Abstract
Purpose of Review
Cyclooxygenase-2 (COX-2) plays a critical role in modulating deleterious actions of angiotensin II (Ang II) where there is an inappropriate activation of the renin-angiotensin system (RAS). This review discusses recent developments regarding the complex interactions by which COX-2 modulates the impact of an activated RAS on kidney function and blood pressure.
Recent Findings
Normal rats with increased COX-2 activity but with different intrarenal Ang II activity due to sodium restriction or chronic treatment with angiotensin converting enzyme (ACE) inhibitors showed similar responses to COX-2 selective inhibition (nimesulide) indicating independence from the intrarenal Ang II activity. COX-2 dependent maintenance of medullary blood flow was consistent and not dependent on dietary salt or ACE inhibition. In contrast, COX-2 influences on sodium excretion were contingent on the prevailing RAS activity. In chronic hypertensive models, COX-2 inhibition elicited similar reductions in kidney function but COX-2 metabolites contribute to rather than ameliorate the hypertension.
Summary
The maintenance of renal hemodynamics reflects direct and opposing effects of Ang II and COX-2 metabolites. The antagonism in water and electrolyte reabsorption is dependent on the prevailing intrarenal Ang II activity. The recent functional experiments demonstrate a beneficial modulation of Ang II by COX-2 except in the presence of inflammation promoted by hypertension, hyperglycemia and oxidative stress.
Keywords: Renin-Angiotensin System, Renal Prostaglandins, Salt Restriction, Angiotensin Converting Enzyme, Hypertension
Introduction
In addition to the beneficial effects of appropriate stimulation of the renin-angiotensin system (RAS) during reductions in sodium intake or extracellular fluid volume, an inappropriately activated RAS, particularly in the kidney, leads to hypertension and sodium retention caused by actions of Ang II on the renal microvasculature and sodium transport[1]. With sustained elevations in intrarenal RAS activity, elevated intrarenal Ang II levels synergize with various paracrine and cytokine factors leading to hypertension and renal injury eventually culminating in chronic kidney disease, diabetic nephropathy and other renal diseases[2]. Without the presence of powerful counteracting protective mechanisms[3], the degree of injury would possibly be much greater. This review is focused on recent developments related to the roles of the metabolites of cyclooxygenase-2 (COX-2) in counteracting the deleterious actions of sustained increases in intrarenal Ang II[4, 5]. Much of our understanding of the interactions between Ang II and COX-2 metabolites has been derived from the patterns of COX-2 expression which are principally targeted by an augmented RAS activity leading to prostaglandin mediated modulation of Ang II actions. Recent functional studies have questioned the firmly held notion that COX-2 derived prostanoids only counteract Ang II effects[6–8].
Expression of COX Enzymes in the Renal Cortex and Medulla
Prostaglandins (PGs) are important mediators of renal vascular tone and sodium balance[9], causing natriuresis due to both its vasodilatory action and inhibition of tubular Na+ reabsorption. The rate-limiting steps in synthesis of PGs are COX-1 and COX-2, which metabolize arachidonic acid to cyclic endoperoxides which are rapidly converted into PGs and thromboxanes by tissue-specific isomerases (prostaglandin synthase)[10].
Agents that specifically block COX-1 or COX-2 have allowed delineation of their quantitative roles in modulating kidney function and blood pressure. COX-1 is termed “constitutive”, because it is normally expressed in a wide range of tissues. In kidneys, COX-1 is present along the distal tubule, and mediates the basal synthesis of prostaglandin E2 (PGE2)[11]. COX-2 is termed “inducible” as stimuli that cause induction of COX-2 are associated with inflammation and cytokine production. However, COX-2 is also constitutively expressed in the kidneys [12] and is differentially regulated by Ang II via distinct actions of AT1 and AT2 receptors[13, 14]. The specific actions of COX-2 metabolites are determined by the restricted expression of COX-2 in cortex and medulla. In the cortex, COX-2 is constitutively expressed in macula densa cells (Fig 1A), which has led to consideration of the roles of COX-2 in regulating tubuloglomerular feedback and renin release via PGE2[15–17]. COX-2 is also expressed in epithelial cells of the thick limb of the ascending loop of Henle [16, 18]. In the medulla and papilla, COX-2 is expressed in interstitial cells [19] (Fig 1B, 1C).
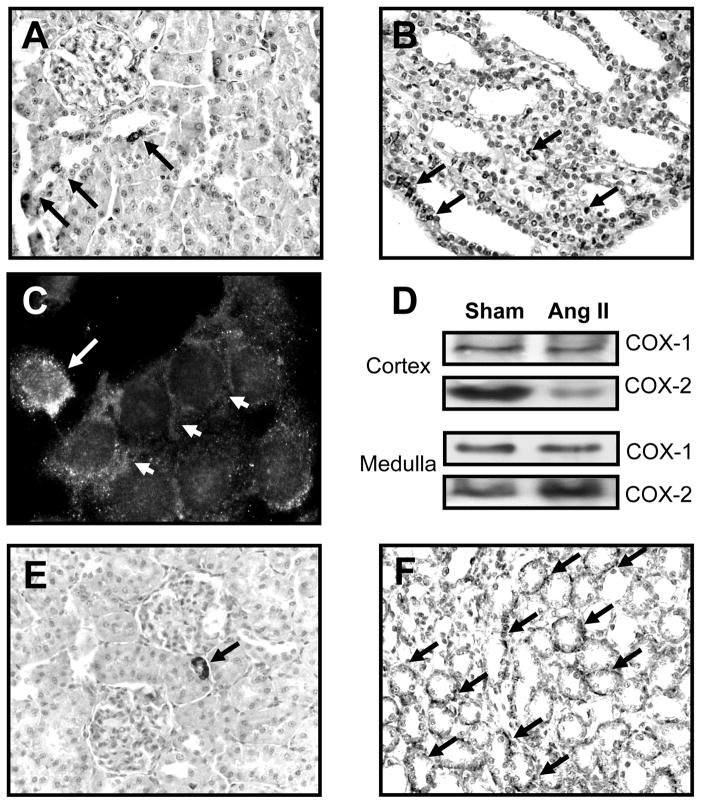
Figure 1.
COX-2 and EP1 receptor in the kidney. A. COX-2 in the macula densa and thick ascending limbs in the cortex (arrows). B. In the inner medulla COX-2 is detected in intercalated and interstitial cells (arrows). C. In primary cultures of inner medullary cells, COX-2 positive cells (white arrow) are closely associated with collecting duct cells (arrowheads). D. In Ang II infused rats (3 days), COX-2 in augmented in the medulla and decreased in cortex with no changes in COX-1. E, F. Immunodetection of EP1 receptor in cortex and medulla (arrows). (A. Gonzalez, unpublished)
COX-2 is differentially regulated in cortex and medulla. Sodium restriction augments cortical COX-2 expression and decreases medullary expression while increased dietary salt leads to increased COX-2 in the inner medulla (IM), but decreased cortical COX-2 expression. This divergent expression reflects distinct functions with medullary production contributing to salt and water reabsorption and medullary blood flow regulation while cortical COX-2 derived PGs modulate renin release and glomerular hemodynamics[14, 16]. Activation of the PGE2 receptor, EP1, antagonizes the Ang II effects on αENaC expression in the IM and aldosterone mediated upregulation of ENaC subunits[20]. EP1 receptors are present in afferent and efferent arterioles (Fig 1E), but are more abundantly expressed in the IM in normal conditions (Fig 1F). Inhibition of COX-2 activity in the IM leads to salt-sensitive hypertension[21], indicating that COX-2 in the renal medulla exerts a key protective anti-hypertensive influence. COX-2 is increased in the IM of Ang II-infused rats but reduced in the cortex without changes in COX-1 (Fig 1D)[20]. There is also augmented mPGES-1 expression resulting in a PGE2-dependent reduction in sodium reabsorption[22]. In aldosterone-infused rats, the initial decrease in sodium excretion wanes in parallel with augmented PGE2 excretion, but persists in mPGES-1 deficient mice[20].
Regulation of Renin Release and Glomerular Vasculature by COX-2 Dependent Prostanoids
COX-2 is a major regulator of renin release via the macula densa mechanism, in particular during sodium restriction[4, 14, 16, 23]. COX-2 is expressed in macula densa cells [4, 24] linking macula densa sensing of sodium delivery to renin synthesis via activation of juxtaglomerular (JG) cell EP4 receptors to increase intracellular cAMP levels[4]. COX-2 deficient mice have reduced plasma renin levels, renin expression and acute stimuli that normally increase renin secretion[25]. Decreases in luminal sodium concentration stimulate PGE2 release to increase renin secretion thus leading to increased Ang II formation[17, 26]. The effects on the microcirculation are less clear because PGE2 may exert either afferent arteriolar vasodilation [27] or vasoconstriction[28], while COX-2 derived thromboxane exerts vasoconstriction[29]. COX-2 contributes to adaptive responses in GFR[30]. In a setting of elevated intrarenal Ang II levels, COX-1 products are predominantly vasoconstrictor while COX-2 products elicit vasodilation[31]. Ang II mediated augmentation of vascular tone is counterbalanced by COX-2 products, presumably PGE2. In addition, COX-2 mediates JG cell renin exocytosis in response to luminal hypotonicity[32].
In contrast to the responses during sodium restriction, the increased plasma renin activity (PRA) subsequent to ACE inhibition is maintained, though significantly attenuated, during COX-2 inhibition[33]. Likewise, sympathetic nervous system activation of β1 adrenergic receptors or blockade with isoproterenol has been disassociated from COX-2 effects on renin[23, 34]. However, dopamine release by the sympathetic nervous system inhibits renin release via attenuation of COX-2 expression[35]. Overall, the extent to which cortical COX-2 promotes or attenuates Ang II mediated vasoconstriction is difficult to ascertain because the consequence of renin release is undefined relative to its potential to reduce renovascular resistance via PGE2 production.
Renal Responses to COX-2 Inhibition in Rats with Varying States of Intrarenal Ang II Activity
While experimental and clinical studies support the renoprotective role of COX-2 metabolites[36], it has been difficult to delineate the actions of COX-2 prostanoids independent of the prevailing local RAS activity. Upregulated renocortical COX-2 expression is associated with increased vasodilatory influence during the augmented Ang II levels induced by sodium restriction[37, 38]. The minimal COX-2 mediated effect in the unperturbed kidney led to speculations that prostaglandin effects depend on down-modulation of the augmented Ang II mediated vasoconstriction observed during sodium restriction. However, COX-2 expression is augmented by sodium restriction in both Sprague Dawley and hypertensive Ren-2 transgenic rats but shows no difference in hemodynamic influence despite greater Ang II in the latter[39].
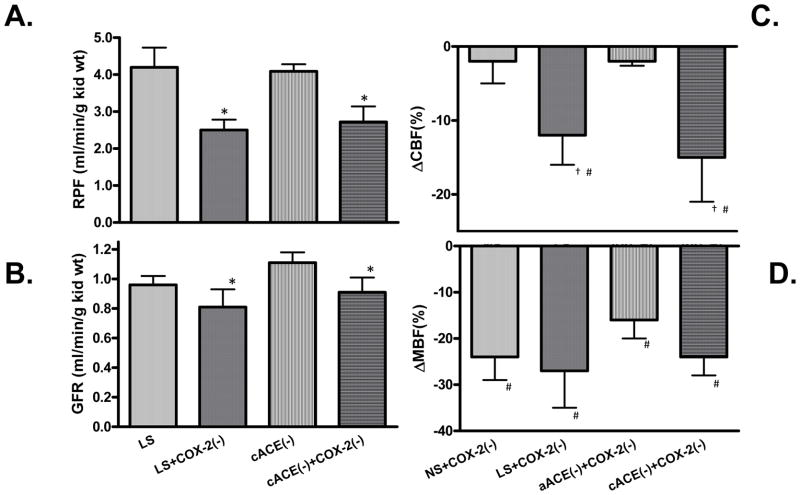
Two models of augmented COX-2 expression were recently studies to provide insight into the dependence of the effects of COX-2 inhibition on the prevailing intrarenal Ang II activity. The sodium restriction model has elevated COX-2 expression as well as augmented intrarenal Ang II activity while the chronic ACE inhibition model has similar augmentation of cortical COX-2 activity but reduced intrarenal Ang II levels[40]. As shown in Figure 2, acute COX-2 inhibition with nimesulide to anesthetized rats subjected to chronic ACE inhibition and sodium restriction elicited similar decreases in glomerular filtration rate (GFR) and renal plasma flow (RPF) thus offsetting any vasoconstrictive potential from renin release[40]. These results are consistent with those obtained during chronic COX-2 inhibition (Roig et al, Ref #38) and indicate that augmented cortical COX-2 promotes a vasodilatory influence on the renal microvasculature independent of the prevailing intrarenal RAS activity[41]. The responses in rats chronically treated with an ACE inhibitor were similar to those in sodium restricted rats and greater than those observed in rats on a normal salt diet.
Figure 2.
Effects of acute COX-2 inhibition. COX-2 (−); (nimesulide 3 mg/kg body wt) on (A) renal plasma flow (RPF) and (B) Glomerular Filtration Rate (GFR) in rats maintained on Low Salt (LS) for 5 days or captopril for 7 days (cACE). *P<0.05 (C) Cortical blood flow (CBF) and (D) medullary blood flow (MBF) changes with COX-2 inhibition (COX-2(−); nimesulide 3 mg/kg body wt) in rats maintained on normal salt (NS) or low salt (LS) for 5 days and after acute captopril (5 mg/kg body wt) alone (aACE) or in combination with chronic captopril (100 mg/kg body wt) for 7 days (cACE). #P<0.05 vs vehicle #P<0.05 LS+COX-2(−) vs. NS+COX-2(−) or aACE+COX-2(−) vs. cACE+COX-2(−) (Data taken from Green et al, 2010, Ref #40).
The responses in medullary blood flow were very similar in rats maintained on normal salt diet, low salt diet or rats treated with ACE inhibitors suggesting a tonic influence of COX-2 to protect medullary blood flow against excessive vasoconstriction under all conditions. COX-2 inhibition significantly reduced sodium excretion only in salt restricted rats indicating a dependence on prevailing Ang II activity. While the effects of COX-2 inhibition on sodium excretion are contingent on RAS activity, they are apparently independent of the medullary blood flow responses.
COX-2 in Ang II-Dependent Hypertension
Another conundrum that has persisted is the functional contribution of COX-2 to the hypertension that results from elevated RAS activity. COX-2 derived prostanoids are thought to exert a vasodilatory influence on the vasculature to help counteract the effects of Ang II-induced vasoconstriction. However, studies in hypertensive rats reported that COX-2 inhibition either decreases [42] or does not affect arterial pressure[43, 44]. Arterial pressure in the aortic coarctation model decreased with COX-2 inhibition in association with decreased PRA suggesting that inhibition of renin release may be responsible[45]. COX-2 inhibition did not augment the reduction in arterial pressure elicited by losartan[46]. Further, the blood pressure lowering effects of furosemide were neutralized by COX-2 inhibition[47]. These results and emerging evidence suggests a COX-2 prohypertensive effect that has been underappreciated. Nonselective COX inhibition lowered blood pressure without affecting renin release in Cx40−/− mice whose genetic alteration manifests a renin-dependent hypertension[48]. In chronic Ang II infused mice where renin levels are suppressed, COX-2 inhibition also decreased blood pressure[31]. These studies suggest that COX-2 contributes to the hypertension independently of the renin or Ang II levels.
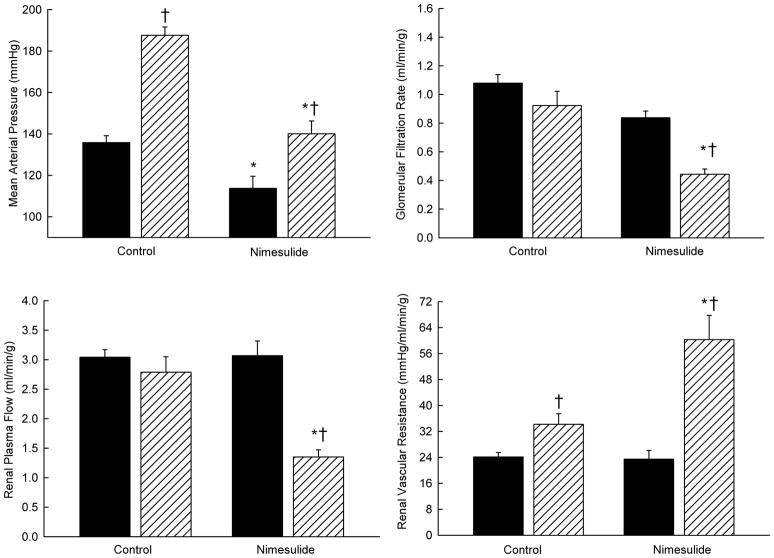
The effects of selective COX-2 blockade with nimesulide on blood pressure and renal hemodynamics were evaluated in transgenic rats [TGR(Cyp1a1-Ren2)] with inducible Ang II-dependent malignant hypertension[6]. In this model, the plasma renin levels are maintained independent of macula densa renin release. Similar to the responses in salt restricted rats, COX-2 inhibition led to pronounced decreases in both GFR and RPF indicating that the enhanced intrarenal COX-2 derived vasodilator metabolites buffer the vasoconstrictor action of Ang II in the kidney and play an important role in maintaining RPF and GFR (Fig 3). However, COX-2 inhibition decreased arterial pressure, indicating that the systemic COX-2 derived metabolites are vasoconstrictive and contribute to the development of hypertension. Similarly, COX-2 inhibition during conditions of neuronal nitric oxide synthase (nNOS) blockade decreased arterial pressure in Cyp1a1-Ren2 rats but increased renal vascular resistance in hypertensive Cyp1a1-Ren2 transgenic rats indicating independence from nitric oxide actions[5]. Thus, elevated arterial blood pressure is due, at least in part, to increased systemic vasoconstrictor prostanoids generated by the COX-2 enzymatic pathway.
Figure 3.
Effects of acute Nimesulide (3 mg/kg, iv) on mean arterial pressure, glomerular filtration rate, renal plasma flow and renal vascular resistance in noninduced Cyp1a1-Ren2 transgenic rats (filled bars; n=7) and Cyp1a1-Ren2 transgenic rats induced with 0.3% indole-3-carbinol for 6–9 days (hatched bars; n=7). * P<0.05 vs. control. † P<0.05 vs. noninduced rats. (Data taken from Opay et al. 2006, Ref #6).
Recent studies reveal oxidative stress as a participant in COX-2-mediated elevation of blood pressure. Local activation of RAS activity, via increased reactive oxygen species (ROS) generation, mediates glomerular COX-2 upregulation during chronic Ang II infusions [49] and in hypertensive salt sensitive rats[50]. Further, ROS might alter the relationship between COX-2 and Ang II from antagonistic to cooperative with regard to the blood pressure effects. The effect of COX-2 inhibition to lower blood pressure in transgenic rats has been attributed to oxidative stress [51] though this dependence has not been consistently demonstrated[52]. These recent experiments suggest the potential for ROS to tip the consequence of COX-2 activity away from attenuating Ang II renovascular resistance regulation and towards augmenting renal inflammation and generation of hypertension.
Diabetic Nephropathy: More than COX-2 Dysregulation of Hemodynamics
COX inhibitors attenuate the development of experimental diabetic nephropathy in rats[53]. Further, COX-2 mediated effects have been implicated in diabetic nephropathy in animal models of diabetes and human diabetic patients. Selective COX-2 inhibition reduces proteinuria in renal patients without reducing systolic blood pressure[54]. Acute and chronic COX-2 inhibition prevents hyperfiltration in diabetic rats and markedly reduces albuminuria independently of changes in plasma glucose levels[55].
Interestingly, changes in RPF have not consistently paralleled the GFR responses to COX-2 inhibition [56, 57] raising the possibility of non-vascular dependent influences of COX-2 inhibitors on GFR. Glomerular effects of COX-2 are supported by the fact that macula densa COX-2 expression peaks at a time earlier than glomerular COX-2[58]. Hyperglycemia augments COX-2 mediated-hyperfiltration but lessens the ability of COX-2 to increase GFR in hyperfiltering patients [56] and might signify increased compromise of the glomerular barrier (in addition to vasodilation) mediated by COX-2. Increased expression of COX-2 in non-macula densa cells and podocytes has been previously demonstrated in streptozotocin diabetic model[57]. Whereas basal COX-2 may be important for podocyte survival, overexpression of podocyte COX-2 increases susceptibility to podocyte injury, possibly via activation of thromboxane receptors[7]. In addition, the hyperfiltration mediated by COX-2 may also contribute to damage of podocytes in that sheer stress increases podocyte COX-2 expression and PGE2 production[59]. Activation of the EP4 receptor has been linked to increased PGE2 production, thus potentially mediating a positive feedback in the course of kidney injury similar to that observed in diabetes. Early after 5/6 nephrectomy, the urinary albumin-creatinine excretion ratio of mice with kidney selective podocyte overexpression of the EP4 receptor was significantly higher than in non-transgenic mice[8].
There is also evidence that COX-2 is a primary mediator of renal injury attributed to RAS activity during hyperglycemia associated with diabetes. In female diabetic patients, Ang II-mediated reductions in GFR were abolished by COX-2 inhibition [60]. Glomerular COX-2 expression is correlated with glomerular sclerosis to a higher degree than renin-positivity[58]. Studies also show association between RAS activity and COX-2 expression. Pro-renin receptor (PRR) overexpression augments cortical COX-2 expression[61]. The relevance of diabetes on RAS promotion of COX-2 activity is demonstrated by glucose increases in mesangial cell COX-2 via the AT1 receptor [62] as well as renin and PRR[63]. Though reactive oxygen species have been implicated in mediating glucose and Ang II augmentation of COX-2 expression in glomerular endothelial cells[64], the mechanism remains to be fully elucidated.
Conclusion
Interactions between the RAS and COX-2 metabolites converge on the kidney through regulation of renin release, renal hemodynamics and excretory function. Recent experiments have revealed a role for COX-2 in the promotion and restriction of the RAS. On one hand, COX-2 metabolites mediate renin release via the EP4 receptor. On the other hand, COX-2 metabolites limit the resulting Ang II mediated increases in renovascular resistance by eliciting direct vasodilation and opposing COX-1/Ang II vascular effects. These studies reveal the potential conversion of COX-2 mediated beneficial actions to a dysfunctional regulator of the RAS involving mechanisms that potentiate Ang II hypertension and kidney injury. Emerging studies implicate oxidative stress in the transition to COX-2 pro-hypertensive effects. Hyperglycemia also appears to transform COX-2 maintenance of hemodynamics to pathophysiologic degradation of the glomerular integrity via a PRR-COX-2-EP4 receptor positive feedback loop. An expanded assessment of prostaglandin synthase and specific receptors may reveal strategies to exploit renal COX-2 activity to ameliorate Ang II-mediated hypertensive and diabetic kidney diseases.
Key Bullet Points.
Low sodium diet and ACE inhibitors upregulate kidney cortical COX-2 activity which elicits direct renoprotective or vasodilator influences independent of RAS activity but altered sodium excretion dependent on prevailing RAS activity.
In angiotensin II dependent hypertension, COX-2 metabolites (PGE2, PGI2) exert vasodilator renoprotective functions but other metabolites (Tx) exert a prohypertensive influence on systemic vascular resistance.
Renal medullary COX-2 maintains medullary perfusion during varying levels of cortical COX-2 and Ang II activities including normal and low salt diet, ACE inhibition, and hypertension.
COX-2 inhibition confers renal protection in diabetic rats unmasking an underappreciated deleterious action of COX-2 in diabetes.
Emerging studies implicate oxidative stress in the transition from beneficial to deleterious glomerular actions of COX-2 in diabetes which leads to podocyte injury via a prorenin receptor-COX-2-EP4 receptor pathway.
Acknowledgments
The authors’ received support from National Heart, Lung, and Blood Institute (HL-18426 and HL-26371) and by the Institutional Award Program of the National Center for Research Resources, Centers of Biomedical Research Excellence (P20 RR-017659). We thank Debbie Olavarrieta for preparation of the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–62. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf G. The renin-angiotensin system and progression of renal diseases. Hamburg: Karger; 2002. pp. 1–268. [Google Scholar]
- 3.Mitchell KD, Botros FT, Navar LG. Intrarenal renin-angiotensin system and counteracting protective mechanisms in angiotensin II-dependent hypertension. Acta Physiologica Hungarica. 2007;94:31–48. doi: 10.1556/APhysiol.94.2007.1-2.5. [DOI] [PubMed] [Google Scholar]
- 4.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 5.Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol. 2008;294:F205–F211. doi: 10.1152/ajprenal.00150.2007. [DOI] [PubMed] [Google Scholar]
- 6.Opay AL, Mouton CR, Mullins J, Mitchell KD. Cyclooxygenase-2 inhibition normalized arterial blood pressure in CYP1A1-Ren2 transgenic rats with inducible Ang II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2006 Apr 18;291(3):F612–F618. doi: 10.1152/ajprenal.00032.2006. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC. Distinct roles for basal and induced COX-2 in podocyte injury. J Am Soc Nephrol. 2009;20:1953–62. doi: 10.1681/ASN.2009010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Stitt-Cavanagh EM, Faour WH, Takami K, Carter A, Vanderhyden B, Guan Y, et al. A maladaptive role for EP4 receptors in podocytes. J Am Soc Nephrol. 2010;21:1678–90. doi: 10.1681/ASN.2009121234. This study demonstrates the role of EP4 in mediating podocyte injury by using podocyte-specific overexpression or deletion of the EP4 receptor. After nephrectomy, albumin-creatinine ratio was high in mice overxpressing EP4 in podocytes and lower than controls in EP4 knockouts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–9. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francois H, Facemire C, Kumar A, Audoly L, Koller B, Coffman T. Role of microsomal prostaglandin E synthase 1 in the kidney. J Am Soc Nephrol. 2007;18:1466–75. doi: 10.1681/ASN.2006040343. [DOI] [PubMed] [Google Scholar]
- 11.Khan KN, Paulson SK, Verburg KM, Lefkowith JB, Maziasz TJ. Pharmacology of cyclooxygenase-2 inhibition in the kidney. Kidney Int. 2002;61:1210–9. doi: 10.1046/j.1523-1755.2002.00263.x. [DOI] [PubMed] [Google Scholar]
- 12.Catella-Lawson F, McAdam B, Morrison BW, Kapoor S, Kujubu D, Antes L, et al. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. Journal of Pharmacology and Experimental Therapeutics. 1999;289:735–41. [PubMed] [Google Scholar]
- 13.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103:16045–50. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RC. An update on cyclooxygenase-2 expression and metabolites in the kidney. Curr Opin Nephrol Hypertens. 2008;17:64–9. doi: 10.1097/MNH.0b013e3282f1bb7d. [DOI] [PubMed] [Google Scholar]
- 15.Harris RC., Jr Cyclooxygenase-2 inhibition and renal physiology. Am J Cardiol. 2002;89:10D–7D. doi: 10.1016/s0002-9149(02)02232-4. [DOI] [PubMed] [Google Scholar]
- 16.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann J, et al. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 17.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, et al. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vio CP, Cespedes C, Gallardo P, Masferrer JL. Renal identification of cyclooxygenase-2 in a subset of thick ascending limb cells. Hypertension. 1997;30 (part 2):687–92. doi: 10.1161/01.hyp.30.3.687. [DOI] [PubMed] [Google Scholar]
- 19.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–77. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez AA, Cespedes C, Villanueva S, Michea L, Vio CP. E Prostanoid-1 receptor regulates renal medullary alphaENaC in rats infused with angiotensin II. Biochem Biophys Res Commun. 2009;389:372–7. doi: 10.1016/j.bbrc.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 21.Zewde T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension. 2004;44:424–8. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- **22.Jia Z, Aoyagi T, Kohan DE, Yang T. mPGES-1 deletion impairs aldosterone escape and enhances sodium appetite. Am J Physiol Renal Physiol. 2010;299:F155–F166. doi: 10.1152/ajprenal.90702.2008. Using mPGES-1 knockout mice, the authors showed that mPGES-1 antagonizes the sodium-retaining action of Aldo at the levels of both the central nervous system and the kidney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matzdorf C, Kurtz A, Hocherl K. COX-2 activity determines the level of renin expression but is dispensable for acute upregulation of renin expression in rat kidneys. Am J Physiol Renal Physiol. 2007;292:F1782–F1790. doi: 10.1152/ajprenal.00513.2006. [DOI] [PubMed] [Google Scholar]
- 24.Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol. 2003;285:F558–F564. doi: 10.1152/ajprenal.00433.2002. [DOI] [PubMed] [Google Scholar]
- 25.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–F422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 26.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, et al. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–9. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 27.Ichihara A, Imig JD, Inscho EW, Navar L. Cyclooxygenase-2 participates in tubular flow-dependent afferent arteriolar tone: interaction with neuronal NOS. Am J Physiol-Renal Physiol. 1998;275:F605–F612. doi: 10.1152/ajprenal.1998.275.4.F605. [DOI] [PubMed] [Google Scholar]
- 28.Badzynska B, Sadowski J. Opposed effects of prostaglandin E2 on perfusion of rat renal cortex and medulla: interactions with the renin-angiotensin system. Exp Physiol. 2008;93:1292–302. doi: 10.1113/expphysiol.2008.043604. [DOI] [PubMed] [Google Scholar]
- 29.Araujo M, Welch WJ. Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide. Am J Physiol Renal Physiol. 2009;296:F790–F794. doi: 10.1152/ajprenal.90446.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng A, Wead LM, Blantz RC. Temporal adaptation of tubuloglomerular feedback: effects of COX-2. Kidney Int. 2004;66:2348–53. doi: 10.1111/j.1523-1755.2004.66033.x. [DOI] [PubMed] [Google Scholar]
- **31.Araujo M, Welch WJ. Tubuloglomerular feedback is decreased in COX-1 knockout mice after chronic angiotensin II infusion. Am J Physiol Renal Physiol. 2010;298:F1059–F1063. doi: 10.1152/ajprenal.00547.2009. The authors tested the roles of COX-1 and COX-2 on the effects of chronic Ang II infusion to enhance tubuloglomerular feedback (TGF). Chronic Ang II infusion increased TGF in WT mice, but reduced it in COX-1 KO mice. Pretreatment with COX-2 inhibitor mice restored the Ang II-associated enhancement in TGF. This study demonstrates that COX-1-generated PGs partially mediate Ang II dependent increases in TGF but that COX-2 PGs counteract this effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanner F, Chambrey R, Bourgeois S, Meer E, Mucsi I, Rosivall L, et al. Increased renal renin content in mice lacking the Na+/H+ exchanger NHE2. Am J Physiol Renal Physiol. 2008;294:F937–F944. doi: 10.1152/ajprenal.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kammerl MC, Nusing RM, Seyberth HW, Riegger GA, Kurtz A, Kramer BK. Inhibition of cyclooxygenase-2 attenuates urinary prostanoid excretion without affecting renal renin expression. Pflugers Arch. 2001;442:842–7. doi: 10.1007/s004240100616. [DOI] [PubMed] [Google Scholar]
- 34.Hocherl K, Kammerl M, Kees F, Kramer BK, Grobecker HF, Kurtz A. Role of renal nerves in stimulation of renin, COX-2, and nNOS in rat renal cortex during salt deficiency. Am J Physiol Renal Physiol. 2002;282:F478–F484. doi: 10.1152/ajprenal.00209.2001. [DOI] [PubMed] [Google Scholar]
- 35.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–70. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arendshorst WJ, Navar LG. Renal circulation and glomerular hemodynamics. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 54–95. [Google Scholar]
- 37.Rodriguez F, Llinas MT, Gonzalez JD, Rivera J, Salazar FJ. Renal changes induced by cyclooxygenase-2 inhibitor during normal and low sodium intake. Hypertension. 2000;36:276–81. doi: 10.1161/01.hyp.36.2.276. [DOI] [PubMed] [Google Scholar]
- 38.Roig F, Llinas MT, Lopez R, Salazar FJ. Role of cyclooxygenase-2 in the prolonged regulation of renal function. Hypertension. 2002;40:721–8. doi: 10.1161/01.hyp.0000036451.76323.29. [DOI] [PubMed] [Google Scholar]
- 39.Vaneckova I, Cahova M, Kramer HJ, Huskova Z, Skaroupkova P, Komers R, et al. Acute effects of cyclooxygenase-2 inhibition on renal function in heterozygous ren-2-transgenic rats on normal or low sodium intake. Kidney Blood Press Res. 2004;27:203–10. doi: 10.1159/000079865. [DOI] [PubMed] [Google Scholar]
- 40.Green T, Rodriguez J, Navar LG. Augmented cyclooxygenase-2 effects on renal function during varying states of angiotensin II. Am J Physiol Renal Physiol. 2010;299:F954–F962. doi: 10.1152/ajprenal.00609.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salazar FJ, Llinas MT. Renal hemodynamic effects elicited by acute cyclooxygenase-2 inhibition are not related to angiotensin II levels. Am J Physiol Renal Physiol. 2010;299:F952–F953. doi: 10.1152/ajprenal.00489.2010. [DOI] [PubMed] [Google Scholar]
- 42.Okumura T, Hayashi I, Ikezawa T, Yamanaka M, Takata T, Fujita Y, et al. Cyclooxygenase-2 inhibitors attenuate increased blood pressure in renovascular hypertensive models, but not in deoxycorticosterone-salt hypertension. Hypertens Res. 2002;25:927–38. doi: 10.1291/hypres.25.927. [DOI] [PubMed] [Google Scholar]
- 43.Richter CM, Godes M, Wagner C, Maser-Gluth C, Herzfeld S, Dorn M, et al. Chronic cyclooxygenase-2 inhibition does not alter blood pressure and kidney function in renovascular hypertensive rats. J Hypertens. 2004;22:191–8. doi: 10.1097/00004872-200401000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Welch WJ, Patel K, Modlinger P, Mendonca M, Kawada N, Dennehy K, et al. Roles of vasoconstrictor prostaglandins, COX-1 and -2, and AT1, AT2, and TP receptors in a rat model of early 2K,1C hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H2644–H2649. doi: 10.1152/ajpheart.00748.2007. [DOI] [PubMed] [Google Scholar]
- 45.Wang J-L, Cheng H-F, Harris RC. Cyclooxygenase-2 inhibition decreases renin content and lowers blood pressure in a model of renovascular hypertension. Hypertension. 1999;34:96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 46.Boshra V, El Wakeel GA, Nader MA. Effect of celecoxib on the antihypertensive effect of losartan in a rat model of renovascular hypertension. Can J Physiol Pharmacol. 2011;89:103–7. doi: 10.1139/y10-112. [DOI] [PubMed] [Google Scholar]
- 47.Kose F, Besen A, Paydas S, Balal M, Gonlusen G, Inal T, et al. Effects of selective Cox-2 inhibitor, rofecoxib, alone or combination with furosemide on renal functions and renal Cox-2 expression in rats. Clin Exp Nephrol. 2010;14:22–7. doi: 10.1007/s10157-009-0214-2. [DOI] [PubMed] [Google Scholar]
- 48.Krattinger N, Alonso F, Capponi A, Mazzolai L, Nicod P, Meda P, et al. Increased expression of renal cyclooxygenase-2 and neuronal nitric oxide synthase in hypertensive Cx40-deficient mice. J Vasc Res. 2009;46:188–98. doi: 10.1159/000156704. [DOI] [PubMed] [Google Scholar]
- 49.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68:2143–53. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 50.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol. 2008;294:F385–F392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 51.Howard LL, Patterson ME, Mullins JJ, Mitchell KD. Salt-sensitive hypertension develops after transient induction of ANG II-dependent hypertension in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol. 2005;288:F810–F815. doi: 10.1152/ajprenal.00148.2004. [DOI] [PubMed] [Google Scholar]
- 52.Kopkan L, Huskova Z, Vanourkova Z, Thumova M, Skaroupkova P, Maly J, et al. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol. 2009;51:175–81. doi: 10.1016/j.vph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Kumar BL, Addepalli V. Minocycline with aspirin: an approach to attenuate diabetic nephropathy in rats. Ren Fail. 2011;33:72–8. doi: 10.3109/0886022X.2010.528117. [DOI] [PubMed] [Google Scholar]
- 54.Vogt L, de Zeeuw D, Woittiez AJ, Navis G. Selective cyclooxygenase-2 (COX-2) inhibition reduces proteinuria in renal patients. Nephrol Dial Transplant. 2009;24:1182–9. doi: 10.1093/ndt/gfn644. [DOI] [PubMed] [Google Scholar]
- **55.Quilley J, Santos M, Pedraza P. Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. Am J Physiol Heart Circ Physiol. 2011;300:H2316–H2322. doi: 10.1152/ajpheart.01259.2010. The authors report beneficial and protective effects of COX-2 inhibition in diabetic rats that were independent of changes in plasma glucose levels. Treatment resulted in reduced urinary excretion of PGE2, 6-ketoPGF1α, and thromboxane B2, all of which were increased in the diabetic rats. The authors suggest that inhibition of COX-2 in diabetic rats confers renal protection and that the induction of COX-2 precedes the increases in cytokines, TNF-α, and TGF-β. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, et al. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes. 2008;57:688–95. doi: 10.2337/db07-1230. [DOI] [PubMed] [Google Scholar]
- 57.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, et al. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest. 2001;107:889–98. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yabuki A, Taniguchi K, Yamato O. Immunohistochemical examination of cyclooxygenase-2 and renin in a KK-A(y) mouse model of diabetic nephropathy. Exp Anim. 2010;59:479–86. doi: 10.1538/expanim.59.479. [DOI] [PubMed] [Google Scholar]
- **59.Srivastava T, McCarthy ET, Sharma R, Cudmore PA, Sharma M, Johnson ML, et al. Prostaglandin E(2) is crucial in the response of podocytes to fluid flow shear stress. J Cell Commun Signal. 2010;4:79–90. doi: 10.1007/s12079-010-0088-9. The authors demonstrate the effects of fluid flow shear stress in cultured podocytes on COX-2 mRNA levels and PGE2 production. Intracellular PGE2 synthesis and expression of COX-2 was increased 2 hours following fluid flow shear stress without changes in COX-1 mRNA. Podocytes are sensitive and responsive to fluid flow shear stress and PGE2 plays an important role in mechanosensing podocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cherney DZ, Scholey JW, Nasrallah R, Dekker MG, Slorach C, Bradley TJ, et al. Renal hemodynamic effect of cyclooxygenase 2 inhibition in young men and women with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2008;294:F1336–F1341. doi: 10.1152/ajprenal.00574.2007. [DOI] [PubMed] [Google Scholar]
- 61.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, et al. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641–6. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 62.Naito M, Shenoy A, Aoyama I, Koopmeiners JS, Komers R, Schnaper HW, et al. High ambient glucose augments angiotensin II-induced proinflammatory gene mRNA expression in human mesangial cells: effects of valsartan and simvastatin. Am J Nephrol. 2009;30:99–111. doi: 10.1159/000203619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150:5557–65. doi: 10.1210/en.2009-0442. This study clarifies the mechanism by which glucose augments the prorenin receptor mediated upregulation of COX-2 via IL-1beta and demonstrates a link between prorenin receptor activation due to glucose treatment and the increased levels of COX-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010;298:F125–F132. doi: 10.1152/ajprenal.00248.2009. This study demonstrates that augmented COX-2 in response to high glucose or increased Ang II is dependent on ROS generation and increased PGI2 synthesis. It supports the concept that increased oxidative stress is a factor in transitioning COX-2 effects from beneficial to deleterious. [DOI] [PMC free article] [PubMed] [Google Scholar]