Abstract
Objective
Examine the association between frailty and cognitive impairment as predictors of mortality over a 10-year period in a selected sample of older Mexican Americans.
Design
Longitudinal analyses using data from the Hispanic Established Populations for the Epidemiologic Study of the Elderly (1995–96/2004–05).
Setting
Five southwestern states: Texas, New Mexico, Colorado, Arizona, and California.
Participants
Mexican Americans aged 67 and older with complete information on the frailty index and the Mini Mental State Examination (MMSE) (n=1,815).
Measurements
Cognitive impairment determined by a score in the MMSE < 21. Frailty defined as three or more of the following components: 1) weight-loss, 2) weakness, 3) self-reported exhaustion, 4) slow walking speed, and 5) low physical activity level. Sociodemographic characteristics and chronic medical conditions were used as covariates. Mortality was determined using the National Death Index or by proxy.
Results
As MMSE score declined over time, the percent of frail individuals increased in a linear fashion. Frailty and cognitive impairment are independent risk factors for mortality after controlling for all covariates (HR 2.03 95% CI 1.57–2.62; HR 1.26 95% CI 1.05–1.52, respectively). When both cognitive impairment and frailty were added to the model, HR for individuals with cognitive impairment was no longer statistically significant.
Conclusion
The relation between frailty and cognitive impairment needs careful analysis in this population to establish pathways increasing mortality and decreasing quality of life. Our results suggest frailty is a stronger predictor of mortality for older Mexican Americans than cognitive impairment.
Keywords: Frailty, cognitive impairment, mortality, Mexican Americans
INTRODUCTION
In the last decade, frailty has been established as a concept that helps identify older adults at risk of adverse events [1]. Different sets of criteria have been used to define frailty. Despite their limitations, these criteria have provided a structured starting point for researchers to study older adults at risk while using a common language. Alterations in physical function have been the main focus of widely used constructs that define frailty [2,3]. Today, frailty is a highly relevant clinical entity with a defined phenotype [2]. Frailty is also a predictor of adverse outcomes including mortality [4–6].
Similarly, cognitive impairment is an independent marker of functional decline and mortality, especially in the presence of dementia [7]. Cognitive impairment also leads to loss of independence affecting individuals, families, and impacting the healthcare system [8]. Investigators have suggested that cognitive function is a predictor for becoming frail; however, measurements of cognitive function are not included in most operational definitions of frailty, despite suggestions by several authors in this regards [9–12]. A recent study has reported that cognitive impairment improves the predictive ability of frailty in association with adverse events [13]. Despite this, consequences of the coexistence of frailty and cognitive impairment are not well understood.
The impact of both frailty and cognitive impairment may be particularly dramatic in members of minority or underserved populations including Mexican Americans [14–19]. This is relevant for two reasons: first, the number of older adults from minority groups is rapidly increasing and variations in clinical entities (i.e. frailty and cognitive impairment) will impact care for these adults in the future [20,21]; second, frailty is associated with development of cognitive impairment [22,23] and similarly, cognitive impairment is associated with becoming frail [19,24]. A better understanding of the relationship between frailty and cognitive impairment and their effect on adverse events will lead to improved interventions for older adults, especially those with limited resources [25].
The purpose of this investigation was to examine how frailty status relates to mortality in the presence of cognitive impairment. We studied a large national sample of older Mexican Americans that has been followed for more than 10 years. Our hypothesis was that mortality risk for older frail adults would change in the presence of cognitive impairment.
METHODS
Sample and Procedures
Data are from the Hispanic Established Populations for the Epidemiological Study of the Elderly (Hispanic EPESE) study. The Hispanic EPESE is an ongoing longitudinal study of Mexican Americans aged 65 and older, residing in Texas, New Mexico, Colorado, Arizona and California. The sample and its characteristics have been described elsewhere [26,27]. The original probability-based sample (N = 3050) was representative of approximately 500,000 older Mexicans Americans living in the Southwest in the mid 1990s. The present study uses data obtained between the second and fifth waves (1995 to 2005). Interviews were conducted every two to three years. Information from the baseline interview is not used since the Physical Activity Scale for the Elderly (PASE), a component of the frailty index (see below), was only administered at the second wave. The PASE scale is a brief and easily scored instrument to assess physical activity in epidemiological studies of persons age 65 years and older [28,29].
Since frailty includes physical and self-reported performance measures, participants who required assistance by a proxy were not included in our sample. Of the 2,438 individuals interviewed in the second wave 2,166 were interviewed in person and 272 by proxy. Of the 2,166 individuals interviewed in person, 303 were subsequently excluded due to missing information on the components used to compute the frailty index and 48 due to missing data for other covariates. The final sample consisted of 1,815 individuals who had complete information on the frailty index and covariates at the 2nd wave (hereafter referred to as baseline [1995/96]), and were re-interviewed in the consecutive waves. By the end of follow-up (year 2005–2006) a total of 84 individuals refused to be interviewed, 124 individuals were lost to follow-up, and 690 individuals were confirmed dead through the National Death Index (NDI) and reports from relatives.
Individuals lost to follow-up, those that died and those excluded due to the criteria presented above were compared to our final sample. Excluded individuals were older, had less education and lower MMSE scores (p<.001). Excluded individuals also had higher prevalence of diabetes, heart attack, stroke, cancer, hip fracture and disability (p<0.05) compared with those included in the study. Finally, a significantly higher percentage of excluded individuals were in the frail category compared to those included in the analysis (18.8% vs. 7.9%; p<.05).
Measures
Frailty was assessed using procedures similar to those developed by Fried and colleagues [2] with the exception of physical activity where we used the PASE scale [29] instead of the Minnesota Leisure Activity Questionnaire [28]. The five components of the frailty measure include: weight loss, exhaustion, walking speed, grip strength and physical activity. Subjects with weight loss > 10 lbs from the previous interview were categorized as positive for the weight loss criterion. Exhaustion was assessed using two items from the Center for Epidemiologic Studies - Depression (CES-D) scale [30]: “I felt that everything I did was an effort” and “I could not get going.” The items asked “How often in the last week did you feel this way?” and were scored on a scale from zero to three depending on the frequency of the symptoms [30]. Subjects answering “2” or “3” to either of these two items were categorized as positive for the exhaustion criterion. Walking speed was assessed over a 16-foot timed walk. Height and gender adjusted time points were used and individuals in the slowest 20 percent were scored as positive for this criterion. Those unable to perform the test were also categorized as positive. Grip strength was assessed with different cut-points for men and women using a Jaymar Hand-held Dynamometer (Model #5030J1- J.A. Preston Corp, Jackson, MI). Subjects unable to perform the grip strength test and those in the lowest 20 percent adjusted for BMI and stratified by gender were categorized as positive for the weakness criterion. Subjects who scored in the lowest 20 percent of the PASE, adjusted by gender, were categorized as positive for the low physical activity criterion.
Individuals with three or more affected components of the frailty measure were considered frail. Individuals with one or two affected components were considered pre-frail and those with zero affected components were considered not frail. This followed the scoring convention developed by Fried and colleagues [2]. A more detailed description of the frailty construct used can be found elsewhere [31,32].
Cognitive impairment was assessed with the Mini Mental State Examination (MMSE) [33]. The English and Spanish versions of the MMSE were adopted from the Diagnostic Interview Scale (DIS) used in prior Hispanic community surveys [34]. This Spanish version of the MMSE met standard criteria for development of translated tests. The MMSE Spanish version has been successfully used in community surveys of Mexican Americans [35]. Scores range from 0 to 30, with lower scores indicating cognitive impairment. MMSE score was used both as a continuous variable and a dichotomized variable (< 21 for cognitive impairment vs. ≥ 21 for adequate cognitive function). We dichotomized the MMSE score based on two factors: total score distribution in our population sample at baseline and reports from previous aging research in similar populations [36]. This cut-point has been used in past studies on cognitive impairment among older populations with low educational attainment and low literacy [34,37,38].
Covariates
Sociodemographic variables included age, gender, marital status and years of formal education. The presence of medical conditions was assessed with a series of questions asking individuals if they had ever been told by a physician that they had diabetes, heart attack, stroke, hypertension, arthritis, cancer, or hip fracture.
Statistical analysis
Chi square and analysis of variance (ANOVA) tests were used to examine differences in the distribution of covariates for individuals by status at follow-up. To determine the relationship between cognitive impairment and frailty, unadjusted mean MMSE score and percent of frail individuals were plotted over time. Cox proportional hazard analysis was then used to estimate 10-year mortality as a function of frailty and cognitive impairment at baseline (MMSE < 21 and MMSE ≥ 21). Three Models were estimated to determine the effect of cognitive impairment and frailty on mortality. Model 1 included sociodemographic characteristics, medical conditions and cognitive impairment. Model 2, included sociodemographic characteristics, medical conditions and the three frailty categories (not frail, pre-frail and frail). In Model 3 (full model), sociodemographic characteristics, medical conditions, and both, cognitive impairment and frailty status were included. Survival curves were estimated according to the Kaplan Meier method and the six different groups were compared using log rank test. The first group included individuals with cognitive impairment that were not frail; the second group included individuals with cognitive impairment that were pre-frail; the third group included individuals with cognitive impairment that were frail. Three additional groups were created using the procedure previously explained, for individuals with adequate cognitive function that were in the three frailty categories. Log rank test was used to compare the survival curve for the six groups. All analyses were performed using the SAS System for Windows, Version 9.2 (SAS Institute, Cary, N.C.).
RESULTS
Table 1 shows the characteristics of the sample of older Mexican Americans by mortality status at follow-up. Being male, older, unmarried, and those with hip fracture and those deemed frail, were significantly more likely to be in the deceased group at follow-up. No other significant differences were observed in the remaining covariates between the three groups.
Table 1.
Descriptive characteristics of the sample by status at follow-up (n=1815)
| Alive (n=917) |
Dead (n=690) |
Lost to Follow-up (n=208) |
p-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age, mean ± SD | 73.3 ± 5.0 | 77.1 ± 6.5 | 73.0 ± 5.0 | <0.0001 |
| Gender (female) | 573 (31.6) | 359 (19.8) | 129 (7.1) | 0.03 |
| Education, mean ± SD | 4.9 ± 3.8 | 4.8 ± 3.9 | 5.5 ± 4.3 | 0.07 |
| Marital Status (married) | 542 (29.9) | 332 (18.3) | 108 (6.0) | 0.0005 |
| Diabetes | 214 (11.8) | 230 (12.7) | 41 (2.3) | 0.20 |
| Heart Attack | 71 (3.9) | 71 (3.9) | 18 (1.0) | 0.25 |
| Hypertension | 392 (21.6) | 346 (19.1) | 94 (5.2) | 0.06 |
| Stroke | 49 (2.7) | 61 (3.4) | 10 (0.6) | 0.27 |
| Cancer | 41 (2.3) | 67 (3.7) | 9 (0.5) | 0.06 |
| Hip Fracture | 3 (0.2) | 17 (0.9) | 2 (0.1) | 0.02 |
| Arthritis | 424 (23.4) | 295 (16.3) | 94 (5.2) | 0.39 |
| Cognitive Impairmenta | 151 (8.3) | 185 (10.2) | 30 (1.7) | 0.06 |
| Frailty Status | ||||
| Non frail | 476 (26.2) | 238 (13.1) | 106 (5.8) | <0.0001 |
| Pre-frail | 405 (22.3) | 355 (19.6) | 91 (5.0) | |
| Frail | 36 (2.0) | 97 (5.3) | 11 (0.6) |
Cognitive impairment = MMSE < 21; SD = Standard Deviation;
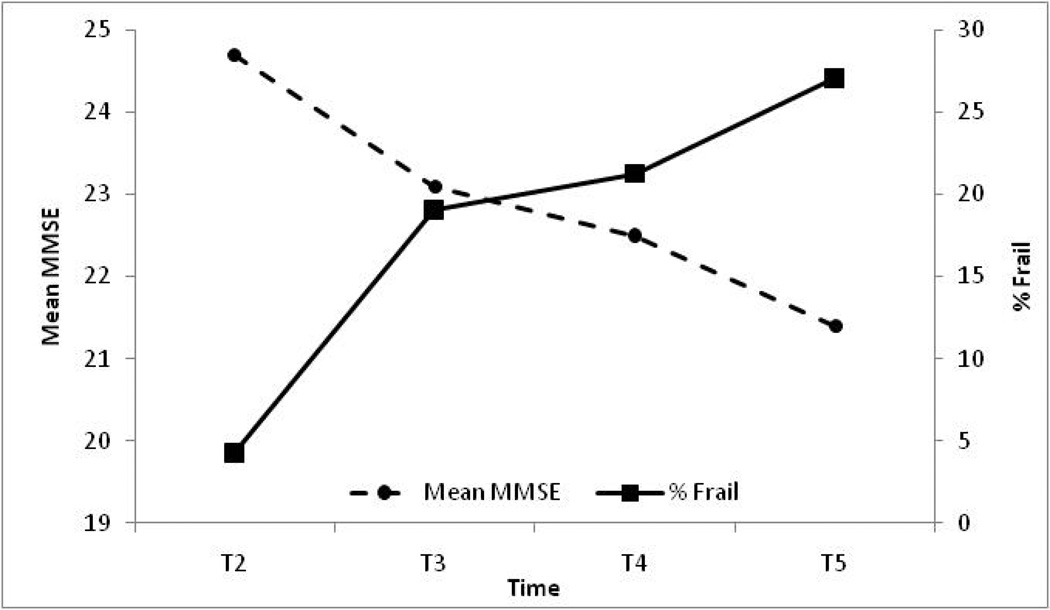
Figure 1 shows the relationship between cognitive impairment and frailty in older Mexican American survivors of our sample. As mean MMSE score declined over time, the percent of frail individuals increased in a linear fashion. There was a 4 point loss in mean MMSE score between wave 2 and wave 5 of the study. In addition, the mean percent of frail older Mexican Americans was more than five times higher in wave 5 compared to wave 2.
Figure 1.
Relationship between frailty and cognitive impairment in survivors between waves 2 to 5 of the Hispanic EPESE (n=1,127)
Table 2 shows Cox proportional hazard ratios of dying during the 10-year period. In Model 1, individuals with cognitive impairment had significantly higher hazard ratios (HR) of dying compared to those with adequate cognitive function after controlling for all sociodemographic variables and medical conditions (HR 1.26, 95% Confidence Interval [95% CI] 1.05–1.52). In model 2, pre-frail and frail individuals had significantly higher HR of dying compared to non frail individuals, after controlling for all covariates (Pre-frail: HR=1.40, 95% CI 1.18–1.66; Frail: HR= 2.03, 95% CI 1.57–2.62). In model 3, when both cognitive impairment and frailty status were added in the Model, the HR of dying for individuals with cognitive impairment was not statistically significant (HR=1.19, 95% CI 0.98–1.43), while pre-frail and frail individuals remained at significantly higher risk of dying compared to non frail individuals despite a reduction in the magnitude of the HR (Pre-frail: HR=1.39, 95% CI 1.17–1.64; Frail: HR= 1.97, 95% CI 1.53–2.55).
Table 2.
Cox proportional hazard models predicting 10-year mortality (N=1815)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age | 1.08 | (1.07–1.10) | 1.08 | (1.06–1.09) | 1.08 | (1.06–1.09) |
| Gender (female) | 0.58 | (0.49–0.69) | 0.58 | (0.49–0.69) | 0.59 | (0.49–0.70) |
| Education (continuous) | 1.02 | (0.99–1.04) | 1.01 | (0.99–1.03) | 1.02 | (1.00–1.04) |
| Marital Status (married) | 0.80 | (0.68–0.96) | 0.79 | (0.66–0.93) | 0.80 | (0.67–0.95) |
| Diabetes | 1.64 | (1.40–1.94) | 1.62 | (1.37–1.90) | 1.62 | (1.38–1.91) |
| Heart Attack | 1.05 | (0.81–1.35) | 1.02 | (0.79–1.32) | 1.02 | (0.80–1.32) |
| Stroke | 1.29 | (0.99–1.68) | 1.20 | (0.92–1.57) | 1.21 | (0.92–1.58) |
| Cancer | 1.69 | (1.31–2.20) | 1.64 | (1.26–2.12) | 1.63 | (1.26–2.11) |
| Hip Fracture | 1.76 | (1.08–2.87) | 1.54 | (0.94–2.51) | 1.55 | (0.95–2.53) |
| Hypertension | 1.29 | (1.11–1.51) | 1.29 | (1.10–1.51) | 1.30 | (1.10–1.51) |
| Arthritis | 0.91 | (0.78–1.07) | 0.86 | (0.73–1.01) | 0.87 | (0.74–1.02) |
| Cognitive Impairmenta | 1.26 | (1.05–1.52) | 1.19 | (0.98–1.43) | ||
| Frailty Status | ||||||
| Non frail | 1.00 | 1.00 | ||||
| Pre-frail | 1.40 | (1.18–1.66) | 1.39 | (1.17–1.64) | ||
| Frail | 2.03 | (1.57–2.62) | 1.97 | (1.53–2.55) | ||
Cognitive impairment = MMSE < 21; HR = Hazard Ratio.
Following procedures used by other researchers to examine percentage reduction in risk [39,40], we estimated the percentage reduction in mortality risk when frailty and cognitive impairment were used separately in a model compared to when they were together in the model. We wanted to know whether the relation between frailty and mortality and cognitive impairment and mortality would change in the presence of the other condition. The mortality risk attributable to cognitive impairment was reduced by 26.9% when frailty was added to the model. Similarly, mortality risk attributable to being pre-frail was reduced by 2.5%, and to being frail by 5.8%, when cognitive impairment was added to the model.
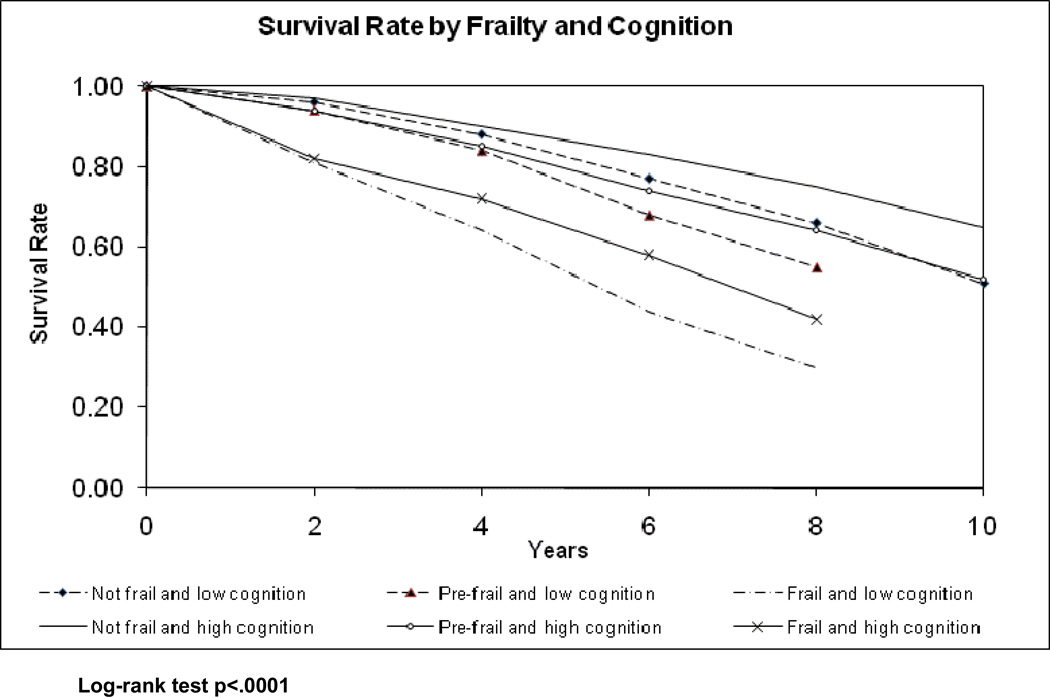
Figure 2 depicts 10-year mortality for all individuals by frailty and cognitive status at baseline. The six groups resulting from combining the three frailty categories (not frail, pre-frail and frail) and the two cognition groups (cognitive impairment and adequate cognitive function based on the MMSE cut-off of 21) are shown in Figure 2. Individuals with adequate cognitive function that were not frail during the observation period had the lowest mortality rates of all the groups with an absolute mortality rate of 35% after 10 years. Individuals with cognitive impairment that were frail had the highest mortality of all the groups with absolute mortality rates of 100% at 10 years and 70% at 8 years.
Figure 2.
Survival curve by frailty and cognitive status at baseline (n=1815)
Both cognitive impairment and frailty status were independent predictors of mortality in this sample. Frail individuals had higher mortality rates compared to pre-frail and not frail individuals regardless of their cognitive status (Cognitive impairment: absolute mortality rate 70% for frail, absolute mortality rate 45% for pre-frail and 34% for not frail individuals; Adequate cognitive function: absolute mortality rate 58% for frail, 36% for pre-frail and 25% for not frail individuals). Similarly, individuals with cognitive impairment had higher mortality rates compared to individuals with adequate cognitive function regardless of their frailty status.
It is worth noting that when the data are analyzed for the 10-year follow-up period, all individuals deemed frail, regardless of their cognitive status, were dead by the final year of follow-up. Similarly, all pre-frail individuals with cognitive impairment died by the 10th year of follow-up.
DISCUSSION
We examined mortality risk for individuals with frailty and cognitive impairment. As mean MMSE score decreased over time, the percent of frail older Mexican Americans increased. Our study suggests that both frailty and cognitive status increase mortality in older Mexican Americans. Mortality risk of individuals with cognitive impairment changes in the presence of frailty. Similarly, mortality risk of pre-frail and frail individuals changes in the presence of cognitive impairment. Thus, coexistence of frailty and cognitive impairment merit further evaluation in this population.
Previous studies show that frail older adults are at higher risk of dying compared to non-frail and even pre-frail older adults [2,41]. This remains true regardless of ethnic differences and, in some cases, regardless of socioeconomic status [42,43]. Additionally, frail older adults are at increased risk of other adverse events like hospitalization, disability, and institutionalization [9]. Frail older adults also have poorer quality of life compared to non-frail individuals [10,44].
Many studies have reported that individuals with cognitive impairment have higher mortality rates compared to individuals with adequate cognitive function [7]. Studies of patients with dementia show that they have higher rates of hospitalization, suffer more complications during hospitalization and end up with more disability [45]. These adverse outcomes result in higher rates of institutionalization, mortality and poorer quality of life.
Frailty and cognitive impairment are distinct clinical syndromes that share some characteristics. There is evidence that the relationship between cognitive status and frailty is based on shared physiologic pathways, and that the clinical presentation of frailty varies if cognitive impairment is added to the equation [46]. As reported previously, frailty is a dynamic condition and individuals may move between frailty categories over time [47]. We believe that frail individuals with cognitive impairment are likely to have limited ability to recover and move out of frailty.
The frailty phenotype proposed by Fried and colleagues [2] was not only a successful attempt at operationalizing a clinical syndrome that had been observed for some time, but represented an innovative approach that highlighted the importance of identifying clinical phenotypes to improve practice. Cognitive status may affect several of the components of the frailty cycle proposed by Fried et al [2]. For example, gait and muscle alterations as well as decreased physical activity are present in patients with cognitive impairment [48–50].
We prospectively analyzed a large number of individuals from a well-defined and comprehensively studied sample of older Mexican Americans. We also included a wide range of covariates related to both frailty and cognitive impairment. However, our study has some limitations. First, the MMSE is a crude measure of cognitive impairment with limited sensitivity to detect small changes in cognitive status in community living older persons [51,52]. Second, because depression and some cognitive disabilities are also related to brain dysregulation, the association between frailty and cognitive impairment can be mediated by other variables [53]. Finally, the information on medical conditions and comorbidities was based on self-reports. We did not have access to medical records, diagnostic images or serum markers to confirm subject self-reports, however, researchers have reported good agreement between self-reported medical conditions and actual medical diagnoses [46,54].
In conclusion, our investigation demonstrated that frailty and cognitive impairment affect mortality differently when they occur alone and when they are present together. Our findings suggest that both cognitive and frailty status are predictors of mortality in older Mexican Americans. Additional studies are necessary to analyze the shared pathways and common mechanisms influencing cognitive impairment and frailty. Pathways leading to death from frailty and cognitive impairment share some characteristics but are ultimately independent. Clinical phenotypes that include frailty and cognitive status and clarify their relationship will help broaden our understanding of the aging process.
AKNOWLEDGEMENTS
This study was supported by grants R03-AG029959, R01-AG017638, R01-AG010939 from the National Institute on Aging and K12 HD052023 of the National Institutes of Health. Infrastructure support provided by the Sealy Center on Aging at the University of Texas Medical Branch.
Reference List
- 1.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston JD, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Abellan VK, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55(8):1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 5.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, Cauley JA, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 6.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41(2):141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Sachs GA. Dying from dementia. N Engl J Med. 2009;361(16):1595–1596. doi: 10.1056/NEJMe0905988. [DOI] [PubMed] [Google Scholar]
- 8.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lally F, Crome P. Understanding frailty. Postgrad Med J. 2007;83(975):16–20. doi: 10.1136/pgmj.2006.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34(5):432–434. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 12.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le GM, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 14.Angel JL, Angel RJ, McClellan JL, Markides KS. Nativity, declining health, and preferences in living arrangements among elderly Mexican Americans: implications for long-term care. Gerontologist. 1996;36(4):464–473. doi: 10.1093/geront/36.4.464. [DOI] [PubMed] [Google Scholar]
- 15.Angel RJ, Angel JL, Lee GY, Markides KS. Age at migration and family dependency among older Mexican immigrants: recent evidence from the Mexican American EPESE. Gerontologist. 1999;39(1):59–65. doi: 10.1093/geront/39.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Hunt KJ, Williams K, Resendez RG, Hazuda HP, Haffner SM, Stern MP. All-cause and cardiovascular mortality among diabetic participants in the San Antonio Heart Study: evidence against the "Hispanic Paradox". Diabetes Care. 2002;25(9):1557–1563. doi: 10.2337/diacare.25.9.1557. [DOI] [PubMed] [Google Scholar]
- 17.Markides KS, Coreil J. The Health of Hispanics in the Southwestern United-States - An Epidemiologic Paradox. Public Health Rep. 1986;101(3):253–265. [PMC free article] [PubMed] [Google Scholar]
- 18.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec No 2):68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 19.Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older mexican americans. J Am Geriatr Soc. 2008;56(10):1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, Dieppe P. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess. 2005;9(38):iii–x. doi: 10.3310/hta9380. 1. [DOI] [PubMed] [Google Scholar]
- 21.Sarkisian CA, Gruenewald TL, John BW, Seeman TE. Preliminary evidence for subdimensions of geriatric frailty: the MacArthur study of successful aging. J Am Geriatr Soc. 2008;56(12):2292–2297. doi: 10.1111/j.1532-5415.2008.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raji MA, Al SS, Ray LA, Patel KV, Markides KS. Early mental ability may predict future ability to live independently. Ethn Dis. 2004;14(1):158–159. [PubMed] [Google Scholar]
- 24.Colombo M, Guaita A, Cottino M, Previdere G, Ferrari D, Vitali S. The impact of cognitive impairment on the rehabilitation process in geriatrics. Arch Gerontol Geriatr Suppl. 2004;(9):85–92. doi: 10.1016/j.archger.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Black SA, Rush RD. Cognitive and Functional Decline in Adults Aged 75 and Older. J Am Geriatr Soc. 2002;50:1978–1986. doi: 10.1046/j.1532-5415.2002.50609.x. [DOI] [PubMed] [Google Scholar]
- 26.Cornoni-Huntley J, Lafferty ME National Institute on Aging. Established populations for epidemiologic studies for the elderly, resource data book. Bethesda, MD: National Institute on Aging; 1986. [Google Scholar]
- 27.Markides KS, Stroup-Benham CA, BS . The health of Mexican-American elderly: selected findings from the Hispanic EPESE. In: Wykle M, Ford A, editors. Serving Minority Elderly in the 21st Century. New York: Springer; 1999. pp. 72–90. [Google Scholar]
- 28.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. 'The CES-D scale: A self report depression scale for research in the general population'. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 31.Al Snih S, Graham JE, Ray LA, Samper-Ternent R, Markides KS, Ottenbacher KJ. Frailty and incidence of activities of daily living disability among older Mexican Americans. J Rehabil Med. 2009;41(11):892–897. doi: 10.2340/16501977-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottenbacher KJ, Ostir GV, Peek MK, Al Snih S, Raji MA, Markides KS. Frailty in older mexican americans. J Am Geriatr Soc. 2005;53:1524–1531. doi: 10.1111/j.1532-5415.2005.53511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State" A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Bird HR, Canino G, Rubio-Stipec M, Shrout P. Use of the mini-mental state examination in a probability sample of a Hispanic population. J Nerv Ment Dis. 1987;175:731–737. doi: 10.1097/00005053-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Escobar J, Burnman A, Karno M, Forsythe A, Landsverk J, Golding JM. Use of the mini-mental status examination (MMSE) in community population of mixed ethnicity: cultural and linguistic artifacts. J Nerv Ment Dis. 1986;174:607–614. doi: 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Raji MA, Al Snih S, Ray LA. Cognitive status and incident disability in older Mexican Americans. Ethn Dis. 2004;14:26–31. [PubMed] [Google Scholar]
- 37.Leveille SG, Guralnik JM, Ferruci L, Corti MS, Kasper J, Fried LP. Black/white differences in the relationship between MMSE scores and disability: the Women's Health and Aging study. The journals of gerontology Series B, Psychological sciences and social sciences. 1998;53(3):201–208. doi: 10.1093/geronb/53b.3.p201. [DOI] [PubMed] [Google Scholar]
- 38.Uhlmann RF, Larson EB. Effect of education on the mini-mental state examination as a screening test for dementia. J Am Geriatr Soc. 1991;39:876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 39.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 40.Szklo M, Nieto F. Epidemiology: beyond the basics. 2nd ed. Jones and Bartlett Publishers; 2006. [Google Scholar]
- 41.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 42.Espinoza SE, Hazuda HP. Frailty in older Mexican-American and European-American adults: is there an ethnic disparity? J Am Geriatr Soc. 2008;56(9):1744–1749. doi: 10.1111/j.1532-5415.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch C, Anderson ML, Newman A, Kop W, Jackson S, Gottdiener J, et al. The association of race with frailty: the cardiovascular health study. Ann Epidemiol. 2006;16(7):545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Bergman H, Hogan DB, Karunananthan S. Frailty: A clinically relevant concept? The Canadian Journal of Geriatrics. 2008;11(3):124–129. [Google Scholar]
- 45.Hogan DB, Fung TS, Ebly EM. Health, function and survival of a cohort of very old Canadians: Results from the second wave of the Canadian Study of Health and Aging. Canadian Journal of Public Health-Revue Canadienne de Sante Publique. 1999;90(5):338–342. doi: 10.1007/BF03404524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchman AS, Boyle PA, Wilson RS, Tnag Y, Bennett DA. Frailty is associated to with incident Alzheimer's disease and cognitive decline in elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 47.Ottenbacher KJ, Graham JE, Al Snih S, Raji M, Samper-Ternent R, Ostir GV, Markides KS. Mexican Americans and frailty: findings from the Hispanic established populations epidemiologic studies of the elderly. Am J Public Health. 2009;99(4):673–679. doi: 10.2105/AJPH.2008.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alfaro-Ancha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61(8):859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alfaro-Ancha A, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Does 8-foot walk time predict cognitive decline in older Mexican Americans? J Am Geriatr Soc. 2007;55:245–251. doi: 10.1111/j.1532-5415.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 50.Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment--the relationship between physical and cognitive function. Neuroepidemiology. 2008;31(3):167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol. 2005;20(4):485–503. doi: 10.1016/j.acn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Wind AW, Schellevis FG, Van Staveren G, Scholten RP, Jonker C, Van Eijk JT. Limitations of the Mini-Mental state examination in diagnosing dementia in general practice. Int J Geriatr Psychiatry. 1997;12:101–108. doi: 10.1002/(sici)1099-1166(199701)12:1<101::aid-gps469>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 53.Royall DR. Executive cognitive impairment: a novel perspective on dementia. Neuroepidemiology. 2000;19:293–299. doi: 10.1159/000026268. [DOI] [PubMed] [Google Scholar]
- 54.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]