Abstract
Adult brain connectivity is shaped by the balance of sensory inputs in early life. In the case of pain pathways, it is less clear whether nociceptive inputs in infancy can have a lasting influence upon central pain processing and adult pain sensitivity. Here, we show that adult pain responses in the rat are ‘primed’ by tissue injury in the neonatal period. Rats that experience hind-paw incision injury at 3 days of age, display an increased magnitude and duration of hyperalgesia following incision in adulthood when compared with those with no early life pain experience. This priming of spinal reflex sensitivity was measured by both reductions in behavioural withdrawal thresholds and increased flexor muscle electromyographic responses to graded suprathreshold hind-paw stimuli in the 4 weeks following adult incision. Prior neonatal injury also ‘primed’ the spinal microglial response to adult injury, resulting in an increased intensity, spatial distribution and duration of ionized calcium-binding adaptor molecule-1-positive microglial reactivity in the dorsal horn. Intrathecal minocycline at the time of adult injury selectively prevented both the hyperalgesia and early microglial reactivity associated with prior neonatal injury. The enhanced neuroimmune response seen in neonatally primed animals could also be demonstrated in the absence of peripheral tissue injury by direct electrical stimulation of tibial nerve fibres, confirming that centrally mediated mechanisms contribute to these long-term effects. These data suggest that early life injury may predispose individuals to enhanced sensitivity to painful events.
Keywords: pain, development, neuron–glia interaction, microglia, sensory processing
Introduction
Sensory information in early childhood provides an important component for wiring the brain during development, and there are critical periods when changing levels of neural activity can alter normal development (Hensch, 2004). While this concept is well established in the visual (Bourne, 2010) and auditory systems (Sanes and Bao, 2009), evidence also suggests that early sensory experience can influence the development of nociceptive pathways (Beggs et al., 2002; Ren et al., 2004; Granmo et al., 2008). Neonates who require intensive care and/or surgery, particularly those born preterm, are subject to diverse alterations in sensory input during early development. Follow-up studies in preterm born cohorts confirm alterations in both structural brain development (Woodward et al., 2006; Tich et al., 2011) and in functional neurodevelopmental outcomes in later life, which are influenced by both the timing (Marlow et al., 2005; Johnson et al., 2009) and by the duration or intensity of perinatal exposures (Brown et al., 2006). Neonatal pain and tissue injury may be contributing factors, as increased neurodevelopmental impairment has been associated with a higher number of painful procedural interventions (Grunau et al., 2009), the need for surgery during the initial hospitalization (Doyle, 2001), and with surgical versus medical management of necrotizing enterocolitis (Rees et al., 2007; Schulzke et al., 2007) or patent ductus arteriosus (Kabra et al., 2007). Despite confounding factors related to intercurrent disease or treatments, evidence that early experience influences pain in later life suggests a specific developmental plasticity in the nocioceptive system. Children who required neonatal intensive care have alterations in sensory function, which are more marked if experienced at an earlier gestational age (Hermann et al., 2006), and if there is increased tissue injury related to surgery (Walker et al., 2009a). Persistent alterations in pain-related behaviour (Grunau et al., 1994; Taddio et al., 1997; Grunau and Tu, 2007; Klein et al., 2009) and in perioperative analgesic requirements (Peters et al., 2005) have also been demonstrated in children with prior neonatal pain experience. Evaluating developmentally regulated effects of peripheral tissue injury and understanding the mechanisms that modulate long-term sensitivity, will allow us to better understand the contribution of early life experience to pain in adulthood.
Persistent alterations in pain sensitivity have been shown in a number of neonatal rodent-injury models, and vary depending on the type and severity of injury and the age at which it occurs (Fitzgerald and Walker, 2009). Hind-paw plantar incision of skin and underlying muscle is a well established and clinically relevant model of surgical injury and postoperative pain in adult rodents (Brennan et al., 1996, 2005). This model also produces clear hyperalgesia at all post-natal ages. Although acute behavioural effects are more transient in younger pups (Ririe et al., 2003, 2008; Walker et al., 2009b), neonatal incision has additional persistent effects and enhances the hyperalgesic response to a subsequent incision 2 weeks later; an effect that is not seen after incision at older ages, and which is dependent on primary afferent activity (Walker et al., 2009b). However, how long the priming effect of this neonatal injury persists, and if this has an impact on the degree and/or duration of sensitivity following painful injury in adulthood is not known.
One mechanism by which early life injury primes future pain sensitivity could be through neuroimmune interactions. In the cortex, reactive glial cells release pro-inflammatory mediators and increase neuronal excitability and seizure activity (Rodgers et al., 2009; Vezzani et al., 2011). Similarly, in the dorsal horn of the spinal cord, interactions between neurons and glia play an important role in modulating sensitivity, particularly in persistent pain states (Beggs and Salter, 2010; Ren and Dubner, 2010; Tsuda et al., 2011). Increased microglial reactivity is implicated in initiating chronic pain in animal models (Ji and Suter, 2007), and there is preliminary evidence of altered microglial reactivity in patients with chronic pain (Alexander et al., 2005; Del Valle et al., 2009; Ritz et al., 2011). It has also been suggested that initiation of a positive feedback loop linking neuronal and microglial reactivity plays a key role in the transition from acute to chronic pain (Suter et al., 2007; Kavelaars et al., 2011), and thus may be a factor in the 10–50% of patients who develop persistent pain following common surgical operations (Kehlet et al., 2006). In adult rats, hind-paw plantar incision causes an acute increase in microglial reactivity in the dorsal horn (Romero-Sandoval et al., 2008a) and inhibitors such as minocycline and fluoro-citrate can partially reverse the accompanying hyperalgesia (Obata et al., 2006; Ito et al., 2009). While the degree of microglial response varies with age and type of injury (Moss et al., 2007; Hathway et al., 2009), it is not known if its magnitude or duration are affected by early life pain experience. As ‘priming’ or ‘neonatal programming’ of adult immune responses by early life immune stressors has been described (Boisse et al., 2004; Mouihate et al., 2010; Spencer et al., 2011), and an early immune challenge can alter pain sensitivity in adulthood (Boisse et al., 2005), we hypothesized that neuroimmune reactivity may be primed by nociceptive inputs in early life, and thus cause a lasting change in the pattern and intensity of pain responses in adulthood.
Materials and methods
Experimental animals
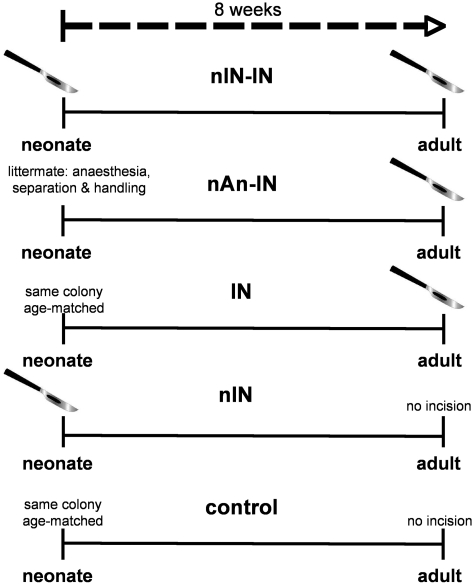
All experiments were performed in accordance with the United Kingdom Animal (Scientific Procedures) Act 1986. Sprague-Dawley rat pups or adult rats were obtained from the Biological Services Unit, University College London, and were maintained on a 12-h light/dark cycle at constant ambient temperature with free access to food and water. In the case of rat pups, handling and maternal separation were kept to a minimum, and litters were weaned into same-sex cages at post-natal Day 21. Treatment comparisons were made between littermates or age- and sex-matched adults. All animals were bred and maintained in-house at University College London and exposed to the same standard caging, handling and diet throughout development. The different experimental groups are represented schematically in Fig. 1 and experimental comparisons and the number of animals are summarized in Tables 1–5.
Figure 1.
Schematic of experimental groups. Groups include: (i) nIN-IN: neonatal incision on post-natal Day 3 and repeat incision 8 weeks later in adulthood; (ii) nAn-IN: littermate control with equivalent anaesthesia, handling and maternal separation on post-natal Day 3 and incision in adulthood (post-natal Day 60); (iii) IN: age- matched animals from the same colony having incision in adulthood; (iv) nIN: animals having neonatal incision and follow-up in adulthood; and (iv) control: age-matched non-incised controls from the same colony (control).
Table 1.
Experimental comparisons: time course and amplitude of priming effect—IN versus nIN-IN
| 1 week | 2 weeks | 4 weeks | |
|---|---|---|---|
| Behaviour | |||
| IN | 8 | 8 | 4 |
| nIN-IN | 8 | 8 | 4 |
| EMGa | |||
| IN | 6 | 5 | 4 |
| nIN-IN | 5 | 5 | 4 |
| Iba1 area | |||
| IN | 4 | 4b | 4b |
| nIN-IN | 4 | 4b | 4b |
a EMG measures in non-incised controls were included for each recording group, n = 6–8.
b Animals were used for Iba1 immunohistochemistry after behavioural tests.
Table 2.
Experimental comparisons: controlling for early life effects—nAN-IN versus IN versus nIN-IN
| Baseline | 24 h |
|||
|---|---|---|---|---|
| EMG | Control | nAn-IN | IN | nIN-N |
| 12 | 6 | 6 | 12 | |
Table 3.
Experimental comparisons: microglial immunoreactivity—baseline and acute incision effects
| Baseline |
24 h |
3 days |
||||
|---|---|---|---|---|---|---|
| Iba1intensity | Control | nIN | IN | nIN-IN | IN | nIN-IN |
| 4 | 4 | 4 | 4 | 4 | 4 | |
Table 4.
Experimental comparisons: effects of minocycline—IN versus nIN-IN
| i.p. saline | i.p. minocycline | Intrathecal artificial CSF | Intrathecal minocycline | |
|---|---|---|---|---|
| EMGa | ||||
| IN | 8 | 6 | 6 | 6 |
| nIN-IN | 6 | 8 | 7 | 8 |
| Control | 6 | 6 | ||
| Iba1 intensity | ||||
| nIN-IN | 4 | 4 | ||
a EMG measures in non-incised controls were included for each recording group, n = 6–8.
i.p. = intraperitoneal.
Table 5.
Experimental comparisons: tibial nerve stimulation—control versus nIN
| 24 h | 48 h | |
|---|---|---|
| Behaviour | ||
| STIM | 8 | 4 |
| nIN-STIM | 8 | 4 |
| Iba1 area | ||
| Tibial nerve stimulation | ||
| STIM | 4a | 4a |
| nIN-STIM | 4a | 4a |
| Sham | ||
| control | 3 | 3 |
| nIN | 3 | 3 |
a Animals were used for Iba1 immunohistochemistry after behavioural tests.
Plantar hind-paw incision
Male and female rat pups aged post-natal Day 3 were anaesthetized with halothane (2–4%) in oxygen. Following application of alcoholic chlorhexidine gluconate 0.5% (Vetasept, Animalcare Ltd) to the plantar aspect of the left hindpaw, a mid-line incision through the skin and fascia was made and the underlying plantaris muscle was elevated and incised longitudinally (Brennan et al., 1996). The same relative length of incision was performed in neonatal and adult animals, extending from the mid-point of the heel to the first footpad (Walker et al., 2009b), which can result in a longer incision in adults than the 1-cm wound originally described by Brennan et al. (1996). Skin edges were closed with 5-0 silk sutures in pups and 3-0 silk in adults (Ethicon). Animals were kept warm during recovery from anaesthesia, returned to their home cage as soon as possible and remaining sutures were removed after 5 days.
Plantar incision (IN) was performed in the same manner in adult rats (170–240 g) from three groups: (i) animals with prior neonatal incision (nIN-IN); (ii) littermates who had anaesthesia only at post-natal Day 3 (nAn-IN); and (iii) age- and gender-matched adults, all born and raised within the same colony and the same environment (IN) (Fig. 1). Animals were coded by an independent colleague after incision and the experimenter was blinded to the treatment group during behavioural or EMG testing.
Behavioural testing
Mechanical withdrawal threshold and thermal withdrawal latency were measured from baseline and at regular intervals to 28 days following adult incision. Eight animals from both IN and nIN-IN groups were followed for 2 weeks to assess the degree of hyperalgesia, and four animals from each group were then followed for a further 2 weeks to assess the duration of hyperalgesia. Following habituation on an elevated mesh platform, a mechanical stimulus (electronic von Frey device; Dynamic Plantar Aesthesiometer, Ugo Basile) was applied to the plantar surface of the hindpaw adjacent to the distal half of the incision. A linear increase in force was applied with a ramp of 20 g/s and maximum of 50 g until a withdrawal reflex was evoked, and the threshold was defined as the mean of three measures. For thermal assessment, animals were habituated in individual Plexiglas cubicles on a glass surface. The time (s) for hind-limb withdrawal from a radiant heat stimulus directed at the mid-plantar surface of the hindpaw was measured and the thermal latency calculated as the mean of three such measures (Plantar Test Analgesia Meter, Harvard Apparatus) (Hargreaves et al., 1988).
Electromyography recordings
Flexor reflex EMG recordings were performed in the three groups of adult animals 24 h after plantar skin incision (nAN-IN, n = 6; IN, n = 6; nIN-IN, n = 12) and compared with measures in non-injured age-matched controls (n = 12). Control non-incised animals were utilized for all subsequent experimental comparisons (n = 6–8 per group). To assess the duration of hyperalgesia, recordings were also performed 1, 2 and 4 weeks following incision in both IN and nIN-IN animals (n = 4–6 per group). EMG recordings from the biceps femoris muscle were performed as previously described (Walker et al., 2007, 2009b). In brief, animals were anaesthetized with halothane (2–4%) in oxygen and a tracheal tube was inserted to allow mechanical ventilation (Small Animal Ventilator, Harvard Apparatus Ltd). Halothane was reduced to 0.9% in oxygen for 30 min to ensure equilibration to a stable plane of anaesthesia, and maintained at this level during EMG recordings. Animals were placed in a spinal frame with the left hind paw secured on a fixed platform. Heart rate was continuously monitored and body temperature was monitored with a rectal probe and maintained with a thermostatically controlled heat source.
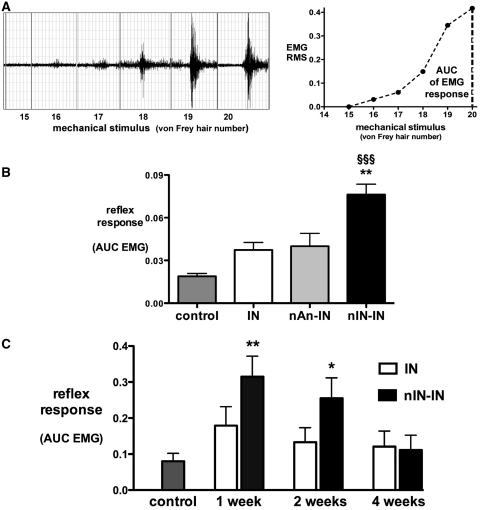
Bipolar EMG electrodes (Ainsworks) comprising stainless steel 30-gauge needles with a central copper wire core were placed through a small skin incision into the belly of the biceps femoris muscle. Von Frey hairs were applied to the plantar surface of the hindpaw for 1 s and the EMG response to the mechanical stimulus was processed (Neurolog, Digitimer) and recorded in 12-s epochs (PowerLab 4S, AD Instruments). Von Frey hairs with logarithmically increasing bending force from 13 to a maximum of 120 g (von Frey hair number 14–20) were sequentially applied to the hindpaw, with at least 60 s between stimuli, to determine the threshold for evoking an EMG response and to quantify the response to suprathreshold stimuli (Fig. 3A).
Figure 3.
Electromyographic quantification of injury-induced changes in reflex sensitivity. (A, left) Example trace of biceps femoris EMG responses to hind-paw mechanical stimuli. Von Frey hairs (number 15–20) deliver forces of 22, 37, 60, 90, 120 and 180 g, respectively. (A, right) The root mean square (RMS) of the EMG response is plotted against the mechanical stimulus and the area under the stimulus-response curve calculated to quantify the reflex response (AUC EMG). (B) The flexor reflex EMG response is shown in age-matched same colony controls (n = 12) and 24 h following incision in IN (age-matched same colony; n = 6), nAN-IN (littermates; n = 6) and in nIN-IN (n = 12) animals. Bars = mean ± SEM; **P < 0.01, nIN-IN versus nAn-IN and nIN-IN versus IN; §§§P < 0.001, nIN-IN versus control and one-way ANOVA with Bonferroni post hoc comparisons. (C) Flexor reflex EMG response 1, 2 and 4 weeks following injury in primed (nIN-IN) and non-primed (IN) age-matched adults. Bars = mean ± SEM; n = 5–6 per group; *P < 0.05 and **P < 0.01, one-way ANOVA with Dunnett's comparison with control.
Immunostaining
Four animals per intervention group were terminally anaesthetized with intraperitoneal pentobarbitone (100 mg/kg) and transcardially perfused with heparinized saline followed by 4% paraformaldehyde for immunohistochemical detection of microglia as previously described (Beggs and Salter, 2007). Briefly, L4 and L5 spinal segments were identified, removed and post-fixed in 10% formalin overnight at 4°C. Following cryoprotection in 30% sucrose, the cords were cut into 40 -µm free-floating sections and washed in phosphate-buffered saline prior to and between subsequent steps. Tissue was blocked for 1 h at room temperature (10% normal donkey serum and 0.3% Triton in phosphate-buffered saline), incubated in rabbit anti-Iba1 antibody (1:2000 Wako) in phosphate-buffered saline with 3% normal donkey serum and 0.3% Triton for 48 h at 4°C, followed by Cy3 donkey anti-rabbit secondary (1:1000 Jackson ImmunoResearch Laboratories) for 3 h at room temperature. FITC-labelled IB4 (1:1000 Sigma-Aldrich) was also added to identify the central terminals of non-peptidergic primary afferents. Finally, sections were mounted on silane-coated slides (Sigma-Aldrich), allowed to dry and coverslipped with Gelmount (Sigma-Aldrich).
Spinal cord sections were imaged using a Zeiss confocal microscope and grey scales adjusted using Adobe CS5 software. Data from several sections were grouped to give a mean value for each animal. The intensity of immunofluorescence in the same size region of interest within the medial dorsal horn was quantified by integrated pixel density generated from high power confocal micrographs. This analysis was utilized for comparison of baseline values in non-incised controls (control), adults with neonatal incision only (nIN) and 3 days following incision in adults with (nIN-IN) and without (IN) neonatal incision; and also for comparison of nIN-IN animals 24 h following intrathecal artificial CSF or minocycline. In addition, comparisons between nIN-IN and IN groups were performed 1, 2 and 4 weeks following incision. To assess the spatial distribution of increased Iba1 immunoreactivity, the area of microglial proliferation in the spinal dorsal horn was quantified using Volocity software (Improvision, PerkinElmer). Proliferative areas were defined as containing more than twice the density of microglia than the equivalent contralateral region.
Minocycline administration
Minocycline was administered during brief anaesthesia with isoflurane 2–4% in oxygen (via a nose cone) in doses and by routes previously shown to produce acute anti-hyperalgesic effects (Hua et al., 2005; Bastos et al., 2007). For intrathecal injections, the lumbar spinous processes were visualized through a small skin incision, and a 30-gauge needle was passed in the midline through the L4/5 or L5/6 intervertebral space. Minocycline was diluted in artificial CSF to a concentration of 2 mg/ml and animals received 0.35 mg/kg (i.e. 70 µg in 35 µl for 200 g rat) 30 min prior to plantar incision and 0.18 mg/kg prior to EMG recording 24 h later (IN, n = 6; nIN-IN, n = 8). Controls received equivalent volumes of intrathecal artificial CSF (IN, n = 6, nIN-IN, n = 7). Spinal cords were removed from four animals from the nIN-IN minocycline and nIN-IN artificial CSF groups for Iba1 immunohistochemistry.
Injections of minocycline (intraperitoneal; IN, n = 6; nIN-IN, n = 8) or saline (IN, n = 8, nIN-IN, n = 6) were delivered into the lower left abdominal quadrant. Minocycline was diluted to 25 mg/ml in sterile saline and animals received 100 mg/kg 1 h before incision and 50 mg/kg 1 h prior to EMG recording the following day. To confirm that minocycline alone did not alter reflex function, EMG measures were also performed in non-incised controls at the same time point following the same schedule of intraperitoneal minocycline dosing or intraperitoneal saline (n = 6 per group).
Electrical stimulation of the tibial nerve
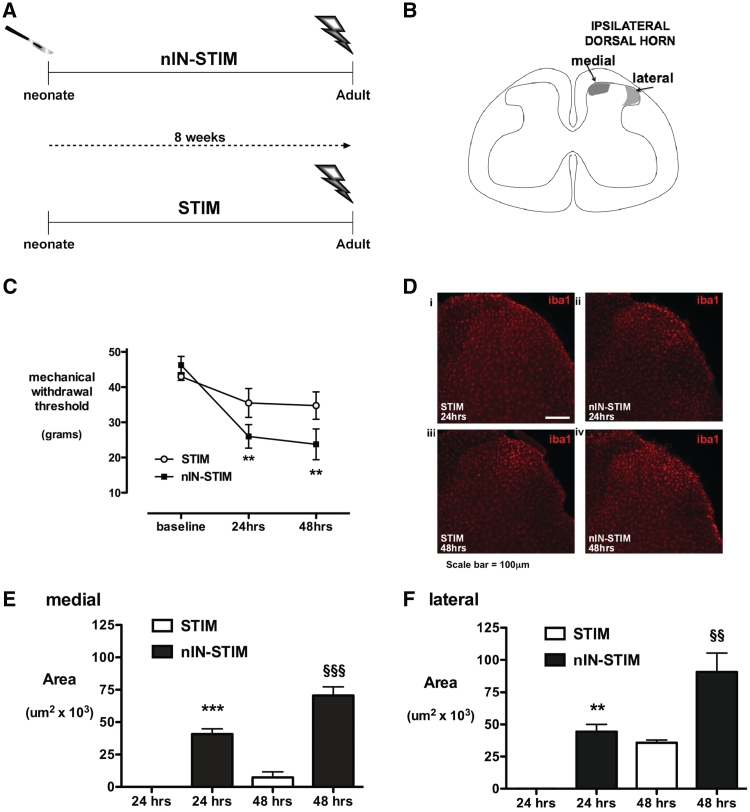
Adult rats with or without prior incision at post-natal Day 3 were anaesthetized with isoflurane 2–4% in oxygen and an incision was made in the mid-thigh to expose the sciatic nerve trifurcation. The tibial branch was dissected free of surrounding connective tissue and elevated without stretch on two silver wire electrodes. Trains of electrical stimuli (500 µs, 10 mA at 10 Hz) were delivered for 5 min to recruit C-fibres in addition to Aα- and Aδ-fibres (nIN-STIM, n = 8; STIM, n = 8; Fig. 6A). Stimulating the sciatic nerve with this protocol has previously been shown to increase microglial reactivity 48 h later in the dorsal horn, without producing peripheral nerve damage, whereas stimuli that activate only Aα- and Aδ-fibres do not significantly increase Iba1 immunoreactivity (Hathway et al., 2009). In sham control animals from both nIN and adult naïve groups (n = 3 per group), the tibial nerve was exposed and placed on the electrodes but the nerve was not electrically stimulated. Wounds were closed with 5/0 Mersilk (Ethicon). Mechanical withdrawal threshold was measured prior to and 24- and 48 h following stimulation, and animals were terminally anaesthetized at 24 (STIM and nIN-STIM, n = 4 per group) or 48 h (STIM and nIN-STIM, n = 4 per group) for immunohistochemistry.
Figure 6.
Response to tibial nerve electrical stimulation at Aβ-, Aδ- and C-fibre intensity. (A) Treatment groups comprise: (i) nIN-STIM: neonatal incision (nIN) and tibial nerve stimulation (STIM) in adulthood; and (ii) STIM: tibial nerve stimulation in age-matched adults from the same colony. (B) Schematic of regions of increased microglial proliferation in the medial and lateral dorsal horn due to tibial stimulation and mid-thigh incision, respectively. (C) Mechanical withdrawal threshold at baseline, 24 and 48 h following tibial nerve electrical stimulation in nIN-STIM and STIM groups. Bars = mean ± SEM; n = 4 per group; **P < 0.01, one-way ANOVA with Dunnet's comparison with baseline. (D) Ipsilateral lumbar dorsal horn sections stained for Iba1 from STIM and nIN-STIM animals 24 (i and ii) and 48 h (iii and iv) following tibial nerve stimulation. The area of microglial proliferation in the medial (E) and lateral (F) dorsal horn is shown 24 and 48 h after tibial nerve electrical stimulation. At 24 h, Iba1 immunoreactivity is increased in the nIN-STIM but not the STIM group (area = 0), and to a greater degree in the nIN-STIM group at 48 h. Enhanced effects are seen in both the medial and lateral dorsal horn in neonatally primed animals (nIN-STIM). Bars = mean ± SEM; n = 4 animals each group; **P < 0.01 and ***P < 0.001, nIN-STIM versus STIM at 24 h; §§P < 0.01 and §§§P < 0.001, nIN-STIM versus STIM at 48 h; one-way ANOVA with Bonferroni post hoc comparisons.
Statistics
Group results are illustrated as means ± SEM. Behavioural data were tested for normality (D'Agostino and Pearson omnibus normality test) and analysed with parametric statistics. Groups were compared using Student's t-test or by ANOVA (Prism Version 5.0 GraphPad). As neonatal injury produces generalized baseline hypoalgesia (Ren et al., 2004), behavioural changes were analysed as the percentage change from baseline to allow each animal to act as its own control. Within-group changes in the duration of hyperalgesia were analysed by one-way repeated measures ANOVA with Dunnett's multiple comparison with baseline. Two-way repeated measures ANOVA and Bonferroni post hoc tests were used to compare the degree of hyperalgesia in IN versus nIN-IN groups, and in male versus female rats. To compare the overall behavioural response in the 2 weeks following incision, the percentage reduction from baseline mechanical withdrawal threshold or thermal latency was plotted against time and the hyperalgesic index (Hua et al., 2005) for each animal was calculated as the area over the curve from 0 to 14 days; such that a larger area over the curve represents a greater change from baseline and greater degree of hyperalgesia.
The duration of the EMG response was outlined from the display of the raw data and the integral of the root mean square of the signal was calculated (EMG response) (Chart, Powerlab AD Instruments). The EMG response was plotted against the von Frey hair number (mechanical stimulus) and the area under the stimulus–response curve calculated to quantify the overall ‘reflex response’ (Walker et al., 2007; Fig. 3A). Results were compared with Student's t-test or one-way ANOVA and post hoc comparisons depending on the number of experimental groups (Prism Version 5.0 GraphPad). P < 0.05 was considered statistically significant.
Results
Early life surgical injury predisposes to persistent allodynia and hyperalgesia in adulthood
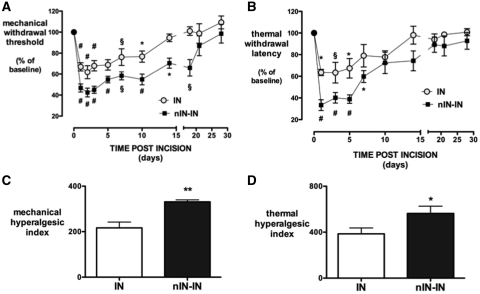
Changes in the degree of behavioural hyperalgesia for 2 weeks following adult incision (IN, n = 8) were compared with animals that also had a priming injury in the neonatal period (nIN-IN, n = 8) (Fig. 2). There was a main effect of treatment (nIN-IN versus IN) for changes in mechanical withdrawal threshold [F(1,98) = 24.1, P = 0.002] and thermal withdrawal latency [F(1,72) = 9.86, P = 0.009]. Treatment (IN versus nIN-IN) accounted for 13% and time for 54% of the total variance in mechanical threshold, and treatment accounted for 12% and time for 43% of total variance in thermal latency, with all effects considered very significant (two-way repeated measures ANOVA). Mechanical withdrawal thresholds at time points up to 14 days and thermal withdrawal latencies up to 5 days post-injury were reduced to a greater degree in the neonatal priming group (nIN-IN versus IN, P < 0.05, two-way repeated measures ANOVA). Both the mechanical and thermal hyperalgesic index (Hua et al., 2005), analysed as the area over the curve of the behavioural change for 2 weeks following incision, were significantly greater in neonatally primed adults (nIN-IN > IN, P < 0.05; Fig. 2C and D).
Figure 2.
Behavioural allodynia following injury in primed (nIN-IN) and non-primed (IN) adult animals. Ipsilateral hind-limb mechanical withdrawal thresholds (A) and thermal withdrawal latencies (B) are expressed as percentage change from baseline for 4 weeks following injury. Bars = mean ± SEM; n = 8 per group, 0–14 days; n = 4 per group, 18–28 days; and *P < 0.05, §P < 0.01 and #P ≤ 0.001, one-way repeated measures ANOVA with Dunnett's comparison with baseline; n = 4 per group, 0–28 days. The mechanical (C) and thermal (D) hyperalgesic index (area over the mechanical threshold or thermal latency curve for each animal for 14 days; area over the curve, 0–14 days) was also increased by neonatal priming. Bars = mean ± SEM; n = 8 per group; and *P < 0.05 and **P < 0.01, unpaired two-tailed Student's t-test.
The duration of mechanical and thermal hyperalgesia was also increased in animals primed by neonatal injury. The threshold for withdrawal from the electronic von Frey mechanical stimulus was reduced below baseline for 10 days following adult incision (IN versus baseline, P < 0.001 to P < 0.05 at different times, one-way repeated measures ANOVA; n = 4 per group, 0–28 days; Fig. 2A). However, in animals primed by prior incision at post-natal Day 3, significant reductions in threshold persisted until 18 days (nIN-IN versus baseline; P < 0.001 to P < 0.05 at different times, one-way repeated measures ANOVA; n = 4 per group, 0–28 days; Fig. 2A). Effects were not modality specific, as significant decreases in thermal latency were also more persistent following neonatal incision (nIN-IN versus IN: 7 versus 5 days, Fig. 2B).
As susceptibility to long-term effects of neonatal injury may differ according to gender (LaPrairie and Murphy, 2010), subgroup analyses of behavioural changes in male and female rats were also performed (n = 4 per group). Enhanced mechanical hyperalgesic responses were evident in both males and females with prior neonatal injury. In animals with or without prior neonatal injury, there was no significant main effect of gender on mechanical threshold [IN: F(1,42) = 5.8, P = 0.052; nIN-IN: F(1,42) = 0.68, P = 0.44] or thermal latency [IN: F(1,20) = 4.46, P = 0.169; nIN-IN: F(1,20) = 14.03, P = 0.065].
Early life surgical injury increases hyperalgesic responses to suprathreshold painful stimuli
To assess spinal nocioceptive reflex excitability, hind-limb flexion EMG activity in response to threshold and suprathreshold mechanical stimuli was recorded under light anaesthesia. Reflex sensitivity was calculated as the area under the mechanical stimulus versus EMG response curve (Fig. 3A). The brief period of maternal separation, handling and neonatal anaesthesia on post-natal Day 3 had no effect on the response to adult incision, as EMG responses did not differ between littermate nAN-IN and IN groups (n = 6 per group, not significant, Fig. 3B). As a result, age- and sex-matched adults from the same colony were used in all other IN and control groups. Twenty-four hours following adult injury, reflex sensitivity was increased (control versus nAN-IN plus IN, P < 0.01, unpaired two-way Student's t-test). The degree of hyperalgesia was greater in neonatally primed adults (nIN-IN > IN and nIN-IN > nAN-IN, P < 0.01; Fig. 3B).
The duration of nociceptive reflex sensitization was prolonged by neonatal priming, as the flexion reflex EMG response to hind-paw mechanical stimulation remained higher than baseline at 1 (P < 0.01) and 2 weeks (P < 0.05), but had returned to control non-incised values by 4 weeks following incision (Fig. 3C).
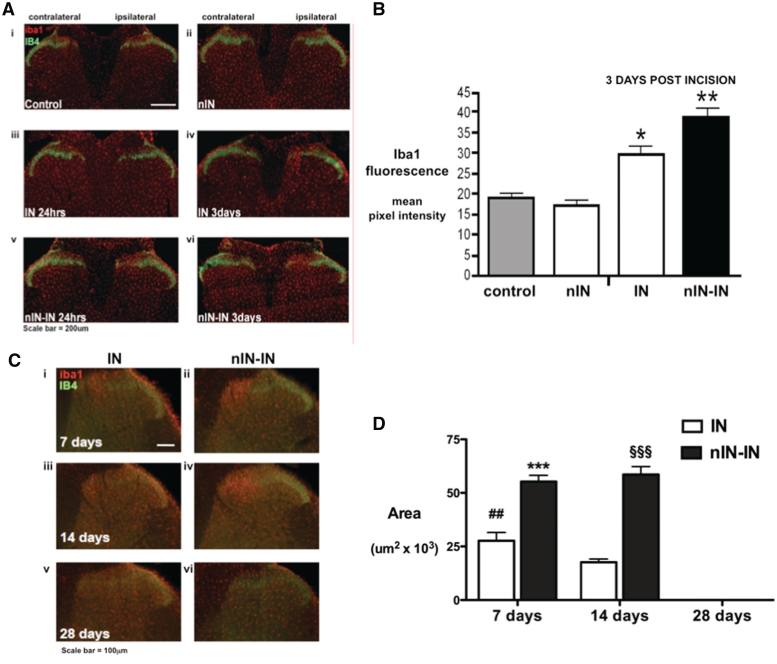
Neonatal priming enhances injury-induced microglial reactivity in the adult dorsal horn
Neonatal priming altered the time course, degree and spatial distribution of the spinal microglial response to adult injury. Neonatal incision did not alter the baseline intensity of Iba1 immunoreactivity in the medial dorsal horn in adulthood [Fig. 4A(i) and (ii), not significant, control versus nIN; Fig. 4B], but had a significant effect on the microglial response to adult injury. In neonatally primed animals, an increased intensity of Iba1 immunofluorescence in the medial dorsal horn was apparent 24 h after incision; Fig. 4A(v). By 3 days, the intensity of Iba1 immunofluorescence was increased in both incision groups (control versus IN, P < 0.05; control versus nIN-IN, P < 0.01), but the degree of change was greater in the neonatally primed group (nIN-IN versus IN P < 0.05, Fig. 4B).
Figure 4.
Iba1 immunoreactivity in the dorsal horn of the spinal cord in primed and non-primed adults. (A) Lumbar spinal cord sections stained with Iba1 (red) and IB4 (green). Examples are shown from adult tissue: (i) control; (ii) neonatal incision only at post-natal Day 3 (nIN); (iii) 24 h following adult IN; (iv) 3 days following IN; (v) 24 h following nIN-IN; and (vi) 3 days following nIN-IN. Iba1 immunoreactivity is increased in the medial aspect of the superficial dorsal horn in nIN-IN at 24 h and 3 days, but only at 3 days in IN. (B) The intensity of Iba1 immunofluorescence in the same area of ipsilateral medial dorsal horn was quantified by integrated pixel density in non-incised controls (control), adults with neonatal incision only (nIN), and 3 days following incision in adults with (nIN-IN) and without (IN) neonatal incision. Bars = mean ± SEM; n = 4 animals per group; *P < 0.05 and **P < 0.01, one-way ANOVA with post hoc comparisons. (C) Spinal cord sections 7, 14 and 28 days following IN (i, iii and v) and nIN-IN (ii, iv and vi). (D) The area of the ipsilateral medial dorsal horn containing more than twice the density of Iba1 immunoreactivity than the equivalent contralateral region is increased 7 days following IN and at 7 and 14 days following nIN-IN. By 28 days there is no difference between ipsilateral and contralateral staining in either group (area of increased staining = 0). Bars = mean ± SEM; n = 4 animals per group, ##P < 0.01 IN versus baseline and ***P < 0.001, nIN-IN versus baseline and nIN-IN versus IN at 7 days; §§§P < 0.001, nIN-IN versus baseline and nIN-IN versus IN at 14 days; one-way ANOVA with Bonferroni post hoc comparisons.
In addition, the spatial distribution or area of increased proliferative change (defined as the dorsal horn area containing more than twice the density of microglia than the equivalent contralateral region) was compared 7, 14 and 28 days following incision (Fig. 4C). The area of enhanced microglial reactivity was significantly increased above baseline at 7 but not 14 days following adult incision (Fig. 4D). In parallel with the extended duration of behavioural hyperalgesia in the neonatally primed group, microglial hyper-reactivity was increased until 14 days, and was distributed over a greater area at both 7 and 14 days (P < 0.001 nIN-IN versus IN; Fig. 4D). By 28 days, proliferative changes had resolved in both groups, as no significant differences in Iba1 immunoreactivity between the ipsilateral and contralateral dorsal horns were present (area = 0, Fig. 4D).
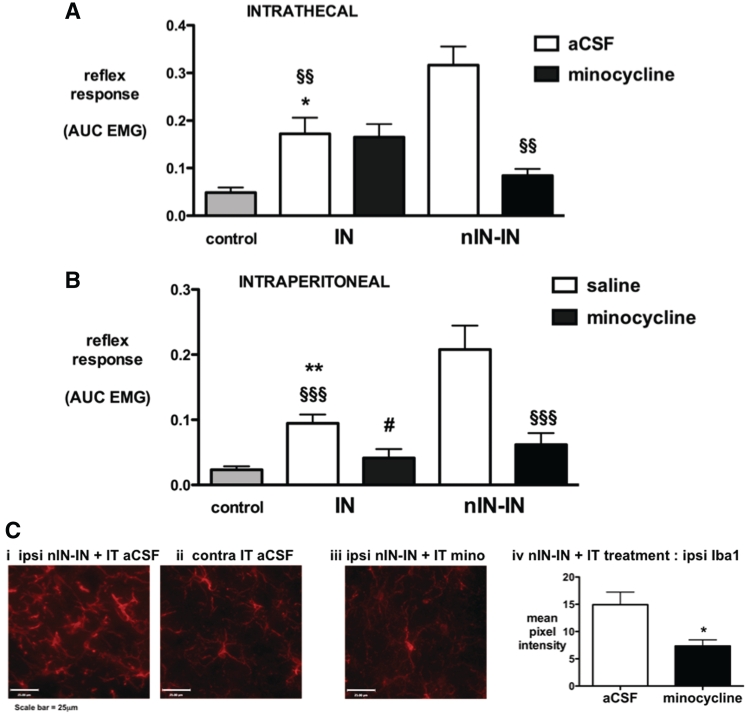
In neonatally primed animals, the enhanced behavioural and microglial responses are selectively prevented by intrathecal minocycline
To assess the contribution of microglial reactivity to neonatal priming, the non-specific microglial inhibitor minocycline was administered 1 h prior to adult incision and prior to EMG recording 24 h later. Intrathecal minocycline selectively prevented hyperalgesia in the neonatally primed group (nIN-IN minocycline versus nIN-IN artificial CSF, P < 0.01, Fig. 5A), but had no effect on adult injury alone (IN minocycline versus IN artificial CSF, not significant, Fig. 5A). In parallel with this anti-hyperalgesic effect in nIN-IN animals, intrathecal minocycline significantly reduced the intensity of Iba1 immunoreactivity in the ipsilateral dorsal horn when compared with intrathecal artificial CSF (P < 0.05; Fig. 5C).
Figure 5.
Effects of minocycline on acute incision-related hyperalgesia. (A) The flexor reflex EMG response is shown in age-matched non-incised controls, and 24 h following incision in IN and nIN-IN groups that received intrathecal minocycline or artificial CSF (vehicle control). Bars = mean ± SEM; n = 6–8 per group; *P < 0.05, IN artificial CSF (aCSF) versus control, §§P < 0.01, nIN-IN artificial CSF versus IN artificial CSF and nIN-IN artificial CSF versus nIN-IN minocyline. (B) The flexor reflex EMG response is shown in age-matched non-incised controls, and 24 h following incision in IN and nIN-IN groups that received intraperitoneal minocycline or saline. Bars = mean ± SEM; n = 6–8 per group; **P < 0.01, IN saline versus control; and §§§P < 0.001, nIN-IN saline versus IN saline and nIN-IN minocycline. #P < 0.05, IN saline versus IN minocycline. (C) Representative high-power images from the medial dorsal horn of nIN-IN animals demonstrate increased Iba1 immunoreactivity in the (i) ipsilateral, but not (ii) contralateral side of the dorsal horn following intrathecal artificial CSF. Ipsilateral increases in Iba1 immunoreactivity are prevented by (iii) intrathecal minocycline and (iv) quantification of Iba1 immunofluorescence in the medial dorsal horn confirms a significant decrease in nIN-IN animals treated with intrathecal minocycline Bars = mean ± SEM; n = 4 per group; *P < 0.05, unpaired two-tailed Student's t-test.
In contrast, intraperitoneal minocycline, which has additional peripheral anti-inflammatory actions, reduced hyperalgesia in both groups (nIN-IN minocycline versus nIN-IN saline, P < 0.001; IN minocycline versus IN saline, P < 0.05; Fig. 5B). In additional control experiments, we confirmed that intraperitoneal minocycline had no non-specific effect on reflex sensitivity, as EMG measures did not differ between non-incised animals receiving intraperitoneal minocyline (doses as above) or intraperitoneal saline (unpaired two-tailed t-test P = 0.48; data not shown).
Vehicle controls for both routes of administration again showed the priming effect of neonatal injury (nIN > IN in artificial CSF and saline groups, P < 0.01; Fig. 5A and B, respectively).
Neonatal priming effects are also revealed by electrical stimulation of the tibial nerve
The evidence suggests that neonatal priming is maintained centrally and involves microglial reactivity. If this is the case, the ‘primed’ central circuit will show enhanced sensitivity in response to the same level of afferent input. Therefore, in adults with and without neonatal incision (nIN-STIM versus STIM; Fig. 6A) effects of tibial nerve stimulation on behavioural hyperalgesia and Iba-1 immunoreactivity in the medial and lateral superficial dorsal horn (Fig. 6B) were compared. In adult animals, mechanical thresholds were reduced following electrical stimulation (STIM 43 ± 1 g at baseline, 35 ± 3.8 g at 48 h), whereas thresholds in the neonatally primed group were significantly reduced below baseline at both 24 and 48 h (nIN-STIM 46 ± 2.4 g versus 26 ± 3.3 g versus 24 ± 4.3 g; P < 0.01; Fig. 6C).
Tibial nerve afferents terminate in the medial dorsal horn. When compared with the contralateral side, a small increase in microglial reactivity in this region was apparent 48 h following electrical stimulation in adult animals [Fig. 6D(iii) and E]. In neonatally primed adults, increased microglial reactivity was apparent from 24 h [Fig. 6D(ii)] and was more widely distributed at both 24 and 48 h (nIN-STM > STIM P < 0.001; Fig. 6E).
Afferents from the mid-thigh incision required to expose the tibial nerve project to a somatotopically distinct region in the lateral dorsal horn (Fig. 6B). As reported previously (Hathway et al., 2009) increased microglial reactivity in the lateral dorsal horn was apparent 48 h following adult incision in both sham (data not shown) and electrical stimulation groups [Fig. 6D(iii)]. In animals with primed central responses (nIN-STIM), increased microglial reactivity occurred earlier (at 24 h) and covered a greater area than the STIM group at both 24 and 48 h (nIN-STIM > STIM, P < 0.05 at 24 h and P < 0.01 at 48 h; Fig. 6F).
Discussion
Here, we show that neonatal injury primes the spinal neuroimmune response, amplifying spinal nociceptive reflex and microglial reactivity to a subsequent noxious input. We have previously shown that incision during the first post-natal week, but not at older ages, enhances the response to subsequent injury 2 weeks later (Walker et al., 2009b). The current results make the key point that the impact of neonatal injury persists, so that both the degree and duration of injury-induced hyperalgesia are still increased in adulthood. These functional changes are mirrored by alterations in the time course and degree of microglial reactivity in the dorsal horn, and are selectively reversed by intrathecal minocycline. While this priming effect is clearly revealed following tissue re-injury, enhanced behavioural and microglial responses can also be demonstrated following direct electrical stimulation of the tibial nerve, which bypasses peripheral nociceptors. These results indicate that the primed state does not arise from altered peripheral terminals or the local tissue environment but rather from centrally mediated changes in dorsal horn sensitivity or connectivity. Thus, the persistent effects of neonatal injury upon adult pain sensitivity are maintained by central mechanisms at least at the level of the spinal cord.
Pain and injury in early life can produce developmentally regulated effects not seen after the same insult at older ages, and result in persistent changes in nociceptive pathways (Fitzgerald and Walker, 2009). Neonatal skin wounds produce peripheral hyperinnervation (Reynolds and Fitzgerald, 1995; Moss et al., 2005), and long-term increases in dorsal horn neuron receptive field size (Torsney and Fitzgerald, 2003). Neonatal inflammation increases the degree of hyperalgesia and neuronal activation in the spinal cord following re-inflammation, incision or capsaicin application in adulthood (Tachibana et al., 2001; Hohmann et al., 2005; Chu et al., 2007), but there is some variability in the chronicity of the initial insult used in these studies (Walker et al., 2003). Furthermore, prolonged maternal separation and repeated handling in the neonatal period (paradigms including up to 3 h of separation daily for 10–14 days) can impact on brain development and the behavioural response to stressors in later life (Kappeler and Meaney, 2010; Korosi and Baram, 2010; Meaney, 2010). This was controlled for in the present study by confirming that incision-related reflex sensitivity in adulthood did not differ between littermates that underwent brief handling and anaesthesia on post-natal Day 3 and age-matched adults bred and raised in the same colony. The impact of gender on long-term changes following neonatal injury continues to be debated (LaPrairie and Murphy, 2010), but in this study, neonatal priming responses were not gender-dependent, and as shown previously in adult mice (Banik et al., 2006), plantar incision reliably produced hyperalgesia in both male and female adult rats.
A key to the priming observed here could have been the nature of the initial priming injury. Hind-paw incision, an established and clinically relevant model of surgical injury (Brennan et al., 2005), damages skin and muscle, induces a local inflammatory response and injures small peripheral nerves; all of which may contribute to the effect. Clinically, as many as 10–50% of patients develop persistent pain states following surgical injury, and identifying patients at risk may improve preventive treatment strategies (Brennan and Kehlet, 2005; Kehlet et al., 2006; Macrae, 2008). The priming effect was tested by injury or peripheral nerve stimulation in the same lumbar spinal segments and it remains to be investigated whether such priming has a more generalized effect. Nevertheless, the finding that neonatal injury increases both the degree and duration of hyperalgesia in adulthood, suggests that it may be predictive of increased pain response in later life.
Dynamic interactions between neurons and glia drive the central response to injury. Throughout the CNS, microglia shift from a surveillant state to become effector microglia (Ransohoff and Perry, 2009), with an increased production and release of cytokine and chemokine mediators, upregulation of receptors and morphological changes. Similarly, in the spinal cord, a pain-related enhanced response phenotype of microglia has been well described (Clark et al., 2007; Romero-Sandoval et al., 2008b; Milligan and Watkins, 2009; Beggs and Salter, 2010). As seen here, plantar incision in adult animals produces somatotopically relevant microglial proliferation in the superficial dorsal horn at 3 days (Obata et al., 2006; Wen et al., 2009). We have now shown that the degree, distribution and time course of injury-induced microglial proliferation in the dorsal horn were enhanced by prior neonatal incision. These long-term effects were not dependent on peripheral re-injury and extended beyond the dermatomal boundary of the initial incision. Electrical stimulation of the tibial nerve, which includes hind-paw afferents, produced a microglial response in the medial dorsal horn, whereas the mid-thigh incision required to expose the nerve altered microglial reactivity in the lateral dorsal horn (Hathway et al., 2009). In both regions, the onset of these responses was more rapid and distribution more widespread in neonatally primed animals. Therefore, rather than being reliant on increased afferent input from the peripheral tissue, the locus of neonatal priming is expressed centrally. Whether this is primarily an alteration specifically in microglial responsivity or in upstream neuroimmune signalling remains to be investigated. It would be useful to confirm that the observed increase in Iba1 immunoreactivity is associated with changes in dorsal horn molecules more directly related to pain modulation, such as increased p38 MAPK activation and increased interleukin 6 messenger RNA expression, as previously shown following plantar incision (Wen et al., 2009) and electrical stimulation (Hathway et al., 2009), respectively. Further experiments will establish the impact of neonatal priming on both the degree and time course of expression of neuroglial modulators of pain sensitivity, and the extent to which the enhanced hyperalgesia is modified by inhibitors of upstream signalling.
It is well established that microglia modulate the response to subsequent injury in the adult CNS. This microglial priming has been described in a number of neurodegenerative and neuroinflammatory diseases, resulting in a greater response (change in morphology, increased cytokine synthesis and increased behavioural response) or more rapid disease progression following exposure to a subsequent systemic inflammatory challenge (Ransohoff and Perry, 2009). Whether primed microglia have persistent changes in morphology and/or function, and the degree to which these changes are evident prior to a subsequent insult, continues to be debated (Moisse et al., 2008; Bland et al., 2010; Perry, 2010). Acute priming of spinal microglia with intrathecal lipopolysaccharide increases ATP-induced release of interleukin-1β 24 h later and phosphorylated p38 MAPK expression in microglia (Clark et al., 2010). Lipopolysaccharide induced a greater degree of hyperalgesia 2 weeks following priming by laparotomy, when microglia were still in a state of enhanced reactivity (Hains et al., 2010). In contrast, in the current study, 8 weeks since the neonatal priming injury, microglia were in an apparent quiescent phenotype, as assessed by Iba1 immunoreactivity. Yet, these animals showed markedly enhanced responses to subsequent noxious stimuli in adulthood.
As microglia are long-lived cells and can retain an innate immune memory (Perry, 2004, 2010; Town et al., 2005), they are well suited to a role in persistent alterations. Microglial cells colonize the developing nervous system pre-natally, and age-related changes in morphology are not seen until late adulthood (Streit and Xue, 2009). The turnover time of microglia in the rodent brain is slow, however, estimated at > 2 years (Lawson et al., 1992), and the ability of some microglia to remain ‘experienced’ may underlie the ability to respond differently when challenged again (Kettenmann et al., 2011). Lipopolysaccharide in the first post-natal week increased the behavioural and microglial response to a subsequent immune challenge in adulthood (Bland et al., 2010). Susceptibility to priming effects may be greater in early life, as effects were more marked following initial lipopolysaccharide administration at post-natal Day 4 than at post-natal Day 30 (Bilbo et al., 2006). Changes in spinal cord signalling and somatosensory function have also been initiated by early systemic immune challenges, as lipopolysaccharide at post-natal Day 14 increased hind-limb reflex sensitivity to mechanical and thermal stimuli in adulthood (Boisse et al., 2005). These developmentally regulated effects have parallels with those following incision injuries. Incision in the first post-natal week, but not at older ages, increases hyperalgesia following subsequent incision indicative of a critical period of post-natal susceptibility for priming in this injury model (Walker et al., 2009b).
Long-term priming effects of systemically administered immune challenges, such as intraperitoneal lipopolysaccharide, involve signalling by humoral (circulating cytokines) and/or autonomic mechanisms (Bilbo et al., 2008; Bland et al., 2010). Here, a peripheral and localized injury is the priming stimulus and the primed hyperalgesia presents unilaterally, suggesting that neural activity has a significant role. Microglia respond to alterations in synaptic activity, and increased Iba1 immunoreactivity in the contralateral ventral horn following middle cerebral artery occlusion is postulated to be due to glutamate release from the descending corticospinal tract (Moisse et al., 2008). Similarly, afferent activity in nociceptive neurons induces microglial reactivity in the dorsal horn (Suter et al., 2009). Primary afferent activity has an important role in the initiation of the priming effect described here, as sciatic nerve blockade at the time of neonatal incision prevented the enhanced response to subsequent incision (Walker et al., 2009b). Although ongoing afferent activity is unlikely to be required to maintain hyperalgesia, it is able to reveal the priming effect in adulthood, as hyperalgesia and microglial reactivity are increased and more persistent following brief electrical stimulation of the tibial nerve.
In addition to its anti-microbial action, the tetracycline minocycline non-selectively inhibits microglia and has neuroprotective effects in models of cerebral injury (Plane et al., 2010). Minocycline inhibits both the morphological changes and the functional release of cytokines associated with increased microglial reactivity (Lai and Todd, 2006). Dose-dependent anti-hyperalgesic effects of minocycline have previously been demonstrated following hind-paw formalin injection and inflammation (Hua et al., 2005; Bastos et al., 2007). Similarly, we found that intraperitoneal minocycline, in doses sufficient to produce peripheral anti-inflammatory effects, had no effect on basal pain sensitivity but reversed the hyperalgesia produced by adult incision, independent of prior injury. In the spinal cord, intrathecal minocycline prevents changes in OX-42 immunolabelling and reduces the cytokine response produced by immune challenges with intrathecal injection of HIV-1 gp120 (Ledeboer et al., 2005) or lipopolysaccharide (Saito et al., 2010). Therefore, we also administered minocycline intrathecally, to more selectively target spinal microglial reactivity. Low dose intrathecal minocycline prevented the increased Iba1 immunoreactivity in the dorsal horn and reduced hyperalgesia only in animals with prior neonatal injury. This suggests that central microglial activation plays a greater role in early hyperalgesia in animals with primed central neuroimmune pathways.
Conclusion
Neonatal injury is associated with an enhanced neuroimmune response to subsequent noxious stimuli that persists into adulthood. Clinically, neonatal surgery and intensive care have been associated with long-term changes in neurodevelopmental outcomes (Johnson et al., 2009), including alterations in sensory processing and the response to future pain (Hermann et al., 2006; Walker et al., 2009a). Repeated surgery and anaesthesia throughout childhood and later life is not uncommon (Wilder et al., 2009; van der Griend et al., 2011), particularly in those with complications of preterm birth or congenital anomalies requiring early intervention. Neonatal surgery has been associated with an increase in stress response, pain and perioperative analgesic requirements following subsequent surgery in infancy (Peters et al., 2005). Our results suggest that early life experience may also have a longer term impact and be predictive of enhanced pain responses to future injury. Modulation of neuroimmune interactions may represent a potential therapeutic option. The neuroprotective effects of minocycline have been assessed in clinical trials, but there is currently insufficient evidence to confirm safety, efficacy and the appropriate timing and dosage in different conditions (Plane et al., 2010). Similarly, the relative risks and benefits of modulating microglial activity to control pain have not been fully evaluated (Romero-Sandoval et al., 2008b). However, the current data suggest that if neuroimmune pathways have been primed by prior experience, targeting these mechanisms may be of particular significance for minimizing persistent pain in later life.
Funding
The Great Ormond Street Hospital Children's Charity and the Royal College of Anaesthetists/British Journal of Anaesthesia UK (to S.W.); the Canadian Institutes of Health Research (CIHR; to S.B. and G.C.); and the Medical Research Council (MRC, UK; to M.F.). M.W.S. is supported by a Canada research chair (tier I) in neuroplasticity and pain, and is an International Research Scholar of the Howard Hughes Medical Institute.
Glossary
Abbreviations
- IN
incision
- nAN
neonatal anaesthesia
- nIN
neonatal incision
- STIM
stimulation
References
- Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–9. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–53. doi: 10.1097/00000542-200612000-00025. [DOI] [PubMed] [Google Scholar]
- Bastos LF, Merlo LA, Rocha LT, Coelho MM. Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur J Pharmacol. 2007;576:171–9. doi: 10.1016/j.ejphar.2007.07.049. [DOI] [PubMed] [Google Scholar]
- Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21:624–33. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010;20:474–80. doi: 10.1016/j.conb.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–58. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, et al. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22:451–5. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–38. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisse L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–34. doi: 10.1523/JNEUROSCI.1077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisse L, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119:133–41. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Bourne JA. Unravelling the development of the visual cortex: implications for plasticity and repair. J Anat. 2010;217:449–68. doi: 10.1111/j.1469-7580.2010.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Kehlet H. Preventive analgesia to reduce wound hyperalgesia and persistent postsurgical pain: not an easy path. Anesthesiology. 2005;103:681–3. doi: 10.1097/00000542-200510000-00004. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. doi: 10.1016/j.atc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Brown NC, Doyle LW, Bear MJ, Inder TE. Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics. 2006;118:2461–71. doi: 10.1542/peds.2006-0880. [DOI] [PubMed] [Google Scholar]
- Chu YC, Chan KH, Tsou MY, Lin SM, Hsieh YC, Tao YX. Mechanical pain hypersensitivity after incisional surgery is enhanced in rats subjected to neonatal peripheral inflammation: effects of N-methyl-D-aspartate receptor antagonists. Anesthesiology. 2007;106:1204–12. doi: 10.1097/01.anes.0000267604.40258.d1. [DOI] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–30. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–82. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun. 2009;23:85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Doyle LW. Outcome at 5 years of age of children 23 to 27 weeks' gestation: refining the prognosis. Pediatrics. 2001;108:134–41. doi: 10.1542/peds.108.1.134. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- Granmo M, Petersson P, Schouenborg J. Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci. 2008;28:5494–503. doi: 10.1523/JNEUROSCI.0651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Tu M. Long-term consequences of pain in human neonates. In: Anand KJ, Stevens BJ, McGrath P, editors. Pain research and clinical management. 3rd edn. Edinburgh: Elsevier; 2007. pp. 45–55. [Google Scholar]
- Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–46. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RVE, Whitfield MF, Petrie JH. Pain sensitivity and temperament in extremely low-birth-weight premature toddlers and preterm and full-term controls. Pain. 1994;58:341–6. doi: 10.1016/0304-3959(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, et al. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–14. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–8. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–79. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–85. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Neely MH, Pina J, Nackley AG. Neonatal chronic hind paw inflammation alters sensitization to intradermal capsaicin in adult rats: a behavioral and immunocytochemical study. J Pain. 2005;6:798–808. doi: 10.1016/j.jpain.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–40. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Ito N, Obata H, Saito S. Spinal microglial expression and mechanical hypersensitivity in a postoperative pain model: comparison with a neuropathic pain model. Anesthesiology. 2009;111:640–8. doi: 10.1097/ALN.0b013e3181b05f42. [DOI] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33: 1–9. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Fawke J, Hennessy E, Rowell V, Thomas S, Wolke D, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics. 2009;124:e249–57. doi: 10.1542/peds.2008-3743. [DOI] [PubMed] [Google Scholar]
- Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. J Pediatr. 2007;150:229–34, 34 e1. doi: 10.1016/j.jpeds.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Kappeler L, Meaney MJ. Epigenetics and parental effects. Bioessays. 2010;32:818–27. doi: 10.1002/bies.201000015. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, Eijkelkamp N, Willemen HL, Wang H, Carbajal AG, Heijnen CJ. Microglial GRK2: a novel regulator of transition from acute to chronic pain. Brain Behav Immun. 2011;25:1055–60. doi: 10.1016/j.bbi.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Klein VC, Gaspardo CM, Martinez FE, Grunau RE, Linhares MB. Pain and distress reactivity and recovery as early predictors of temperament in toddlers born preterm. Early Hum Dev. 2009;85:569–76. doi: 10.1016/j.earlhumdev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. Plasticity of the stress response early in life: mechanisms and significance. Dev Psychobiol. 2010;52:661–70. doi: 10.1002/dev.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia. 2006;53:809–16. doi: 10.1002/glia.20335. [DOI] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ. Long-term impact of neonatal injury in male and female rats: sex differences, mechanisms and clinical implications. Front Neuroendocrinol. 2010;31:193–202. doi: 10.1016/j.yfrne.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–15. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisse K, Welch I, Hill T, Volkening K, Strong MJ. Transient middle cerebral artery occlusion induces microglial priming in the lumbar spinal cord: a novel model of neuroinflammation. J Neuroinflammation. 2008;5:29, 1–9. doi: 10.1186/1742-2094-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A, Alvares D, Meredith-Middleton J, Robinson M, Slater R, Hunt SP, et al. Ephrin-A4 inhibits sensory neurite outgrowth and is regulated by neonatal skin wounding. Eur J Neurosci. 2005;22:2413–21. doi: 10.1111/j.1460-9568.2005.04452.x. [DOI] [PubMed] [Google Scholar]
- Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway GJ, Salter MW, et al. Spinal microglia and neuropathic pain in young rats. Pain. 2007;128:215–24. doi: 10.1016/j.pain.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J Neurosci. 2010;30:7975–83. doi: 10.1523/JNEUROSCI.6078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–22. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–13. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–86. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- Peters JW, Schouw R, Anand KJ, van Dijk M, Duivenvoorden HJ, Tibboel D. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain. 2005;114:444–54. doi: 10.1016/j.pain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67:1442–8. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193–8. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, et al. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–96. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–76. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ML, Fitzgerald M. Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol. 1995;358:487–98. doi: 10.1002/cne.903580403. [DOI] [PubMed] [Google Scholar]
- Ririe DG, Bremner LR, Fitzgerald M. Comparison of the immediate effects of surgical incision on dorsal horn neuronal receptive field size and responses during postnatal development. Anesthesiology. 2008;109:698–706. doi: 10.1097/ALN.0b013e3181870a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe DG, Vernon TL, Tobin JR, Eisenach JC. Age-dependent responses to thermal hyperalgesia and mechanical allodynia in a rat model of acute postoperative pain. Anesthesiology. 2003;99:443–8. doi: 10.1097/00000542-200308000-00027. [DOI] [PubMed] [Google Scholar]
- Ritz BW, Alexander GM, Nogusa S, Perreault MJ, Peterlin BL, Grothusen JR, et al. Elevated blood levels of inflammatory monocytes (CD14(+) CD16(+)) in patients with complex regional pain syndrome. Clin Exp Immunol. 2011;164:108–17. doi: 10.1111/j.1365-2249.2010.04308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. 2009;132:2478–86. doi: 10.1093/brain/awp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008a;1219:116–26. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs. 2008b;9:726–34. [PMC free article] [PubMed] [Google Scholar]
- Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, et al. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol. 2010;160:1754–64. doi: 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol. 2009;19:188–99. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161:583–90. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Galic MA, Pittman QJ. Neonatal programming of innate immune function. Am J Physiol Endocrinol Metab. 2011;300:E11–8. doi: 10.1152/ajpendo.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–9. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53, 1–14. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron Glia Biol. 2007;3:255–68. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T, Ling QD, Ruda MA. Increased Fos induction in adult rats that experienced neonatal peripheral inflammation. Neuroreport. 2001;12:925–7. doi: 10.1097/00001756-200104170-00012. [DOI] [PubMed] [Google Scholar]
- Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- Tich SN, Anderson PJ, Hunt RW, Lee KJ, Doyle LW, Inder TE. Neurodevelopmental and perinatal correlates of simple brain metrics in very preterm infants. Arch Pediatr Adolesc Med. 2011;165:216–22. doi: 10.1001/archpediatrics.2011.9. [DOI] [PubMed] [Google Scholar]
- Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol. 2003;550:255–61. doi: 10.1113/jphysiol.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24, 1–10. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134:1127–39. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Griend BF, Lister NA, McKenzie IM, Martin N, Ragg PG, Sheppard SJ, et al. Postoperative mortality in children after 101 885 anesthetics at a tertiary pediatric hospital. Anesth Analg. 2011;112:1440–7. doi: 10.1213/ANE.0b013e318213be52. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–9. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009a;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–95. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Lickiss T, Moss A, Fitzgerald M. Primary and secondary hyperalgesia can be differentiated by postnatal age and ERK activation in the spinal dorsal horn of the rat pup. Pain. 2007;128:157–68. doi: 10.1016/j.pain.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Walker SM, Tochiki KK, Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain. 2009b;147:99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–65. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]