Abstract
Microdera punctipennis Kasz (Coleoptera: Tenebrionidae) is a unique species that lives in the desert region of Central Asia and has adopted a nocturnal habit to survive the desert environment. Female adults are larger in size than male adults. The female/male ratio was 1.04:1. A rearing method using reused plastic bottles was used. The rearing conditions were 30 ± 0.5°C, 30 ± 6% relative humidity (RH), and 16:8 L:D photoperiod. Cabbage was provided as food. Cannibalism was avoided by rearing one larva in a bottle. A complete life cycle was obtained under these conditions. The viability of eggs, larvae, prepupae, pupae, and teneral adults was 93.54%, 83.71%, 84.76%, 87.64%, and 93.59%, respectively. Embryogenesis took 7.35 days on average. The larval duration in each instar was 2.25 days. The mean duration of the larvae, prepupae, pupae, and teneral adult was 49.27, 7.05, 9.95, and 10.12 days, respectively. The coloration of each developmental stage gradually changed from creamy white to light brownish or black. Females commenced oviposition when their body color became black. On average, each female produced 568 eggs.
Keywords : Central Asia, developmental duration, immature stages, Tenebrionidae
Introduction
Beetles, especially Tenebrionidae, are among the most successful animals of the desert, and are called “indicators of desertization” (Ren and Yu 1999). They adopt several strategies to survive hostile environments. Behaviorally and morphologically the majority of desert beetles are active at night. During the day they bury themselves deeply in the substrate to avoid high temperatures and low humidity (Wharton 1983; Cloudsley-Thompson 1990) and take up fog-water as a water source (Seely 1979; Parker and Lawrence 2001; Hamilton et al. 2003; Adam 2004). They tend to have a flattened body and short legs, meaning they are well adapted to burrowing in sand. Body size is an important feature for adaptation to microclimate and substrate factors (Thomas 1983; Doyen and Slobodchikoff 1984). Certain structural and physiological regulations developed by the desert beetles also play an important role in desert adaptation. For instance, the subelytral cavity, an airtight space formed by the fusion of the elytra (Draney 1993; Gorb 1998), is found especially in desert Tenebrionidae (Cloudsley-Thompson 2001) and it helps to lower cuticular water permeability in desert beetles (Zachariassen 1991, 1996). Desert tenebrionid beetles also adopt seasonal behavioral changes to avoid hostile conditions; most species exhibited 7–10 month of activity with one or two peaks of abundance (Krasnov and Ayal 1995).
Microdera punctipennis Kasz (Coleoptera: Tenebrionidae), is a small, flightless beetle adapted to live in the Gurbantonggut desert (Huang et al. 2005), the second largest desert in northwest China. Antifreeze protein genes from M. punctipennis have been cloned (Zhao et al. 2005) and functionally characterized (Wang et al. 2008; Qiu et al. 2010), but little else is known of its biology and immature stages.
This article aims to establish: (i) the morphological and behavioral features of M. punctipennis adults collected from its natural environment; (ii) a rearing method for M. punctipennis so as to identify the immature stages of M. punctipennis under rearing conditions; and (iii) the biological parameters of development of M. punctipennis under rearing conditions.
Materials and Methods
Adult collection site and observation
Microdera punctipennis adults were collected in 2008 from Fukang (44° 24′ N, 087° 51′E, 444 m), which is about 100 km northeast of the geological center of Asia. The annual average air temperature is 5–7.5° C. The highest air temperature is more than 40° C and the lowest is lower than -40° C (Wei and Liu 2000; Qian et al. 2004). Body parameters including cephalic capsule width, pronotum width, elytra width, and body length were measured by vernier calipers. Body weight was measured on a fine electronic scale (AL104, Mettler Toledo).
Egg collection and observation
Overwintered adults collected in March were used for mating and oviposition. Male and female adults were distinguished when they were mating. One hundred thirteen pairs of field-collected adults in total were reared at uncontrolled laboratory conditions in a plastic container (40 cm length × 24 cm width × 12 cm depth) containing 5 cm deep of dry sand, fed with fresh cabbage. Eggs were collected by sifting the sand to separate eggs from the sand. Egg numbers were counted daily.
Eggs were placed in Petri dishes (15 cm diameter) at 30° C, and hatching time and hatched eggs were daily recorded. To measure egg weight, 50 eggs were weighed in total using an electronic balance (exact within ± 0.1 μg). Before being weighed, eggs were cleaned individually with a drop of distilled water to remove attached sand. The cleaned eggs were observed under an optical stereomicroscope or scanning electronic microscopic (LEO1430VP, LEO, www.zeiss.com). Egg length and width were measured under stereomicroscope equipped with Elements 3.0 software (Nikon SMZ-800, www.nikon.com), and calibrated by an objective micrometer.
Larval rearing and duration
Discarded plastic bottles (600 ml) were cut off at 4 cm from the mouth. 70ml of water was first added, then 800g of sand, to form a wet sand gradient. Newly hatched 3rd instar larvae were singly placed in the set-up bottle for rearing. Total weight of the rearing bottle was measured, and the water loss was supplied at about 20 day's intervals by syringing from the bottom of the bottle. Larval rearing was maintained at 30±0.5° C, 30±6% RH and 16:8 L:D photoperiod conditions. Larval molting was daily checked to record the duration of each instar, indicated by the molts. The instar number was also determined based on the frequency distribution of body parameters, including larval cephalic capsule width and length, pronotum width and length, body length, and weight. To observe and measure larvae, they were first chilled on ice for 3 minutes, and then photographed and measured under stereomicroscope. The measured larvae were no longer recorded for the developmental duration.
Prepupal, pupal, and teneral period
Prepupa and pupa were respectively kept in Petri dishes (15cm diameter) under the rearing conditions; the number of pupa was daily recorded. Prepupal body length, body weight, and body thickness of the 8th body segment were measured. Pupal cephalic capsule width, pronotum width and length, body width and length, body weight, and urogomphi length were measured. The teneral period refers to the length of days that the newly emerged adult stays inactive in sand. Teneral cephalic capsule width, pronotum width, elytral width, body length, and weight were measured.
Statistical analysis
The relationship between larval instars and developmental durations were analyzed by linear regression. Adult body parameters were submitted to student's t-test at two tailed with 5% significance level. The analysis was conducted by GraphPad Prism 4 software.
Results
Microdera punctipennis adult
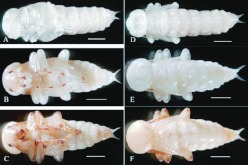
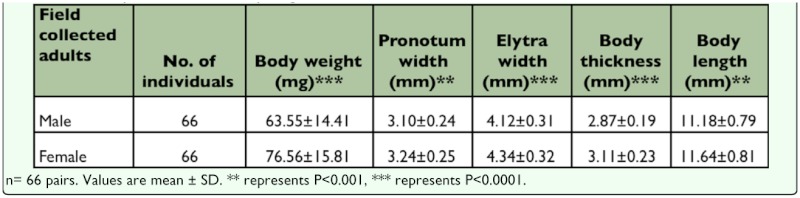
Mature M. punctipennis adults collected from their natural environment were black and small (Figure 1A). Table 1 shows the body dimensions and body weight of field collected adults (n = 66pairs). The body length difference between the male and female was statistically significant (P<0.05), so were the other body parameters (body length, t130 = 3.315, P = 0.0012; pronotum width, t130 = 3.149, P = 0.0020; elytral width, t130 = 4.943, P < 0.0001; body thickness, t130 = 4.032, P < 0.0001; body weight, t130 = 6.450, P < 0.0001). The ratio of females to males was 1.04:1 (n = 430).
Figure 1.
Male and female adults of Microdera punctipennis. (A) dorsal view of male (left) and female (right); (B) mating; bar represents 2 mm. High quality figures are available online.
Table 1.
The body dimensions and body weight of field collected adults.
The desert beetle M. punctipennis is night active; it feeds, mates, and oviposits at night. Under laboratory conditions, adults were found to feed on immature individuals, damaged eggs, and adults, but not intact eggs. On encountering danger, it contracted its legs, straightened its antennae, and pretended to be dead. For courtship, the male mounted on the female from behind (Figure 1B). Courtship lasted 2–10 minutes, and mating lasted 1–3 minutes.
Oviposition and egg stages
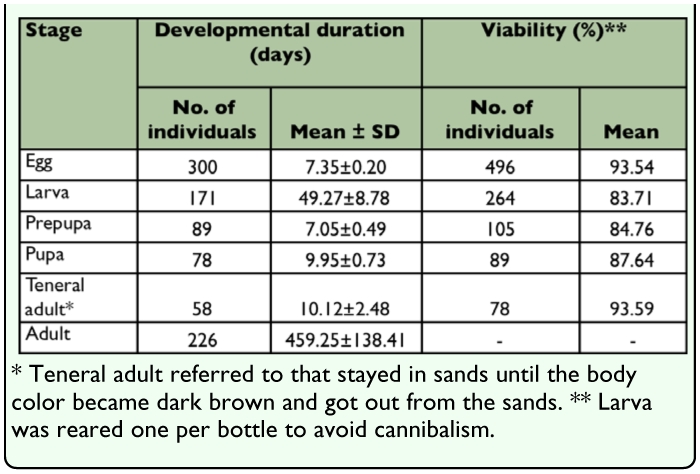
Newly emerged adults began to lay eggs about 20 days after emergence until the end of their lifetime. When laying eggs females inserted the end of their abdomen into the sand, the angle between the body axis and the sand surface was about 20°. After oviposition the female withdrew its ovipositor, kicking sand with its postpedes to bury the eggs. Eggs were laid individually in sand, 3.05 ±1.16 eggs per day, ranging from 1 to 6 (n = 113 pairs). The average number of eggs laid in the first year was 455 per female, and in the second year was 113. The average mass of 50 eggs was 21.70 ± 0.48 mg, and varied from 20.90 to 22.70 mg (n = 400). The egg stage lasted 7.35 ± 0.18 days, varied from 6 to 8 days (n=400). The percentage egg hatchability under the rearing conditions was 93.54% (n = 496) (Table 2).
Table 2.
Viability and developmental duration of M. punctipennis reared at 30 ± 0.5°C, 30±6% RH, 16L: 8D.
The newly laid egg was elliptic, creamy white, very delicate, and sticky with sand often attached (Figure 2A). The eggshell became hard and one or two air-chambers within the egg formed after 10 hours (Figure 2B). After 6–7 days, the egg became obtuse and yellowish in appearance. Prior to hatching, red-brown tarsungulus of prothoracic legs and ochre ocelli of the developed larva were visible under the eggshell under the stereomicroscope (Figure 2C). The eggshell surface was homogeneous and compact under scanning electronic microscope.
Figure 2.
Microdera punctipennis egg. (A) natural view of newly laid egg with sand covered; (B) 12-hour-old egg washed with water, an air chamber are seen at one end; (C) 7-day-old egg - short arrow indicates the ochre ocelli, long arrow indicates the tarsungulus of the prothoracic legs of the embryonic larva; bars represent 2mm. High quality figures are available online.
Larval stages
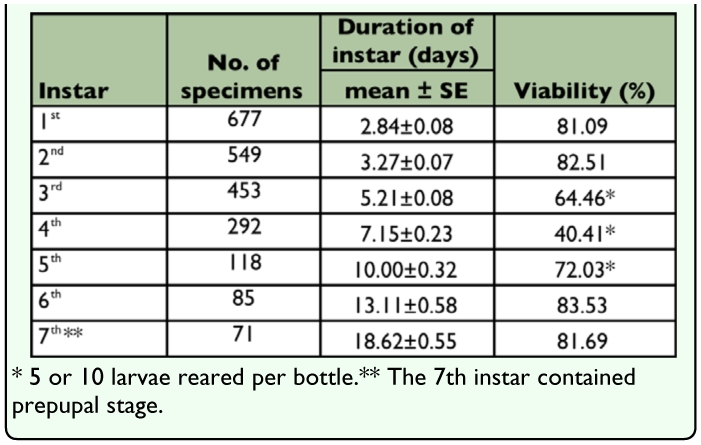
Seven instars in total were determined in M. punctipennis by the number of molts as shown in Figure 3. Frequency distributions of larval body parameters, except cephalic capsule length, displayed seven frequency peaks indicating seven instars. Developmental duration (in days) and viability of each larval instar are shown in Table 3. The relative low viability from the 3rd to 5th instars was due to the cannibalism when dozens of larvae were reared per bottle early in this investigation.
Figure 3.
Frequency distributions of logarithmed cephalic capsule width and pronotum width during the larval stages of Microdera punctipennis. The most frequently occurring sizes (arrows) of cephalic capsules width and pronotum width identified 7 instars. High quality figures are available online.
Table 3.
Developmental duration and viability of M. punctipennis larval instars reared at 30±0.5°C, 30± 6% RH and 16L:8D.
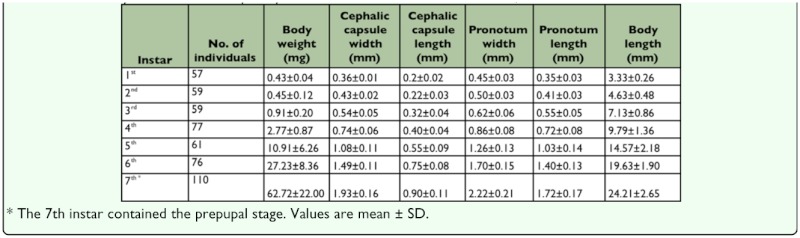
The larval body in all instars, except the newly emerged 1st instar and prepupa, was flat and elongated. Body parameters and body weight of each instar are shown in Table 4. Except cephalic capsule length, which showed slow growth rate, all the other body parameters showed a similar growth pattern. Moreover, larval body weight increased very slowly in the first four instars, but dramatically increased in the later instars. Under the rearing conditions, the duration time in each instar proceeded in a linear way. The linear function was Y = 2.25x - 0.83 (R2 = 0.97), indicating that the developmental time in each successive instar was 2.25 days longer than in the previous instar. The overall larval stage from the 1st to the 7th instar lasted about 56 days and ranged from 36-78 days (n = 71).
Table 4.
The body dimensions of M. punctipennis larval instars reared at 30±0.5°C,30± 6% RH and 16L:8D.
Larval color from the 1st to the 7th instar changed gradually from creamy white to yellowish. Head capsule and thorax color changed from creamy white to brown. Meanwhile, the body wall became hard. The 1st instar was delicate; body segments beads as in Figure 4A. The 2nd instar became elongated and transparent; the alimentary tract was visible under a stereomicroscope (Figure 4B).
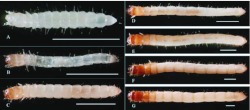
Figure 4.
Larval stages of Microdera punctipennis. (A) 1st instar; (B) 2nd instar; (C) 3rd instar; (D) 4th instar; (E) 5th instar; (F) 6th instar; (G) 7th instar. Bar represents 2 mm. High quality figures are available online.
The 3rd instar was opaque, the head capsule was orange, and a cuplike pigmentation on the pronotum could be viewed (Figure 4C) which gradually intensified in the 4th and 5th instar (Figure 4D, 4E). The 6th instar had two bands on the dorsal side of each abdomen segment; and the cuplike pigmentation disappeared (Figure 4F). The 7th instar was similar with the 6th instar, but much stronger (Figure 4G).
Larval molting
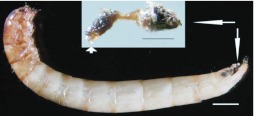
The first instar emerged either from the head or pygidium. It molted to the 2nd instar without food, but 80% of the starved 2nd instar larvae (n=50) died in the process of exuviation to the 3rd instar. During exuviation the skin split first along the tergal suture of the head and thorax, and then the thorax, head, legs, and abdomen emerged (Figures 5A). This process lasted for about 10 minutes. The newly emerged larva puckered up at the thorax (Figure 5B) and kept inactive for a few minutes. After the thorax became flattened (Figure 5C), larva burrowed into sand. Cannibalism was observed in larvae older than the 2nD instars.
Figure 5.
Eclosion of Microdera punctipennis larvae. (A) head and thorax emerged first with the help of pygopodium (lateral view); (B) lateral view of the just molted larva with its thorax puckering up and the molted cuticle still on (inset); (C) dorsal view of the larva 10 minutes after molting; bar represents 2 mm. High quality figures are available online.
Larval structures for sand living
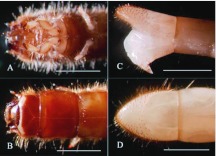
Under the rearing conditions, the larva lived in the boundary between dry and wet sand and was active on the surface in dark. Larval prothoracic legs were larger and stronger than the other legs (Figure 6A) and the cephalic capsule was hard (Figure 6B). These structures together, with their two pygopods and ossified spines on the last body segments (Figures 6C, 6D), help the larva tunnel into sand. Upon pupation, the full-grown larva burrowed a hole in the wet sand for pupation.
Figure 6.
Details of body morphology of Microdera punctipennis larva for sand living. (A) sharp and hard tips of prothoracic legs used to excavate sand (ventral view); (B) strongly chitinized labrum, cephalic capsule and pronotum used to thrust sand (dorsal view); (C) strong and sharp pygopods used as center of effort to draw back or molt (lateral view); (D) ossified spines on the last body segment (dorsal view); bar represents 2 mm. High quality figures are available online.
Prepupa and pupa stages
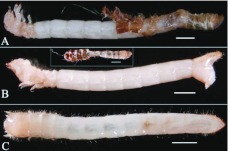
In the prepupal stage, the body was cylindrical L-shaped, yellowish, and motionless (Figure 7). Pygopods withdrew and attached to the tergite. The anus was plugged with solid meconium. Exuviation also started when the skin split along the tergal suture of the head and thorax (Figure 8). Body parameters of the prepupa are showed in Table 5. The prepupal period lasted 7.05 ± 0.49 days, ranged from 6–8 days, and viability was 84.76% (n = 105).
Figure 7.
Prepupa of Microdera punctipennis with its anus blinded with meconium (lateral view). Bar represents 2 mm. Inset shows the dissected meconium (bar represents 1 mm), short arrow indicates the part inside body and long arrow indicates the part outside body. High quality figures are available online.
Figure 8.
Pupating of Microdera punctipennis. Inset shows the initial stage. Bars represent 2 mm. High quality figures are available online.
Table 5.
The body dimensions of M. punctipennis prepupa and pupa reared at 30±0.5°C, 30± 6% RH and 16L:8D.
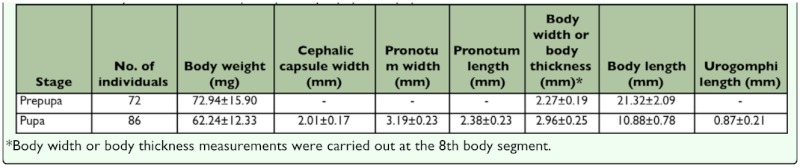
The newly emerged pupa (Figure 9A) was totally creamy white and semitransparent. It folded all its appendages on the ventral surface. A pair of antennae and prominent eyes appeared. Two concavities on the pronotum of the newly emerged pupa were observed (Figure 9D), but disappeared about 2 hours later (Figure 9E). Two days after emergence the color of the eyes, antennae, mandibles, and claws gradually changed from white to black (completely black on day 7) (Figure 9B, 9C). Antenna segmentation was visible at this time (Figures 9C, 9F). Pupal duration lasted 9.95 ± 0.73 days, ranged from 8–12 days, and viability was 87.64% (n = 89). Compared with prepupa, pupal body weight and length were decreased, but body width increased (Table 5).
Figure 9.
Color change of Microdera punctipennis pupa. (A) ventral view of a just emerged pupa; (B) ventral view of a 5-day pupa; (C) ventral view of a pre-molt pupa; (D) dorsal view of a just emerged pupa; (E) dorsal view of a 2-hour pupa; (F) dorsal view of a pre-molt pupa; bars represent 2 mm. High quality figures are available online.
Teneral adult stages
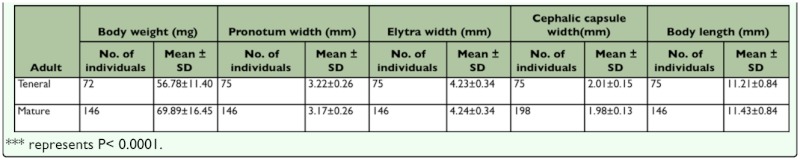
Adult molting occurred at night. The newly emerged adult was creamy white, except the mandibles, tarsal claws, and the joints of antennae and legs (Figures 10A, 10B). During the teneral stage, body color changed progressively from white to black (Figure 10D, 10E, 10F); meanwhile, the elytra and body wall became hard. The newly emerged terneral adult was very delicate with wrinkled elytra (Figure 10B). One hour later, the wrinkled elytra became smooth (Figure 10C), and elytral veins were visible in the dorsal view. The inactive adult stayed in the pupal chamber for 6–17 days (n = 58) before it emerged from the sand. It was dark-brown in color and began to court and mate. The teneral adult was able to survive for at least 20 days without food. There were no significant differences in body parameters between teneral and mature adults (Table 6), except body weight as the ternal adult was significantly lower in weight than the mature adult (t216 = 6.08, P < 0.0001). The teneral adult period lasted 10.12 ± 2.46 days, and viability was 93.59% (n = 78).
Figure 10.
Teneral adult of Microdera punctipennis. (A) lateral view of an adult emerging from its pupa with pupal case attached; (B) dorsal view of a just emerged adult; (C) dorsal view of an one-hour adult; (D) dorsal view of an one-day adult; (E) dorsal view of a 3-day adult; (F) dorsal view of a 7-day adult; bars represent 2 mm. High quality figures are available online.
Table 6.
Body dimension and body weight of laboratory reared teneral and mature adults.
Discussion
Microdera punctipennis adults were collected from Fukang, the southern fringe of the Gurbantonggut desert, which is about 100 km north of the geological center of Asia. M. punctipennis is a unique species in the Gurbantonggut desert (Huang et al. 2005). It adopts several strategies to survive desert environmental extremes. M. punctipennis has become nocturnal thereby avoiding the excessive heat and low humidity of their environment; they burrowed in sand or the Substrate around roots of shrubs during the day. In dusk, M. punctipennis began to feed, mate, and oviposit. This is a primary response of desert tenebriond beetles to heat and dryness (Wharton 1983). M. punctipennis has evolved the flattened body and short legs that are suitable for burrowing into sand, and a fused subelytral cavity for body water conservation, which are the typical morphological features of desert beetles (Draney 1993). The homogeneous and compact eggshell and the sticky layer on the egg surface may be other adaptations for desiccation resistance. The researchers also found that the female kicked sand to bury eggs after oviposition. In addition, M. punctipennis larvae have developed morphological characters for sand living, such as sharp and strong chitinized prothoracic legs, pygopods, a hard cephalic capsule, and flat body shape. Active absorption of atmospheric water vapor through rectum has been found in Tenebrio molitor larvae (Coutchié and Machin, 1984). On the contrary, M. punctipennis prepupa anus was plugged with meconium, which may function in the protection of body water from loss.
Seven instars in total were identified in M. punctipennis under the rearing conditions both by the number of molts and frequency distribution of larval body parameters. The later method was used in the instar determination in other insects (Bailez et al. 2003, Maria Do Rosário, 2007; Panzavolta, 2007). It was reported that Tenebrionid beetles Sternoplax setosa had 6–8 instars (Zhang et al. 2005a), Platyscelis hauseri had 9 instars (Yu et al. 2000), Blaps femoralis had 11 instarts (Yu and Zhang 2005), and Blaps kiritshenkoi had 12 instars (Zhang et al. 2005b). Draney (1993) reported that for all known desert tenebrionids the number of instars often exceeds 10, influenced by intrinsic and extrinsic factors, while Cloudsley- Thompson (2001) suggested that a reduced number of instars is an adaptation for desert beetles to their arid environment. Further investigations is needed to obtain life cycle data for this beetle in its natural environment. The influence of environmental factors on the number of instars and the duration of each instar need to be elucidated.
The duration of each instar under the rearing conditions could be predicted by the function of Y = 2.25 × -0.83, indicating that the duration in each successive instar was 2.25 days. The overall larval stage from the 1st to the 7th instar lasted about 56 days. This rate of development was greatly reduced compared to other desert tenebrionids (Zhang et al. 2004; Yu and Zhang 2005; Yu and Zhang 2004) reared under uncontrolled laboratory conditions. The reduced duration time in the rearing system described in the present work may suggest a profound influence of temperature on larva development.
The M. punctipennis pupa was initially creamy white, which was different from the brownish-yellow pupa of Dasylepida sp. (Coleoptera: Scarabaeidae) (Oyafuso et al. 2002). The newly eclosed M. punctipennis adult was similar to the teneral adult of beetle Luprops tristis (in Tenebrionidae) in that both were inactive until the body color turned dark (Sabu et al. 2008). But the just emerged M. punctipennis adult was white, instead of the brown color of Luprops tristis and Librodor japonicus (Coleoptera: Nitidulidae) (Okada and Miyatske 2007). The adult M. punctipennis became brown 48 hours after eclosion,.
The main problem in M. punctipennis rearing is the larval cannibalism, and it can be overcome by rearing one larva per bottle, which also provides a convenient way to observe the development of successive larval instars. Sabu et al. (2008) reported a similar rearing method for beetle Luprops tristis. In addition, the method discussed in this work provides a way to control sand moisture, which was a key factor for M. punctipennis (Bailez et al. 2003). This method also was effective in rearing other desert beetles in the laboratory. There are huge changes from egg to larva to pupa and the mature adult. The establishment of the rearing method not only provides a complete system for observing the biology of this beetle, but also the possibility for investigating these changes using molecular techniques.
Acknowledgements
We would like to thank Professor Renxin Huang for insect identification. We thank Rui Han, Ling Chen, Xuetao Zhang, Fen Li, and Jiajia Xing for their assistance with egg collection and larvae rearing. We also thank Feng Hou with the field survey. This research was supported by the National Natural Science Foundation of China (31060292) and the Open Fund from Xinjiang Key Laboratory of Biological Resources and Genetic Engineering (XJDX0201–2010–05) to Dr. Ji Ma
References
- Adam S. Like water off a beetle's back. Natural History. 2004;2:26–27. [Google Scholar]
- Bailez OE, Viana-Bailez AM, Lima JOG, Moreira DDO. Life-history of the Guava weevil, Conotrachelus psidii Marshall (Coleoptera: Curculionidae), under laboratory conditions. Neotropical Entomology. 2003;32:203–207. [Google Scholar]
- Cloudsley-Thompson JL. Thermal ecology and behaviour of Physadesmia globosa (Coleoptera: Tenebrionidae) in the Namib Desert. Journal of Arid Environments. 1990;19:317–324. [Google Scholar]
- Cloudsley-Thompson JL. Thermal and water relations of desert beetles. Naturwissenschaften. 2001;88:447–460. doi: 10.1007/s001140100256. [DOI] [PubMed] [Google Scholar]
- Coutchié PA, Machin J. Allometry of water vapor absorption in two species of tenebrionid beetle larvae. American Journal of Physiology A. 1984;42:521–531. doi: 10.1152/ajpregu.1984.247.2.R230. [DOI] [PubMed] [Google Scholar]
- Doyen JT, Slobodchikoff CH. Evolution of microgeographic races without isolation in a coastal dune beetle. Journal of Biogeography. 1984;11:13–25. [Google Scholar]
- Draney ML. The subelytral cavity of desert tenebrionids. Florida Entomologist. 1993;76:539–549. [Google Scholar]
- Gorb SN. Frictional surfaces of the elytra-to-body arresting mechanism in tenebrionid beetles (Coleoptera: Tenebrionidae): design of co-opted fields of microtrichia and cuticle ultrastructure. International Journal of Insect Morphology and Embryology. 1998;27:205–225. [Google Scholar]
- Hamilton WJ, III, Henschel JR, Seely MK. Fog collection by Namib Desert beetles. South African Journal of Science 2003. 4:181–182. [Google Scholar]
- Huang RX, Wu W, Mao XF, Hu HY, Fan ZT, Hou YJ, Li XP, Du CH, Shao HG, Huang X, OUYang T. The Fauna of the Desert Insects of Xiniang and Its Formation and Evolution. . Xinjiang Science and Technology Publishing House (in Chinese with English abstract). 2005.
- Krasnov B, Ayal Y. Seasonal changes in darkling beetle communities (Coleoptera: Tenbrionidae) in the Ramon Erosion Cirque Negev Highlands, Israel. Journal of Arid Environments. 1995;31:335–347. [Google Scholar]
- Maria DRT, Edleide LDS, Adriana LM, Carlos EDS, Ana PPD, Alana DLM, José DSS, Ruthr DN, Antônio EGS. The biology of Dlatraea flavipennella (Lepidoptera: Crambidae) reared under laboratory conditions. Florida Entomologist. 2007;90:309–313. [Google Scholar]
- Okada K, Miyatske T. Librodor japonicus (Coleoptera: Nitidulidae): life history, effect of temperature on development, and seasonal abundance. Applied Entomology Zoology. 2007;42:411–417. [Google Scholar]
- Oyafuso A, Arakaki N, Sadoyama Y, Kishita M, Kawamura F, Ishimine M, Kinjo M, Hirai Y. Life history of the white grub Dasylepida sp. (Coleoptera: Scarabaeidae), a new and severe pest on sugarcane on the Miyako Islands, Okinawa. Applied Entomology Zoology. 2002;37:595–601. [Google Scholar]
- Parker AR, Lawrence CR. Water capture by a desert beetle. Nature. 2001;414:33–34. doi: 10.1038/35102108. [DOI] [PubMed] [Google Scholar]
- Qian YB, Xu XW, Lei JQ, Wu ZN. Physical environmental comparison between two biological sand-control system constructions along the linear project areas of the two large deserts in Xinjiang. Journal of Arid Land Resources and Environment. 2004;18:33–37. [Google Scholar]
- Qiu LM, Ma J, Wang J, Zhang FC, Wang Y. Thermal stability properties of an antifreeze protein from the desert beetle Microdera punctipennis. Cryobiology. 2010;60:192–197. doi: 10.1016/j.cryobiol.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Ren GD, Yu YZ. The Desert and Semi-Desert Tenebrionids of CHINA. Hebei University Publishing House (in Chinese with English abstract); 1999. [Google Scholar]
- Sabu TK, Vinod KV, Jobi MC. Life history, aggregation and dormancy of the rubber plantation litter beetle, Luprops tristis, from the rubber plantations of moist south Western Ghats. Journal of Insect Science. 2008;8:01. doi: 10.1673/031.008.0101. Available online http://www.insectscience.org/8.01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely M.K. Irregular fog as a water source for desert dune beetles. Oecologia. 1979;42:213–227. doi: 10.1007/BF00344858. [DOI] [PubMed] [Google Scholar]
- Thomas DB. Tenebrionid beetles diversity and habitat complex in the eastern Mojave Desert. Coleopterists Bulletin. 1983;37:135–147. [Google Scholar]
- Wang Y, Qiu LM, Dai CY, Wang J, Luo JM, Zhang FC, Ma J. Expression of insect (Microdera puntipennis dzungarica) antifreeze protein MpAFP 149 confers the cold tolerance to transgenic tobacco. Plant Cell Report. 2008;27:1349–1358. doi: 10.1007/s00299-008-0562-5. [DOI] [PubMed] [Google Scholar]
- Wei WS, Liu MZ. Modern desert environment and climate change-A case study in Gurbantonggut Desert. Journal of Desert Research. 2000;20:178–184. [Google Scholar]
- Wharton RA. Dispersal, diel periodicity, and longevity of Stips stali (Haag) (Coleoptera: Tenebrionidae). Coleopterists Bulletin. 1983;37:27–33. [Google Scholar]
- Yu YZ, Zhang FJ. The biological characters of Blaps opacareitter ( Coleoptera: tenebrionidae) Journal of Ningxia University (Natural Science Edition) 2004;25:5–7. (in Chinese with English abstract). [Google Scholar]
- Yu YZ, Zhang JY. The biological characteristics of Blaps femoralis. Chinese Bullentin of Entomology. 2005;42:290–294. [Google Scholar]
- Yu YZ, Ren GD, Zhang DZ. First record of biological characters of Platyscelis hauseri Reitter 1889 (Coleoptera:Tenebrionidae). Journal of Hebei University. 2000;20:110–114. [Google Scholar]
- Zhang JY, Jia L, Yu YZ. The Biological Characteristics of Blaps gobiensis (Coleoptera: Tenebrionidae). Journal of Ningxia University (Natural Science Edition) 2004;25:264–267. [Google Scholar]
- Zhang JY, Yu YZ, Jia L. Biological characteristic of Blaps kiritshenkoi (Coleoptera: Tenebrionidae). Plant Protection. 2005b;31:44–47. [Google Scholar]
- Zhang JY, Yu YZ, Zhang FJ. The biological characters of Sternoplax setosa setosa. Journal of Agricultural Sciences. 2005a;26:10–13. [Google Scholar]
- Zhao G, Ma J, Xue N, Yang CG, Zhang FF, Zhang FC. Cloning of a cDNA encoding antifreeze protein in Microdera punctipenis dzunarica (Coleoptera: Tenebrionidae) and its activity assay. Acta Entomologica Sinica. 2005;48(5):667–673. [Google Scholar]
- Zachariassen KE. Routes of transpiratory water loss in a dryhabitat tenebrionid beetle. Journal of Experimental Biology. 1991;157:425–437. [Google Scholar]
- Zachariassen KE. The water conserving physiological compromise of desert insects. European Journal of Entomology. 1996;93:359–367. [Google Scholar]