Abstract
Human recombinant arginase I cobalt coupled to polyethylene glycol 5000 (HuArg I [Co]-PEG5000) achieved potent in vitro depletion of arginine from tissue culture medium and cytotoxicity to many cancer cell lines. The recombinant enzyme also produced tumor growth inhibition of hepatocellular carcinoma and pancreatic carcinoma xenografts. Although these results were promising, the therapeutic index was narrow. Toxicities were seen in normal cells in tissue culture. In vivo normal tissue injury occurred at doses twice the effective dose. The current study was conducted to define, in greater detail, the maximum tolerated dose (MTD), pharmacodynamics, and dose-limiting toxicities (DLTs) of twice-weekly intraperitoneal HuArg I [Co]-PEG5000 in Balb/c mice. Animal weight and survival were monitored, serum arginine levels measured, and complete blood cell counts, chemistries, necropsies, and histologies were performed. In addition, methods to ameliorate the HuArg I [Co]-PEG5000 adverse effects were tested. Supplemental l-citrulline was given concurrently with the arginase drug. The HuArg I [Co]-PEG5000 MTD in mice was 5 mg/kg twice weekly, and DLTs included weight loss and marrow necrosis. No other organ damage or changes in blood cell counts or chemistries were observed. Arginase reduced serum arginine levels from 60 µM to 4 to 6 µM. Supplemental l-citrulline given per os or daily subcutaneously reduced and delayed toxicities, and l-citrulline given twice daily subcutaneously completely prevented animal toxicities. On the basis of these results, we hypothesize that HuArg I [Co]-PEG5000, particularly with supplemental l-citrulline, may be an attractive therapeutic agent for argininosuccinate synthetase-deficient tumors.
Introduction
Metastatic carcinoma patients receive limited benefit from chemotherapy, and therapeutic agents that target cancer cell-dependent molecular pathways have been developed. Nonessential amino acid auxotrophy has been observed in a number of different malignancies, and tumor cell dependence on arginine has been correlated with tumor cell deficiency of the urea cycle enzymes—ornithine transcarbamylase (OTC) and/or argininosuccinate synthetase (ASS) [1]. Two different arginine-depleting proteins—arginine deiminase and arginase—have been produced and tested clinically in patients with metastatic melanoma and/or hepatocellular carcinoma [2,3]. Although remissions were observed in some cases, immunogenicity of the arginine deiminase and low potency and stability of the arginase inhibited efficacy. Recently, we prepared a pegylated recombinant human arginase I containing two Co2+ atoms instead of the Mn2+ atoms [4]. The molecule had improved catalytic activity, stability, and reduced immunogenicity relative to the other arginine-depleting molecules. We then examined the sensitivity of human normal and malignant cells [5]. Human recombinant arginase I cobalt coupled to polyethylene glycol 5000 (HuArg I [Co]-PEG5000) was cytotoxic to all normal and tumor cell lines. This finding correlated with the absence of OTC from most normal and malignant cells. In the presence of excess l-citrulline, selective cytotoxicity was observed for ASS-deficient tumors and a few normal tissues. The results were consistent with the rescue of arginine synthesis in normal tissues with the l-citrulline (Figure 1). A pilot animal study evaluated the safety and efficacy of HuArg I [Co]-PEG5000 alone [6]. Single doses or weekly doses of enzyme yielded a maximum tolerated dose (MTD) of 8 mg/kg intraperitoneal (i.p.) and dose-limiting toxicities (DLTs) of weight loss and marrow damage. Tumor growth inhibition of 46% and 58% was observed for hepatocellular carcinoma and pancreatic carcinoma xenografts, respectively. On the basis of our tissue culture results, in this study, we sought to test the in vivo effects of supplemental l-citrulline on HuArg I [Co]-PEG5000 toxicity. We also assessed different l-citrulline doses and delivery methods and a twice-weekly HuArg I [Co]-PEG5000 dose regimen, performed pharmacodynamic studies of serum arginine concentrations, and did complete blood cell counts, blood chemistries, necropsies, and histopathology on animals.
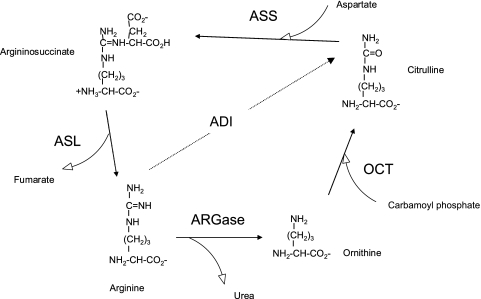
Figure 1.
Urea cycle pathway and arginine deiminase. ADI indicates arginine deiminase; ASL, argininosuccinate lyase; ARGase, arginase. Tumors often lack ASS. Normal tissues frequently lack OTC. ADI is from microbial source. ARGase are intracellular enzymes of liver and other organs.
Materials and Methods
Reagents
HuArg I [Co]-PEG5000 was produced from purified HuArg I expressed in Escherichia coli [6]. Briefly, enzyme in 100 mM sodium phosphate pH 8.3 was reacted with a 40-fold excess of methoxy PEG succinimidyl carboxymethyl ester 5000 MW (JemKem Technology, Allen, TX) for 1 hour at 25°C. The mixture was then incubated with 10 mM cobalt chloride hexahydrate (MP Biomedicals, Solon, OH) and heated to 50°C for 10 minutes. HuArg I [Co]-PEG5000 was then centrifuged, dialyzed into phosphate-buffered saline (PBS) with 10% glycerol, sterile filtered, aliquoted, and stored at -80°C until used. l-Citrulline was purchased from Sigma-Aldrich (St Louis, MO).
Animals
Six-to eight-week-old female Balb/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in ventilated cages equipped with municipal water and standard rodent chow containing 1.4% arginine (Purina LabDiet 5001). Animals were weighed daily and examined twice daily for activity, eating, posture, and coat condition. Mice received 1 ml of Dulbecco PBS subcutaneously daily for weight loss exceeding 10%. In addition, supplemental nutrition with DietGel, water gels, and dry cereal mix was provided. Moribund animals or animals losing more than 25% body weight were killed by CO2 asphyxiation, and cardiac puncture was performed to collect blood. Full necropsies were done, and tissue histopathology was performed with formalin-fixed, paraffin-embedded 6-µm hematoxylin/eosin-stained sections of brain, lung, liver, heart, stomach, intestine, colon, kidney, pancreas, bladder, skin, uterus, muscle, and bone marrow. Blood cell counts and chemistries were performed on an Abaxis (Union City, CA) VetScan HM2 and VS, respectively.
HuArg I [Co]-PEG5000 Dose Studies
Cohorts of 10 to 20 mice received 1, 5, or 10 mg/kg HuArg I [Co]-PEG5000 twice weekly for up to 4 weeks. Animals were monitored for weight, overall condition, and survival. One hundred microliters of blood was collected from facial vein of surviving animals on days 7 and 28. Blood was mixed with equal volume 0.1 M citrate buffer pH 6.6, plasma was separated, and the solution was stored at -80°C. Moribund animals and animals completing the study were assayed for tissue histology and blood cell counts and chemistries as described.
l-Citrulline Supplementation Experiments
Cohorts of 20 animals received 10 mg/kg HuArg I [Co]-PEG5000 i.p. twice weekly for 2 weeks with daily 1 ml of PBS subcutaneously daily, with 1 ml of 100 mg/ml l-citrulline in PBS subcutaneously daily, with 1 ml of 100 mg/ml l-citrulline in PBS subcutaneously twice daily, or with l-citrulline in drinking water at 100 mg/ml. Animals were again monitored for weight, overall condition, and survival.
Serum Arginine Concentrations
Freshly obtained or thawed plasma was mixed with perchloric acid, neutralized with potassium carbonate, and centrifuged. Supernatants were reacted with 30 mM o-phthalaldehyde (OPA), 50 mM 2-mercaptoethanol, 40 mM sodium borate, and 3.1% Brij-35 pH 9.5. Samples and standards (Sigma-Aldrich) were run immediately on a Supelcosil LC-18 (Sigma-Aldrich) high-performance liquid chromatography (Shimadzu, Tokyo, Japan) with a gradient of 86% 0.1 M sodium acetate pH 7.2/14% methanol to 100% methanol as described by Wu and Meininger [7]. Fluorescence is monitored at excitation and emission wavelengths of 340 and 455 nm, respectively. Area under the curve was calculated and correlated with standards. The assay range was 4 to 100 µM arginine.
Statistics
Mean and SDs were used to summarize continuous variables. Different dose groups were compared with control groups using a Mann-Whitney test. Survival analyses were performed using Kaplan-Meier survival curves, and median survival time was calculated. The comparison of two or more groups was performed using the log-rank test, considering the threshold value P ≤ .05. Analyses were performed in R 2.12.1 (R development, 2010).
Results
HuArg I [Co]-PEG5000 Mouse Toxicity
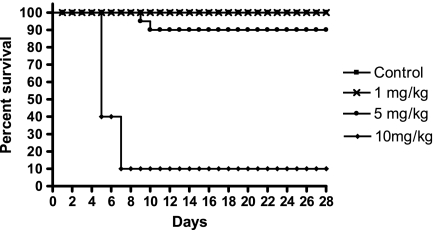
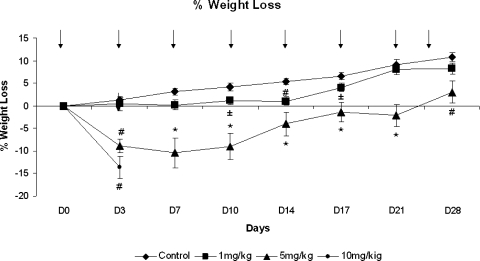
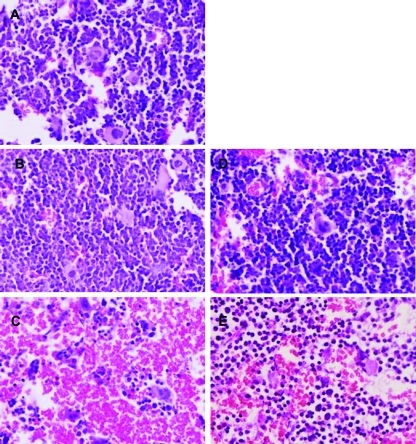
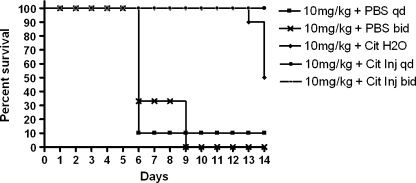
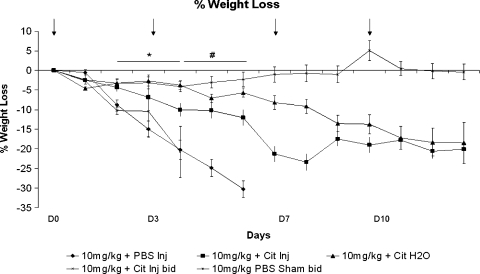
The MTD of HuArg I [Co]-PEG5000 administered i.p. twice weekly to Balb/c mice was 5 mg/kg. As shown in Figure 2, control and 1 mg/kg protein yielded 100% survival, 5 mg/kg produced 90% survival, and 10 mg/kg led to 10% survival. Lethality occurred within 10 days of treatment initiation. The difference in survival between the control, 1 and 5 mg/kg were not significant; the differences in survival between control and 10 mg/kg and between the 5- and 10-mg/kg dose levels were significant (P < .001) as determine by log-rank tests. The DLT was extreme weight loss (Figure 3) and marrow necrosis (Figure 4). No abnormalities in blood cell counts, chemistries, or other organ histopathology were seen.
Figure 2.
Kaplan-Meier plot of mice survival at different dose levels of HuArg I [Co]-PEG5000 therapy. Animals were dosed i.p. twice weekly for 4 weeks.
Figure 3.
Percent mean weight loss from baseline for HuArg I [Co]-PEG5000-treated mice at different times of postinitiation therapy. Error bars represent SEM. Arrows indicate days of arginase injections. *P < .001, #P < .005, ±P < .05 compared with control.
Figure 4.
Bone marrow histopathology. Iliac wings removed, fixed in paraformaldehyde, dehydrated, embedded in paraffin, rehydrated, and stained with hematoxylin and eosin. Representative photomicroscopic images at 150x magnification. (A) Control PBS. (B) HuArg I [Co]-PEG5000 5 mg/kg. (C) HuArg I [Co]-PEG5000 10 mg/kg. (D) HuArg I [Co]-PEG5000 10 mg/kg + l-citrulline 100 mg subcutaneously daily. (E) HuArg I [Co]-PEG5000 10 mg/kg + oral l-citrulline 100 mg/ml. Note absence of nuclei and extensive red cell replacement of necrotic bone marrow in C. Mice in D and E were allowed to recover for 2 weeks before histologic analysis.
Plasma Arginine Concentrations
Control mouse plasma arginine levels averaged 61 ± 19 and 44 ± 15 µM on days 7 and 28 of the experiment, respectively (Table 1). HuArg I [Co]-PEG5000 1 mg/kg treated animals yielded arginine concentrations of 32 ± 30 and 54 ± 22 µM on days 7 and 28, respectively. In contrast, HuArg I [Co]-PEG5000 5 mg/kg gave 7 ± 2 and 18 ± 20 µM on days 7 and 28, respectively; HuArg I [Co]-PEG5000 10 mg/kg produced 6 ± 2 µM arginine levels on day 7. There were no surviving mice at this dose on day 28. Both the 5- and 10-mg/kg-treated plasma arginine levels were significantly lower than the control and 1-mg/kg-dose-level plasma levels on day 7 (P < .001) and day 28 for the surviving 5-mg/kg mice (P < .01).
Table 1.
Serum Arginine Levels after Treatment with Different Doses of HuArg I [Co]-PEG5000 on Days 7 and 28.
| Day 7 | Day 28 | |||
| Mean | SD | Mean | SD | |
| Control | 60.61 | 19.01 | 43.71 | 14.92 |
| 1 mg/kg | 32.19* | 29.93 | 53.91 | 21.84 |
| 5 mg/kg | 6.63† | 2.49 | 18.09‡ | 20.48 |
| 10 mg/kg | 5.54† | 2.01 | - | - |
P < .05.
P < .001.
P < .01.
l-Citrulline Protection
Because all deaths from HuArg I [Co]-PEG5000 occurred within 10 days, we designed the protection study to last 2 weeks. l-Citrulline was given either as 100-mg (5 g/kg) bolus subcutaneously injection daily or twice daily or at 100 mg/ml in drinking water to HuArg I [Co]-PEG5000 10-mg/kg-treated animals for 2 weeks. l-Citrulline supplementation ameliorated the HuArg I [Co]-PEG5000 toxicities in all treated cohorts. Animals treated with either daily or twice-daily subcutaneous l-citrulline survived the entire 2 weeks (Figure 5). Some orally supplemented animals stopped taking fluids and thus lost l-citrulline protection and were killed because of extreme weight loss. The difference in survival was highly significant for all citrulline interventions compared with control or animals treated with PBS subcutaneously twice daily (P < .01). Weight loss was reduced from an average of more than 30% to 0% or 17% (Figure 6). Animals treated with l-citrulline supplementation showed improved bone marrow histology after being allowed to recover for 2 weeks (Figure 3).
Figure 5.
Kaplan-Meier plot of mice survival of HuArg I [Co]-PEG5000 10 mg/kg treated mice with l-citrulline supplementation. Mice were treated twice weekly for 2 weeks.
Figure 6.
Percent mean weight loss from baseline for 10-mg/kg HuArg I [Co]-PEG5000-treated mice at different times of postinitiation therapy with or without l-citrulline supplementation. Error bars represent SEM. Arrows indicate days of arginase injections. *P < .01. #P < .001.
Discussion
Arginine depletion therapy for arginine auxotrophic malignancies is an active area of clinical investigation. We are producing HuArg I [Co]-PEG5000 under Good Manufacturing Practice guidelines for clinical studies. Thus, accurate information on likely MTD, DLTs, and pharmacodynamics are of great importance for clinical protocol design and identification of interventions to reduce adverse effects. Further, in vivo toxicity studies with HuArg I [Co]-PEG5000 may shed further light on the dependence of various normal organs on circulating plasma arginine.
Previous work with arginine deiminase in hepatocellular carcinoma patients yielded rare transient, reversible encephalopathy, hyponatremia, hyperkalemia, anemia, hypoalbuminemia, hyperuricemia, hypofibrinogenemia, and elevated serum lipase level [2]. Many of these complications may be been secondary to hepatocellular carcinoma. In a separate study, arginine deiminase was administered systemically to metastatic melanoma patients, and no grade 3 or 4 toxicities occurred [8]. Hyperuricemia and rare, mildly elevated serum lipase and amylase levels were seen without clinical pancreatitis. Dose was limited by intramuscular injection volume. Thus, conversion of arginine to citrulline by the arginine deiminase achieved low serum arginine levels with few—if any—major adverse effects.
Human Mn2+-containing arginase given to hepatocellular carcinoma patients generated hyperbilirubinemia, transaminasemia, nausea, diarrhea, and abdominal pain [9]. The investigators reported prolonged serum arginine levels of less than 8 µM. Again, the relationship of the adverse effect profile to the patient's disease versus drug was undefined. No studies of this agent in other cancer patients have been reported. Hence, it is difficult to directly compare the safety of arginine deiminase and Mn2+ arginase. In our studies of the Co2+ arginase both in tissue culture and in rodents, significant toxicity was seen at high doses. In our prior tissue culture work, HuArg I [Co]-PEG5000 was cytotoxic to almost all normal and malignant human cell lines [5]. In a prior mouse study, 15 mg/kg HuArg I [Co]-PEG5000 given weekly produced weight loss, hunched posture, lethargy, and bone marrow necrosis [6]. In our current experiments, we verified the toxicity in mice. HuArg I [Co]-PEG5000 given at 10 mg/kg twice weekly produced uniform lethality, tremendous weight loss, and bone marrow necrosis. The greater toxicity of the arginase relative to arginine deiminase may be due to failure of normal tissues to rescue arginine from ornithine—the product of arginase. The conversion from ornithine to arginine requires OTC, which we previously showed was low in many normal primary cell cultures [5].
The isolated injury to bone marrow and loss of animal weight are curious toxicities. As noted above, our results substantiate the previous HuArg I [Co]-PEG5000 mouse study [6]. The lack of changes in blood cell counts confounds the marrow histology. The poor oral intake associated with weight loss without changes in serum chemistry or major organ histology is not explainable by us but may be alleviated in humans with fluid and/or caloric supplementation. It is also unclear if arginine deprivation therapy will have as severe an impact on non-rodents such as primates based on variations in species sensitivity [10]. Additional studies in nonhuman primates may shed additional light on the DLT before clinical trials.
Mouse plasma arginine concentrations fell after HuArg I [Co]-PEG5000 infusion. Plasma arginine remained low for 7 days at the medium and high dose levels. We were not able to see a significant difference in circulating arginine concentration to account for the increased toxicity of the highest dose. A likely explanation is the modest sensitivity of the high-performance liquid chromatography assay method with a lower limit of detection close to 4 to 6 µM and both medium- and high-dose HuArg I [Co]-PEG5000 depleted arginine at or below this level. In contrast, liquid chromatography-mass spectrometry provides a lower limit of quantification of 1 µM and may permit differentiation of posttherapy arginine concentrations [11]. Another reason may be that the higher HuArg I [Co]-PEG5000 dose impacts more the extracellular tissue concentrations of arginine, which we have not measured. Nevertheless, HuArg I [Co]-PEG5000 achieved excellent plasma arginine depletion at a safe dose.
If the HuArg I [Co]-PEG5000 in vivo toxicity is due to the lack of OTC in normal tissues, supplementation of animals with l-citrulline may alleviate normal tissue injuries. Our results support this hypothesis. There was dramatic improvement in the 10-mg/kg-dose-level survival, weight, and marrow histology with i.p. or oral l-citrulline. l-Citrulline has been safely administered to humans and, in fact, is associated with improved erection hardness in patients with mild erectile dysfunction, right ventricular function in patients with heart failure, reduced arterial stiffness, decreased ECG QT interval, and absent effects on whole body protein metabolism [12–16]. Our study suggests that l-citrulline supplementation with HuArg I [Co]-PEG5000 may improve the therapeutic index—particularly in patients with ASS-negative metastatic cancers. Confirmation of the maintenance of in vivo efficacy in a mouse xenograft model and safety in a nonhuman primate model should be done to extend these results and facilitate clinical development. Overall, HuArg I [Co]-PEG5000 is an exciting new therapeutic compound for arginine auxotrophic malignancies.
Footnotes
This study was supported by Love Cures Foundation Grant (A.E.F.), Sweetest Loop Grant (A.E.F.), Tula Lee Stone Endowed Chair of Cancer Research (A.E.F.), Cancer Prevention and Research Institute of Texas Grant RP100890 (G.G.), Scott & White Memorial Hospital Foundation (A.E.F.), TI3D/Welch Foundation grant HF0032 (G.G.), and National Institutes of Health grant 139059 (G.G.).
References
- 1.Feun LG, You M, Wu C, Kuo MT, Waugpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Design. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yau CC, Chan P, Pang R, Chan W, Cheng PN, Poon R. A phase I study of recombinant human arginase (rhArgI) for patients with advanced hepatocellular carcinoma. J Clin Oncol. 2010;28(suppl):e13503. [Google Scholar]
- 3.Feun LG, You M, Wu C, Wangpaichitr M, Kuo MT, Marini A, Jungbluth A, Savaraj N. Final results of phase II trial of pegylated arginine deiminase (ADI-PEG20) in metastatic melanoma (MM) J Clin Oncol. 2010;28(suppl):15s. [Google Scholar]
- 4.Stone EM, Glazer E, Chantranupong L, Cherukuri P, Breece RM, Tierney DL, Curley SA, Iverson BL, Georgiou G. Replacing Mn(2+) with Co(2+) in human arginase I enhances cytotoxicity toward l-arginine auxotrophic cancer cell lines. ACS Chem Biol. 2010;5:333–342. doi: 10.1021/cb900267j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal V, Woo JH, Mauldin JP, Jo C, Stone EM, Georgiou G, Frankel AE. Cytotoxicity of human recombinant arginase I [Co]-PEG5000 in the presence of supplemental l-citrulline is dependent on decreased argininosuccinate synthetase expression in human cells. Anticancer Drugs. 2012;23:51–64. doi: 10.1097/CAD.0b013e32834ae42b. [DOI] [PubMed] [Google Scholar]
- 6.Glazer ES, Stone EM, Zhu C, Massey KL, Hamir AN, Curley SA. Bioengineered human arginase I with enhanced activity and stability controls hepatocellular and pancreatic carcinoma xenografts. Transl Oncol. 2011;4:138–146. doi: 10.1593/tlo.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G, Meininger CJ. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008;440:177–189. doi: 10.1016/S0076-6879(07)00810-5. [DOI] [PubMed] [Google Scholar]
- 8.Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23:7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- 9.Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V, Petrillo A, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol. 2010;28:2220–2226. doi: 10.1200/JCO.2009.26.7765. [DOI] [PubMed] [Google Scholar]
- 10.Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Exp Opin Invest Drugs. 2006;15:815–822. doi: 10.1517/13543784.15.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harder U, Koletzko B, Peissner W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:495–504. doi: 10.1016/j.jchromb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Cormio L, De Siati M, Lorusso F, Selvaggio O, Mirabella L, Sanguedolce F, Carrieri G. Oral l-citrulline supplementation improves erection hardness in men with mild erectile dysfunction. Urology. 2011;77:119–122. doi: 10.1016/j.urology.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Orozco-Gutierrez JJ, Castillo-Martinez L, Orea-Tejeda A, Vazquez-Diaz O, Valdespino-Trejo A, Narvaez-David R, Keirns-Davis C, Carrasco-Ortiz O, Navarro-Navarro A, Sanchez-Santillan R. Effect of l-arginine or l-citrulline oral supplementation on blood pressure and right ventricular function in heart failure patients with preserved ejection fraction. Cardiol J. 2010;17:612–618. [PubMed] [Google Scholar]
- 14.Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, Watanabe F, Morishita K. Short-term effects of l-citrulline supplementation on arterial stiffness in middle-aged men. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.10.004. E-pub ahead of print November 8. [DOI] [PubMed] [Google Scholar]
- 15.Kameda N, Okigawa T, Kimura T, Fujibayashi M, Asada T, Kinoshita R, Baba S, Morita M, Morishita K, Moritani T. The effect of l-citrulline ingestion on ECG QT interval and autonomic nervous system activity. J Physiol Anthropol. 2011;30:41–45. doi: 10.2114/jpa2.30.41. [DOI] [PubMed] [Google Scholar]
- 16.Thibault R, Flet L, Vavasseur F, Lemerle M, Ferchaud-Roucher V, Picot D, Darmaun D. Oral citrulline does not affect whole body protein metabolism in healthy human volunteers: results of a prospective, randomized, double-blind, cross-over study. Clin Nutr. 2011;30:807–811. doi: 10.1016/j.clnu.2011.06.005. [DOI] [PubMed] [Google Scholar]