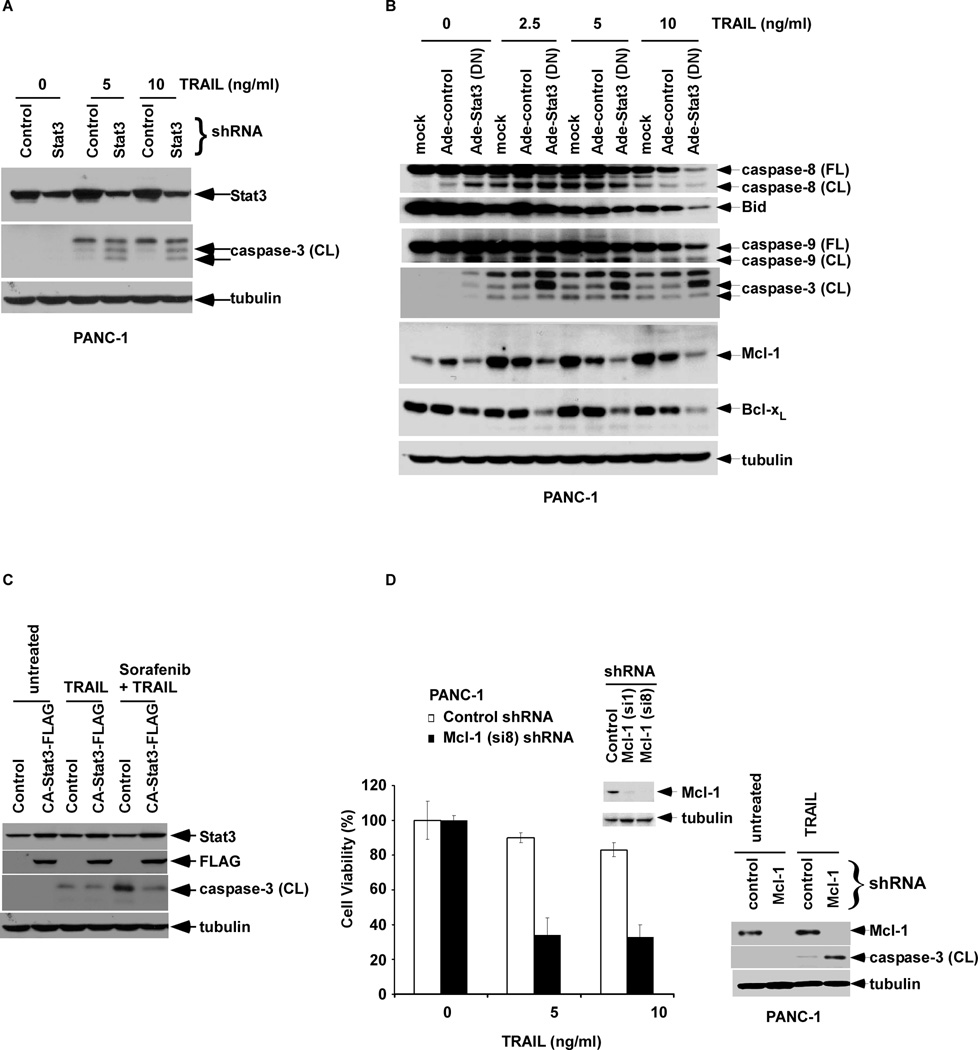
Sorafenib monotherapy was shown to induce a dose-dependent apoptosis in pancreatic cancer cell lines, as shown by annexin V (+) FITC staining (Fig. 2). Using TRAIL-sensitive (BxPC-3) and -resistant (PANC-1) cell lines (42), sorafenib treatment was shown to significantly enhance TRAIL-induced apoptosis (Fig. 2). Apoptosis induction was associated with increased caspase cleavage, Bid truncation, and release of the mitochondrial apoptogenic proteins (cytochrome c and AIF) compared to either drug alone (Fig. 3A, B), indicating enhanced cross-talk between the DR- and mitochondria-mediated apoptotic pathways (26, 27). The ability of sorafenib to enhance TRAIL-mediated apoptotic signaling could be blocked by a pan-caspase inhibitor (z-VAD-fmk) [data not shown]. We then determined whether targeting STAT3 can modulate TRAIL-mediated apoptotic signaling. Stable STAT3 knockdown by shRNA was shown to enhance TRAIL-induced caspase-3 cleavage (Fig. 4A). Similarly, cells transduced with a DN STAT3 showed enhanced caspase cleavage and reduced full length Bid when treated with TRAIL compared to empty vector-alone cells (Fig. 4B). DN STAT3 was also shown to down-regulate Mcl-1 and Bcl-xL proteins in TRAIL-treated cells (Fig. 4B). Conversely, cells transduced with constitutively active STAT3 were protected from caspase-3 cleavage triggered by sorafenib plus TRAIL (Fig. 4C). Mcl-1 knockdown by shRNA was shown to sensitize PANC-1 cells to TRAIL-mediated cytotoxicity and caspase-3 cleavage compared to shRNA control cells (Fig. 4D). Together, these data demonstrate that suppression of p-STAT3 can down-regulate Mcl-1 and Bcl-xL proteins to enhance TRAIL-induced apoptosis.
Figure 2.
Sorafenib enhances TRAIL-mediated apoptosis. PANC-1 (A) or BxPC-3 (B) cells were pre-treated with sorafenib for 24 h or 12 h, respectively, followed by TRAIL for 24 h. Apoptosis was analyzed by annexin V labeling and FACS analysis. Annexin V(+) cells were quantified (see Methods) and shown in a bar graph. Experiments were conducted in triplicate and mean values ± SD (bars) are shown.
Figure 3.
Sorafenib enhances TRAIL-mediated apoptotic signaling. PANC-1 (A) and BxPC-3 cells (B) cells were pre-treated with sorafenib (16 µM) for 24 h or 12 h, respectively, followed by TRAIL (10 ng/ml for PANC-1; 2.5 ng/ml for BxPC-3) for 5 h. Then, the WCLs (left panels) were analyzed by Western blotting for caspase-8, -9, -3 and Bid. The cytosolic fractions (right panels) were analyzed for mitochondrial cytochrome c and AIF release. COX IV was utilized as a mitochondrial marker and β-tubulin served as a loading control.
Figure 4.
STAT3 shRNA or dominant negative (DN) STAT3 sensitizes cells to TRAIL-mediated apoptosis whereas constitutively active (CA)-STAT3 attenuates apoptosis induction by sorafenib and TRAIL. A, PANC-1 cells containing lentiviral STAT3 shRNA or control were treated with TRAIL for 5 h and caspase-3 cleavage in WCLs was determined by Western blotting. B, PANC-1 cells were transduced with an adenoviral DN STAT3 (MOI = 2000 pfu/cell) for 48 h, and then treated with TRAIL for 5 h. Cleavage of caspase-8, -9, -3 and expression of Bid were analyzed by Western blotting in the WCL. C, Retroviral CA-STAT3-FLAG overexpression or control BxPC-3 cells were treated with sorafenib (0, 16 µM) for 12h followed by TRAIL (0, 2.5 ng/ml) for 3h. Cleaved caspase-3 was then probed. D, PANC-1 cells were transduced with Mcl-1 shRNA (si1 and si8 constructs) or control shRNA and the efficiency of Mcl-1 knockdown was analyzed in cell lysates by Western blotting (inset). Knockdown efficiency was higher in the si8 construct that was used for subsequent experiments. Cells were treated with TRAIL (0, 5, 10 ng/ml) for 48 h and cell viability was analyzed using the MTS assay. Experiments were conducted in triplicate and mean values ± SD (bars) are shown. Next, PANC-1 cells containing Mcl-1 shRNA (si8) or control shRNA were treated in the absence or presence of TRAIL (10 ng/ml, 5 h) and Mcl-1 expression and caspase-3 cleavage (CL) were analyzed in cell lysates by Western blotting (right panel). β-tubulin was utilized as a control for protein loading.
Pro-apoptotic Bim regulates apoptotic signaling by sorafenib plus TRAIL
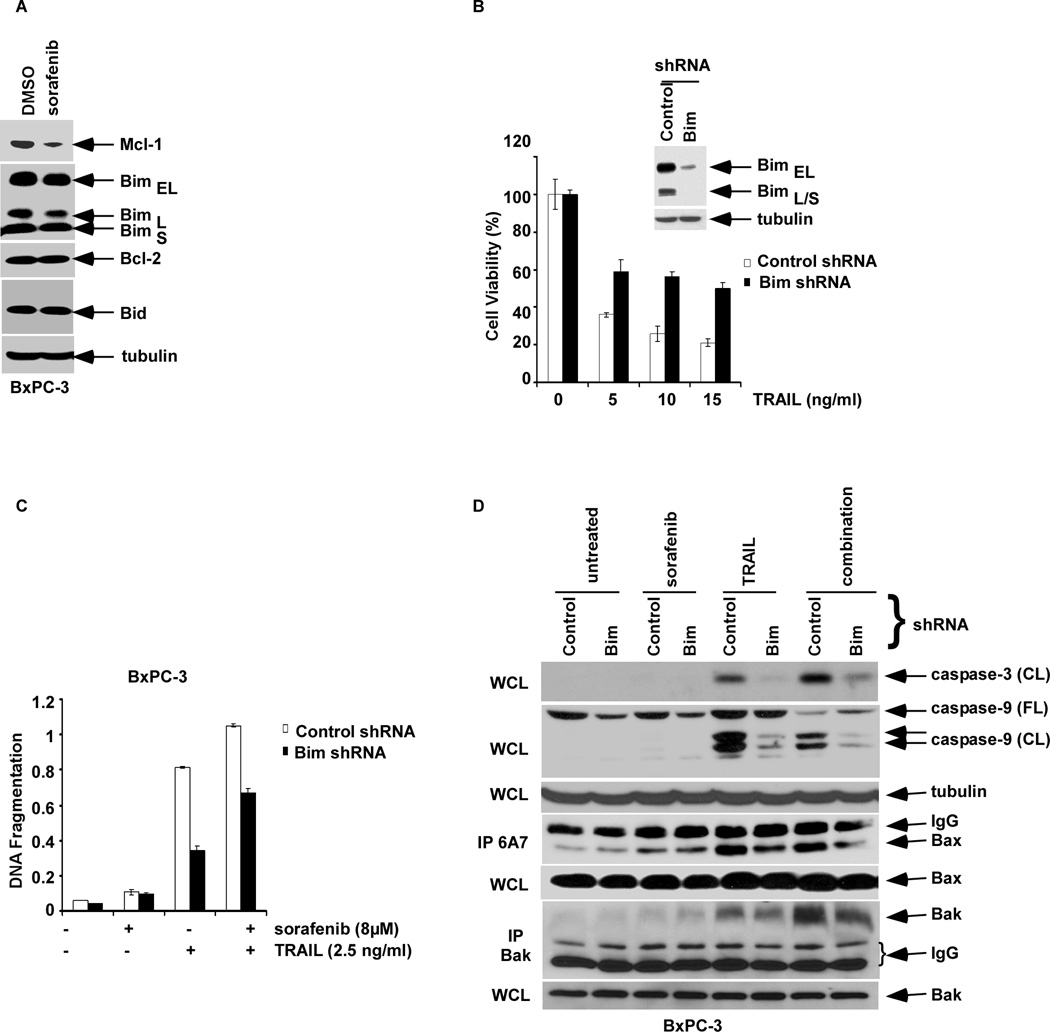
The BH3-only Bim protein can bind to and neutralize all pro-survival Bcl-2 family proteins and is, therefore, a potent inducer of apoptosis (19). We determined the role of Bim in the apoptotic response to sorafenib and TRAIL. In contrast to AML cells (20), sorafenib treatment did not alter Bim expression (Fig. 5A). Knockdown of Bim using a lentiviral shRNA in BxPC-3 cells was shown to significantly attenuate apoptosis induction by TRAIL alone and combined with sorafenib (Fig. 5B, C), and reduced caspase-9 and -3 cleavage (Fig. 5D). Sorafenib potentiated a TRAIL-mediated activation of Bak, but not Bax, as shown by its conformational change that was attenuated by Bim knockdown (Fig. 5D).
Figure 5.
BH3-only Bim mediates sorafenib and/or TRAIL-induced apoptosis. A, BxPC-3 cells were treated with vehicle or sorafenib (16 µM) for 24h and WCLs were probed for the indicated proteins by Western blotting. B, BxPC-3 cells were transduced with shRNA to Bim or control and the WCL was analyzed for Bim proteins by Western blotting (inset). Cells with Bim knockdown or control shRNA were then incubated with TRAIL for 48 h, and cell viability was determined using the MTS assay. Experiments were conducted in triplicate and mean values ± SD (bars) are shown. C, Bim knockdown and shRNA control BxPC-3 cells were pre-treated with sorafenib (0, 8 µM; 4 h) followed by incubation with TRAIL (2.5 ng/ml; 2 h), and DNA fragmentation (see Methods) was analyzed. Experiments were conducted in triplicate and mean values ± SD (bars) are shown. D, Bim knockdown or control BxPC-3 cells were pretreated with vehicle or sorafenib (8µM; 12 h) alone and in combination with TRAIL (2.5 ng/ml; 2 h). Caspase activation was analyzed in WCL by Western blotting. In addition, a treatment-induced Bak or Bax conformational change was analyzed by immunoprecipitation (IP) using conformation-specific antibodies. β-tubulin was used as a control for protein loading.
Discussion
STAT3 can promote tumor cell growth and apoptosis resistance that contribute to tumor progression and treatment failure (38). We found that sorafenib can suppress p-STAT3 that was associated with down-regulation of Mcl-1 and Bcl-xL proteins, apoptosis induction, and sensitization to TRAIL-mediated apoptosis. Phosphorylation of STAT3 on Tyr705 has been shown to control its dimerization, nuclear translocation, DNA binding, and transcriptional activation of target genes, including Mcl-1 (9, 38) . Mcl-1 and Bcl-xL are known to play a critical role in tumor cell survival and sorafenib has been consistently shown to down-regulate Mcl-1 expression in diverse tumor cell types (16, 17). To determine whether STAT3 can regulate Mcl-1 or Bcl-xL expression in pancreatic cancer cells, we suppressed STAT3 using shRNA or a dominant negative (DN) STAT3 that resulted in suppression of Mcl-1 and Bcl-xL expression. STAT3 knockdown was also shown to inhibit expression of its known transcriptional target, VEGF (6, 41). In stable STAT3 knockdown cells, an enhanced basal caspase-3 cleavage was observed compared to control knockdown cells. In addition, overexpression of constitutively active STAT3 was shown to attenuate sorafenib-mediated Mcl-1 down-regulation and to potently inhibit caspase-3 cleavage. While sorafenib was shown to inhibit STAT3 activation and to down-regulate Mcl-1, STAT3-independent pathways may also contribute to its pro-apoptotic effect. In this regard, other mechanisms of sorafenib-induced Mcl-1 down-regulation have been reported and include alterations in NF-κB-mediated transcription, inhibition of eIF4E-associated translation, and accelerated proteosomal degradation (16, 17, 22). Sorafenib inhibited p-ERK, but not p-AKT, expression consistent with its ability to block Ras/Raf/MEK/ERK activation (11, 12), and both ERK and Src/Jak signaling have been shown to activate STAT3 (6). Other studies have also shown that sorafenib or sunitinib can reduce cellular levels of p-STAT3 Tyr705 in cancers of the bile duct (43) and kidney (44) as well as in medulloblastoma cells (45). Together, our data indicate that sorafenib induces apoptosis and inhibits Mcl-1 through blockade of the STAT3 signaling pathway in human pancreatic cancer cells.
Suppression of p-STAT3 and Mcl-1 by sorafenib enhanced TRAIL-induced apoptosis that was associated with Bid truncation and release of mitochondrial cytochrome c and AIF, consistent with cross-talk between the DR-mediated and mitochondrial apoptotic pathways. STAT3 was shown to negatively regulate TRAIL-mediated apoptosis in that STAT3 knockdown or a DN STAT3 sensitized cells to TRAIL-induced caspase-3 cleavage. In contrast, a constitutively active STAT3 attenuated caspase-3 cleavage induced by sorafenib alone and combined with TRAIL. Given that STAT3 can down-regulate Mcl-1, we confirmed the pro-survival role of Mcl-1 in our pancreatic cancer cell lines. Specifically, cells with Mcl-1 knockdown showed increased TRAIL-mediated apoptosis in accordance with prior studies by our laboratory and others demonstrating that ectopic Mcl-1 or Bcl-2 expression can confer TRAIL resistance (28–31). Sorafenib potentiated a TRAIL-mediated Bak, but not Bax, conformational change consistent with its activation. Similarly, the combination of sorafenib and TRAIL activated Bak in acute myelogenous leukemia (AML) cells (23). Mcl-1 has been shown to preferentially inhibit Bak versus Bax activation (24), and this finding along with the release of Bak from Mcl-1 may be important contributors to the ability of sorafenib to enhance TRAIL-mediated apoptosis. Sorafenib has been shown to enhance TRAIL-mediated apoptosis in other tumor cell types in association with suppression of Mcl-1, NF-κB (22) or c-FLIP (24), indicating that sorafenib exerts its anti-tumor effects via multiple mechanisms.
Studies in human leukemia cells have shown that sorafenib can induce Bim expression and that Bim can regulate sorafenib-mediated apoptosis (20). Bim can mediate a mitochondrial apoptotic response when Mcl-1 is suppressed (46). We examined the ability of Bim to regulate apoptosis induction by sorafenib plus TRAIL. In contrast to leukemic cells, sorafenib did not modulate Bim expression in pancreatic cancer cells. In cells treated with sorafenib and/or TRAIL, however, Bim knockdown attenuated caspase cleavage and reduced Bak/Bax activation, indicating that apoptosis induction by sorafenib plus TRAIL is partially dependent upon Bim. Bim may play a key role in Bak/Bax activation given that Bim, tBid or Bad can displace the mitochondrial outer-membrane protein VDAC2 from Bak to enable its homo-oligomerization and resultant apoptosis (47).
In conclusion, sorafenib treatment can suppress STAT3 phosphorylation at Tyr705 and inhibit STAT3-regulated Mcl-1 and Bcl-xL expression to enhance TRAIL-mediated apoptosis in pancreatic cancer cell lines. These findings demonstrate that targeting STAT3 using sorafenib, or potentially with a STAT3 inhibitor, can enhance apoptotic susceptibility and suggest a strategy to increase therapeutic efficacy against pancreatic cancers.
Acknowledgments
This work was supported by a Pilot Award from the Mayo Clinic Pancreatic Cancer SPORE (NCI P50 CA10270) and a Fraternal Order of Eagles Foundation Award (both to FAS).
The authors express their appreciation to Terri Johnson and Jonelle Morales for their very capable secretarial assistance.
Abbreviation List
- STAT3
Signal Transducer and Activator of Transcription 3
- TRAIL
TNF-related apoptosis inducing ligand
- Mcl-1
Myeloid Cell Leukemia 1
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005 Jan–Feb;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nature clinical practice. 2006 Jul;3(7):388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 3.Scholz A, Heinze S, Detjen KM, et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003 Sep;125(3):891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004 Feb;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 5.Darnell JE., Jr STATs and gene regulation. Science. 1997 Sep 12;277(5332):1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 6.Gray MJ, Zhang J, Ellis LM, et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005 Apr 28;24(19):3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 7.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999 Jan;10(1):105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 8.Grandis JR, Drenning SD, Zeng Q, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epling-Burnette PK, Liu JH, Catlett-Falcone R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001 Feb;107(3):351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gritsko T, Williams A, Turkson J, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006 Jan 1;12(1):11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004 Oct 1;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 12.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003 Jan;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 13.Mariyama M, Kishi K, Nakamura K, Obata H, Nishimura S. Frequency and types of point mutation at the 12th codon of the c-Ki-ras gene found in pancreatic cancers from Japanese patients. Jpn J Cancer Res. 1989 Jul;80(7):622–626. doi: 10.1111/j.1349-7006.1989.tb01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008 Feb;134(2):379. doi: 10.1053/j.gastro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006 Dec 15;12(24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 16.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005 Oct 21;280(42):35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 17.Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005 Oct 20;24(46):6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 18.Le Gouill S, Podar K, Harousseau JL, Anderson KC. Mcl-1 regulation and its role in multiple myeloma. Cell cycle (Georgetown, Tex. 2004 Oct;3(10):1259–1262. doi: 10.4161/cc.3.10.1196. [DOI] [PubMed] [Google Scholar]
- 19.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007 Feb 26;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008 Apr;22(4):808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 21.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007 Feb 9;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 22.Ricci MS, Kim SH, Ogi K, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer cell. 2007 Jul;12(1):66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Meng XW, Lee SH, Dai H, et al. Mcl-1 as a buffer for proapoptotic Bcl-2 family members during TRAIL-induced apoptosis: a mechanistic basis for sorafenib (Bay 43-9006)-induced TRAIL sensitization. J Biol Chem. 2007 Oct 12;282(41):29831–29846. doi: 10.1074/jbc.M706110200. [DOI] [PubMed] [Google Scholar]
- 24.Rosato RR, Almenara JA, Coe S, Grant S. The multikinase inhibitor sorafenib potentiates TRAIL lethality in human leukemia cells in association with Mcl-1 and cFLIPL down-regulation. Cancer Res. 2007 Oct 1;67(19):9490–9500. doi: 10.1158/0008-5472.CAN-07-0598. [DOI] [PubMed] [Google Scholar]
- 25.Koschny R, Walczak H, Ganten TM. The promise of TRAIL--potential and risks of a novel anticancer therapy. J Mol Med. 2007 Sep;85(9):923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003 Nov 24;22(53):8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 27.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998 Mar 16;17(6):1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008 Apr 1;68(7):2062–2064. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Chen W, Zeng W, et al. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Molecular cancer therapeutics. 2008 May;7(5):1156–1163. doi: 10.1158/1535-7163.MCT-07-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun SY, Yue P, Zhou JY, et al. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun. 2001 Jan 26;280(3):788–797. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004 Dec 15;10(24):8284–8292. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009 Jan 1;15(1):150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Morgan-Lappe S, Huang X, et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007 Jun 7;26(27):3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 34.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008 Apr 15;68(8):2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling X, Arlinghaus RB. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 2005 Apr 1;65(7):2532–2536. doi: 10.1158/0008-5472.CAN-04-2425. [DOI] [PubMed] [Google Scholar]
- 36.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999 Aug 6;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 37.Behrend L, Mohr A, Dick T, Zwacka RM. Manganese superoxide dismutase induces p53-dependent senescence in colorectal cancer cells. Mol Cell Biol. 2005 Sep;25(17):7758–7769. doi: 10.1128/MCB.25.17.7758-7769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002 Apr;8(4):945–954. [PubMed] [Google Scholar]
- 39.Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007 Mar 2;282(9):6192–6200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lufei C, Koh TH, Uchida T, Cao X. Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene. 2007 Dec 6;26(55):7656–7664. doi: 10.1038/sj.onc.1210567. [DOI] [PubMed] [Google Scholar]
- 41.Wei D, Le X, Zheng L, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003 Jan 23;22(3):319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 42.Khanbolooki S, Nawrocki ST, Arumugam T, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Molecular cancer therapeutics. 2006 Sep;5(9):2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 43.Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009 Aug 5; doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009 Mar 15;69(6):2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F, Van Meter TE, Buettner R, et al. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Molecular cancer therapeutics. 2008 Nov;7(11):3519–3526. doi: 10.1158/1535-7163.MCT-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006 Apr 14;281(15):10153–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 47.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003 Jul 25;301(5632):513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]