Abstract
Existing standards for screening and management of late effects occurring in children who have undergone hematopoietic cell transplantation (HCT) include recommendations from pediatric cancer networks and consensus guidelines from adult-oriented transplantation societies applicable to all recipients of HCT. While these approaches have significant merit, they are not pediatric-HCT focused and they do not address post-HCT challenges faced by children with complex non-malignant disorders. In this article we discuss the strengths and weaknesses of current published recommendations and conclude that pediatric-specific guidelines for post-HCT screening and management would be beneficial to the long-term health of these patients and would promote late-effects research in this field. Our panel of late effects experts also provides recommendations for follow up and therapy of selected post-HCT organ and endocrine complications in pediatric patients.
Introduction
In April 2011 the NCI, NHLBI and the Pediatric Blood and Marrow Transplant Consortium (PBMTC) sponsored a consensus conference of international experts in clinical and biological research into late effects after hematopoietic cell transplantation (HCT) in children. The goal of the conference was to review the current state of knowledge and define gaps in the field, develop consensus on critical areas for future research, and determine the best study designs to effectively address these questions. This is the final manuscript in a published series that addresses these goals in the following areas for children post-HCT:1 1) genetic risks of experiencing late effects,2 2) methodological challenges in late effects study designs,2 3) specific organ effects,3 4) metabolic disorders,3 5) endocrine issues,4 6) immune dysfunction and reconstitution,5 and 7) quality of life (QoL), functional, and neurocognitive outcomes.6
The goal of this final manuscript is to review and compare current recommendations published by several important groups for long-term follow up in some of the above-mentioned areas, adding updated recommendations from the panel of experts that participated in the conference. We will also review the need for pediatric-specific post-HCT guidelines. In doing this we recognize that many of these recommendations require further study to validate their clinical utility. This issue is especially prescient in children. Although several studies have defined increased risks of mortality and a high incidence of late effects after HCT in cohorts comprised largely of adults,7–9 only a handful of relatively small studies have focused on children.10–14 Many of the recommendations we will review are either based upon studies comprised mainly of adults or upon survivor studies in children that were not designed to specifically address unique issues associated with HCT (allogenicity, prolonged altered immunity, graft vs. host-disease, specific effects of megadose therapy, etc.).
Published Guidelines: Children’s Oncology Group (COG)
The COG has published a comprehensive series of recommendations entitled, Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers (version 3.0, 2008 available at www.survivorshipguidelines.org). These guidelines are risk-based and exposure related. They come with a “Patient Specific Guideline Identification Tool,” that allows patients to define their treatment era, tabulate cumulative exposures to chemotherapeutic doses, identify sites and doses of radiation exposure, verify whether they have undergone HCT, list specific surgeries performed, and designate whether they have receive other therapies (radio-iodine therapy or systemic MIBG therapy). The tool then guides patients to sections that review specific late effects associated with specific exposures the patient has experienced. These sections describe the following: associated risk factors for the effect, other potential late effects associated with the described effect, the nature and recommended frequency of specific evaluations by health care professionals, recommended health counseling and links to recommendations for cancer screening.
A number of late affects after HCT are included in sections covering specific therapeutic exposures (i.e. endocrine effects are included in the TBI section). In addition, there are fifteen sections included that focus on complications of HCT that do not fit well into the therapeutic exposures model. The HCT-specific sections address risks of secondary cancers (AML, solid tumors, lymphoma), hepatic toxicity, osteonecrosis, and reduced bone mineral density experienced by HCT survivors. Next, problems associated with chronic GVHD are addressed: complications of skin, eye, and mouth, chronic pulmonary disease, immunological dysfunction (with active GVHD), esophageal and vaginal strictures/fibrosis, and joint contractures.
Published Guidelines: Center for International Blood and Marrow Transplant Research (CIBMTR), American Society of Blood and Marrow Transplantation (ASBMT), European Group for Blood and Marrow Transplantation (EBMT), Asia-Pacific Blood and Marrow Transplantation Group (APBMT), Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ), East Mediterranean Blood and Marrow Transplantation Group (EMBMT) and Sociedade Brasileira de Transplante de Medula Ossea (SBTMO)
In 2011 the CIBMTR, ASBMT, EBMT, APBMT, BMTSANZ, EMBMT and SBTMO published Recommended Screening and Preventive Practices for Long-term Survivors after Hematopoietic Cell Transplantation,15 an update of guidelines originally published in 2006 by the EBMT, CIBMTR, and ASBMT.16 Because these guidelines are applicable specifically to patients who have undergone HCT, the authors chose to organize screening recommendations by tissues and organs affected as well as risks of secondary malignancies and psychosocial harm, rather than the specific therapeutic exposures approach taken by COG. Possible late complications specific to each organ are listed along with risk factors such as age, radiation, steroid exposure, etc.; recommendations for frequency of screening are also included.
European Efforts—PanCareSurFup: Sorting out Evidence vs. Opinion
The UK Children’s Cancer Study Group (now known as Children’s Cancer and Leukaemia Group, CCLG) Late Effects Group Therapy-Based Long-Term Follow-Up Practice Statement (2nd edition, 2005; http://www.cclg.org.uk/dynamic_files/LTFU-full.pdf) includes an appendix devoted exclusively to providing comprehensive recommendations for follow-up of survivors of childhood HCT. It was written using literature review and expert opinion. It is divided into systems (eg endocrine), organs (eg skin) and functions (eg quality of life), and includes specific sections for secondary malignancies and chronic GVHD. Causes and higher risk factors for chronic toxicities are listed, and recommendations provided for the nature of clinical evaluation, frequency of follow-up and initial further actions. Cross-references to standard (ie non-HCT) guidelines are provided where appropriate. Although this document has the advantage of being focused on the details and complex issues of follow-up of pediatric HCT survivors, its current utility is limited by the absence of formal evidence-based methodology and of a recent update.
The Pan-European Network for Care of Survivors after Childhood and Adolescent Cancer (PanCare) was established in 2008 and has successfully obtained European Union funding for the PanCare Childhood and Adolescent Cancer Survivor Care and Follow-up Studies (PanCareSurFup) collaborative project. Among several large epidemiological studies, it will develop evidence-based pan-European guidelines for long-term follow-up care of survivors of childhood malignancy and of HCT. These guidelines will cover clinical practice and organization of follow-up care, as well as transition to age-appropriate care for survivors approaching adulthood, and finally the promotion of a healthy lifestyle. The overall intention is to address topics that are most important for survivors and their families, and to facilitate optimal follow-up for each survivor in a variety of countries with different levels of healthcare resources and systems. The guidelines will be developed with evidence-based methodology that includes selection of appropriate topics and clinically important questions to guide relevant literature searches that will then inform the production of evidence summaries. These summaries will then be used to construct draft and, after appropriate peer review, refine final practice recommendations. Although these guidelines will cover survivors of childhood malignancy in general, particular attention will be paid to ensure that late adverse effects suffered by and the follow-up needs of all pediatric HCT survivors (both with malignant and non-malignant diseases) will be included.
International Harmonization of Late Effects Follow Up
Recent and ongoing initiatives are seeking to achieve international harmonization of long-term follow-up and surveillance recommendations for survivors of childhood cancer. The existing pediatric clinical guidelines mentioned above (COG, CCLG), as well as the Scottish Intercollegiate Guidelines Network (SIGN) long-term follow up of survivors of childhood cancer (2004; www.sign.ac.uk/pdf/sign76.pdf), and the Dutch Childhood Oncology Group (DCOG, SKION) LATER guidelines (long term effects after childhood; http://later.skion.nl/) differ in their scope and methodology. This has led to a variety of different recommendations. Despite the potentially different needs of different countries and healthcare settings, there are advantages and efficiencies in collaborative efforts to share evidence, workload and conclusions. Recent work between the above groups and several other national late effects group representatives has led to the development of harmonized breast cancer surveillance recommendations for survivors of childhood cancer, identifying which survivors should be screened, how, when and how often. These recommendations have been developed in such a manner as to permit implementation in a variety of different healthcare and resource settings. The next step planned is to develop analogous recommendations for cardiomypathy screening, which is clearly of major importance for many survivors of HCT. Other future topics are being selected in an ongoing Delphi survey.
Comparison of Screening Recommendations: Do They Meet the Needs of the Pediatric HCT Survivor?
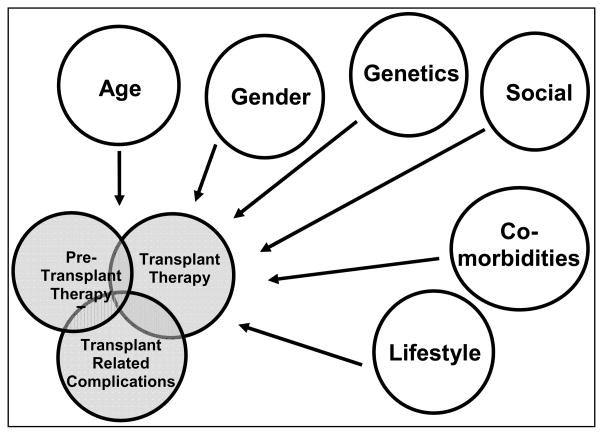
The COG and CCLG guidelines are designed to comprehensively address all modalities of cancer therapy and recognize differences in late effects noted based upon therapeutic technique, treatment era, dose, and developmental stage in children. They are very straightforward for children who receive a small number of agents followed by local surgery or radiation. The major challenge with applying these guidelines to the pediatric HCT population is that the majority of patients undergoing HCT have been treated with a large number of agents both prior to and after a first relapse and sometimes subsequent relapses. This may mean that risk of worsening organ damage with the megadose therapy of HCT is higher in some children due to pre-existing treatments, therefore, listing effects due to a single agent does not do justice to the total effect of an HCT regimen. Furthermore, over time, the ability to delivery HCT safely to higher risk children who have received more regimens and potentially multiple transplants has increased. Thus, adverse effects of HCT are expected to be worse in these heavily pre-treated children. In addition, genetics, age, gender, lifestyle, and existent comorbidities can have an effect. Finally, transplant-specific effects such as altered immunity and GVHD along with prolonged exposure to immune suppressive agents significantly changes patients’ long-term risk profiles (i.e. prolonged prednisone exposure dramatically affects growth and bone health, prolonged intense immune suppression affects second malignancy risk, etc.: Figure 1). While the COG and CCLG guidelines partially address some of these issues in their HCT-specific sections, the complexity of the problem makes addressing these issues in a comprehensive way challenging.
Figure 1.
Late effects after HCT are a result of the interaction between pre-HCT exposures to chemotherapy, radiation, and surgery with the transplant conditioning regimen and acute transplant complications and finally with transplant specific complications such as acute and chronic GVHD, persistent immunodeficiency, etc.. The risk for these outcomes are also modified by other intrinsic and extrinsic factors (age, gender, genetics, social, co-morbidities, lifestyle) that can alter these risks in either a positive or negative manner.
A further practical challenge with using the COG guidelines for transplant-specific late effects is that they are very difficult to glean from this enormous work. For example, challenges post-HCT patients experience with growth failure are spread out amongst the many therapeutic exposures that pre-HCT patients have, and the task of pulling things together for these very complex patients is daunting. Finally, the COG and CCLG guidelines are strongly oriented toward children with cancer. More than a third of pediatric HCT patients undergo the procedure due to non-malignant indications.
The CIBMTR/ASBMT/EBMT/APBMT/BMTSANZ/EMBMT/SBTMO guidelines (hereafter called “joint transplant society guidelines”) approach the subject in a very practical, although less comprehensive fashion. Broad guidelines applicable to the most common late effects noted after HCT are included. Specific types and timing of follow up are outlined and a large number of transplant-specific references are included. It is easier for both patients and practitioners to look at these guidelines and know the major issues that should be covered in a follow up visit of a patient who has undergone HCT. Caveats include the fact that the guidelines do not address the many differences in outcomes that can occur after HCT based upon pre-HCT therapies and comorbidities. In addition, the simplicity offered by these general guidelines does not allow one to look at differences in degree of intensity of preparative regimens used, extent of GVHD and post-HCT immune suppression undergone, different outcomes based upon use of different stem cell sources, and risks based upon age, specific non-malignant disorder of the recipient, etc..
With the strengths and weaknesses of the published recommendations in mind, Table 1 offers a comparison between COG, Joint Transplant Society, and CCLG recommendations for specific areas. Table 1 also includes specific recommendations from our panel of experts based upon updated evidence. There are many areas that are not addressed in this table that would need to be included in comprehensive guidelines (screening for second malignancies, neurocognitive, functional and quality of life effects, consequences of prolonged immune deficiency, etc.).
Table 1.
Selected Screening Recommendations for Late Effects After HCT in Pediatric Patients
| Children’s Oncology Group (COG) Recommendations | Joint Transplant Society* Recommendations | UK Children’s Cancer and Leukaemia Group (CCLG) Practice Statement, BMT section** | Expert Panel Recommendations | |||
|---|---|---|---|---|---|---|
| Screening | Management | |||||
| Iron Overload | HCT section 95 AST, ALT, Bilirubin, Ferritin screening at entry into f/u and prn. Biopsy, chelation, phlebotomy as indicated | Serum ferritin at 1 year after HCT in patients who have received RBC transfusions; consider liver biopsy or imaging study for abnormal results based on magnitude of elevation and clinical context; subsequent monitoring is suggested for patients with elevated LFTs, continued RBC transfusions, or presence of HCV infection |
History, examination Liver function tests yearly, ferritin as needed | Annual serum Ferritin; if elevated, consider T2* MRI | Phlebotomy or chelation | |
| GI | HCT section 95 AST, ALT, Bilirubin, Ferritin screening at entry into f/u and prn Hepatology consultation for persistent abnormal LFTs Hep B,C viral testing as indicated |
LFTs every 3–6 months in the first year, then individualized, but at least yearly thereafter Monitor viral load by PCR for patients with known hepatitis B or C, with liver and infectious disease specialist consultation Consider liver biopsy at 8– 10 years after HCT to assess cirrhosis in patients with chronic HCV infection |
Only needed in presence of overt symptoms History, examination High index of suspicion for chronic GVHD in patients with cholestasis or acute hepatitis Microbiological, virological,biochemic al investigation of malabsorption if present |
Annual screening for chronic GVHD Hepatitis virus infection screening Annual hepatocellular carcinoma screening for high risk patients:
|
||
| Renal | Chemotherapy, alkylating agents section 13. Risks—therapy with ifosfamide, cisplatin, carboplatin, amino- glycosides, amphotericin, immunosuppressant s, XRT of kidney. Screen with lytes/BUN/Cr urinalysis at entry into f/u and prn. Rx electrolyte supplementation, nephrology for hypertension, proteinuria, progressive renal insufficiency. | Blood pressure assessment at every clinic visit, with aggressive hypertension management Assess renal function with BUN, creatinine and urine protein at 6 months, 1 year and at least yearly thereafter Consider further workup (kidney biopsy or renal ultrasound) for further workup of renal dysfunction as clinically indicated |
Blood pressure, screen for hematuria/proteinuria (if protein positive, check urine protein: creatinine ratio), BUN/Cr yearly Consider GFR measurement if high creatinine High index of suspicion for chronic GVHD in patients with proteinuria/nephrotic syndrome |
Monitor urine for albumin:creatinine ratio at day 80 and then annually. If ratio ≥ 30 and<300mg/gm, confirm with two + tests in 3–6 months and monitor every 3–6 months. If ratio >300mg/g, monitor every 3–6 months. | Treat with ACE inhibitor or ARB if albumin:creatinin e ratio is >300mg/gm on one occasion or if patient has persistent ratio above 30gm/kg on 3 occasions in a 6 month period and has hypertension | |
| Pulmonary | HCT section 101 Risks—chest XRT/TBI, Bleomycin, Busulfan, BCNU, CCNU, cGVHD, Screen with CXR and PFTs at entry into f/u and prn. Avoid smoking, caution with SCUBA, anesthesia. Give influenza and pneumococal vaccines |
Routine clinical evaluation at 6 months and 1 year after HCT and at least yearly thereafter Assessment of tobacco use and couselling against smoking PFTs and focused radiologic assessment for allogeneic HCT recipients |
History, examination PFTs at 1 year (subsequent frequency depends on results and presence/absence of symptoms) Chest X-ray/consider high resolution CT if symptomatic or if severely abnormal PFTs High index of suspicion for chronic GVHD Advise against tobacco Advise influenza and pneumococcal immunizations |
Pulmonary function testing for allogeneic recipients twice per year for two years with consideration for more frequent screening in recipients of mismatched or unrelated donors grafts, or patients with active chronic GVHD After two years, consider yearly f/u PFTs based upon symptoms and past measurements |
If patients experience a decrease in PFTs >15% or a new pulmonary infiltrate, evaluate for infection/ GVHD. Refer to pulmonology for disease-specific care as needed. |
|
| Cardiac | XRT Section 71— multiple cardiac effects (CHF, cardiomyopathy, etc.) Risks higher with previous anthracyclines or combined with cyclo-phosphamide as conditioning for HCT. Screen with baseline echo/EKG, fasting glucose/lipid profile q2yrs. Recommendations regarding screening and treatment based upon condition and total anthracycline dose. |
Routine clinical assessment of cardiovascular risk factors as per general health maintenance at 1 year and at least yearly thereafter Education and counseling on “heart “ healthy lifestyle (regular exercise, healthy weight, no smoking, dietary counseling) Early treatment of cardiovascular risk factors such as diabetes, hypertension, and dyslipidemia Administration of antibiotics for endocarditis prophylaxis according to American Heart Association guidelines |
Echo – annually if abnormal, every 3–5 years if normal If heart included in radiotherapy field at any time (ie including TBI), consider review of other risk factors (eg measure fasting lipids) Advise against tobacco No other recommendations specific to HCT*** |
Annual CV risk assessment Blood Pressure each visit and at least annually; ECG/ECHO at least every 5 years, more frequently if anthracyclines, TBI or chest irradiation was given |
Referral to cardiology for abnormal or declining cardiac function | |
| Metabolic | XRT Section 49— Metabolic syndrome as a possible late effect of TBI. Screen with ht/wt/bp/BMI yearly plus fasting glucose/lipid profile every two years. Rx with diet, counseling, physical activity. | Screening for cardiovascular risk factors as outlined under “cardiac” | Measure fasting blood glucose, fasting lipids, HbA1c yearly Perform glucose tolerance test if fasting glucose elevated |
Lipid profile & fasting glucose at least every 5 years; if abnormal, screen annually | No transplant- specific recommendation s available | |
| Thyroid Dysfunction | XRT Section 64/65— hypo/hyperthyroidis m. Risks—XRT =10Gy, thyroid in field. Yearly screening, more frequently during rapid growth. |
Thyroid function testing yearly post-HCT, or if relevant symptoms develop | TSH and FT4 yearly Palpate thyroid yearly Measure thyroid autoantibodies if TFTs abnormal Perform US scan and refer for fine needle biopsy if thyroid nodule palpated |
TSH and FT4 annually:
|
If TSH is high and FT4 normal, either treat or repeat in 2 months. Replace thyroid as indicated for low levels. Rare secondary thyroid tumors post-TBI are can be cured with surgery. |
|
| Growth Impairment | XRT Section 50— Growth Hormone Deficiency Risks—young age, TBI≥ 10Gy single fx, ≥12 Gy fractionated. Screen with dietary assessment, ht/wt/BMI every 6m until growth completed. Refer to endocrine for ht<3rd %, drop ≥ 2 % rankings, growth velocity<4–5cm/yr, lack of growth spurt. |
Pediatric recipients: Monitor growth velocity in children annually; assessment of thyroid, and growth hormone function if clinically indicated | Measure height and weight, calculate height velocity 3–6 monthly until puberty and growth completed Measure IGF-1 and bone age in TBI recipients if concern about growth Refer to pediatric endocrinologist for consideration of dynamic GH testing in TBI recipients with slow growth (height velocity<25th centile) |
Accurate measure of growth yearly through full growth (age 17 girls, 19 boys). Bone age as needed. | Bone age and referral to endocrine for patients not growing appropriately. GH therapy may unmask hypothyroidism. |
|
| Low Bone Mineral Density | HCT section 97 Risks—young age, caucasion, low BMI, steroids, calcineurins, cranial XRT/TBI, GH deficiency, delayed puberty, hyper- thyroidism, poor exercise, poor nutrition, smoking, alcohol use, carbonated beverages. Dexa at entry into long-term f/u and prn. Rx with Vit D, Ca, exercise. Endocrine consultation for osteoporosis/history of fractures. |
Dual photon densitometry at 1 year for adult women, all allogeneic HCTrecipients and patients who are at high risk for bone loss; subsequent testing determined by defects or to assess response to therapy Physical activity, vitamin D, and calcium supplementation to prevent loss of bone density -Patients with cGVHD: Consider dual photon densitometry at an earlier date in patients with prolonged corticosteroid or calcineurin inhibitor exposure. |
History, examination (fractures, back pain) Consider measurement of BMD by DEXA scan, especially in patients treated for GH deficiency or hypogonadism |
Dexa-scan pre- HCT, 1 year post- HCT, yearly if Z- score<-1. |
Patients with Z- score<−2, history of fractures refer to endocrine. Supplement Ca & Vit. D, weight- bearing exercise, avoid smoking, alcohol, caffeine | |
| Osteonecrosis | HCT section 96 Risks—age ≥10 at HCT, steroids, TBI, focal XRT, allogeneic > autologous, cGVHD. Screen yearly with exam, MRI as clinically indicated. |
MRI to evaluate patients with joint symptoms | History, examination Perform MRI if suspicion of ON Refer patients with ON to orthopedic surgeon |
Consider MRI screening of asymptomatic patients on high- dose steroids. Early MRI screening of any patients with symptoms of joint pain, pain in groin or anterior thigh, limping. |
Minimize steroids and alcohol consumption, offer analgesics, non-weight- bearing exercise, PT. Refer to Orthopedics. | |
| Reproductive Risks | Chemotherapy, Alkylating agents section 7. Risks—combined doses of alylators/heavy metals/ DTIC/temazolamide with XRT to cranium or gonads. Screen FSH/LH/testosterone, Tanner staging ages 13–14 and as clinically indicated for delayed puberty, irregular menses. Semen analysis, repeat as indicated as resumption can occur 10 yrs after rx |
Consider referral to appropriate specialists for patients who are contemplating a pregnancy or are having difficulty conceiving Counsel sexually active patients in the reproductive age group about birth control post-HCT |
Assess pubertal stage 3–6 monthly until puberty and growth completed Measure sex hormones (testosterone or oestradiol), FSH, LH and inhibin B (if available) yearly from 10 years age Suggest semen analysis when appropriate At appropriate time: Discuss risk of impaired fertility, adverse pregnancy outcome and early menopause Discuss advisability of using contraception even with impaired fertility Discuss referral to Reproductive Medicine specialist for consideration of assisted reproduction technology when appropriate Refer patients with Leydig cell or ovarian failure to endocrinologist for hormone treatment |
Women:monitor of ovarian failure (FSH, assess cycling) Men: semen analysis |
Women: Anti- Mullerian Hormone (AMH) may assess ovarian reserve. Treat ovarian failure with hormone replacement therapy. Men: If oligospermia noted, could offer intracytoplasmic sperm injection. |
|
Recommendations from the Center for International Blood and Marrow Transplant Research (CIBMTR), the American Society of Blood and Marrow Transplantation (ASBMT), European Group for Blood and Marrow Transplantation (EBMT), Asia-Pacific Blood and Marrow Transplantation Group (APBMT), Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ), East Mediterranean Blood and Marrow Transplantation Group (EMBMT) and Sociedade Brasileira de Transplante de Medula Ossea (SBTMO)
Further information about higher risk factors and further actions is provided in each section.
Cross-references to Cardiac section of the Practice Statement
Post-HCT Follow-Up Guidelines Specific to Pediatric Patients
We have outlined strengths and weaknesses of current published recommendations for long-term follow up of children who have undergone HCT. The most significant weakness of both the pediatric-specific cancer recommendations and the Joint Transplant Society guidelines for pediatric HCT patients is that they do not contain detailed information needed for long-term follow up of children transplanted for non-malignant disorders. Many non-malignant conditions require unique disease-specific follow up (eg bone and neurocognitive challenges in Hurler syndrome, increased second cancer risk in Fanconi Anemia, etc.). Absence of these recommendations in current guidelines is a major gap, given that up to a third of pediatric HCT is for children with these disorders. With these issues in mind, ideal pediatric-specific post-HCT follow up guidelines should accomplish the following: 1) Provide evidence-based recommendations for screening and intervention that consider both pre-HCT treatment and underlying genetic disorders; 2) be accessible enough to be used by local physicians, yet have depth and secondary resources available that will nurture research and cater to the needs of specialized late effects clinics; 3) use vigorous methodology according to recognized standards such as AGREE (appraisal of guidelines research and evaluation, www.agreecollaboration.org) in order to foster international utilization and comparative studies aimed at treating or preventing late effects, and 4) undergo revisions on a scheduled basis as ongoing studies lead to new recommendations for intervention. We recommend a coordinated effort through the PBMTC, the COG SCT Committee, the CCLG BMT subgroup, the EBMT Pediatric Diseases Working Group and other pediatric-oriented HCT-specific groups, working in parallel with larger pediatric cancer late effects groups (COG Late Effects Task Force, PanCareSurFup, the International Harmonization Collaboration, etc.) to formulate these guidelines.
Recommendations for Follow up in Selected Areas from our Consensus Panel Experts
IRON OVERLOAD,GASTROINTESTINAL, AND HEPATOBILIARY ISSUES
Secondary iron overload is a nearly universal complication of HCT causing liver cardiac, pancreatic, pituitary and thyroid-related morbidity. Risk factors include high numbers of red cell transfusions and increased GI absorption of iron due to inflammatory conditions, including GVHD.17 Studies using serum ferritin as a marker suggest that iron levels fall slowly over time after transplant, reaching normal levels years later.18, 19 In heavily iron-overloaded patients, iron reduction therapy may improve transplantation outcomes20 and cardiac function.21 Because of this, it is important to screen for iron overload after HCT using serum ferritin.
Although liver biopsy has often been recommended to quantify iron overload, recent standardization of the T2* MRI method of quantifying tissue iron will likely replace this for managing patients. The mainstay of treatment of iron overload is phlebotomy in patients with recovered normal erythropoiesis.22
The majority of GI late effects are related to protracted acute GVHD and chronic GVHD. As GVHD is controlled and tolerance is developed, most symptoms resolve. Major hepatobiliary concerns include the consequences of viral hepatitis acquired before or during the transplant, biliary stone disease, and focal liver lesions.23 Screening and management of viral hepatitis should distinguish this from GVHD presenting with hepatocellular injury, and should involve a hepatologist.
RENAL DISEASE
Hypertension and renal function screening should occur at all long term follow up visits. As outlined in our earlier description of renal dysfunction,3 albuminuria and proteinuria may reflect GVHD-induced endothelial injury, inflammatory tubular and interstitial damage and progressive chronic kidney disease (CKD); however, it is not known if albuminuria or proteinuria by themselves cause the increased morbidity and mortality of HCT, or merely reflect other processes. Regardless, because they may predict poor late outcomes, it is worthwhile to screen for albuminuria and proteinuria and consider referral to a nephrologist for patients with this and other signs of ongoing renal disease. It is also important to aggressively treat hypertension in patients post-HCT, especially when they have been treated with prolonged courses of calcineurin inhibitors. Whether post-HCT patients with albuminuria and hypertension benefit from treatment with ACE inhibitors or angiotensin receptor blockers requires further study, but careful control of hypertension with captopril did show a benefit in a small study24 and larger studies are underway.
PULMONARY DISEASE
Early recognition and treatment of chronic pulmonary conditions after HCT may be important to successful outcomes, hence, increased surveillance for lung dysfunction by serial PFTs for the first two years following HCT should be considered whenever feasible. Screening prior to symptoms is important; by the time patients become symptomatic, the disease is generally advanced.25 Comprehensive evaluation is recommended when PFTs are decreased by more than 15% or when signs or symptoms of pulmonary dysfunction are detected.26, 27 Testing should include a high-resolution CT scan of the chest and broncho-alveolar lavage to exclude opportunistic infections if applicable. In consultation with Pulmonology, lung biopsy may helpful in making definitive diagnoses when necessary.
Standard treatment for obstructive lung disease combines enhanced immunosuppression with supportive care including antimicrobial prophylaxis, bronchodilator therapy and supplemental oxygen when indicated. Unfortunately, the response in patients with restrictive lung disease to multiple agents including corticosteroids, cyclosporine, tacrolimus and azathioprine is limited.28 The potential role for TNFa in the pathogenesis of both obstructive and restrictive lung disease suggests that neutralizing agents such as etanercept may have promise.29 The combination of azithromycin, montelukast and inhaled fluticasone is currently being investigated to prevent progression of newly-diagnosed bronchiolitis obliterans.30
CARDIAC DISEASE/METABOLIC SYNDROME
Although cardiac dysfunction has been studied extensively in non-HCT settings, less is known regarding the incidence and predictors of CHF following HCT in childhood. Potentially cardiotoxic exposures unique to HCT include conditioning with high-dose chemotherapy (especially cyclophosphamide) and total body irradiation (TBI).31 In addition, HCT survivors are at increased risk of developing cardiovascular risk factors such as hypertension and diabetes due, in part, to exposure to TBI, prolonged immunosuppressive therapy following allogeneic HCT, or other health conditions such as hypothyroidism or growth hormone deficiency.31, 32 In addition, pre-HCT exposures and cardiac function have been shown to have significant impact on post-HCT cardiac function, so levels of pre-HCT anthracycline and chest irradiation should be known as post-HCT patients are evaluated for long-term issues.33 Although more specific work needs to be done to verify this, current evidence suggests that the risk for late-occurring cardiovascular complications following HCT may be largely due to pre-HCT therapeutic exposures, with little additional risk from conditioning-related exposures or GVHD.33–35
Screening and follow up for cardiac/metabolic syndrome issues should include blood pressure assessment, measurement of lipid profile and fasting glucose every 5 years or yearly if abnormal, and treatment of lipid abnormalities based on current cardiovascular guidelines.
ECG/Echocardiograms should be obtained at appropriate intervals based upon pre-HCT exposures to anthracyclines and chest-wall irradiation.
THYROID DYSFUNCTION
Since we do not yet know the full impact of the various reduced intensity and non-myeloablative HCT regimens upon the development of thyroid dysfunction (TD), all patients should be monitored. The current recommendations are that thyroid function studies (TSH and FT4) should be performed annually post-HCT36 and patients with abnormal values should be referred to pediatric endocrinology. If TSH is elevated with a normal FT4, thyroid hormone replacement could be initiated,36 but since subclinical hypothyroidism may resolve spontaneously,37 the studies could also simply be repeated in approximately 2–6 months. Patients starting on thyroid replacement should be followed with a repeat level 6 weeks later and then twice yearly in post-pubertal patients and every 3–4 months in pre-pubertal or pubertal patients.36 In patients receiving BU-based conditioning, surveillance needs to continue for at least 10 years post-HC.38 However, in patients that received TBI, there is no clear plateau in the incidence out to 30 years,38 suggesting that screening may need to continue life-long. Fortunately, the development of thyroid tumors (median onset 10 years post-HCT [range 4.5 – 22.3 years]) is a rare event, and seems to only occur in patients who received TBI (incidence 3%).38 In patients who received TBI, careful physical examination of the thyroid gland in mandatory, and thyroid ultrasound should be performed as clinically indicated.38
GROWTH IMPAIRMENT
The current recommendations are that growth should be measured with an accurate stadiometer annually in children and growth velocity calculated.36 Patients experiencing slow growth should also have their bone age obtained annually until their epiphyses close (generally around age 17 for girls and age 19 for boys). For those children who do not appear to be tracking along an appropriate growth curve, referral to pediatric endocrinology consultation is appropriate.
The use of recombinant GH therapy to treat growth impairment due to GH deficiency has not been universal. Clearly, the administration of GH appears to improve final height compared to similarly-transplanted controls that chose not to receive GH.39, 40 The treatment effect was strongest in those less than 10 years of age at time of HCT, whereas an effect of GH on older children was difficult to ascertain.40 One concern regarding the use of GH is that utilizing an agent that stimulates the growth of cells may play a role in the development of relapse of the patient’s underlying malignant disease or of a secondary malignancy. Fortunately, the data does not support this concern, though clearly the development of benign osteochondromas or exostoses are more common in GH-treated patients.40 Interestingly, GH-treatment may also reveal previously unrecognized hypothyroidism, highlighting the importance of routine monitoring of all aspects of the endocrine system.40
BONE HEALTH: LOW BONE MINERAL DENSITY
Given the limitations of existing studies specific to pediatric HCT, the recommendations for follow-up are based on a general knowledge of modifiable risk factors, similar to those recently published.41, 42 Patients should be encouraged to maintain an adequate intake of calcium and vitamin D,43, 44 and should be counseled about adverse effects of cigarette smoking as well as alcohol and caffeine consumption.42 Weight-bearing exercise, early mobilization and return to normal activities should also be encouraged.
It is currently unclear when screening for low bone mineral density (BMD) should be initiated and what is the appropriate frequency of screening after HCT. Until more data are available, general guidelines for monitoring BMD in children and adolescents should be followed,45 which includes annual age-appropriate monitoring of growth, thyroid, and pubertal development. We also recommend DXA scan pre-HCT, 1 year after HCT, then once a year if BMD Z-score is<−1 or every 5 years if BMD Z-score is normal. Patients exposed to high doses of methotrexate and corticosteroids, patients with a significant bone loss (BMD Z-score<−2), history of fractures, or endocrine deficiencies should be referred to a pediatric endocrinologist. Correction of hormonal deficiencies (hypogonadism, GH deficiency) should be done if deemed appropriate based on age and the risk-to-benefit ratio. We also recommended checking serum calcium, magnesium, and 25-hydroxyvitamin D level in HCT recipients, particularly in those patients who are shown to have low BMD.46
Bisphosphonates have been shown to be effective in preventing bone loss after HCT in adult patients.47, 48 The primary mode of action of bisphosphonates is inhibition of bone resorption by osteoclasts.49 Although it is not known at present if bone resorption is increased in children after HCT, one study showed that bisphosphonate therapy can improve BMD in pediatric HCT recipients as well.46 More studies are needed to determine if the use of bisphosphonates can be recommended as a routine measure in children who received HCT who either have low BMD or are at increased risk for continued bone loss (eg treatment with corticosteroids for GVHD). Potential concerns about the use of bisphosphonates in children have been recently reviewed by Ward et al.50
BONE HEALTH: OSTEONECROSIS
MRI screening of patients receiving high dose steroids has allowed identification of osteonecrosis (ON) in the early, asymptomatic stages.51 Although the disease progresses in the vast majority of cases,52 small, central lesions, away from the weight-bearing area may decrease or remain stable over time53, 54 or rarely, spontaneous regression has been reported.55
Involvement of a pediatric orthopedic surgeon is key to the management of patients with ON. Prior to subchondral fracture and collapse, in asymptomatic patients, whether or not to treat is controversial as the natural history of asymptomatic disease is poorly understood. Several temporary measures have been recommended,56–58 which include minimizing the exposure to corticosteroids, minimizing alcohol consumption, analgesics for pain relief, limited weight bearing, and physical therapy to design appropriate non-weight bearing exercise (e.g. swimming). However, prolonged limited weight bearing in the pediatric population may not be practical.
In symptomatic patients, treatment is targeted at joint preservation and pain control. Core decompression consists of opening a tract into the necrotic bone to reduce intraosseous pressure and stimulate healing. While effective at relieving pain, a large percentage of patients will progress to subchondral fracture. Several pharmacologic approaches hold promise for more effective prevention of ON occurrence or its progression, although the data are limited. Lipid-lowering agents, such as statins, may be beneficial, with evidence pointing to hyperlipidemia playing a pathogenic role 59–61 but other data fail to find this association.62 Additionally, statins may increase bone formation and improve bone mineral density.63 Low-molecular-weight heparin may also be considered to target hypercoagulability in ON,64 although there is concern that it might increase the risk of osteoporosis.65 Bisphosphonates may be used to improve the biomechanical properties of bone and prevent or delay bone collapse early in the disease course, as well as for pain control.66–68 However, the safety and efficacy of bisphosphonates in children at risk for ON has not been studied except for traumatic ON in adolescents.69 Recently, cellular therapy using autologous marrow derived mesenchymal stem cells70, 71 has been proposed based upon the findings of a reduced population of stem cells in the marrow adjacent to osteonecrotic sites.72
After subchondral fracture and collapse, arthritis leading to joint destruction is inevitable with debilitating loss of ambulatory function. Unfortunately, the only reliable means of eliminating pain and restoring function at this juncture is replacement of the affected joint. Joint replacement offers an excellent functional outcome and is the standard of care in skeletally mature patients, however, the limited long term durability of prosthetic joints in young adults makes this an unattractive option. In the skeletally immature patient, joint replacement is deferred and attempts are made to control pain with analgesics and activity modifications. The effectiveness of these non-surgical interventions is poor.
Given these potential treatments, annual hip and knee MRI should be considered for high risk children > 8 years of age for up to 3 years after HCT or when indicated by clinical symptoms (joint pain, pain in the groin or anterior thigh, limping).58, 73 Once ON is diagnosed, however, guidelines and consensus on treatment are lacking.
REPRODUCTIVE RISKS
For males, reproductive function should be evaluated by assessing sexual function and by performing a semen analysis. In women, it is important to monitor menstrual function, though hormonal contraception will mask any signs of ovarian failure. Even women who maintain cyclic menses after therapy are at risk for early menopause, infertility, and long-term health problems related to early ovarian failure.74–77 Several hormones and ultrasound measures have been utilized to evaluate a woman’s fertility potential and response to fertility treatments. Specifically, these include early follicular phase measures of serum Follicle Stimulating Hormone (FSH), Inhibin B, and Anti-Mullerian Hormone (AMH), as well as ultrasound measures of ovarian volume and Antral Follicle Counts (AFC). Years after treatment for cancer, these measures may be impaired in survivors.78–82 Of these, it appears that AMH and AFC are the most sensitive measures of ovarian reserve.83 Ongoing prospective studies of ovarian reserve at the University of Pennsylvania have found ovarian reserve is impaired in menstruating HCT survivors who were exposed to more intense conditioning regimens. While these tests may help to determine ovarian reserve in transplant recipients, it is not yet clear if they will ultimately help to predict the likelihood of pregnancy or time to menopause in this population.
Early loss of ovarian function is associated with menopausal symptoms and long-term health risks including cardiovascular disease and osteoporosis. Estrogens are the most effective therapy for the treatment of menopausal symptoms and genitourinary atrophy, and these symptoms dramatically improve in transplant patients started on hormone therapy (HT).84 In addition, there are data in women with premature ovarian failure due to bone marrow transplantation that HT improves bone mineral density.85 While therapeutic options for the treatment of early ovarian failure in females who have completed puberty may include combined hormonal contraception or hormone replacement,86 there are no clear guidelines regarding the optimal method of hormone replacement therapy in this population since little data exist comparing the long term safety and efficacy of various different forms of HT in cancer survivors. Importantly, the results of large HT trials such as the Women’s Health Initiative cannot be generalized to the population of young cancer survivors with premature ovarian failure where the benefits of HT usually outweigh potential risks. Prepubertal transplant patients should be monitored closely for development of secondary sexual characteristics after 10–11 years of age. Patients with evidence of gonadal failure should be cared for by a pediatric endocrinologist who can administer a physiologic regimen of hormone replacement therapy to ensure optimal development of secondary sex characteristics and adult stature.87
Males with oligospermia as a result of previous cancer therapy may be able to donate for intrauterine insemination or IVF with intracytoplasmic sperm injection (ICSI). In the setting of azoospermia due to testicular failure after cancer therapy, donor sperm may be the only available option. Menstruating women found to have decreased ovarian reserve after cancer therapies may be candidates for undergoing fertility therapies with ovulation induction, intrauterine inseminations, or in-vitro fertilization. However, existing data suggest that cancer survivors have a diminished response to ovarian stimulation and lower IVF success rates compared to couples without a history of cancer.88 After a woman has experienced reproductive dysfunction as a result of cancer treatment, the option with the highest chance of a delivering a live born infant is donor egg. In cases where the HCT recipient is not sufficiently healthy to consider pregnancy herself,89 or if she is unable to carry a pregnancy successfully because of prior cancer treatments, gestational surrogacy may be considered. Lastly, embryo donation and adoption are additional options that survivors of HCT may consider.
While HCT often leads to ovarian failure and infertility, unintended pregnancies may occur even in patients who are presumed to have ovarian failure.90 Because HCT survivors may have co-morbidities making pregnancy complicated, discussion of family planning and contraceptive options should be a priority. Contraceptive choices may be limited for this population since a history of thromboembolic disease, significant liver dysfunction, or other co-morbidities may make estrogen containing hormonal contraception unsafe. While barrier contraceptives and progestin containing methods may be reasonable choices for such patients, other effective methods include intrauterine devices and sterilization procedures.91 Whether intended or unintended, pregnancies in HCT-survivors should be considered “high-risk” because of increased risk of miscarriage and high risk of premature delivery.90 Children born to survivors do not appear to be at increased risk of having birth defects.92
Conclusions
Outstanding resources exist that assist practitioners in caring for pediatric survivors of HCT. Significant efforts to standardize late effects follow up and foster late effects research are underway. These efforts would be greatly enhanced by pediatric-specific guidelines for follow up after HCT designed with the goal of improving long-term care and overall health of children who have undergone this procedure.
Acknowledgments
Funding for this work was made possible in part by the following National Institute of Health grants: 1R13CA159788-01 (MP, KSB), U01HL069254 (MP), R01 CA112530-05 (KSB). The views expressed in this manuscript do not reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government. Further support was provided by a generous grant from the St. Baldrick’s Foundation and the Lance Armstrong Foundation, as well as the following pharmaceutical companies: Genzyme, Otsuka America Pharmaceutical, Inc., and Sigma-Tau Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of those that provided funding.
Footnotes
Conflicts of Interest: The following authors have indicated commercial support relationship(s): George McDonald: Soligenix, Calistoga, Gentium, EMD Serono, Pfizer - Consultant, Soligenix - speakers bureau member, Soligenix, Xenoport - Stock holder; Joanne Kurtzberg: Aldagen, NMDP, HRSA, NHLBI, Chimerix - grant/research support; Kenneth Cooke: Athersys Inc - grant/research support, Amgen Inc - study drug and investigational pharmacy services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker KS, Bhatia S, Bunin N, Nieder M, Dvorak CC, Sung L, et al. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: state of the science, future directions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(10):1424–7. doi: 10.1016/j.bbmt.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Davies SM, Scott Baker K, Pulsipher MA, Hansen JA. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: etiology and pathogenesis of late effects after HCT performed in childhood--methodologic challenges. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(10):1428–35. doi: 10.1016/j.bbmt.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieder ML, McDonald GB, Kida A, Hingorani S, Armenian SH, Cooke KR, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(11):1573–84. doi: 10.1016/j.bbmt.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dvorak CC, Gracia CR, Sanders JE, Cheng EY, Scott Baker K, Pulsipher MA, et al. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: Endocrine Challenges-Thyroid Dysfunction, Growth Impairment, Bone Health, & Reproductive Risks. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(12):1725–38. doi: 10.1016/j.bbmt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunin N, Small T, Szabolcs P, Baker KS, Pulsipher MA, Torgerson T. NCI, NHLBI/PBMTC First International Conference on Late Effects After Pediatric Hematopoietic Cell Transplantation: Persistent Immune Deficiency in Pediatric Transplant Survivors. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: Health-Related Quality of Life, Functional, and Neurocognitive Outcomes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–22. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin PJ, Counts GW, Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(6):1011–6. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresters D, Van Gils IC, Dekker FW, Lankester AC, Bredius RG, Schweizer JJ. Abnormal liver enzymes two years after haematopoietic stem cell transplantation in children: prevalence and risk factors. Bone Marrow Transplant. 2008;41(1):27–31. doi: 10.1038/sj.bmt.1705887. [DOI] [PubMed] [Google Scholar]
- 11.Faraci M, Barra S, Cohen A, Lanino E, Grisolia F, Miano M, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys. 2005;63(5):1568–75. doi: 10.1016/j.ijrobp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Ferry C, Gemayel G, Rocha V, Labopin M, Esperou H, Robin M, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007;40(3):219–24. doi: 10.1038/sj.bmt.1705710. [DOI] [PubMed] [Google Scholar]
- 13.Leahey AM, Teunissen H, Friedman DL, Moshang T, Lange BJ, Meadows AT. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32(3):163–9. doi: 10.1002/(sici)1096-911x(199903)32:3<163::aid-mpo1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Perkins JL, Kunin-Batson AS, Youngren NM, Ness KK, Ulrich KJ, Hansen MJ, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49(7):958–63. doi: 10.1002/pbc.21207. [DOI] [PubMed] [Google Scholar]
- 15.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended Screening and Preventive Practices for Long-Term Survivors after Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 [Google Scholar]
- 16.Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12(2):138–51. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 18.Lucarelli G, Angelucci E, Giardini C, Baronciani D, Galimberti M, Polchi P, et al. Fate of iron stores in thalassaemia after bone-marrow transplantation. Lancet. 1993;342(8884):1388–91. doi: 10.1016/0140-6736(93)92753-g. [DOI] [PubMed] [Google Scholar]
- 19.Chotsampancharoen T, Gan K, Kasow KA, Barfield RC, Hale GA, Leung W. Iron overload in survivors of childhood leukemia after allogeneic hematopoietic stem cell transplantation. Pediatric transplantation. 2009;13(3):348–52. doi: 10.1111/j.1399-3046.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 20.Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, et al. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation. Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90(3):994–8. [PubMed] [Google Scholar]
- 21.Mariotti E, Angelucci E, Agostini A, Baronciani D, Sgarbi E, Lucarelli G. Evaluation of cardiac status in iron-loaded thalassaemia patients following bone marrow transplantation: improvement in cardiac function during reduction in body iron burden. British journal of haematology. 1998;103(4):916–21. doi: 10.1046/j.1365-2141.1998.01099.x. [DOI] [PubMed] [Google Scholar]
- 22.Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, et al. Treatment of iron overload in the “ex-thalassemic”. Report from the phlebotomy program. Annals of the New York Academy of Sciences. 1998;850:288–93. doi: 10.1111/j.1749-6632.1998.tb10485.x. [DOI] [PubMed] [Google Scholar]
- 23.McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51(4):1450–60. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano JA, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. International journal of radiation oncology, biology, physics. 2008;70 (5):1546–51. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chien JW, Martin PJ, Flowers ME, Nichols WG, Clark JG. Implications of early airflow decline after myeloablative allogeneic stem cell transplantation. Bone marrow transplantation. 2004;33(7):759–64. doi: 10.1038/sj.bmt.1704422. [DOI] [PubMed] [Google Scholar]
- 26.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(1 Suppl):S106–14. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildebrandt GC, Fazekas T, Lawitschka A, Bertz H, Greinix H, Halter J, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone marrow transplantation. 2011;46(10):1283–95. doi: 10.1038/bmt.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke KR, Yanik G, editors. Lung Injury Following Hematopoietic Stem Cell Transplantation. 2009. [Google Scholar]
- 29.Yanik GA, Mineishi S, Levine JE, Kitko CL, White ES, Vander Lugt MT, et al. Soluble Tumor Necrosis Factor Receptor: Enbrel (Etanercept) for Subacute Pulmonary Dysfunction Following Allogeneic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman BC, Jacobsohn DA, Williams KM, Au BK, Au MA, Lee SJ, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone marrow transplantation. 2011;46(10):1369–73. doi: 10.1038/bmt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42(18):3191–8. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. The New England journal of medicine. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 33.Armenian SH, Sun CL, Francisco L, Steinberger J, Kurian S, Wong FL, et al. Late congestive heart failure after hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(34):5537–43. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armenian SH, Sun CL, Kawashima T, Arora M, Leisenring W, Sklar CA, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118(5):1413–20. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armenian SH, Sun CL, Mills G, Teh JB, Francisco L, Durand JB, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(8):1138–44. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo J, Wingard J, Tichelli A, Lee S, Van Lint M, Burns L, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12(2):138–51. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Katsanis E, Shapiro R, Robison L, Haake R, Kim T, Pescovitz O, et al. Thyroid dysfunction following bone marrow transplantation: long-term follow-up of 80 pediatric patients. Bone Marrow Transplant. 1990;5(5):335–40. [PubMed] [Google Scholar]
- 38.Sanders J, Hoffmeister P, Woolfrey A, Carpenter P, Storer B, Storb R, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years’ experience. Blood. 2009;113(2):306–8. doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huma Z, Boulad F, Black P, Heller G, Sklar C. Growth in children after bone marrow transplantation for acute leukemia. Blood. 1995;86(2):819–24. [PubMed] [Google Scholar]
- 40.Sanders J, Guthrie K, Hoffmeister P, Woolfrey A, Carpenter P, Appelbaum F. Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood. 2005;105(3):1348–54. doi: 10.1182/blood-2004-07-2528. [DOI] [PubMed] [Google Scholar]
- 41.McClune BL, Polgreen LE, Burmeister LA, Blaes AH, Mulrooney DA, Burns LJ, et al. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone Marrow Transplant. 2011;46(1):1–9. doi: 10.1038/bmt.2010.198. [DOI] [PubMed] [Google Scholar]
- 42.Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–13. doi: 10.1542/peds.2007-1396. [DOI] [PubMed] [Google Scholar]
- 43.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 44.Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93(7):2716–21. doi: 10.1210/jc.2007-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop N, Braillon P, Burnham J, Cimaz R, Davies J, Fewtrell M, et al. Dual-energy X-ray aborptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11(1):29–42. doi: 10.1016/j.jocd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Carpenter P, Hoffmeister P, Chesnut C, Storer B, Charuhas P, Woolfrey A, et al. Bisphosphonate therapy for reduced bone mineral density in children with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(6):683–90. doi: 10.1016/j.bbmt.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Kananen K, Volin L, Laitinen K, Alfthan H, Ruutu T, Valimaki MJ. Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J Clin Endocrinol Metab. 2005;90(7):3877–85. doi: 10.1210/jc.2004-2161. [DOI] [PubMed] [Google Scholar]
- 48.Grigg AP, Shuttleworth P, Reynolds J, Schwarer AP, Szer J, Bradstock K, et al. Pamidronate reduces bone loss after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2006;91(10):3835–43. doi: 10.1210/jc.2006-0684. [DOI] [PubMed] [Google Scholar]
- 49.Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119 (Suppl 2):S150–62. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 50.Ward LM, Petryk A, Gordon CM. Use of bisphosphonates in the treatment of pediatric osteoporosis. Int J Clin Rheumatol. 2009;4(6):657–672. [Google Scholar]
- 51.Marston S, Gillingham K, Bailey R, Cheng E. A Prospective Study of Osteonecrosis of the Femoral Head in Solid Organ Transplantation Patients. J Bone and Joint Surg-Am. 2002;84A(12):2145–51. doi: 10.2106/00004623-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Musso ES, Mitchell SN, Schink-Ascani M, Bassett CA. Results of conservative management of osteonecrosis of the femoral head. A retrospective review. Clin Orthop Relat Res. 1986;(207):209–15. [PubMed] [Google Scholar]
- 53.Hungerford DS, Jones LC. Asymptomatic osteonecrosis: should it be treated? Clin Orthop Relat Res. 2004;(429):124–30. [PubMed] [Google Scholar]
- 54.Wiesmann A, Pereira P, Bohm P, Faul C, Kanz L, Einsele H. Avascular necrosis of bone following allogeneic stem cell transplantation: MR screening and therapeutic options. Bone Marrow Transplant. 1998;22(6):565–9. doi: 10.1038/sj.bmt.1701374. [DOI] [PubMed] [Google Scholar]
- 55.Cheng E, Thongtrangan I, Laorr A, Saleh K. Spontaneous Resolution of Osteonecrosis of the Femoral Head. J Bone Joint Surg Am. 2004;86(12):2594–9. doi: 10.2106/00004623-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32(2):94–124. [PubMed] [Google Scholar]
- 57.Burger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)--experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44(3):220–5. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 58.Kaste SC, Shidler TJ, Tong X, Srivastava DK, Rochester R, Hudson MM, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33(4):435–41. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Zhang N, Yue D. Experimental steroid osteonecrosis in rabbits and pathologic findings. Zhonghua Wai Ke Za Zhi. 1995;33(8):485–7. [PubMed] [Google Scholar]
- 60.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;(344):8–19. [PubMed] [Google Scholar]
- 61.Pritchett JW. Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res. 2001;(386):173–8. doi: 10.1097/00003086-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 62.Ajmal M, Matas AJ, Kuskowski M, Cheng EY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40(2):235–9. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355(9222):2218–9. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 64.Glueck CJ, Freiberg RA, Sieve L, Wang P. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;(435):164–70. doi: 10.1097/01.blo.0000157539.67567.03. [DOI] [PubMed] [Google Scholar]
- 65.Lefkou E, Khamashta M, Hampson G, Hunt B. Review: Low-molecular-weight heparin-induced osteoporosis and osteoporotic fractures: a myth or an existing entity? Lupus. 2010;19(1):3–12. doi: 10.1177/0961203309353171. [DOI] [PubMed] [Google Scholar]
- 66.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87(10):2155–9. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen T, Zacharin MR. Pamidronate treatment of steroid associated osteonecrosis in young patients treated for acute lymphoblastic leukaemia--two-year outcomes. J Pediatr Endocrinol Metab. 2006;19(2):161–7. doi: 10.1515/jpem.2006.19.2.161. [DOI] [PubMed] [Google Scholar]
- 68.Nishii T, Sugano N, Miki H, Hashimoto J, Yoshikawa H. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Relat Res. 2006;443:273–9. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- 69.Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am. 2007;89(8):1727–34. doi: 10.2106/JBJS.F.00964. [DOI] [PubMed] [Google Scholar]
- 70.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A(6):1153–60. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. Journal of Bone & Joint Surgery - British Volume. 2005;87(7):896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 72.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81 (2):349–55. doi: 10.1302/0301-620x.81b2.8818. [DOI] [PubMed] [Google Scholar]
- 73.Leung W, Ahn H, Rose SR, Phipps S, Smith T, Gan K, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86(4):215–24. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 74.Sklar C. Reproductive physiology and treatment-related loss of sex hormone production. Med Pediatr Oncol. 1999;33(1):2–8. doi: 10.1002/(sici)1096-911x(199907)33:1<2::aid-mpo2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 75.Byrne J, Fears TR, Gail MH, Pee D, Connelly RR, Austin DF, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. 1992;166(3):788–93. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 76.Byrne J. Infertility and premature menopause in childhood cancer survivors. Med Pediatr Oncol. 1999;33(1):24–8. doi: 10.1002/(sici)1096-911x(199907)33:1<24::aid-mpo5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 77.Kreuser ED, Felsenberg D, Behles C, Seibt-Jung H, Mielcarek M, Diehl V, et al. Long-term gonadal dysfunction and its impact on bone mineralization in patients following COPP/ABVD chemotherapy for Hodgkin’s disease. Ann Oncol. 1992;3 (Suppl 4):105–10. doi: 10.1093/annonc/3.suppl_4.s105. [DOI] [PubMed] [Google Scholar]
- 78.Lie Fong S, Lugtenburg PJ, Schipper I, Themmen AP, de Jong FH, Sonneveld P, et al. Anti-mullerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod. 2008;23(3):674–8. doi: 10.1093/humrep/dem392. [DOI] [PubMed] [Google Scholar]
- 79.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–14. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 80.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18(11):2368–74. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 81.Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD) Hematology. 2007;12(2):141–7. doi: 10.1080/10245330600954072. [DOI] [PubMed] [Google Scholar]
- 82.van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin’s lymphoma during childhood. J Clin Endocrinol Metab. 2007;92(10):3869–74. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 83.Larsen EC, Muller J, Rechnitzer C, Schmiegelow K, Andersen AN. Diminished ovarian reserve in female childhood cancer survivors with regular menstrual cycles and basal FSH<10 IU/l. Hum Reprod. 2003;18(2):417–22. doi: 10.1093/humrep/deg073. [DOI] [PubMed] [Google Scholar]
- 84.Piccioni P, Scirpa P, D’Emilio I, Sora F, Scarciglia M, Laurenti L, et al. Hormonal replacement therapy after stem cell transplantation. Maturitas. 2004;49(4):327–33. doi: 10.1016/j.maturitas.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Castelo-Branco C, Rovira M, Pons F, Duran M, Sierra J, Vives A, et al. The effect of hormone replacement therapy on bone mass in patients with ovarian failure due to bone marrow transplantation. Maturitas. 1996;23(3):307–12. doi: 10.1016/0378-5122(95)00991-4. [DOI] [PubMed] [Google Scholar]
- 86.Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113(6):1355–63. doi: 10.1097/AOG.0b013e3181a66843. [DOI] [PubMed] [Google Scholar]
- 87.Sanders JE. Growth and development after hematopoietic cell transplant in children. Bone Marrow Transplant. 2008;41(2):223–7. doi: 10.1038/sj.bmt.1705875. [DOI] [PubMed] [Google Scholar]
- 88.Ginsburg ES, Yanushpolsky EH, Jackson KV. In vitro fertilization for cancer patients and survivors. Fertil Steril. 2001;75(4):705–10. doi: 10.1016/s0015-0282(00)01802-1. [DOI] [PubMed] [Google Scholar]
- 89.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 90.Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87(7):3045–52. [PubMed] [Google Scholar]
- 91.Gracia CR. Reproductive health after cancer. Cancer Treat Res. 2010;156:3–9. doi: 10.1007/978-1-4419-6518-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanders JE, Woolfrey AE, Carpenter PA, Storer BE, Hoffmeister PA, Deeg HJ, et al. Late effects among pediatric patients followed for nearly 4 decades after transplantation for severe aplastic anemia. Blood. 2011;118(5):1421–8. doi: 10.1182/blood-2011-02-334953. [DOI] [PMC free article] [PubMed] [Google Scholar]