Abstract
Post-mortem ganglion cell dropout has been observed in multiple sclerosis; however, longitudinal in vivo assessment of retinal neuronal layers following acute optic neuritis remains largely unexplored. Peripapillary retinal nerve fibre layer thickness, measured by optical coherence tomography, has been proposed as an outcome measure in studies of neuroprotective agents in multiple sclerosis, yet potential swelling during the acute stages of optic neuritis may confound baseline measurements. The objective of this study was to ascertain whether patients with multiple sclerosis or neuromyelitis optica develop retinal neuronal layer pathology following acute optic neuritis, and to systematically characterize such changes in vivo over time. Spectral domain optical coherence tomography imaging, including automated retinal layer segmentation, was performed serially in 20 participants during the acute phase of optic neuritis, and again 3 and 6 months later. Imaging was performed cross-sectionally in 98 multiple sclerosis participants, 22 neuromyelitis optica participants and 72 healthy controls. Neuronal thinning was observed in the ganglion cell layer of eyes affected by acute optic neuritis 3 and 6 months after onset (P < 0.001). Baseline ganglion cell layer thicknesses did not demonstrate swelling when compared with contralateral unaffected eyes, whereas peripapillary retinal nerve fibre layer oedema was observed in affected eyes (P = 0.008) and subsequently thinned over the course of this study. Ganglion cell layer thickness was lower in both participants with multiple sclerosis and participants with neuromyelitis optica, with and without a history of optic neuritis, when compared with healthy controls (P < 0.001) and correlated with visual function. Of all patient groups investigated, those with neuromyelitis optica and a history of optic neuritis exhibited the greatest reduction in ganglion cell layer thickness. Results from our in vivo longitudinal study demonstrate retinal neuronal layer thinning following acute optic neuritis, corroborating the hypothesis that axonal injury may cause neuronal pathology in multiple sclerosis. Further, these data provide evidence of subclinical disease activity, in both participants with multiple sclerosis and with neuromyelitis optica without a history of optic neuritis, a disease in which subclinical disease activity has not been widely appreciated. No pathology was seen in the inner or outer nuclear layers of eyes with optic neuritis, suggesting that retrograde degeneration after optic neuritis may not extend into the deeper retinal layers. The subsequent thinning of the ganglion cell layer following acute optic neuritis, in the absence of evidence of baseline swelling, suggests the potential utility of quantitative optical coherence tomography retinal layer segmentation to monitor neuroprotective effects of novel agents in therapeutic trials.
Keywords: optical coherence tomography, retinal segmentation, optic neuritis, multiple sclerosis, demyelinating disease, neuro-ophthalmology
Introduction
Acute optic neuritis, an inflammatory attack of the optic nerve, is a common early manifestation of multiple sclerosis and occurs in ∼30–70% of patients with multiple sclerosis during the course of their illness (Frohman et al., 2005; Balcer, 2006). Further, post-mortem analyses reveal that 94–99% of patients with multiple sclerosis have plaques in their optic nerves, irrespective of a clinical history of optic neuritis, reflecting the frequent tendency of multiple sclerosis to afflict the optic nerves both clinically and subclinically (Ikuta and Zimmerman, 1976). Given the frequent involvement of the anterior visual pathway during the disease course, it has been proposed as a model within which to study the neurodegenerative processes associated with multiple sclerosis (Frohman et al., 2008; Barkhof et al., 2009).
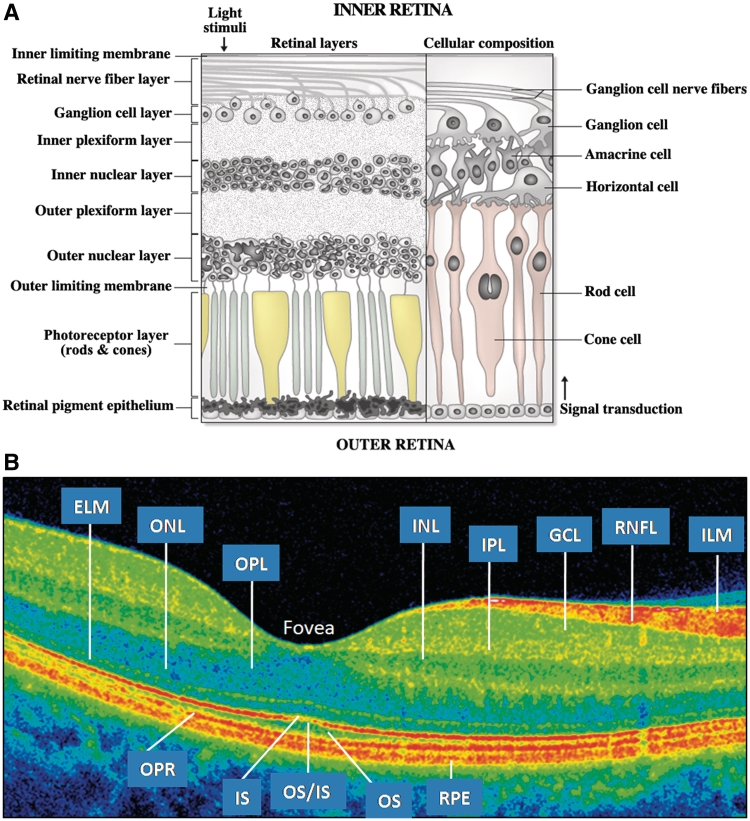
The retinal nerve fibre layer, the innermost retinal layer (Fig. 1), is comprised of unmyelinated axons originating from ganglion cell neurons that form the constituent fibres of the optic nerve (Perry and Lund, 1990). Peripapillary retinal nerve fibre layer and retinal macular thickness measurements can be quantified objectively with optical coherence tomography, a non-invasive, reproducible, office-based imaging technique (Huang et al., 1991; Hee et al., 1995; Syc et al., 2010). Optical coherence tomography quantification of axonal integrity, reflected by retinal nerve fibre layer thickness has been studied both cross-sectionally and longitudinally in patients with multiple sclerosis and clinically isolated syndrome (Pulicken et al., 2007; Talman et al., 2010). Retinal nerve fibre layer thickness (estimated by third generation time-domain optical coherence tomography) has been shown to decrease by ∼10–40 µm in the 3–6 months following an episode of acute optic neuritis (Costello et al., 2006). An additional measure obtained from optical coherence tomography scans centred on the fovea (rather than the optic disc) is the average macular thickness, which represents the thickness of tissue between the inner limiting membrane and the junction between the inner segments and outer segments of the photoreceptors within a 3 mm radius of the fovea. The retinal nerve fibres originate from retinal ganglion cells, and degeneration of the retinal nerve fibre layer may lead to average macular thickness reductions, at least in part secondary to ganglion cell death resulting from retrograde axonal degeneration (Burkholder et al., 2009). However, average macular thickness measures represent multiple layers of the macula, and thereby include distinctive cell types with diverse retinal functions. Thus, quantification of the retinal ganglion cell layer may provide a more specific assessment of a discrete retinal neuronal population for ascertaining structural and functional integrity within the retina in multiple sclerosis.
Figure 1.
(A) Illustration of the retina. The optic nerve is formed by the fibres of the retinal nerve fibre layer (RNFL) which exit the retina at the optic disc. The nerve fibres of the RNFL originate from the ganglion cells in the ganglion cell layer. (B) Cirrus HD-OCT B-scan. A false colour scheme is shown, which is generated by the differences between the retinal layers in tissue reflectivity. Please note that the reflectivity of the ganglion cell layer (GCL) and inner plexiform layer (IPL) are very similar, which makes these layers almost indistinguishable from one another. ELM = external limiting membrane; ILM = inner limiting membrane; INL = inner nuclear layer; IS = inner photoreceptor segments; IS/OS = IS/OS junction; ONL = outer nuclear layer; OPL = outer plexiform layer; OPR = outer photoreceptors; OS = outer photoreceptor segments; RPE = retinal pigment epithelium.
Recent advances in optical coherence tomography technology now allow segmentation of discrete retinal layers (Fig. 1) (Hood et al., 2009; Tan et al., 2009). Although post-mortem ganglion cell dropout is described in >70% of multiple sclerosis eyes, only limited in vivo assessment of the ganglion cell layer, and the deeper retinal layers following acute optic neuritis has been performed (Kerrison et al., 1994; Green et al., 2010). A small study of acute optic neuritis in seven individuals revealed that ganglion cell layer thickness decreased after the baseline visit in affected acute optic neuritis eyes and was not influenced by the presence of initial disc or retinal nerve fibre layer oedema (Garas et al., 2011). To date, however, in vivo assessment of the retinal neuronal layers in other demyelinating diseases remains largely unexplored.
Neuromyelitis optica, a debilitating neuroinflammatory disease, is characterized by episodes of severe acute optic neuritis (often resulting in persistent visual loss) and longitudinally extensive transverse myelitis (Wingerchuk and Weinshenker, 2003). Optic neuritis episodes in neuromyelitis optica result in greater axonal and possibly neuronal loss, as evidenced by greater reductions in retinal nerve fibre layer thickness and average macular thickness when compared with acute optic neuritis in patients with multiple sclerosis (Naismith et al., 2009a; Ratchford et al., 2009). Retinal segmentation may be utilized to compare specific regions of neuronal loss after acute optic neuritis in multiple sclerosis and neuromyelitis optica, and may elucidate whether retrograde degeneration following acute optic neuritis in neuromyelitis optica leads to greater loss in the ganglion cell layer.
In addition, this comparison will allow evaluation of the deeper retinal layers (specifically the inner and outer nuclear layers) and may determine whether the severity of axonal injury associated with acute optic neuritis contributes to not only the extent, but also predicts the pattern of retinal neuronal thinning (i.e. if the deeper neuronal layers of neuromyelitis optica eyes demonstrate greater thinning than those layers of multiple sclerosis eyes following acute optic neuritis). Indeed, determination of whether inner or outer nuclear layer thinning may occur as a consequence of acute optic neuritis in general has not been elucidated. Amidst recent reports of inner and outer nuclear layer thinning in multiple sclerosis, and the proposed possibility that such changes may reflect primary retinal pathology in multiple sclerosis, as opposed to sequelae of acute optic neuritis, the endeavour to characterize retinal neuronal layer changes following acute optic neuritis (in multiple sclerosis and neuromyelitis optica) may help determine if thinning in these deeper retinal layers is secondary to acute optic neuritis (indicating mechanisms related to retrograde degeneration) (Saidha et al., 2011).
Acute optic neuritis has been proposed as a disease model to study potential neuroprotective and neurorestorative agents, with optical coherence tomography measures of retinal nerve fibre layer thickness and average macular thickness representing the primary outcome measures. The evaluation of neuronal injury using optical coherence tomography-based retinal segmentation may provide a more robust outcome measure for clinical trials. For instance, during the acute stage of optic neuritis, inflammation may lead to swelling of the optic disc and the retinal nerve fibre layer (Hickman et al., 2002; Costello et al., 2006; Henderson et al., 2010). This swelling can be a confounding variable as it may mask the early stages of axonal thinning, thus limiting accurate quantification of retinal nerve fibre layer thickness. In essence, concomitant swelling during the earliest stages of acute optic neuritis precludes an accurate baseline measure of retinal nerve fibre layer thickness from which to utilize as a point of reference for the longitudinal assessment and confirmation of axonal degeneration or axonal neuronal protection. Statistical methods have been proposed to adjust for this inflammation; however, these methods require the use of the fellow eye, which may not be a legitimate comparator given that many patients with multiple sclerosis harbour occult retinal damage within the ‘unaffected’ eye at the time of acute optic neuritis in the ‘affected’ eye (Henderson et al., 2010).
An ideal outcome measure for neuroprotective and regenerative trials would be a measure that can be utilized at baseline without the confounding effect of inflammation of the retinal nerve fibre layer and the intrinsic noise that is introduced by utilizing the fellow eye as a surrogate for baseline quantification measures. Herein, we propose that the ganglion cell layer may be utilized as a primary outcome measure in trials of neuroprotective agents, and may be superior to the retinal nerve fibre layer due to the absence of ganglion cell layer swelling during acute optic neuritis. In this study, we sought: (i) to determine if patients with multiple sclerosis develop ganglion cell layer, as well as inner or outer nuclear layer pathology following acute optic neuritis, and characterize and quantify such changes in vivo over time; (ii) to compare the effects of optic neuritis in patients with multiple sclerosis and patients with neuromyelitis optica utilizing spectral-domain optical coherence tomography segmentation; and (iii) propose sample size calculations for clinical trials of neuroprotective or remyelinating agents in acute optic neuritis, with ganglion cell layer thickness as the primary outcome measure.
Subject and methods
Study population
Participants were recruited from the Johns Hopkins University and University of Texas-Southwestern multiple sclerosis and neuromyelitis optica clinics. All participants provided informed, written consent prior to the beginning of study procedures. The protocol was approved by the Institutional Review Boards of the Johns Hopkins University and the University of Texas-Southwestern. Participants between the ages of 18 and 65 without a known history of ocular disease, glaucoma, diabetes and/or a refractive error of greater than ±6 diopters were enrolled. Patient history and medical records were reviewed in order to determine medical history including disease duration, treatment (current and past) and history of optic neuritis events (including the date and affected side). Healthy controls without a history of neurological and ophthalmological disease were recruited from among the Johns Hopkins staff. The study was performed in accordance with Health Insurance Portability and Accountability Act guidelines.
Twenty participants with acute optic neuritis completed optical coherence tomography and visual testing within 4 weeks of optic neuritis onset (onset was defined as onset of visual dysfunction), 3 months after acute optic neuritis, and 6 months after acute optic neuritis. Three study participants were unable to attend their 3-month visit. Eight of the study participants underwent further optical coherence tomography imaging 1 year after acute optic neuritis onset. In addition, optical coherence tomography imaging and visual testing was performed in 98 participants with multiple sclerosis, 22 participants with neuromyelitis optica and neuromyelitis optica spectrum (as defined by revised Wingerchuk criteria) and 72 healthy controls (Wingerchuk et al., 2006). The healthy controls were divided into two cohorts; one cohort of 50 individuals that were age- and sex-matched to the multiple sclerosis cohort, and a cohort of 22 individuals that were age- and sex-matched to the neuromyelitis optica cohort. Two participants with neuromyelitis optica with severe visual impairment in one eye were unable to fixate which precluded optical coherence tomography examination of those eyes. Clinically isolated syndrome, relapsing–remitting multiple sclerosis and secondary progressive multiple sclerosis, but not primary progressive patients with multiple sclerosis were enrolled in this study since we sought to examine retinal changes after acute optic neuritis.
Optical coherence tomography
Optical coherence tomography evaluation was performed using the Cirrus HD-OCT machine (Carl Zeiss Meditec) software version 5.0. Retinal nerve fibre layer thickness measures were obtained using the Optic Disc 200 × 200 protocol (200 horizontal scan lines each composed of 200 A-scans), which generates a 6 × 6 × 2 mm volume cube. Software available on the Cirrus machine automatically detects the centre of the optic disc and provides the mean retinal nerve fibre layer thickness around a circumpapillary circle with a 1.73 mm radius from the centre of the optic disc. Macular measurements were determined from the Macular Cube 518 × 128 protocol (128 horizontal scan lines each composed of 512 A-scans and one central vertical and horizontal scan composed of 1024 A-scans). The analysis software identifies the inner limiting membrane and the inner boundary of the retinal pigment epithelium within the 6 × 6 × 2 mm volume cube scan to determine total macular volume, average macular thickness and the average thickness of nine regions defined by the Early Treatment Diabetic Retinopathy Study group (1985).
Imaging was performed by trained technicians on undilated pupils. After scan acquisition, all scans were reviewed to ensure proper fixation and appropriate scan quality. Scans with signal strengths <7 were excluded from analysis.
Retinal segmentation analysis
In addition to optic nerve and macular measurements, further retinal segmentation analysis was completed in a blinded fashion on macular cube data as has been previously published by our group (Saidha et al., 2011). The segmentation software detects and retains the curvature of the retina during the segmentation process. Segmentation was performed in 3D utilizing information from neighbouring A-scans and B-scans to determine the anatomical boundaries of interest. The region of interest for the algorithm includes retinal tissue between the inner limiting membrane and retinal pigment epithelium (both boundaries indentified by the conventional Cirrus HD-OCT algorithm) and excludes the choroid and vitreous. Since the optical coherence tomography image is generated by a coherent detection process, the signal within the region of interest contains speckle noise (analogous to that found in ultrasound images). In order to suppress this speckle noise, median filtering was used as an efficient and effective method of reducing the speckle while still preserving the edges between the layers.
The resulting images were then filtered to enhance the edges between the layers. Tissue boundaries were characterized by changes in the bulk scattering in the tissues with gradients generally in the axial direction (Fig. 1B). After filtering, the resulting edge images were sigmoid transformed. After transformation, the identification of the boundaries of interest—the outer boundary of the retinal nerve fibre layer, the outer boundary of the inner plexiform layer, and the outer boundary of the outer plexiform layer—was completed by combining anatomical information with the enhanced images to create cost functions for each boundary. This combination allowed for the simultaneous encoding of edge locations using local information and boundary identification using global information such as layer continuity, fovea location, relative layer thicknesses and ordering. A minimum cost graph traversal algorithm was used. The cost images combined the edge image and the positional cost images based on the retinal layers already identified. The order in which the layers were identified is important as each new layer identified provides positional cost information to the subsequent layer(s). Using the cost functions for each boundary, the algorithm traversed the 3D data set to identify the boundary locations that minimize the cumulative cost for each layer. Retinal measures including the combined thicknesses of the ganglion cell layer and inner plexiform layer, the inner nuclear layer and outer plexiform layer and the outer nuclear layer (including inner and outer photoreceptor segments) were calculated from the defined layer boundaries. Thickness maps were calculated from the axial distance between the layer boundaries at each A-scan location in the region where retinal layers appear the thickest, an annulus of inner radius 0.54 mm and outer radius 2.4 mm centred on the fovea (Fig. 2). All scans were reviewed for algorithm failures of the segmentation protocol; however, no scans were excluded from the cohort. This segmentation protocol has been shown to be reproducible in both patients with multiple sclerosis and healthy controls (intra-class correlation: 0.91–0.99 for all optical coherence tomography segmentation measurements as described above) (Saidha et al., 2011). This software is a prototype algorithm that is intended to be incorporated into the Cirrus 6.0 software.
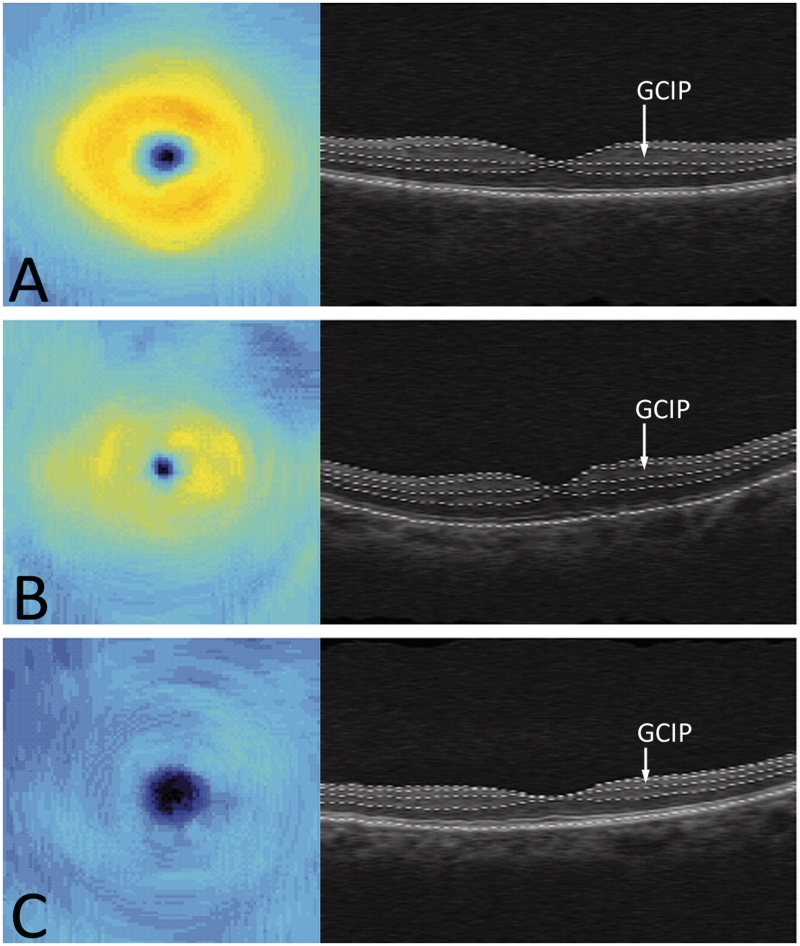
Figure 2.
Ganglion cell layer plus inner plexiform layer (GCIP) thickness maps and retinal B-scans in the horizontal direction showing layer segmentations for a healthy control (A), a multiple sclerosis eye with a history of optic neuritis (B), and a neuromyelitis optica eye with a history of optic neuritis (C).
Visual testing
Visual testing (including high and low-contrast letter acuity) was completed in a darkened room using retro-illuminated charts. The number of letters read correctly was recorded for both Early Treatment Diabetic Retinopathy Study charts and Sloan charts (2.5 and 1.25% contrast, repectively). Testing was performed monocularly.
Statistical analysis
All statistical analyses were completed using STATA Version 11 (StataCorp). A two-sided t-test was used to determine the difference in retinal measures (retinal nerve fibre layer, average macular thickness and retinal layer thicknesses) as well as visual acuity between the affected acute optic neuritis eye and the contralateral eye, as the examined variables followed a normal distribution. Spearman rank correlation was used to determine correlations between visual acuity and retinal measures. For the cross sectional analysis examining multiple sclerosis and neuromyelitis optica eyes with and without a history of optic neuritis with healthy controls, multivariate linear regression was used to adjust for age and sex. Sample size calculations were completed based on the mean difference (and corresponding standard deviation) of the baseline and 6-month value of combined ganglion cell layer plus inner plexiform layer thickness in the affected eye. Confidence intervals were obtained using a non-parametric bootstrap estimator of the coefficient of variation (standard deviation divided by the absolute value of the mean) of observed thinning based on 100 000 samples. Statistical significance was defined as P < 0.05.
Results
Demographics
Of the 20 longitudinal acute optic neuritis participants (mean age 37.5 ± 10.1 years, 18 females), seven were diagnosed with clinically isolated syndrome, four were diagnosed with relapsing–remitting multiple sclerosis when they presented with acute optic neuritis, and nine had a previous diagnosis of relapsing–remitting multiple sclerosis (disease duration 5.1 ± 3.6 years). Seven participants were on multiple sclerosis disease-modifying therapies at study enrolment, while 17 were on treatment by study conclusion (6 months after acute optic neuritis). All but one participant with acute optic neuritis received 3–5 days of high dose intravenous methylprednisolone. Two relapsing–remitting multiple sclerosis participants had an inadequate response to steroid therapy; therefore, they underwent subsequent plasmapheresis. Two participants in the longitudinal cohort had a past history of one event of acute optic neuritis in their affected eye. Four participants reported a remote history of optic neuritis (at least >1 year prior to enroling in the study) in their contralateral eye.
In addition, 98 patients with multiple sclerosis (mean age 41.7 ± 10.8 years, 70 females, 76 with relapsing–remitting multiple sclerosis, 22 with secondary progressive multiple sclerosis) were recruited for the cross-sectional study. Of these participants, 47 had no previous history of optic neuritis, 33 had a remote history of unilateral optic neuritis and 18 had a remote history of optic neuritis in each eye. Of the 22 participants with neuromyelitis optica (16 neuromyelitis optica-IgG positive) enrolled to the cross-sectional study (mean age 48.6 years ± 9.9 years, 19 females), five had clinically definite neuromyelitis optica (with a history of bilateral optic neuritis) and 17 had neuromyelitis optica spectrum disorder. Fifty age- and sex-matched healthy individuals without known neurological or ophthalmological disease (mean age 40.5 ± 7.7 years, 35 females) served as the comparison control group for the multiple sclerosis cohort, and another 22 age- and sex-matched healthy individuals (mean age 46.7 ± 7.6 years, 16 females) served as the comparison control group for the neuromyelitis optica cohort, as the neuromyelitis optica cohort was significantly older than the multiple sclerosis cohort. (Summary of demographics illustrated in Table 1).
Table 1.
Summary of demographics
| Multiple sclerosis | Acute optic neuritis | Neuromyelitis optica | Healthy control matched to multiple sclerosis cohort | Healthy control matched to neuromyelitis optica cohort | |
|---|---|---|---|---|---|
| Subjects, n | 98 | 20 | 22 | 50 | 22 |
| Age at OCT scan (years) (SD) | 41.7 (10.8) | 37.5 (10.1)a | 48.3 (9.9) | 40.5 (7.7) | 46.7 (7.6) |
| Sex, n (%) | |||||
| Male | 28 (29) | 2 (10) | 3 (14) | 15 (30) | 6 (27) |
| Female | 70 (71) | 18 (90) | 19 (86) | 35 (70) | 16 (73) |
| Average disease duration (SD) | 12.4 (8.6) | 4.7 (5.3) | 7.1 (7.4) | ||
| Eyes with history of acute optic neuritis, n (%) | 73 (37) | 24 (60) | 19 (45) |
a At baseline.
OCT = optical coherence tomography; SD = standard deviation.
Longitudinal acute optic neuritis
In the acute phase of optic neuritis, there was no observed difference in combined ganglion cell layer plus inner plexiform layer thickness between affected optic neuritis and contralateral unaffected eyes, whereas, average retinal nerve fibre layer thickness was significantly higher in affected optic neuritis eyes reflecting retinal nerve fibre layer oedema (Table 2). Aside from retinal nerve fibre layer swelling, no other differences in optical coherence tomography measures were observed between affected acute optic neuritis and contralateral eyes. High and low-contrast visual acuities were both significantly reduced in affected acute optic neuritis compared with contralateral eyes.
Table 2.
Longitudinal acute optic neuritis optical coherence tomography segmentation
| Baseline (n = 20) |
3 months (n = 17) |
6 months (n = 20) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Affected eye | Contralateral eye | P-value | Affected eye | Contralateral eye | P-value | Affected eye | Contralateral eye | P-value | |
| ARFNLT (µm) (SD) | 106.3 (23.7) | 89.8 (11.9) | 0.008 | 86.5 (12.6) | 91.1 (13.1) | 0.85 | 83.3 (11.9) | 90.7 (11.6) | 0.05 |
| AMT (µm) (SD) | 276.25 (12.5) | 275.3 (15.2) | 0.83 | 269.2 (15.6) | 272.1 (16.2) | 0.70 | 266.7 (15.3) | 275.6 (15.7) | 0.08 |
| AT GCL + IPL, µm (SD) | 76.4 (6.3) | 76.5 (8.9) | 0.97 | 66.3 (10.5) | 75.2 (9.5) | 0.01 | 67.1 (10.3) | 76.2 (8.9) | 0.005 |
| AT RNFL+GCL+IPL, (µm) (SD) | 107.5 (7.9) | 107.0 (12.3) | 0.44 | 92.8 (15.2) | 105.7 (13.2) | 0.01 | 93.7 (14.7) | 106.7 (12.5) | 0.005 |
| AT INL + OPL (µm) (SD) | 64.4 (3.8) | 64.7 (4.0) | 0.81 | 66.2 (3.7) | 64.1 (4.0) | 0.12 | 64.8 (3.6) | 65.2 (4.3) | 0.75 |
| AT ONL + PRL (µm) (SD) | 119.0 (7.2) | 118.4 (6.6) | 0.79 | 122.2 (9.2) | 116.8 (7.1) | 0.06 | 119.8 (7.7) | 118.3 (7.2) | 0.53 |
| Letters read correctly at | |||||||||
| 100% (SD)a | 36.1 (23.6) | 56.9 (6.9) | <0.001 | 52.2 (16.2) | 58.8 (5.4) | 0.12 | 54.5 (15.9) | 58.6 (7.3) | 0.30 |
| 2.5% (SD)a | 4.3 (7.9) | 28.7 (9.1) | <0.001 | 17.4 (14.9) | 28.9 (10.7) | 0.02 | 21.4 (13.9) | 26.3 (11.5) | 0.26 |
| 1.25% (SD)a | 1.9 (3.8) | 17.4 (10.2) | <0.001 | 9.2 (11.1) | 19.9 (10.9) | 0.008 | 10.6 (10.0) | 16.4 (9.7) | 0.07 |
The P-values displayed compare the affected eye and contralateral eye at each time point.
a Baseline n = 19, 3 months n = 15, 6 months n = 18.
AMT = average macular thickness; ARNFLT = average peripapillary retinal nerve fiber layer thickness; AT = average thickness; GCL = ganglion cell layer; INL = innernuclear layer; IPL = inner plexiform layer; ONL = outer nuclear layer; OPL = outer plexiform layer; PRL = photoreceptor segments layer; SD = standard deviation.
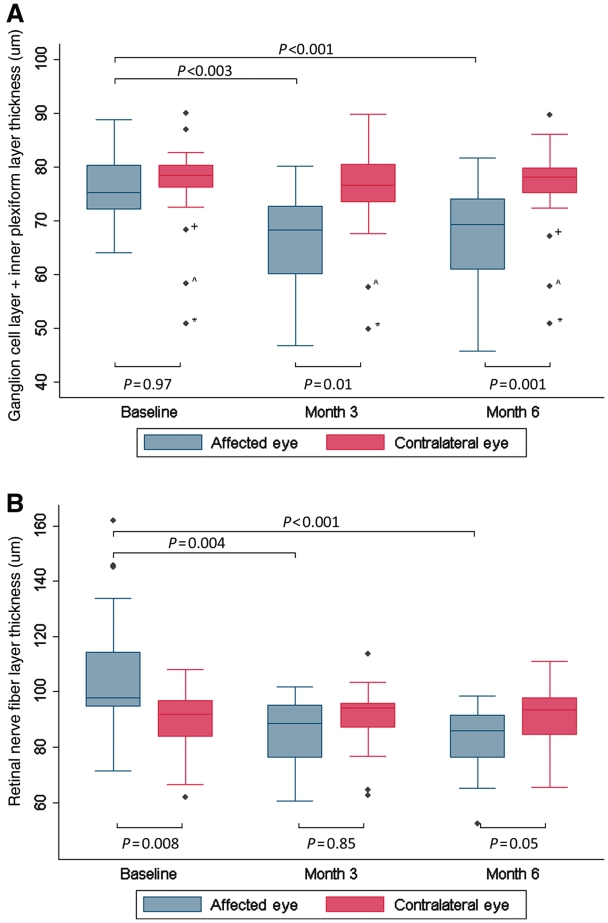
Thinning of the ganglion cell layer plus the inner plexiform layer, was evident in affected optic neuritis eyes at 3 months, in comparison with both ipsilateral baseline ganglion cell layer plus inner plexiform layer thickness (P = 0.003) and the contralateral eyes at 3 months (P = 0.01). Retinal nerve fibre layer thickness was also reduced in affected optic neuritis eyes at 3 months when compared with baseline swollen ipsilateral retinal nerve fibre layer thicknesses (P = 0.004). As three individuals missed their 3-month visit, 3-month analyses were only completed on the baseline and 3-month follow-up values of the individuals that presented for their 3-month visit (n = 17). Low-contrast visual acuity at 3-months improved in affected eyes compared with baseline (2.5%, P = 0.006; 1.25%, P = 0.02). Summary of longitudinal acute optic neuritis optical coherence tomography segmentation analyses are illustrated in Table 2 and Fig. 3.
Figure 3.
Ganglion cell layer plus inner plexiform layer (GCIP, A) and retinal nerve fibre layer (RNFL, B) thickness are significantly reduced 3 and 6 months after acute optic neuritis in the affected eyes when compared with baseline. Note the significant swelling of the RNFL of the affected eye when compared with the contralateral eye at baseline. No significant longitudinal changes were seen in the contralateral eye for GCIP or RNFL. All 20 participants are included in the baseline and Month 6 data and 17 individuals were scanned at Month 3. The P-values presented at Month 3 represent the significant change in optical coherence tomography measures from those 17 individuals at baseline to Month 3. The P-values presented at Month 6 represent the significant change from the entire cohort at baseline to the entire cohort at Month 6. The error bars represent the 5th and 95th percentiles of the measure. Asterisk indicates 45-year-old female, relapsing–remitting multiple sclerosis for 10 years, two remote episodes of optic neuritis in the contralateral eye; wedge symbol indicates 35-year-old female, relapsing–remitting multiple sclerosis for 7 years, one remote episode of optic neuritis in the contralateral eye; plus symbol indicates 24-year-old female, clinical isolated syndrome, no prior history of optic neuritis.
Ganglion cell layer plus inner plexiform layer thickness in affected eyes 6 months after acute optic neuritis remained reduced compared with affected eyes at baseline (P < 0.001), and contralateral eyes at 6 months (P = 0.005). Average retinal nerve fibre and average macular thickness in affected eyes were reduced compared with their baseline (P < 0.001 and P = 0.04, respectively). High- and low-contrast visual acuity were improved in affected eyes compared with their baseline (100%, P = 0.02; 2.5%, P = 0.002; 1.25%, P = 0.009), and baseline visual acuity measures were not predictive of ganglion cell layer thickness at 6 months after acute optic neuritis. Several participants were followed for a greater period of time and the ganglion cell layer of the affected eye of these participants remained thinned 12 months after their episode of acute optic neuritis (n = 8, baseline: 80.4 ± 6.1 µm, 12 month: 72.7 ± 6.0 µm, P = 0.02). Ganglion cell layer plus inner plexiform layer thickness and retinal nerve fibre layer thicknesses of unaffected eyes remained constant throughout the entire follow-up period.
Analysis of deeper retinal layers, the inner and outer nuclear layers, revealed that thicknesses remained constant in the affected optic neuritis eyes throughout the study and also did not differ between affected optic neuritis and unaffected contralateral eyes at any of the time points during longitudinal follow-up. Further analysis was also completed to stratify the longitudinal acute optic neuritis cohort into those with relapsing–remitting multiple sclerosis and clinically isolated syndrome at baseline. The change in ganglion cell layer plus inner plexiform layer thickness in the affected eye from baseline to the 6-month visit was not significantly different between diagnoses (relapsing–remitting multiple sclerosis: 9.08 ± 7.2 µm, clinically isolated syndrome: 9.8 ± 8.2 µm, P = 0.84). In addition, the change in ganglion cell layer plus inner plexiform layer thickness in the affected eye from baseline to the 6-month visit in those eyes with a past history of optic neuritis (n = 2, recurrent optic neuritis) when compared with those eyes without a prior history of optic neuritis (n = 18) were not significantly different (recurrent optic neuritis: 9.74 ± 12.1 µm, first optic neuritis attack: 9.29 ± 7.2 µm, P = 0.94).
Remote optic neuritis in multiple sclerosis and neuromyelitis optica
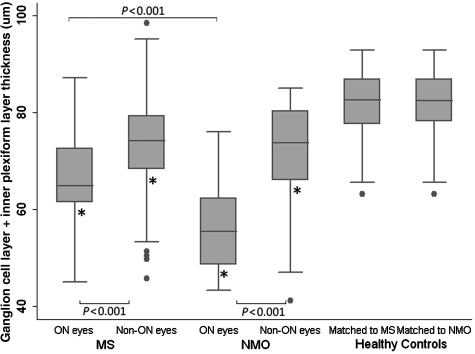
Ganglion cell layer plus inner plexiform layer thickness was lower in multiple sclerosis eyes with a remote history of optic neuritis compared with multiple sclerosis eyes without a history of optic neuritis (P < 0.001) after adjusting for age and gender (Table 3, Fig. 4). Further, ganglion cell layer plus inner plexiform layer thickness of multiple sclerosis eyes with and without history of remote optic neuritis were lower than in controls (P < 0.001 for each comparison). Retinal nerve fibre layer and average macular thickness were also reduced in multiple sclerosis optic neuritis eyes compared with non-optic neuritis multiple sclerosis eyes and healthy controls (P < 0.001 for each comparison for both measures). No significant difference was seen between inner and outer nuclear layer thickness in the multiple sclerosis optic neuritis eyes and the multiple sclerosis non-optic neuritis eyes. Ganglion cell layer plus inner plexiform layer thickness was associated with 100% high-contrast, 2.5 and 1.25% low-contrast letter acuity in relapsing–remitting multiple sclerosis in eyes with and without a history of acute optic neuritis, however, the correlation coefficients were highest in the cohort with prior optic neuritis (optic neuritis cohort: 100% contrast: r = 0.45, P < 0.001; 2.5% contrast: r = 0.50, P < 0.001; 1.25% contrast: r = 0.57, P < 0.001; non-optic neuritis cohort: 100% contrast: r = 0.30, P < 0.001; 2.5% contrast: r = 0.46, P < 0.001; 1.25% contrast: r = 0.52, P < 0.001).
Table 3.
Cross-section optical coherence tomography segmentation analysis
| Multiple sclerosis |
Neuromyelitis optica |
HC matched to MS cohort | HC matched to NMO cohort | |||||
|---|---|---|---|---|---|---|---|---|
| ON eyes | Non-ON eyes | P-value | ON eyes | Non-ON eyes | P-value | |||
| Number of eyes | n = 73 | n = 123 | n = 17 | n = 23 | n = 100 | n = 44 | ||
| ARFNLT (µm) (SD) | 78.7 (11.7) | 84.9 (12.2) | <0.001 | 68.5 (13.9) | 87.6 (16.4) | <0.001 | 93.4 (10.4) | 93.2 (9.5) |
| AMT (µm) (SD) | 263.5 (15.2) | 272.2 (14.9) | <0.001 | 248.5 (13.2) | 265.0 (16.0) | <0.001 | 282.7 (14.7) | 285.3 (14.9) |
| AT GCL + IPL (µm) (SD) | 65.6 (10.4) | 73.2 (9.3) | <0.001 | 56.4 (9.7) | 71.6 (12.1) | <0.001 | 81.9 (6.5) | 81.9 (6.8) |
| AT RNFL + GCL + IPL (µm) (SD) | 91.6 (15.5) | 102.9 (13.0) | <0.001 | 75.8 (15.7) | 100.0 (16.6) | <0.001 | 115.3 (8.5) | 114.8 (10.0) |
| AT INL + OPL (µm) (SD) | 65.3 (4.0) | 65.1 (5.0) | 0.82 | 65.0 (5.5) | 61.6 (3.2) | 0.02 | 65.1 (4.8) | 65.5 (4.1) |
| AT ONL + PRL (µm) (SD) | 119.4 (8.6) | 118.9 (6.9) | 0.58 | 120.3 (7.8) | 115.7 (5.2) | 0.03 | 121.4 (10.0) | 121.3 (7.8) |
| Letters read correctly at | ||||||||
| 100% (SD)a | 58.2 (11.9) | 59.2 (7.0) | 0.51 | 28.1 (25.3) | 48.2 (17.1) | 0.001 | 61.7 (7.3) | 61.3 (7.5) |
| 2.5% (SD)a | 27.4 (13.3) | 27.9 (11.3) | 0.82 | 6 (8.1) | 16.2 (16.0) | 0.004 | 32.7 (8.7) | 32.3 (10.3) |
| 1.25% (SD)a | 13.1 (11.5) | 13.5 (11.2) | 0.81 | 1.7 (2.7) | 8.1 (9.9) | 0.004 | 20.6 (9.6) | 17.7 (9.0) |
The P-values displayed compare the optic neuritis eyes to the non-optic neuritis eyes for each disease.
a MS ON eyes: n = 67, MS non-ON eyes n = 121, NMO ON eyes: n = 15, NMO non-ON eyes n = 16, HC matched to MS eyes n = 87, HC matched to NMO eyes n = 39.
AMT = average macular thickness; ARNFLT = average peripapillary retinal nerve fiber layer thickness; AT = average thickness; GCL = ganglion cell layer; HC = healthy control; IPL = inner plexiform layer; INL = inner nuclear layer; MS = multiple sclerosis; NMO = neuromyelitis optica; ON = optic neuritis; ONL = outer nuclear layer; OPL = outer plexiform layer; PRL = photoreceptor segments layer; SD = standard deviation.
Figure 4.
Cross sectional analysis of multiple sclerosis (MS) and neuromyelitis optica (NMO) participants with and without a history of optic neuritis and healthy controls. The ganglion cell layer (GCL) plus inner plexiform layer (IPL) thickness of optic neuritis (ON) and non-optic neuritis (non-ON) eyes of participants with multiple sclerosis and with neuromyelitis optica were reduced when compared with controls (P < 0.001 for all comparisons-indicated by an asterisk). Optic neuritis eyes of both multiple sclerosis and neuromyelitis optica eyes were significantly reduced when compared with non-optic neuritis eyes and optic neuritis eyes of patients with neuromyelitis optica were reduced when compared with optic neuritis eyes of participants with multiple sclerosis (P < 0.001). The error bars represent the 5th and 95th percentiles of the measure.
Similar findings were seen between optic neuritis eyes and non-optic neuritis eyes in the neuromyelitis optica cohort. Ganglion cell layer plus inner plexiform layer thickness was decreased in neuromyelitis optica optic neuritis eyes compared with neuromyelitis optica non-optic neuritis eyes (P < 0.001) and both neuromyelitis optica with and without remote optic neuritis history had reduced ganglion cell layer plus inner plexiform layer thicknesses compared with healthy controls (P < 0.001 for both comparisons). Both retinal nerve fibre layer and average macular thickness were reduced in neuromyelitis optica optic neuritis eyes compared to neuromyelitis optica non-optic neuritis eyes and healthy controls (P < 0.001), and average macular thickness was decreased in neuromyelitis optica non-optic neuritis eyes compared with healthy controls (P < 0.001). Inner and outer nuclear layer thicknesses were also decreased in neuromyelitis optica non-optic neuritis eyes compared to neuromyelitis optica optic neuritis eyes (inner nuclear layer, P = 0.02; outer nuclear layer, P = 0.03) and healthy controls (inner nuclear layer, P = 0.001; outer nuclear layer, P = 0.03), while there was no difference for these measures between neuromyelitis optica optic neuritis eyes and healthy controls. Ganglion cell layer plus inner plexiform layer thickness was significantly associated with 1.25% low-contrast letter acuity in neuromyelitis optica optic neuritis eyes (r = 0.52, P = 0.04) and 1.25 and 2.5% low-contrast letter acuity in neuromyelitis optica non-optic neuritis eyes (2.5% contrast: r = 0.76, P = 0.004; 1.25% contrast: r = 0.61, P < 0.001).
Ganglion cell layer plus inner plexiform layer, retinal nerve fibre layer and average macular thickness of neuromyelitis optica optic neuritis eyes were all reduced compared with multiple sclerosis optic neuritis eyes and non-optic neuritis multiple sclerosis eyes (P < 0.001 for all comparisons). No difference was observed between ganglion cell layer plus inner plexiform layer and retinal nerve fibre layer thickness of non-optic neuritis neuromyelitis optica and non-optic neuritis multiple sclerosis eyes, but average macular thickness was reduced for this comparison (P = 0.04). Inner and outer nuclear layers were also reduced in non-optic neuritis neuromyelitis optica eyes when compared with non-optic neuritis multiple sclerosis eyes (inner nuclear layer, P = 0.006; outer nuclear layer, P = 0.007).
Power analysis for acute optic neuritis trials
Sample sizes were calculated for clinical trials of hypothetical neuroprotective or remyelinating compounds that may reduce the observed rate of change in the ganglion cell layer plus inner plexiform layer after acute optic neuritis. The number of participants needed per arm of a 6-month placebo-controlled trial with a 1:1 randomization rate was calculated for reductions of 20, 30, 40, 50 and 60% of the observed change in ganglion cell layer plus inner plexiform layer after acute optic neuritis with 80, 85 and 90% power (Table 4). For example, a study with a neuroprotective agent that would reduce the observed neuronal damage by 50% would need 44 subjects per arm at 80% power [95% confidence interval (CI) 20–85]. A neuroprotective agent that would reduce the observed thinning by 30% would require 119 participants per arm at 80% power (95% CI 55–236). Confidence intervals were obtained using a non-parametric bootstrap estimator of the coefficient of variation (standard deviation divided by the absolute value of the mean) of observed thinning based on 100 000 samples: (0.56–1.16).
Table 4.
Proposed sample sizes (n per trial arm) for hypothetical 6-month clinical trial of neuroprotection in optic neuritis using ganglion cell layer plus inner plexiform layer as an outcome measure
| Effect Size | 20% | 30% | 40% | 50% | 60% |
|---|---|---|---|---|---|
| 80% power | 258 | 119 | 68 | 44 | 31 |
| 85% power | 295 | 136 | 78 | 51 | 36 |
| 90% power | 346 | 159 | 92 | 59 | 42 |
Effect size was defined as the per cent reduction of the observed mean change from baseline to 6 months.
Discussion
Results of this study demonstrate human in vivo evidence that serial ganglion cell layer plus inner plexiform layer thinning occurs following acute optic neuritis, and provides convincing in vivo support that ganglion cell layer pathology may be the derivative of optic nerve pathology, as has been proposed with prior animal and post-mortem studies, but not previously demonstrated with in vivo investigations. One of the key findings of this study is the absence of detectable swelling within the ganglion cell layer plus inner plexiform layer during the acute phase of optic neuritis, as is frequently observed within the retinal nerve fibre layer. This advantageous feature of the ganglion cell layer plus inner plexiform layer overcomes one of the major limitations associated with the retinal nerve fibre layer and highlights the utility of ganglion cell layer plus inner plexiform layer measures over retinal nerve fibre layer measures. Beyond this, ganglion cell layer plus inner plexiform layer thinning was not only evident in multiple sclerosis and neuromyelitis optica eyes with a history of optic neuritis, but also in multiple sclerosis and neuromyelitis optica eyes without a history of optic neuritis. While subclinical disease activity (with affinity to afflict the optic nerves) is well recognized in multiple sclerosis, subclinical disease activity as part of the neuromyelitis optica disease process is not a widely appreciated phenomenon. This finding may have significant implications for neuromyelitis optica pathophysiology, and tests conventional paradigms regarding our understanding of this disorder (although further studies will be needed to confirm our finding).
Thinning of the ganglion cell layer plus inner plexiform layer was detectable within 3 months of acute optic neuritis and persisted at 6–12 months, and is therefore likely to predominantly reflect permanent neuronal loss within the ganglion cell layer and damage to ganglion cell layer dendrites within the inner plexiform layer. Thinning of the deeper retinal neuronal layers was not seen longitudinally after acute optic neuritis. Retinal nerve fibre layer and ganglion cell layer plus inner plexiform layer thinning were also observed in non-optic neuritis multiple sclerosis eyes and is likely to be the result of subclinical optic neuropathy, given the predilection of multiple sclerosis to involve the optic nerves. Taken together, these in vivo findings provide support that ganglion cell layer atrophy results from optic neuropathy in multiple sclerosis. Our findings are consistent with, and explain the aetiology of findings of previous post-mortem studies, which demonstrate ganglion cell layer atrophy in >70% of multiple sclerosis eyes (Kerrison et al., 1994; Green et al., 2010). A previous smaller study of seven individuals with acute optic neuritis likewise revealed that the combined measure of retinal nerve fibre layer and ganglion cell layer plus inner plexiform layer thickness decreased in acute optic neuritis eyes and similarly demonstrated the absence of oedema affecting this layer during the acute phase of the disease (Garas et al., 2011). Our current study is distinguished from this prior study by including a larger study cohort, and by examining ganglion cell layer plus inner plexiform layer thickness separately from macular retinal nerve fibre layer thickness, which might also be prone to oedema during acute optic neuritis similar to the peripapillary retinal nerve fibre layer. Although the majority of our cohort (19 of 20 individuals) received high dose intravenous methylprednisolone for treatment of their acute optic neuritis, it is unlikely that this influenced axonal or neuronal optical coherence tomography measurements since previous studies have failed to show a beneficial effect of steroid therapy in acute optic neuritis on long-term visual function, cross-sectional optic nerve area or retinal nerve fibre layer thickness (Beck and Cleary, 1993; Hickman et al., 2004; Naismith et al., 2009b).
An extensive and thorough analysis examining the utility of optical coherence tomography as a primary outcome measure in acute optic neuritis clinical trials has suggested that baseline retinal nerve fibre layer measures from the fellow or contralateral eye could be used as a comparison for retinal nerve fibre layer measures from affected eyes at study end. This approach overcomes potential confounders related to initial retinal nerve fibre layer swelling during the acute phase of the disease, provided statistical adjustments and analyses are performed (Henderson et al., 2010). However, this approach is complex and it is suggested the fellow eye should not be used as a baseline measure if it has experienced prior optic neuritis. Our findings suggest that due to the absence of swelling in the ganglion cell layer plus inner plexiform layer at baseline, true baseline optical coherence tomography measures of the affected eye may be used to calculate the change in thickness for this layer after acute optic neuritis. This approach abolishes the need to utilize the contralateral eye for estimation of surrogate baseline measures and overcomes the limitations associated with this alternative strategy as outlined above. This may allow greater flexibility in the recruitment for such trials since it enables the recruitment of individuals who may have a known history of optic neuritis in the contralateral eye, who may have otherwise been excluded from the study. Further, both clinically isolated syndrome and patients with multiple sclerosis with acute optic neuritis could potentially be recruited to the same study of a neuroprotective agent as each of these cohorts demonstrated similar losses in ganglion cell layer plus inner plexiform layer thickness over the course of 6 months.
Additional studies assessing optical coherence tomography segmentation findings at 6 weeks post-acute optic neuritis may help further elucidate the specific time course of ganglion cell layer plus inner plexiform layer loss after acute optic neuritis. Utilizing an outcome measure in a study such as a weighted average of the 6-month time point and additional optical coherence tomography scans such as at 3 months or 6 weeks post acute optic neuritis may decrease the sample size needed for a 6-month clinical trial of a neuroprotective agent. Further evaluation of acute optic neuritis subjects may need to be completed to determine the optimal combination of time points to be used for ganglion cell layer plus inner plexiform layer outcome measure in acute optic neuritis clinical trials.
Beyond the determination that optical coherence tomography segmentation-derived measures of ganglion cell layer plus inner plexiform layer thickness may be superior to retinal nerve fibre layer thickness as a candidate outcome measure in acute optic neuritis neuroprotective study models, we speculate that the application of this technique may also help shed light on the immunophenotypic heterogeneity underlying acute optic neuritis, when coupled with specific immunological analyses. The capacity to quantitatively assess not only axonal, but now also neuronal integrity with optical coherence tomography segmentation meets a much wanted need. Apart from varying degrees of retinal axonal and neuronal loss, which are observed at an individual patient level following acute optic neuritis, it appears that some patients do not experience any retinal axonal or neuronal loss. Yet the basis of such differences remains largely unexplained. One possibility underlying these differences might be heterogeneity in T helper immunological profiles, as has recently been described in multiple sclerosis and the extent of cytotoxic T cell infiltrates, which may mediate direct neuronal damage (Giuliani et al., 2003; Axtell et al., 2010; Wang et al., 2010). This may also be a regional phenomenon as a recent study has shown evidence for neuronal injury in the cerebral cortex but not in the hippocampus (Dutta, 2011). Coupling such immunological analyses with optical coherence tomography segmentation findings (for example in acute optic neuritis) may help further our understanding of the pathophysiology of multiple sclerosis, other causes of acute optic neuritis, as well as for acute optic neuritis in general.
Retinal segmentation of the neuromyelitis optica optical coherence tomography scans revealed neuronal layer changes not only in those with, but interestingly also in those without a history of optic neuritis. In keeping with earlier studies, neuromyelitis optica participants with a history of optic neuritis had greater retinal nerve fibre layer and macular thickness reductions compared with neuromyelitis optica participants without a history of optic neuritis, as well as participants with multiple sclerosis with and without a history of optic neuritis (Naismith et al., 2009a; Ratchford et al., 2009). While participants with neuromyelitis optica and a history of optic neuritis demonstrated significant ganglion cell layer plus inner plexiform layer thinning, they did not have significant inner or outer nuclear layer changes. This may suggest that retrograde degeneration of optic nerve axons results in ganglion cell layer plus inner plexiform layer thinning, but not deeper retinal neuronal pathology in the inner or outer nuclear layer. This same finding in both multiple sclerosis and neuromyelitis optica optic neuritis eyes supports previous observations from animal and electrophysiological studies showing that retinal atrophy following optic nerve transection is limited to the inner retina (retinal nerve fibre layer, ganglion beta and alpha cells) (Hollander et al., 1984). Additional histological and electrophysiological studies show no atrophy or dysfunction in the deeper retinal layers (Dawson et al., 1982; Seiple et al., 1983; Kaufman and Celesia, 1985; Williams et al., 2001). One animal study did identify reorganization of the inner nuclear layer after optic nerve transection, however, this reorganization was not accompanied by cell loss (Williams et al., 2001).
In this study, retinal changes were not limited to neuromyelitis optica eyes with a history of optic neuritis. Thinning in the ganglion cell layer plus inner plexiform layer, inner nuclear layer, and outer nuclear layer were all present in non-optic neuritis neuromyelitis optica eyes. This finding suggests that subclinical disease activity might occur in a proportion of patients with neuromyelitis optica, similar to that observed in multiple sclerosis (Toussaint et al., 1983; Parisi et al., 1999; Trip et al., 2005; Fisher et al., 2006). These retinal neuronal changes may be the derivative of subclinical optic neuropathy or might reflect anti-aquaporin-4-mediated retinal pathology directed against retinal astrocytes, since inner or outer nuclear layer changes are not thought to result from optic neuropathy (as supported by this study). The principle aquaporin-4 expressing astroglial cells of the retina, Müller cells, span the thickness of the retina (with their cell bodies located in the inner nuclear layer), are thought to highly express aquaporin-4 (Nagelhus et al., 1998), and are critical for the health of all retinal neurons including those in the ganglion cell, inner nuclear and outer nuclear layers. Aquaporin-4 is also expressed on glial membranes contacting the ganglion cell layer and retinal nerve fibre layer. Deletion of aquaporin-4 has been shown to result in a retinal inflammatory response (upregulation of interleukin-1β, interleukin-6 and inducible nitric oxide synthase and a downregulation of cyclo-oxygenase-2) in animal models (Pannicke et al., 2010). This retinal inflammatory response may mediate retinal neuronal changes in neuromyelitis optica. As such, direct anti-aquaporin-4-mediated mechanisms of retinal pathology should be borne in mind when considering potential pathophysiological mechanisms that might underlie the changes we observed in the non-optic neuritis neuromyelitis optica eyes assessed in this study. Evaluation of a large, independent cohort of neuromyelitis optica eyes with and without a history of acute optic neuritis is warranted to confirm our findings and further explore potential mechanisms resulting in the retinal neuronal changes observed.
This study has a number of limitations that warrant discussion. In the segmentation algorithm utilized in this study, the ganglion cell layer and inner plexiform layer are combined to form a composite thickness. The boundary between the ganglion cell layer and inner plexiform layer is difficult to robustly differentiate due to similar reflectivity of these layers on Cirrus HD-OCT. Although this composite measure is thought to predominantly reflect the ganglion cell neurons, the inner plexiform layer thickness may vary by location depending on the number of displaced ganglion cell and amacrine neurons, as well as the number of traversing blood vessels. In addition, the thickness of inner nuclear layer is combined with that of the outer plexiform layer, while the outer nuclear layer thickness also includes the inner and outer photoreceptor segments. These delineations were used to examine neuronal changes after optic neuritis as they have been shown to be reproducible in multiple sclerosis participants and healthy controls (Saidha et al., 2011). Perhaps further advances in the resolution of optical coherence tomography acquisition or in optical coherence tomography segmentation technology may allow for more discrete segmentation of these layers.
Some individuals had a past history of acute optic neuritis in the affected eye or in the contralateral eye. Despite a past history of acute optic neuritis, there was no significant difference detected between the mean difference from baseline to 6 months of those individuals with and without a prior history of acute optic neuritis. Due to the small number of participants with a past history of acute optic neuritis in the affected eye, a larger sample size is needed to confirm this finding. There may also be variability in the effect of an acute optic neuritis event on ganglion cell layer plus inner plexiform layer thickness between clinically isolated syndrome and participants with relapsing–remitting multiple sclerosis. While no difference in ganglion cell layer plus inner plexiform layer loss between baseline and 6 months was observed between clinically isolated syndrome and relapsing–remitting multiple sclerosis participants in our study cohort, larger cohorts are needed to prove there is no difference between these groups. In addition, three participants in the longitudinal cohort did not attend their 3-month study visit. In order to account for this, the retinal thickness measurements of those who attended the 3-month study visits were only compared with their own baseline thickness and not with the mean baseline thickness of the cohort of 20.
It is possible that the thickness of the retinal neuronal layers may be confounded by perivascular inflammation and gliosis after optic neuritis. A pathological study of retinal tissue and corresponding optical coherence tomography scans will be needed to understand perivascular inflammation and gliosis and how the presence of such pathology might influence optical coherence tomography scans. In our cohort no macular B-scans were identified with focal areas of increased thickness, which might have suggested the presence of overt perivascular inflammation or gliosis. However, if perivascular inflammation or gliosis were present (and not identified upon review) they would likely increase the thickness of the retinal layers. As we are showing reductions of the combined thickness of the ganglion cell layer and inner plexiform layer in this study, our results would likely be more robust if underlying inflammation and gliosis are falsely increasing the thickness of retinal layers.
Furthermore, while the multiple sclerosis and healthy control cohorts were age matched, the neuromyelitis optica cohort was significantly older than both the participants with multiple sclerosis and healthy controls. In order to account for this difference, all analyses comparing these cohorts were adjusted for age as a term in the regression model. A second age- and sex-matched cohort of healthy controls was also used to compare participants with neuromyelitis optica to healthy individuals.
In summary, segmentation of retinal optical coherence tomography scans enables the in vivo quantification of the integrity of retinal neuronal layers and demonstrates retinal neuronal layer thinning following acute optic neuritis, as well as in those individuals without a history of optic neuritis in both neuromyelitis optica and multiple sclerosis, consistent with subclinical disease activity in these disorders. As such, ganglion cell layer plus inner plexiform layer thickness as measured by retinal segmentation of optical coherence tomography scans may be an ideal marker for monitoring neurodegeneration within the eye and may provide a feasible, specific primary outcome measure for evaluating neuroprotective agents in clinical trials. The importance and relevance of our findings are underpinned by imminent trials of potential neuroprotective agents in acute optic neuritis, and the identification of truly neuroprotective agents through such a study may not only be relevant to multiple sclerosis, neuromyelitis optica or other causes of optic neuropathy, but might have major implications for neurological diseases in general. Optical coherence tomography segmentation may afford the capability to quantitatively assess retinal axons and neurons within the anterior visual pathway, an anatomically discrete system that is frequently targeted by multiple sclerosis, neuromyelitis optica and other disorders. We anticipate that future studies utilizing this technology in the assessment of these conditions, as well as in a breadth of other neurological disorders, will help further our understanding of these diseases and shed light on their pathophysiology and potential heterogeneity.
Funding
National Multiple Sclerosis Society (TR 3760-A-3 to P.A.C and RG 4212-A-4 to Laura J. Balcer subcontracted to P.A.C.); the National Eye Institute (R01 EY 014993 and R01 EY 019473 to Laura J. Balcer subcontracted to P.A.C.); the Braxton Debbie Angela Dillon and Skip (DADS) Donor Advisor Fund (to P.A.C., E.M.F.). S.B.S. reports no disclosures. S.S. has received consulting fees from MedicalLogix for the development of continuing medical education programs. S.D.N. has received speaker honoraria and consulting fees from Biogen Idec. J.N.R. receives research support for clinical trials from Novartis, Biogen-Idec and University of California-Los Angeles. M.L. and E.F. report no disclosures. C.M.C. has received consulting fees from On-X Life technologies and speaking and consulting fees from Merck. J.D.O., M.K.D. and S.A.M. are employed by Carl Zeiss Meditec Inc. E.M.F. has received speaking and consulting fees from Biogen Idec, TEVA Neuroscience, Acorda, Bayer and Novartis and consulting fees Abbott Laboratories. P.A.C. has provided consultation services to Novartis, EMD-Serono, Teva, Biogen-IDEC; and has received grant support from EMD-Serono, Teva, Biogen-IDEC, Genentech, Bayer, Abbott and Vertex.
Acknowledgements
We wish to thank Maureen Mealy, Michaela Seigo, and Aleksandra Stankiewicz for assisting with data collection.
References
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–12. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354:1273–80. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–66. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol. 1993;111:773–5. doi: 10.1001/archopht.1993.01090060061023. [DOI] [PubMed] [Google Scholar]
- Burkholder BM, Osborne B, Loguidice MJ, Bisker E, Frohman TC, Conger A, et al. Macular volume determined by optical coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009;66:1366–72. doi: 10.1001/archneurol.2009.230. [DOI] [PubMed] [Google Scholar]
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- Dawson WW, Maida TM, Rubin ML. Human pattern-evoked retinal responses are altered by optic atrophy. Invest Ophthalmol Vis Sci. 1982;22:796–803. [PubMed] [Google Scholar]
- Dutta R, Chang A, Doud MK, Kidd GJ, Ribaudo MV, Young EA, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69:445–54. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Frohman TC, Zee DS, McColl R, Galetta S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol. 2005;4:111–21. doi: 10.1016/S1474-4422(05)00992-0. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4:664–75. doi: 10.1038/ncpneuro0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garas A, Simo M, Hollo G. Nerve fiber layer and macular thinning measured with different imaging methods during the course of acute optic neuritis. Eur J Ophthalmol. 2011;21:473–83. doi: 10.5301/EJO.2010.5844. [DOI] [PubMed] [Google Scholar]
- Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171:368–79. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- Henderson AP, Altmann DR, Trip AS, Kallis C, Jones SJ, Schlottmann PG, et al. A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain. 2010;133:2592–602. doi: 10.1093/brain/awq146. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 2002;360:1953–62. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Toosy AT, Jones SJ, Altmann DR, Miszkiel KA, MacManus DG, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127:2498–505. doi: 10.1093/brain/awh284. [DOI] [PubMed] [Google Scholar]
- Hollander H, Bisti S, Maffei L, Hebel R. Electroretinographic responses and retrograde changes of retinal morphology after intracranial optic nerve section. A quantitative analysis in the cat. Exp Brain Res. 1984;55:483–93. doi: 10.1007/BF00235279. [DOI] [PubMed] [Google Scholar]
- Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2328–36. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26:26–8. doi: 10.1212/wnl.26.6_part_2.26. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Celesia GG. Simultaneous recording of pattern electroretinogram and visual evoked responses in neuro-ophthalmologic disorders. Neurology. 1985;35:644–51. doi: 10.1212/wnl.35.5.644. [DOI] [PubMed] [Google Scholar]
- Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina. 1994;14:445–51. doi: 10.1097/00006982-199414050-00010. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, et al. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–19. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Tutlam NT, Xu J, Klawiter EC, Shepherd J, Trinkaus K, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009a;72:1077–82. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Tutlam NT, Xu J, Shepherd JB, Klawiter EC, Song SK, et al. Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 2009b;73:46–52. doi: 10.1212/WNL.0b013e3181aaea32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannicke T, Wurm A, Iandiev I, Hollborn M, Linnertz R, Binder DK. Deletion of aquaporin-4 renders retinal glial cells more susceptible to osmotic stress. J Neurosci Res. 2010;88:2877–88. doi: 10.1002/jnr.22437. [DOI] [PubMed] [Google Scholar]
- Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7. [PubMed] [Google Scholar]
- Perry VH, Lund RD. Evidence that the lamina cribrosa prevents intraretinal myelination of retinal ganglion cell axons. J Neurocytol. 1990;19:265–72. doi: 10.1007/BF01217304. [DOI] [PubMed] [Google Scholar]
- Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–92. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302–8. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134:518–33. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- Seiple W, Price MJ, Kupersmith M, Siegel IM, Carr RE. The pattern electroretinogram in optic nerve disease. Ophthalmology. 1983;90:1127–32. doi: 10.1016/s0161-6420(83)80057-8. [DOI] [PubMed] [Google Scholar]
- Syc SB, Warner CV, Hiremath GS, Farrell SK, Ratchford JN, Conger A, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16:829–39. doi: 10.1177/1352458510371640. [DOI] [PubMed] [Google Scholar]
- Talman LS, Bisker ER, Sackel DJ, Long DA, Jr, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–60. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–14. doi: 10.1016/j.ophtha.2009.05.025. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint D, Perier O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3:211–20. [PubMed] [Google Scholar]
- Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–91. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- Wang T, Lee MH, Johnson T, Allie R, Hu L, Calabresi PA, et al. Activated T-cells inhibit neurogenesis by releasing granzyme B: rescue by Kv1.3 blockers. J Neurosci. 2010;30:5020–7. doi: 10.1523/JNEUROSCI.0311-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RR, Cusato K, Raven MA, Reese BE. Organization of the inner retina following early elimination of the retinal ganglion cell population: effects on cell numbers and stratification patterns. Vis Neurosci. 2001;18:233–44. doi: 10.1017/s0952523801182088. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology. 2003;60:848–53. doi: 10.1212/01.wnl.0000049912.02954.2c. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]