Abstract
Objective. To determine the relationship between serum vitamin D and markers of subclinical cardiovascular disease (CVD) in patients with SLE.
Methods. We recruited SLE patients (≥4 ACR 1997 criteria) from outpatient clinics between January 2007 and January 2009. Vitamin D deficiency was defined as serum 25(OH)D <20 ng/ml measured by ELISA. Disease activity was measured using the SLEDAI-2K score. Aortic pulse wave velocity (aPWV) was measured using PulseTrace 3600 (Micromedical) and carotid plaque (CP) and intima–media thickness (IMT) assessed using B-mode Doppler US.
Results. Seventy-five women with SLE were recruited with a median (interquartile range) disease duration of 16 (8–27) years. Patients with vitamin D deficiency had higher BMI (P = 0.014) and insulin resistance (P = 0.023) than those with 25(OH)D >20 ng/ml. Subjects with SLEDAI-2K ≥4 had lower 25(OH)D than those with SLEDAI-2K <4 (median 12.9 vs 20.3 ng/ml, P = 0.031). Aortic stiffness was significantly associated with serum 25(OH)D [log(aPWV) β (95% CI) −0.0217 (−0.038, −0.005), P = 0.010] independently of BMI, CVD risk factors and serum insulin. Adjustment for disease activity reduced the strength of the association. There was no association between 25(OH)D and CP or IMT.
Conclusions. Vitamin D deficiency is associated with increased aortic stiffness in SLE, independent of CVD risk factors and insulin. Increased inflammatory disease activity may be the mechanism by which vitamin D deficiency mediates vascular stiffness in this patient group.
Keywords: systemic lupus erythematosus, vitamin D, vascular stiffness, cardiovascular risk, disease activity
Introduction
SLE an autoimmune systemic inflammatory disease predominantly affecting women. Mortality in SLE has a bimodal distribution, with a second peak due to cardiovascular disease (CVD) [1]. The excess cardiovascular risk, which may be up to 52 times in SLE, is not explained by traditional cardiovascular risk factors [2–4]. The identification of these novel risk factors is important to ensure development of targeted therapies to reduce cardiovascular morbidity and mortality in patients with SLE.
Recently, the roles of vitamin D beyond calcium homeostasis have been widely studied. The vitamin D receptor has been identified in both cells of the immune system (monocytes, antigen-presenting cells, macrophages, T cells) and the cardiovascular system (endothelial cells and vascular smooth muscle cells) [5, 6]. Epidemiological studies within the general population have demonstrated that vitamin D deficiency is an independent risk factor for adverse cardiovascular events [7–9]. Furthermore, lower serum 25(OH)D is associated with the presence of subclinical CVD, including carotid IMT, coronary artery calcification and endothelial dysfunction [10–15].
Vitamin D deficiency is more common in patients with SLE than in age- and gender-matched controls [16–19]. This is perhaps unsurprising given that 90% of serum 25(OH)D is obtained from sunlight, and that photosensitivity is a common feature of lupus. Other studies have also suggested contributions from renal disease and the use of CSs [17, 20–23].
The importance of vitamin D deficiency beyond its role in bone health in SLE is unclear and is the subject of current debate [24]. Vitamin D has important immunomodulatory roles in vitro raising the possibility of disease-specific roles in SLE. Indeed, vitamin D deficiency has been associated with increased lupus disease activity in some [18, 20, 25, 26] but not all studies [27–31].
If vitamin D deficiency were to contribute to CVD in SLE, it would represent a readily modifiable novel risk factor. In this study, we aimed to determine whether vitamin D deficiency was associated with markers of subclinical CVD in patients with SLE.
Methods
We recruited SLE patients aged 18–70 years from regional outpatient clinics between January 2007 and January 2009. All patients fulfilled ≥4 ACR Revised 1997 Classification criteria for SLE. A full clinical history and physical examination was undertaken in all patients. Lupus disease activity was measured using the SLEDAI-2000 scale [32]. Fasting blood samples were taken for measurement of lipid profiles and glucose, serum insulin, serum C3 and C4 complement, biochemical profile, full blood count and anti-dsDNA titre. Insulin resistance was calculated using the homeostatic model assessment (HOMA-IR) method [33]. Vascular cell adhesion molecules (VCAM-1, E-selectin) were measured using DuoSet ELISA development kits (R&D Systems, Abingdon, UK). Serum 25(OH)D and PTH were measured in randomly selected patients using ELISA. Serum vitamin D deficiency was defined as 25(OH)D ≤20 ng/ml according to regional guidelines (J. Berry, personal communication). The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation and normal renal function was defined as an eGFR > 90 ml/min/1.73 m2 [34].
Aortic pulse wave velocity (aPWV) was measured using the automated PulseTrace 3600 (Micro Medical, UK) by sequentially recording ECG-gated carotid and femoral artery waveforms as described by Asmar et al. [35]. Briefly, the distance from the sternal notch to the femoral artery was measured as a straight line between the points on the body surface using a tape measure. A 4 Mhz Doppler pencil probe was placed at 45° to the skin surface of the artery and measured with the probe facing towards the flow direction (towards the heart). The median (IQR) aPWV in a group of healthy female controls was 4.2 (2.9–9.2) m/s (S. Haque, personal communication). Carotid intima–media thickness (cIMT) and carotid plaque (CP) were measured using B-mode Doppler US by an experienced vascular sonographer. The mean carotid IMT was determined from three separate measurements on the far wall of the common carotid artery along a section 1 cm proximal to the carotid bulb on the left and right as previously described [36]. CP was defined as present if two of the three following conditions were met: (i) a distinct area of protrusion >50% into the lumen compared with the surrounding wall; (ii) increased echogenicity; and (iii) IMT > 0.15 cm [37]. Neither assessor was aware of the vitamin D status of the patient.
Statistical analysis was conducted using STATA v10.0 (StataCorp LP, TX, USA). Differences between vitamin D-deficient and non-deficient groups were determined using non-parametric tests (Mann–Whitney U-test). Linear and logistic regression models were used to investigate linear associations between 25(OH)D and markers of subclinical CVD. For linear regression, aPWV was log transformed to satisfy assumptions of normality. Logistic regression was used to analyse binomial variables.
Ethical approval for this study was obtained from the local research ethics committee (Northwest 5 REC, reference number 05/MRE08/62) and written consent was obtained in accordance with the Declaration of Helsinki.
Results
Characteristics of patients
We recruited 75 women with SLE. The median [interquartile range (IQR)] age of the study population was 53 (46–60) years with a median disease duration of 16 (8–27) years. Sixty-nine patients (92.0%) were Caucasian. The majority of the patients had stable disease with median (IQR) SLEDAI-2000 score of 0 (0–4). Thirty-three (51.6%) had a SLEDAI-2000 score of 0.
CSs were being taken by 29/75 (38.7%) patients with a median (IQR) daily dose of 7.5 (5–14.5) mg. Anti-malarial therapy (predominantly HCQ) was being taken by 38/75 (50.7%) patients. Immunosuppressants were used concurrently in 27/75 (36%) patients; AZA, n = 13 (17%); ciclosporin, n = 2 (3%); MMF, n = 7 (9%); MTX, n = 7 (9%); LEF, n = 1 (1%); rituximab, n = 1 (1%). The demographic and disease characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of the study population
| n (%) or median (IQR) | |
|---|---|
| Demographics | |
| Female gender | 75 (100) |
| Age, yearsa | 53.4 (45.8, 60.4) |
| Ethnicity | 69 (92.0) |
| Caucasian | |
| Afro-Caribbean | 1 (1.33) |
| South Asian | 1 (1.33) |
| South-East Asian | 1 (1.33) |
| Middle Eastern | 2 (2.67) |
| Mixed race | 1 (1.33) |
| Disease characteristics | |
| Disease duration, yearsa | 16.1 (8.8, 27.3) |
| SLEDAI scorea | 0 (0, 4) |
| C3, g/la | 1.07 (0.88, 1.36) |
| C4, g/la | 0.2 (0.13, 0.24) |
| hs-CRP, mg/la | 3.1 (1.6, 7.0) |
| Currently receiving steroids | 29 (38.6) |
| Current daily steroid dose, mga | 7.5 (5, 14.5) |
| Currently receiving HCQ | 38 (50.7) |
| Currently receiving immunosuppressants | 27 (36.0) |
| Traditional cardiovascular risk factors | |
| Systolic BP, mmHga | 125 (116, 140) |
| Diastolic BP, mmHga | 71 (66, 77) |
| Use of anti-hypertensive medication | 24 (32.4) |
| BMIa, k/gm2 | 26.7 (24.1, 30.5) |
| Waist : hip ratioa | 0.88 (0.85, 0.93) |
| eGFR (MDRD)a, ml/min/1.73m2 | 88.2 (71.2, 100.6) |
| eGFR <60 ml/min/1.73 m2 | 8 (10.7) |
| Total cholesterol, mmol/la | 4.67 (4.13, 5.36) |
| HDL cholesterol, mmol/la | 1.72 (1.44, 2.01) |
| Serum triglycerides, mmol/la | 1.11 (0.83, 1.53) |
| Fasting insulin, μIU/mla | 11.7 (8.5, 19.3) |
| HOMA-IRa | 1.7 (1.3, 2.8) |
| Current smoker | 7 (9.7) |
| Smoking history (pack-years)a | 0.9 (0, 10.8) |
| History of diabetes mellitus | 4 (5.3) |
| History of any CVD (myocardial infarction, angina pectoris, peripheral vascular disease, transient ischaemic attack, stroke) | 18 (24) |
| History of ischaemic heart disease (myocardial infarction, angina pectoris) | 3 (4) |
aMedian (IQR).
Prevalence of vitamin D deficiency
The median (IQR) 25(OH)D was 19.7 (15.1–29.6) ng/ml for the whole population. Thirty-nine (52%) patients were vitamin D deficient. Serum 25(OH)D levels were higher in the summer than the winter (24.2 vs 19.3 ng/ml), although this was not statistically significant (P = 0.352), and even in the summer months 25/50 (50%) were vitamin D deficient. Patients prescribed calcium/vitamin D had no significant difference in their vitamin D levels and 14/29 (48%) of those prescribed supplements were vitamin D deficient.
Vitamin D deficiency and SLE disease
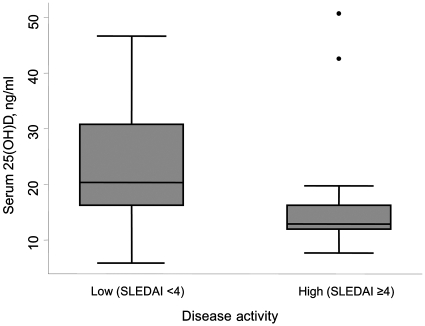
Patients with active disease (SLEDAI-2000 ≥4, n = 13) had significantly lower 25(OH)D than those without [12.9 (12.0–16.2) vs 20.3 (16.2–30.8) ng/ml, P = 0.031] as shown in Fig. 1. There was no association between serum vitamin D and anti-dsDNA antibodies or serum C3 or C4 complement. Vitamin D levels did not differ in those patients receiving CSs or in those with renal impairment (data not shown).
Fig. 1.
Comparison of 25(OH)D levels according to clinical disease activity using the SLEDAI-2000. Patients with more active disease (defined as being within the upper quartile, corresponding to a SLEDAI score of ≥4) had significantly lower 25(OH)D than those with inactive or low disease activity (P = 0.03). Box represents 25th and 75th percentiles, horizontal line respresents the median, error bars show the 5th and 95th percentiles, outside values are shown as dots.
Vitamin D deficiency and cardiovascular risk factors
Three patients (4%) had ischaemic heart disease as manifest by a history of angina pectoris. Two of these three patients also reported a previous myocardial infarction. Only seven (9.7%) patients were current smokers, and the median (IQR) smoking history for the whole group was 0.9 (0–10.8) pack-years. Most patients were overweight with a median (IQR) BMI of 26.7 (24.1–30.5) kg/m2. The prevalence of traditional cardiovascular risk factors is presented in Table 1.
Patients who were vitamin D deficient had significantly higher BMI [28.7 (24.7–31.1) vs 25.7 (22.8–28.1), P = 0.014], fasting serum insulin [12.6 (9.2–23.4) vs 10.8 (7.8–13.8) μIU/ml] and insulin resistance [HOMA-IR 1.95 (1.4–3.2) vs 1.6 (1.2–1.9), P = 0.023] than those with 25(OH)D >20 ng/ml. There was no significant association between serum 25(OH)D and other traditional cardiovascular risk factors, although vitamin D deficiency was associated with a trend towards increased waist circumference, waist : hip ratio and diastolic blood pressure (Table 2).
Table 2.
Association between vitamin D deficiency and cardiovascular risk factors
| CVD risk factor | 25(OH)D ≤20 ng/ml | 25(OH)D >20 ng/ml | P-value |
|---|---|---|---|
| Age, years | 52.0 (42.3–60.4) | 54.0 (47.3–60.35) | 0.35 |
| Current smokers, n (%) | 5 (14) | 2 (6) | 0.26 |
| BMI, kg/m2 | 28.7 (24.7–31.1) | 25.7 (22.8–28.1) | 0.01 |
| Waist circumference, cm | 92.4 (84.5–105.9) | 87.1 (80.1–96.9) | 0.05 |
| Waist : hip ratio | 0.89 (0.85–0.97) | 0.87 (0.833–0.91) | 0.07 |
| Systolic BP, mmHg | 123 (116–142) | 127.5 (116–140) | 0.98 |
| Diastolic BP, mmHg | 73 (66–80) | 70.5 (61–75) | 0.08 |
| Total cholesterol, mmol/l | 4.68 (4.13–5.56) | 4.57 (4.1–5.26) | 0.50 |
| HDL cholesterol, mmol/l | 1.67 (1.42–1.975) | 1.75 (1.45–2.015) | 0.97 |
| Fasting glucose, mmol/l | 4.8 (4.35–5.2) | 4.5 (4.2–5.1) | 0.47 |
| Serum insulin, μIU/ml | 12.6 (9.1–23.4) | 10.7 (7.7–13.8) | 0.03 |
| Insulin resistance (HOMA-IR) | 1.95 (1.4–3.2) | 1.6 (1.2–1.9) | 0.02 |
| eGFR, ml/min/1.73 m2 | 87.6 (64.3–101.7) | 90.7 (74.7–100.6) | 0.61 |
Results are expressed as median (IQR) unless otherwise stated. BP: blood pressure. Statistically significant results are shown in bold type.
Vitamin D deficiency and markers of subclinical CVD
The median (IQR) aPWV in our whole population was 6.2 (3.7–11.0) m/s. Patients who were vitamin D deficient had a significantly increased aPWV [8.2 (4.7–11.6) vs 5.4 (3.3–8.9), P = 0.033]. There was no significant association between 25(OH)D deficiency and carotid IMT, CP or soluble vascular cell adhesion molecules (E-selectin and VCAM-1) (Table 3).
Table 3.
Association between vitamin D deficiency and markers of subclinical CVD
| Marker of CVD | All subjects | 25(OH)D ≤20 ng/ml (n = 39) | 25(OH)D >20 ng/ml (n = 36) | P-value |
|---|---|---|---|---|
| aPWV, m/s | 6.2 (3.7–11.0) | 8.2 (4.7–11.6) | 5.4 (3.3–8.9) | 0.03 |
| CP, n (%)a | 35 (51.4) | 14 (20.6) | 21 (30.8) | 0.09 |
| Carotid IMT, mma | 0.07 (0.06–0.08) | 0.06 (0.05–0.07) | 0.07 (0.06–0.08) | 0.21 |
| VCAM-1, ng/ml | 289 (232.3–369.1) | 265.4 (226.1–313.1) | 309.65 (245.75–389.7) | 0.06 |
| E-selectin, ng/ml | 10.38 (6.89–13.5) | 10.38 (7.14–12.8) | 10.34 (6.54–14.15) | 0.69 |
Results are expressed as median (IQR) unless otherwise stated. Statistically significant results are shown in bold type. aTotal n = 68 (n = 34 in each group).
In a univariate linear regression model, lower serum 25(OH)D was associated with a significantly increased aPVW [log(aPWV) β (95% CI) −0.021 (−0.038, −0.005), P = 0.010] but not CP [odds ratio (95% CI) 1.03 (0.98, 1.08), P = 0.205) or cIMT [odds ratio, ≥75th percentile (95% CI) 1.02 (0.96, 1.07), P = 0.57]. Adjusting for age, traditional cardiovascular risk factors and serum insulin levels, this association remained significant [β (95% CI) −0.023 (−0.043, −0.003), P = 0.021] (Table 4). Adjustment for the SLEDAI-2000 score reduced the strength of the association such that it was no longer significant [β (95% CI) −0.012 (−0.031, 0.007), P = 0.200] (Table 4).
Table 4.
Vitamin D deficiency and subclinical markers of CVD
| aPWVa |
CPb |
cIMTc |
||||
|---|---|---|---|---|---|---|
| Model | β (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Unadjusted | −0.021 (−0.038, −0.005) | 0.010 | 1.03 (0.98, 1.08) | 0.205 | 1.02 (0.96, 1.07) | 0.571 |
| Adjusted | ||||||
| Model 1d | −0.020 (−0.040, −0.0003) | 0.047 | 1.03 (0.97, 1.09) | 0.366 | 1.02 (0.95, 1.09) | 0.585 |
| Model 2e | −0.023 (−0.043, −0.003) | 0.021 | 1.04 (0.97, 1.11) | 0.236 | 1.02 (0.95, 1.10) | 0.561 |
| Model 3f | −0.012 (−0.031, 0.007) | 0.200 | 1.03 (0.96, 1.10) | 0.382 | 1.00 (0.92, 1.09) | 0.999 |
aLinear regression of log(aPWV). bLogistic regression model for the presence of CP. cLogistic regression model for cIMT above upper quartile. dAdjusted for age, season (summer or winter), BMI, systolic blood pressure, pack-years smoking, disease duration, eGFR, fasting glucose. eAs Model 1 above plus serum insulin. fAs Model 1 above plus SLEDAI score. β: β-coeffecient, OR: odds ratio.
Discussion
This is the first study to demonstrate an association between 25(OH)D and subclinical CVD in patients with SLE. Serum vitamin D was inversely associated with aortic stiffness in a linear regression model, but not with either CP or IMT. This association persisted after adjustment for traditional CVD risk factors and BMI. Our results confirm those of Wu et al. [38], who also found no association between vitamin D and CP or IMT.
Increased arterial stiffness, as measured by aPWV, predicts cardiovascular outcomes in the general population. A recent meta-analysis of 17 studies revealed that a 1 m/s increase in aPWV can increase the risk of a cardiovascular event by 14% [39]. In our study, the difference in median aPWV between the vitamin D-deficient and -replete groups was 2.8 m/s. This suggests that vitamin D deficiency may significantly increase the risk of future CVD within the lupus population.
Vascular stiffness can be partly driven by inflammation, and better disease control in patients with inflammatory arthritis results in a reduction in pulse wave velocity [40]. Selzer et al. [41] noted that inflammatory biomarkers in SLE were particularly associated with aPWV. In our study, the association between 25(OH)D and stiffness was at least in part accounted for by disease activity since in a regression model that includes SLEDAI score the association was no longer significant.
Patients with more active disease (SLEDAI score in the upper quartile of the range) had significantly lower 25(OH)D. There was no association, however, between 25(OH)D and serum C3 and C4 complement or anti-dsDNA titre. Furthermore, there was no association between 25(OH)D and use of immunosuppressants, anti-malarials or CSs. The precise mechanism by which this association may be mediated requires further study.
Our results suggest that the association between 25(OH)D and disease activity is strongest in those patients with the most active disease/lowest vitamin D. Given that 90% of vitamin D is synthesised in ultraviolet (UV) light-exposed skin, sunlight avoidance may contribute significantly to the association between D and disease activity. Photosensitivity is a common feature of SLE and the use of high-factor sun block is a mainstay of conservative management. Patients with active disease may also be less inclined to spend time outdoors due to feeling unwell, an example of reverse causality.
Vitamin D has, however, been shown to have important roles in the regulation of the inflammatory response. In vitro studies have shown that the active metabolite of vitamin D can modulate the inflammatory response by driving a Th1 cell response towards a Th2 response via inhibition of Th1 cell proliferation, reducing the production of inflammatory cytokines (IL-2 and IFN-γ), and the induction of regulatory T cells [42–44]. Furthermore, autoantibody production from peripheral blood mononuclear cells isolated from patients with SLE is inhibited by vitamin D [45]. Vitamin D deficiency may therefore augment the inflammatory response in SLE, underpinning both increased disease activity and vascular stiffness.
Insulin resistance (elevated serum insulin and HOMA-IR) and obesity were the only traditional cardiovascular risk factors to be significantly associated with vitamin D status. Increased BMI and insulin resistance have been associated with 25(OH)D in other studies of both adults and adolescents with SLE [37, 38] and within the general population [46–48]. We have shown, however, that aPWV was associated with vitamin D independently of either BMI or serum insulin levels.
Vitamin D deficiency was common in our population of patients with established SLE [52% of patients had serum 25(OH)D <20 ng/ml]. This confirms the findings of others, although a direct comparison of serum 25(OH)D between studies is difficult due to variation in the study populations, study location and perhaps more importantly, in the methods used to measure vitamin D. In patients with SLE, mean levels of 25(OH)D have been reported from 11.5 to 44.6 ng/ml between different studies. Regardless of the absolute serum values reported, levels of vitamin D are invariably lower in SLE patients than in controls [16–19].
Our study is limited by a relatively small study population and may therefore lack power to identify other associations between vitamin D and risk factors for CVD. Patients with vitamin D deficiency had a trend towards increased diastolic blood pressure, central obesity, lower HDL cholesterol and renal impairment, although none of these parameters reached statistical significance. Although an association was seen between aPWV and 25(OH)D following adjustment for season and traditional risk factors, we did not adjust for ethnicity in this study. Indo-Asians and Afro-Caribbeans have more severe SLE [49, 50], and in the general population, these ethnicities are also associated with lower vitamin D concentrations [51, 52]. Whether the relationship between vitamin D and vascular disease differs in these subgroups beyond its influence on lupus disease activity cannot be answered in this study. A larger study will be needed to examine whether the relationships we have noted varies in different ethnic subgroups. Furthermore, due to the cross-sectional design of the study, only the 25(OH)D status at the time of assessment was measured.
Others have found no association between vitamin D and subclinical CVD in patients with SLE and our results are in apparent contradiction to this [38]. In this context, it is, however, important to consider temporal differences in the development of vascular stiffness compared with carotid IMT/plaque. Whereas atherosclerosis develops slowly over a period of many years, vascular stiffness is a dynamic process that can change in response to therapy over a relative short period of time [40]. A single measurement of 25(OH)D is unlikely to reflect its contribution to the development of increased IMT, which occurs over many years, resulting in an apparent lack of association. Furthermore, different risk factors appear to be associated with the development and progression of aPWV, CP and cIMT [41, 53, 54], suggesting different pathological processes. It could be concluded that our observations of aPWV reflect the study of an earlier and more dynamic pathological process than either CP or cIMT, and that aPWV may therefore be more responsive to current vitamin D status.
Conclusions
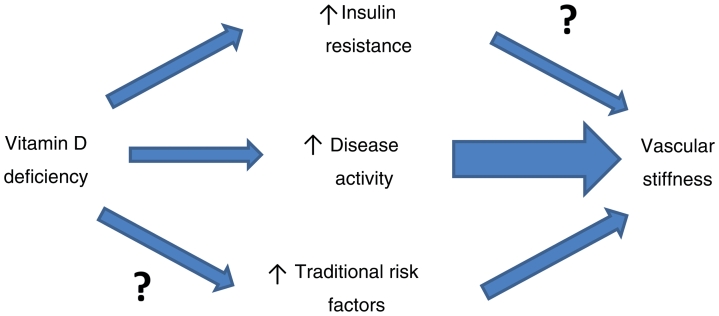
Vitamin D deficiency is common in SLE and is associated with vascular stiffness independently of traditional cardiovascular risk factors and insulin resistance. There is a strong inverse association between vitamin D deficiency and lupus disease activity which may, in part, drive this process in vitamin D deficient patients (Fig. 2). Prospective interventional studies and randomized controlled trials should focus on the effect of treating vitamin D deficiency on both SLE disease activity and vascular stiffness.
Fig. 2.
Proposed model of the relationships between vitamin D and vascular stiffness in SLE.

Acknowledgements
I.N.B. is supported by Arthritis Research UK, the Manchester Academic Health Sciences Centre and the NIHR Manchester Biomedical Research Centre.
Funding: This work was supported by an Arthritis Research UK Clinical Research Fellowship (grant ID 17574, to S.H.) and NIHR Manchester Biomedical Research Centre Clinical Fellowship (to J.A.R.).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Urowitz MB, Bookman AAM, Koehler BE, et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–5. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 2.Bruce IN, Urowitz MB, Gladman DD, et al. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48:3159–67. doi: 10.1002/art.11296. [DOI] [PubMed] [Google Scholar]
- 3.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365–79. doi: 10.1016/j.ecl.2010.02.010. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D Levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 8.Scragg R, Jackson R, Holdaway IM, et al. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–63. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty TM, Tang W, Dascalos S, et al. Ethnic origin and serum levels of 1{alpha},25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96:1477–81. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- 11.Jablonski KL, Chonchol M, Pierce GL, et al. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilz S, Henry R, Snijder M, et al. 25-Hydroxyvitamin D is not associated with carotid intima-media thickness in older men and women. Calcif Tissue Int. 2009;84:423–4. doi: 10.1007/s00223-009-9238-6. [DOI] [PubMed] [Google Scholar]
- 13.Reis JP, von Muhlen D, Michos ED, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207:589–90. doi: 10.1016/j.atherosclerosis.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 15.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 16.Damanhouri LH. Vitamin D deficiency in Saudi patients with systemic lupus erythematosus. Saudi Med J. 2009;30:1291–5. [PubMed] [Google Scholar]
- 17.Kamen DL, Cooper GS, Bouali H, et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Borba V, Vieira J, Kasamatsu T, et al. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20:427–33. doi: 10.1007/s00198-008-0676-1. [DOI] [PubMed] [Google Scholar]
- 19.Muller K, Kriegbaum NJ, Baslund B, et al. Vitamin-D-3 Metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D-3 in patients with systemic lupus erythematosus. Clin Rheumatol. 1995;14:397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 20.Amital H, Szekanecz Z, Szucs G, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis. 2010;69:1155–7. doi: 10.1136/ard.2009.120329. [DOI] [PubMed] [Google Scholar]
- 21.Broder A, Tobin J, Putterman C. Disease-specific definitions of vitamin D deficiency need to be established in autoimmune and non-autoimmune chronic diseases: a retrospective comparison of three chronic diseases. Arthritis Res Ther. 2010;12:R191. doi: 10.1186/ar3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Irastorza G, Egurbide MV, Olivares N, et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology. 2008;47:920–3. doi: 10.1093/rheumatology/ken121. [DOI] [PubMed] [Google Scholar]
- 23.Klein RG, Arnaud SB, Gallagher JC, et al. Intestinal calcium absorption in exogenous hypercortisonism. Role of 25-hydroxyvitamin D and corticosteroid dose. J Clin Invest. 1977;60:253–9. doi: 10.1172/JCI108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds JA, Bruce IN. Vitamin D in systemic lupus erythematosus: potential beyond bone health. Int J Clin Rheum. 2009;4:297–309. [Google Scholar]
- 25.Szodoray P, Tarr T, Bazso A, et al. The immunopathological role of vitamin D in patients with SLE: data from a single centre registry in Hungary. Scand J Rheumatol. 2010:1–5. doi: 10.3109/03009742.2010.507220. [DOI] [PubMed] [Google Scholar]
- 26.Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17:6–10. doi: 10.1177/0961203307085879. [DOI] [PubMed] [Google Scholar]
- 27.Orbach H, Zandman-Goddard G, Amital H, et al. Novel biomarkers in autoimmune diseases. Ann N Y Acad Sci. 2007;1109:385–400. doi: 10.1196/annals.1398.044. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Robles C, Rios-Fernandez R, Callejas-Rubio J, et al. Vitamin D deficiency in a cohort of patients with systemic lupus erythematous in the South of Spain. Lupus. 2011:330–1. doi: 10.1177/0961203310378670. [DOI] [PubMed] [Google Scholar]
- 30.Kim HA, Sung JM, Jeon JY, et al. Vitamin D may not be a good marker of disease activity in Korean patients with systemic lupus erythematosus. Rheumatol Int. 2011;31:1189–94. doi: 10.1007/s00296-010-1442-1. [DOI] [PubMed] [Google Scholar]
- 31.Toloza SMA, Cole DEC, Gladman DD, et al. Vitamin D insufficiency in a large female SLE cohort. Lupus. 2010;19:13–9. doi: 10.1177/0961203309345775. [DOI] [PubMed] [Google Scholar]
- 32.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 35.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studies. Hypertension. 1995;26:485–90. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu PS, Desai SR. A simple and reproducible method for assessing intimal-medial thickness of the common carotid artery. Br J Radiol. 1997;70:85–9. doi: 10.1259/bjr.70.829.9059301. [DOI] [PubMed] [Google Scholar]
- 37.Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–83. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 38.Wu PW, Rhew EY, Dyer AR, et al. 25-Hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Care Res. 2009;61:1387–95. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 40.Angel K, Provan SA, Gulseth HL, et al. Tumor necrosis factor-{alpha} antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. 2010;55:333–8. doi: 10.1161/HYPERTENSIONAHA.109.143982. [DOI] [PubMed] [Google Scholar]
- 41.Selzer F, Sutton-Tyrrell K, Fitzgerald S, et al. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–82. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 42.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 44.Overbergh L, Decallonne B, Waer M, et al. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543) Diabetes. 2000;49:1301–7. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 45.Linker-Israeli M, Elstner E, Klinenberg JR, et al. Vitamin D3 and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol. 2001;99:82–93. doi: 10.1006/clim.2000.4998. [DOI] [PubMed] [Google Scholar]
- 46.Vilarrasa N, Maravall J, Estepa A, et al. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–8. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- 47.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, et al. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine. 2009;36:205–10. doi: 10.1007/s12020-009-9216-9. [DOI] [PubMed] [Google Scholar]
- 48.Gannage-Yared MH, Chedid R, Khalife S, et al. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009;160:965–71. doi: 10.1530/EJE-08-0952. [DOI] [PubMed] [Google Scholar]
- 49.Studenski S, Allen NB, Caldwell DS, et al. Survival in systemic lupus erythematosus. A multivariate analysis of demographic factors. Arthritis Rheum. 1987;30:1326–32. doi: 10.1002/art.1780301202. [DOI] [PubMed] [Google Scholar]
- 50.Alarcon GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. Arthritis Rheum. 1998;41:1173–80. doi: 10.1002/1529-0131(199807)41:7<1173::AID-ART5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 51.Lowe NM, Mitra SR, Foster PC, et al. Vitamin D status and markers of bone turnover in Caucasian and South Asian postmenopausal women living in the UK. Br J Nutr. 2010;103:1706–10. doi: 10.1017/S0007114509993850. [DOI] [PubMed] [Google Scholar]
- 52.Ford L, Graham V, Wall A, et al. Vitamin D concentrations in an UK inner-city multicultural outpatient population. Ann Clin Biochem. 2006;43:468–73. doi: 10.1258/000456306778904614. [DOI] [PubMed] [Google Scholar]
- 53.Thompson T, Sutton-Tyrell K, Wilman RP, et al. Progression of carotid intima-media thickness and plaque in women with systemic lupus erythematosus. Arthritis Rheum. 2008;58:835–42. doi: 10.1002/art.23196. [DOI] [PubMed] [Google Scholar]
- 54.Sabio JM, Vargas-Hitos J, Zamora-Pasadas M, et al. Metabolic syndrome is associated with increased arterial stiffness and biomarkers of subclinical atherosclerosis in patients with systemic lupus erythematosus. J Rheumatol. 2009;36:2204–11. doi: 10.3899/jrheum.081253. [DOI] [PubMed] [Google Scholar]