Abstract
Objectives
Olfactory dysfunction is described in several neuropsychiatric disorders but there is little research on olfactory processing in bipolar disorder.
Methods
We assessed odor detection threshold (sensitivity) and smell identification test scores along with symptoms, cognition, and social function in 20 DSM-IV bipolar disorder patients and 44 control subjects.
Results
The patient and control groups had similar demographic measures, intelligence, and mean olfaction scores, but significantly differed in social domains, including adjustment, function, and anxiety. Odor detection sensitivity showed significantly opposite correlations for the depressive and manic mood domains in bipolar disorder (r to z = 2.83, p = 0.005). Depressive symptoms were related to increased sensitivity (the ability to detect odors at a lower concentration) and mania symptoms were related to decreased sensitivity for odor detection. Increased sensitivity for odor detection also predicted significantly better employment (r = −0.642, p = 0.024), whereas less sensitivity was associated with social avoidance (r = 0.702, p = 0.024) and social fear (r = 0.610, p = 0.046).
Conclusions
Diminished odor detection sensitivity predicted mania and social avoidance, whereas more sensitive odor detection predicted more depressive symptoms but better employment functioning in bipolar disorder patients. Odor acuity may be an illness state marker of mood syndromes in bipolar disorder. Alternatively, differences in odor acuity may identify heterogeneous subgroups within the bipolar spectrum. Longitudinal assessments in a large, sex-stratified sample are needed to understand the implications of odor sensitivity in patients with bipolar disorder.
Keywords: bipolar disorder, olfaction, social function
Bipolar disorder affects an estimated 2.6% of the adult population in the United States (1). It is characterized by impaired executive function and severe emotional dysregulation with episodes of mania and depression (2, 3). Furthermore, it is associated with significant morbidity and mortality (3, 4); half of patients attempt suicide at least once and up to 20% ultimately commit suicide (4–6). Bipolar disorder is also associated with substantial disruptions in occupational and social functioning (3, 7, 8). The psychosocial difficulties lead to impairments in social and vocational functioning, further contributing to symptomatic expression of the illness (9). Notably, only 20% of bipolar disorder patients are married as compared with 60% of the general population, and up to 19–58% do not live independently (9). Subthreshold mood symptoms in technically euthymic patients are common and contribute to increased risk for additional affective episodes and increased rates of comorbidity (3, 10–13).
The etiology of bipolar disorder is unknown, but imaging studies have found functional and anatomic differences in the amygdala, anterior cinguate, striatum, ventromedial prefrontal cortex, and orbitofrontal cortex of patients with bipolar disorder (14–17). Of interest, a number of these regions play important roles in olfactory processing; the amygdala, hippocampus, and orbitofrontal cortex receive projections from the piriform cortex and are key secondary olfactory areas (18, 19). These associations raise the possibility that olfactory ability, which can be easily and quickly assessed during an office visit, may correspond to bipolar disorder. Other features of olfactory processing that heighten this possibility include the reciprocal interconnections between diverse limbic regions and olfactory regions that modulate odor detection and higher olfactory processing (20). Notably, smell is the only sense that directly projects to the prefrontal cortex without thalamic processing (18, 19).
There are a number of olfactory function studies in mood disorders, but relatively few that focus on bipolar disorder. Several studies that included patients with bipolar disorder did not clearly differentiate between bipolar disorder and other affective psychoses (21–23). Of the studies on bipolar disorder with rigorous diagnostic procedures, Swiecicki et al. (24) reported intact sensitivity for odor detection and no smell identification deficits in both bipolar and unipolar depressed patients, although Kruger et al. (25) reported more sensitive acuity in a subset of bipolar disorder patients with event-related episode. Studies of olfaction in unipolar depression have provided conflicting results. There are reports of normal (26, 27) and of diminished ability to detect and identify odors (28, 29), as well as reports of enhanced odor acuity; decreased smell identification for the right nostril is also reported for seasonal affective disorder patients (30, 31).
We undertook a study to comprehensively examine both right and left nostril sensitivity for odor detection and smell identification performance in stable DSM-IV bipolar disorder patients. We also examined the olfactory data with respect to psychiatric symptoms, social anxiety, functionality, and cognitive performance.
Methods
Subjects
The study sample was comprised of 20 patients with bipolar disorder and 44 healthy controls. The patients were recruited from research and clinical settings at the New York State Psychiatric Institute, and healthy controls were recruited from medical center postings and internet advertisements. The study was Institutional Review Board approved in accordance with the Helsinki Declaration of 1975 and Health Insurance Portability and Accountability Act (HIPAA) compliant; all participants signed informed consent forms.
Clinical measures
Subjects were diagnosed based on DSM-IV Axis-I criteria using data from clinical histories and structured interviews completed by experienced clinical raters using the Diagnostic Interview for Genetic Studies (DIGS) (32). Psychiatric symptoms were assessed using clinician ratings from the Positive and Negative Syndrome Scale (PANSS) (33). The PANSS contains three scales that measure positive, negative, and general psychopathology. We also computed the five factors derived from the White et al. (34) factor analysis assessing positive symptoms, negative symptoms, dysphoric mood, activation, and autistic preoccupation. The 11-item Young Mania Rating Scale (YMRS) (35), a clinician-administered mania rating scale with acceptable reliability, validity, and sensitivity, was used to assess manic symptoms.
Social function and social anxiety measures
The Social Functioning Scale (SFS) (36) assessed functioning in the areas of work, school, daily activities, and family and peer relationships. The Liebowitz Social Anxiety Scale (LSAS) (37) assessed self-report social anxiety, social fear, performance anxiety, and performance fear.
Olfactory processing
Odor sensitivity was determined as the detection threshold based on the Smell Threshold Test (STT) (Sensonics, Inc., Haddon Heights, NJ, USA). For the STT, the subject was presented with two squeeze bottles; one containing phenyl ethyl alcohol (PEA) and one containing mineral oil, which was used as a blank. The subject was then asked to identify which concentration of PEA smelled stronger. PEA concentrations ranged from −10 log vol/vol to −2 log vol/vol and increased in half log steps. Testing was based on a single staircase model. STT scores were then converted to absolute values so that higher STT scores represent that lower concentrations are necessary for the subject to detect PEA, corresponding to increased odor acuity or sensitivity. Odor identification was assessed using the University of Pennsylvania Smell Identification Test (UPSIT) (38). The UPSIT contains 40 scratch-and-sniff odor strips and uses a forced multiple choice format.
Cognition
The Wechsler Adult Intelligence Scale–third edition (WAIS-III) (39) was used to measure full scale, verbal, and performance IQs. The revised Wechsler Memory Scale–revised (WMS-R) (40) was used to assess verbal, visual, and general memory, as well as attention and delayed recall memory. Visual attention, task switching, and processing speed were assessed with the Trail Making Tests A and B (41).
Statistical analyses
SIR Database Management Software (SIR 2002, SIR Pty Ltd, Terrey Hills, NSW, Australia) was used for data management and entry, and IBM/SPSS (PASW Statistics 17) was used for statistical analyses. Descriptive statistics (means, standard deviations, distributions of measures) were examined to identify salient characteristics for further analysis. The UPSIT and STT scores were normally distributed within each of the study groups. Demographics, olfactory, social adjustment, function, anxiety, and cognitive measures were examined using either a two-by-two [diagnosis (healthy controls and bipolar disorder patients) by gender (male and female)] analysis of variance (ANOVA), or a two-by-two multivariate analysis of variance (MANOVA) for multiple measure domains. To examine the associations between olfactory measures and measures from each domain, we calculated Pearson correlation coefficients. To compare the correlations we then performed Fisher’s r to z transformations using free online software [Calculation for the test of the difference between two independent correlation coefficients (42)]. Due to the unique nature of this data we did not apply Bonferroni correction to the correlation coefficients, hoping to generate hypotheses for future research.
Results
The 20 patients diagnosed with bipolar disorder (5 males and 15 females) ranged in age from 20 to 53 years [34.5 ± 8.9 (mean ± standard deviation)], and had a mean illness onset age of 21.2 ± 8.0 years. Among the bipolar disorder patients: 12 cases were in a depressed episode, 5 were in a manic episode, 2 were in a hypomanic episode, and 1 was in a mixed state. At the time of testing, all participants were clinically stable and the mean YMRS score was 4.3 ± 3.1 ranging from 0 to 9 (clinically significant YMRS mania = 12). The 44 comparison subjects (18 males and 26 females) ranged in age from 18 to 61 years (mean = 32.6 ± 11.8). The patient and control groups did not significantly differ in age or in mean olfactory measures, right or left nostril mean odor sensitivity, or smell identification test scores. There were also no significant effects for sex, nor any sex-by-group interactions for these measures (Table 1). There was no difference between the groups in level of education (χ2 = 6.66, df = 4, p = 0.155), or full scale IQ (Table 2).
Table 1.
Demographic data and measures of olfaction in healthy controls and bipolar disorder patients by gendera
| Healthy controls Mean (SD) |
Bipolar disorder Mean (SD) |
Statistics (Diagnosis) | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | F | p-value | |
| Demographics | n = 18 | n = 26 | n = 5 | n = 15 | ||
| Age | 29.5 (8.3) | 34.7 (13.4) | 31.1 (5.7) | 35.6 (9.7) | 0.15 | 0.705 |
| Education (category) | 3.6 (0.7) | 4.0 (0.8) | 3.5 (1.3) | 3.4 (1.1) | 1.19 | 0.280 |
| n = 2 | n = 14 | |||||
| Onset of symptoms | NA | NA | 17.0 (5.7) | 21.8 (8.3) | NA | NA |
| Duration | NA | NA | 17.9 (2.3) | 13.9 (10.0) | NA | NA |
| Smell identification | n = 17 | n = 20 | n = 3 | n = 13 | ||
| UPSIT score | 31.5 (3.6) | 33.5 (4.4) | 34.3 (1.2) | 32.5 (3.4) | 0.50 | 0.484 |
| Odor thresholdb | n = 17 | n = 17 | n = 2 | n = 10 | 0.06 | 0.940 |
| Right STT score | −4.62 (1.40) | −4.83 (1.60) | −4.73 (1.40) | −4.33 (0.66) | 0.12 | 0.735 |
| Left STT score | −4.64 (1.60) | −4.71 (1.10) | −4.24 (0.00) | −4.84 (1.90) | 0.05 | 0.826 |
| Mean STT scorec | −4.63 (1.50) | −4.77 (0.98) | −4.49 (0.70) | −4.58 (0.92) | 0.11 | 0.737 |
There were no significant effects for sex or interactions between group and sex. NA = not applicable; UPSIT = University of Pennsylvania Smell Identification Test; STT = Smell Threshold Test.
Test statistic: analysis of variance (ANOVA) or multivariate ANOVA where noted.
Multivariate Wilks’ Lambda (all dfs = 2/41).
ANOVA, df = 1/42.
Table 2.
Neuropsychological assessments in healthy controls and bipolar disorder patients by gendera
| Healthy controls Mean (SD) |
Bipolar disorder Mean (SD) |
Statistics (Diagnosis) | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | F | p-value | |
| WAIS-III IQb | n = 17 | n = 1 | n = 3 | n = 14 | 0.43 | 0.653 |
| Verbal IQ | 110.4 (13.1) | 106.4 (12.9) | 104.5 (22.4) | 103.9 (14.1) | 0.78 | 0.381 |
| Performance IQ | 97.8 (12.6) | 99.9 (14.3) | 94.5 (11.8) | 96.4 (11.8) | 0.62 | 0.436 |
| Full scale IQc | 105.2 (12.3) | 103.4 (12.1) | 99.3 (20.4) | 100.7 (12.5) | 0.96 | 0.332 |
| WAIS-III indicesd | n = 17 | n = 18 | n = 4 | n = 14 | 0.84 | 0.508 |
| Verbal comprehension | 110.3 (14.0) | 110.9 (15.0) | 104.3 (22.2) | 109.6 (15.4) | 0.51 | 0.478 |
| Perceptual organization | 97.1 (15.1) | 98.9 (15.8) | 93.8 (11.9) | 97.9 (12.0) | 0.20 | 0.661 |
| Working memory | 110.7 (16.2) | 101.0 (9.3) | 102.8 (30.0) | 97.9 (11.9) | 1.24 | 0.272 |
| Processing speed | 103.7 (10.7) | 104.0 (12.2) | 94.0 (16.8) | 98.7 (13.9) | 3.12 | 0.084 |
| WMS-R indicese | n = 13 | n = 14 | n = 3 | n = 14 | 0.84 | 0.511 |
| Verbal | 98.8 (14.0) | 92.1 (13.8) | 80.7 (7.6) | 98.9 (20.9) | 0.88 | 0.355 |
| Visual | 97.5 (13.7) | 100.4 (16.9) | 87.0 (16.4) | 96.7 (17.3) | 1.42 | 0.241 |
| Attention | 99.9 (17.6) | 93.9 (16.6) | 108.7 (17.9) | 89.6 (12.9) | 0.14 | 0.712 |
| Delayed recall | 102.1 (18.7) | 97.1 (10.6) | 83.3 (6.8) | 96.4 (21.8) | 2.31 | 0.137 |
| General indexf | 99.0 (14.6) | 101.6 (19.7) | 79.7 (11.0) | 98.1 (20.9) | 2.89 | 0.097 |
| Trail Making Testg | n = 14 | n = 15 | n = 3 | n = 14 | 3.59 | 0.037h |
| Trails A time | 27.6 (12.4) | 30.5 (11.8) | 46.0 (8.2) | 37.1 (17.3) | 6.09 | 0.018h |
| Trails B time | 77.5 (60.8) | 77.4 (26.7) | 79.3 (17.7) | 87.5 (50.4) | 0.12 | 0.731 |
There were no significant effects for sex or interactions between group and sex except where noted in the text. WAIS-III = Wechsler Adult Intelligence Scale–third edition; WMS-R = Wechsler Memory Scale–revised.
Test statistic: multivariate analysis of variance (MANOVA).
Multivariate Wilks’ Lambda (all dfs = 2/44).
ANOVA, df = 1/45.
Multivariate Wilks’ Lambda (all dfs = 4/42).
Multivariate Wilks’ Lambda (all dfs = 4/37).
ANOVA, df = 1/41.
Multivariate Wilks’ Lambda (all dfs = 2/41).
p < 0.05.
The few cognitive deficits for the patient group were slower processing speed (specific to Trails A times), and lower verbal memory index scores for the male cases as evidenced by the significant interaction term. There was a significant sex effect for the WMS-R Attention Index, with females exhibiting lower scores than males (ANOVA: F = 4.51, p = 0.040). There was also a significant diagnosis-by-sex interaction for the WMS-R Verbal Index, with lower scores in the male bipolar disorder patients as compared to the female bipolar disorder patients, and healthy controls (both male and female) (ANOVA: F = 4.24, p = 0.046). None of the cognitive measures were significantly associated with odor identification or the mean odor sensitivity in the bipolar disorder patients.
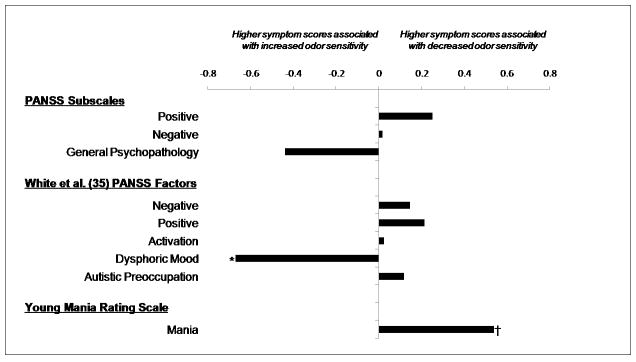
There were significant relationships between symptoms and olfaction (Fig. 1); increased odor detection sensitivity, equivalent to greater acuity, was associated with higher symptom levels on the PANSS dysthymia factor, which is a depression measure (r = −0.67, p = 0.024). The direction of the association of odor sensitivity with depression was significantly different than the relationship of sensitivity with mania (z = 2.83, p = 0.005), although the magnitide of the correlation between less sensitivity and high mania scores reached only a trend level of significance (r = 0.541, p = 0.085). There were no significant associations between smell identification and any of the symptom scales.
Fig. 1.
Correlations between mean odor threshold scores and psychiatric symptoms in the bipolar disorder group. Lower odor threshold scores signify increased odor sensitivity. *p < 0.05; **p < 0.10. Correlation coefficient probabilities were not Bonferroni corrected.
While the overall MANOVA examining the four subscales of the LSAS was not significant, each of the subscales exhibited a significant diagnosis effect (Table 3). LSAS scores were significantly higher in the bipolar disorder group (F = 8.78, p = 0.005), an effect that was predominantly explained by the female patients exhibiting the highest levels for each subscale (all p < 0.011). There was a strong positive correlation between LSAS measures and the sensitivity of the mean and left nostril for odor detection, respectively, for social avoidance (r = 0.702, p = 0.016; r = 0.692, p = 0.018), performance avoidance (r = 0.671, p = 0.024; r = 0.716, p = 0.013), total avoidance (r = 0.697, p = 0.017; r = 0.713, p = 0.014), and social fear (r = 0.610, p = 0.046; r = 0.620, p = 0.042). In all cases, lower levels of fear and avoidance were associated with better odor acuity. In contrast, right nostril threshold did not correlate with these subscales, nor did odor identification.
Table 3.
Social adjustment and function measures in healthy controls and bipolar disorder patients by gendera
| Healthy controls Mean (SD) |
Bipolar disorder Mean (SD) |
Statistics (Diagnosis) | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | F | p-value | |
| Liebowitz Social Anxiety Scaleb | n = 17 | n = 18 | n = 4 | n = 12 | 2.12 | 0.095 |
| Social fear | 3.5 (4.3) | 5.9 (6.0) | 8.8 (7.0) | 13.4 (10.6) | 7.15 | 0.009e |
| Performance fear | 3.9 (4.2) | 6.6 (5.1) | 9.0 (6.6) | 14.0 (10.4) | 8.00 | 0.007e |
| Social avoidance | 3.4 (3.8) | 4.8 (6.2) | 7.5 (7.0) | 12.6 (10.0) | 7.00 | 0.011f |
| Performance avoidance | 3.4 (4.7) | 4.7 (5.2) | 7.3 (5.7) | 12.8 (9.1) | 8.36 | 0.006e |
| LSAS total | 14.2 (13.5) | 21.9 (21.1) | 32.5 (25.0) | 52.8 (38.8) | 8.78 | 0.005e |
| Social Function Scalec | n = 17 | n = 19 | n = 4 | n = 14 | 3.53 | 0.004e |
| Social withdrawal | 111.3 (11.3) | 112.8 (8.9) | 110.9 (9.4) | 99.1 (6.7) | 5.39 | 0.001e |
| Relationship | 132.2 (18.1) | 141.1 (11.7) | 123.0 (31.6) | 137.3 (15.4) | 1.43 | 0.171 |
| Social activities | 131.4 (8.5) | 130.9 (8.2) | 122.1 (16.2) | 113.0 (15.7) | 13.39 | 0.001e |
| Recreation | 111.0 (11.3) | 119.6 (13.3) | 114.0 (18.8) | 109.9 (14.0) | 0.57 | 0.152 |
| Independent competence | 119.6 (6.1) | 118.8 (5.1) | 119.8 (6.5) | 114.1 (8.6) | 1.16 | 0.097 |
| Independent performance | 116.7 (6.2) | 119.3 (9.9) | 117.9 (8.7) | 111.1 (12.8) | 1.23 | 0.126 |
| Employment | 118.0 (6.4) | 118.5 (5.8) | 107.4 (11.4) | 114.4 (9.4) | 8.96 | 0.038f |
| SFS global meand | 119.2 (7.0) | 123.0 (5.3) | 116.4 (11.2) | 114.1 (4.2) | 8.34 | 0.006e |
There were no significant effects for sex or interactions between group and sex except where noted in the text. LSAS = Liebowitz Social Anxiety Scale; SFS = Social Function Scale.
Test statistic: multivariate analysis of variance (MANOVA).
Multivariate Wilks’ Lambda (all dfs = 4/44).
Multivariate Wilks’ Lambda (all dfs = 7/44).
ANOVA, df = 1/51.
p < 0.01.
p < 0.05.
For social functon, a MANOVA examining the seven domains of the SFS yielded a significant multivariate result for diagnosis [Wilks’ Lambda F(7/44) = 3.53, p = 0.004; Table 3], as well as a marginally significant gender effect [Wilks’ Lambda F(7/44) = 3.16, p = 0.009 (not tabled)]. An examination of the SFS domains revealed significant bipolar deficits for social activities (p = 0.001), and employment (p = 0.038). A significant diagnosis effect (p = 0.001) and interaction of diagnosis and gender (p = 0.034) were present for the social withdrawal domain. These effects were specific to deficits among the female bipolar disorder patients, since the other three subgroups were similar. The SFS Global mean also exhibited significant deficits among the bipolar disorder group [ANOVA F(1/50) = 8.34, p = 0.006]. Significant deficits among the bipolar disorder group were not present for the following SFS domains: relationships, recreation, independent competence, and performance.
With respect to olfaction among the bipolar disorder patients, higher levels on the SFS employment scale, indicative of health, were significantly associated with greater odor sensitivity (mean threshold r = −0.642, p = 0.024; right nostril threshold r = −0.586, p = 0.045); and higher level of independent performance was marginally correlated with more sensitive odor acuity (mean threshold r = −0.545, p = 0.067, left nostril threshold r = −0.548, p = 0.065). Better odor identification (UPSIT) was marginally associated with higher levels of independent competence (r = 0.478, p = 0.061).
Discussion
This study suggests the possibility that odor detection sensitivity is dichotomously related to the different mood syndromes in stable bipolar disorder patients. Depressive symptoms corresponded to greater sensitivity for detecting odors at lower concentrations, whereas symptoms of mania were associated with diminished odor detection sensitivity. Odor sensitivity also showed opposite associations with measures of functioning in the patients; social fear and social anxiety were related to reduced sensitivity, whereas better employment and independent function were strongly related to increased odor sensitivity.
Odor acuity may be a state measure of mood syndromes in bipolar disorder patients, with decreasing acuity identifying the risk for mania symptoms and social anxiety. If so, a heightened or increasing sensitivity to odor detection may mark the risk for depressive episodes. Known risk factors for new depressive episodes include both subthreshold depressive symptoms (12, 43) and higher occupational status (3, 13) which shared their association with acuity. If this model is confirmed, odor acuity may be useful in clinical treatment.
Another possibility is that odor acuity may be a trait measure in bipolar disorder patients that identifies separate subtypes of the condition within the bipolar disease spectrum. This explanation is supported by the finding by Kruger et al. (25) of increased sensitivity in a subset of bipolar disorder patients with a history of even-related episodes compared to those without such history. The relationships between mania and social anxiety with acuity are consistent with the association of more social anxiety symptoms and social sensitivity in mania (44, 45).
This preliminary study could not distinguish between these possibilities. Additional longitudinal studies in larger, sex-stratified samples are necessary to confirm the current findings and determine their relevance for treatment studies and genetic research. The dichotomous relationship of acuity to illness features could underlie the conflicting findings in the literature (25, 28, 29, 46).
Previous studies of odor sensitivity and major depressive disorder found either no difference (24, 26), or decreased acuity (29, 46, 47). The initial presentation of bipolar disorder is often a depressive episode, leading to frequent misdiagnosis as unipolar depression (48). As treatment differs for the two disorders and many antidepressants can precipitate mania, it is important to get the correct diagnosis (48) and odor threshold could plausibly be a useful diagnostic indicator if it is differentially related to depressive symptoms in bipolar and unipolar depression.
The linkage of depressive symptoms in these bipolar disorder cases to hyperacuity is comparable to our recent findings linking hyperacuity to emotional expression impairments in schizophrenia (49). It is within consideration that hypersensitivity to olfactory stimuli could be related to diminished positive affect across different disorders, perhaps because of diminished central inhibitory processes. Some sufficient amount of olfactory function may also subserve independent function and employment, perhaps unconscious chemoperception for social signaling molecules or their associated higher pathways are involved in discerning social expectations, as lesser acuity for odor detection was associated with social anxiety symptoms in the bipolar disorder group.
In keeping with the previous bipolar disorder study (24), smell identification scores were similar in the patient and comparison groups, and smell identification was unrelated to the clinical features of bipolar disorder in this preliminary study. The normal smell identification in bipolar disorder cases may indicate that higher cortical processing of olfactory data is intact in bipolar disorder versus many schizophrenia patients, for whom smell identification deficits are commonly reported (50). We previously showed that a group of schizophrenia patients failed to increase hippocampal activity for a smell identification task compared to control subjects (51). Odor detection threshold is considered a marker of olfactory epithelium and olfactory bulb function, and also of the higher limbic regions that modulate perception (19).
It is also notable that the left nostril was associated with social fear and avoidance, while the right nostril threshold was associated with better employment function. Lateralization is a common feature of neurobiological circuitry and lateralized findings are reported in bipolar disorder subjects (52, 53). In their study of amygdala-orbitofrontal functional connectivity in response to emotional faces, Versace et al. (53) proposed that increased left sides functional connectivity for sad faces represented a state marker for bipolar depression. A similar experiment found elevated left amygdala activity in response to sad faces to be a state marker for bipolar depression (52). In this context, the left lateralization of our findings are of particular interest.
It is also of interest that the olfactory system, along with the hippocampus, is a confirmed site of ongoing adult human neurogenesis (18, 19), and abnormal neurogenesis is increasingly implicated in mental illness (54, 55). Plasticity in the olfactory circuitry suggests that olfactory tasks may have particular utility in elucidating or tracking episodic mental disorders. Olfactory dysfunction is already linked to several neuropsychiatric disorders, most notably Alzheimer’s disease, Parkinson’s disease, and schizophrenia (18, 19, 56, 57). Studies have found alterations in brain derived neurotrophic factor (BDNF) levels in bipolar disorder patients during manic and depressive episodes and in the euthymic state (15, 58, 59). As the olfactory bulb is a known site of neurogenesis, these findings also encourage future longitudinal studies considering mood, growth factor changes, and olfaction. BDNF is particularly noteworthy given its role in neurogenesis (15, 59).
In summary, while this study found similar mean smell identification scores and odor detection threshold for bipolar disorder and comparison subject groups, it shines a new and exciting light on the possible utility of odor detection acuity as a biomarker for the disorder. Depressive symptoms corresponded to greater sensitivity, or hyperacuity—as noted by the ability to detect odors at lower concentrations—and the symptoms of mania corresponded with blunted odor detection, or hypoacuity. Like mania, social avoidance, social fear, and anxiety were also related to decreased odor detection. By contrast, better employment and independent function were strongly related to better odor detection, or hyperacuity. These two double dichotomies for odor detection threshold, wherein mania and social anxiety were linked with less odor acuity while depressive symptoms and better function were linked with more sensitive acuity, may explain the previous conflicting literature on odor acuity in bipolar disorder cases.
Importantly, the significant correlation between odor detection threshold with both affective syndromes and social symptoms and function may be a finding with enormous potential for clinical translation. Limitations of this preliminary study include small sample size with under-representation of males in the patient group, which did not permit sex-specific analyses. As olfactory function has been noted to vary with gender (60), caution must be used when applying these findings to the bipolar disorder population as a whole. We also did not control for medication effects, however, previous studies examining olfactory function in schizophrenia did not find medication effects (61–64). While we would expect similar results in bipolar disorder, the effect of medication on olfactory function in this population is an area for further exploration. Finally, it should be noted that the correlation coefficients were not Bonferroni corrected in hopes of fostering new hypotheses and future research.
Acknowledgments
This work was supported by the National Institute of Mental Health Grants R01-MH066428-05 and 2 K24 MH01699 to DM. The authors thank Dr. Maria Okendo for her assistance in recruitment for this study.
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.National Institute of Mental Health. The Numbers Count: Mental Disorders in America. 2010 Retrieved August 23, 2010 from www.nimh.nih.gov/health/publications/the-numbers-count-mental-disorders-in-america/index.shtml.
- 2.Miller CJ, Johnson SL, Eisner L. Assessment tools for adult bipolar disorder. Clin Psychol. 2009;16:188–201. doi: 10.1111/j.1468-2850.2009.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treuer T, Tohen M. Predicting the course and outcome of bipolar disorder: a review. Eur Psychiatry. 2010;25:328–333. doi: 10.1016/j.eurpsy.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Zalsman G, Braun M, Arendt M, et al. A comparison of the medical lethality of suicide attempts in bipolar and major depressive disorders. Bipolar Disord. 2006;8:558–565. doi: 10.1111/j.1399-5618.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 5.Novick DM, Swartz HA, Frank E. Suicide attempts in bipolar I and bipolar II disorder: a review and meta-analysis of the evidence. Bipolar Disord. 2010;12:1–9. doi: 10.1111/j.1399-5618.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pompili M, Rihmer Z, Innamorati M, Lester D, Girardi P, Tatarelli R. Assessment and treatment of suicide risk in bipolar disorders. Expert Rev Neurother. 2009;9:109–136. doi: 10.1586/14737175.9.1.109. [DOI] [PubMed] [Google Scholar]
- 7.Clark L, Goodwin GM. State- and trait-related deficits in sustained attention in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254:61–68. doi: 10.1007/s00406-004-0460-y. [DOI] [PubMed] [Google Scholar]
- 8.Montoya A, Tohen M, Vieta E, et al. Functioning and symptomatic outcomes in patients with bipolar I disorder in syndromal remission: a 1-year, prospective, observational cohort study. J Affect Disord. 2010;127:50–57. doi: 10.1016/j.jad.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar Disord. 2007;9:183–196. doi: 10.1111/j.1399-5618.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Glenn T, Grof P, Schmid R, Pfennig A, Whybrow PC. Subsyndromal mood symptoms: a useful concept for maintenance studies of bipolar disorder? Psychopathology. 2010;43:1–7. doi: 10.1159/000255957. [DOI] [PubMed] [Google Scholar]
- 11.Keller MB, Lavori PW, Kane JM, et al. Subsyndromal symptoms in bipolar disorder. A comparison of standard and low serum levels of lithium. Arch Gen Psychiatry. 1992;49:371–376. doi: 10.1001/archpsyc.1992.01820050035005. [DOI] [PubMed] [Google Scholar]
- 12.Tohen M, Bowden CL, Calabrese JR, et al. Influence of sub-syndromal symptoms after remission from manic or mixed episodes. Br J Psychiatry. 2006;189:515–519. doi: 10.1192/bjp.bp.105.020321. [DOI] [PubMed] [Google Scholar]
- 13.Tohen M, Zarate CA, Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal N, Port JD, Bazzocchi M, Renshaw PF. Update on the use of MR for assessment and diagnosis of psychiatric diseases. Radiology. 2010;255:23–41. doi: 10.1148/radiol.09090339. [DOI] [PubMed] [Google Scholar]
- 15.Berk M, Malhi GS, Hallam K, et al. Early intervention in bipolar disorders: clinical, biochemical and neuroimaging imperatives. J Affect Disord. 2009;114:1–13. doi: 10.1016/j.jad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Brambilla P, Hatch JP, Soares JC. Limbic changes identified by imaging in bipolar patients. Curr Psychiatry Rep. 2008;10:505–509. doi: 10.1007/s11920-008-0080-8. [DOI] [PubMed] [Google Scholar]
- 17.Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9:512–520. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kivity S, Ortega-Hernandez OD, Shoenfeld Y. Olfaction–a window to the mind. Isr Med Assoc J. 2009;11:238–243. [PubMed] [Google Scholar]
- 19.Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull. 2009;35:1117–1131. doi: 10.1093/schbul/sbp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doty RL. The olfactory system and its disorders. Semin Neurol. 2009;29:74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- 21.Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22:1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz T, Kopala L, Clark C, Jones B. Olfactory deficits in schizophrenia. Biol Psychiatry. 1988;23:123–128. doi: 10.1016/0006-3223(88)90081-9. [DOI] [PubMed] [Google Scholar]
- 23.Striebel KM, Beyerstein B, Remick RA, Kopala L, Honer WG. Olfactory identification and psychosis. Biol Psychiatry. 1999;45:1419–1425. doi: 10.1016/s0006-3223(98)00245-5. [DOI] [PubMed] [Google Scholar]
- 24.Swiecicki L, Zatorski P, Bzinkowska D, Sienkiewicz-Jarosz H, Szyndler J, Scinska A. Gustatory and olfactory function in patients with unipolar and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:827–834. doi: 10.1016/j.pnpbp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Kruger S, Frasnelli J, Braunig P, Hummel T. Increased olfactory sensitivity in euthymic patients with bipolar disorder with event-related episodes compared with patients with bipolar disorder without such episodes. J Psychiatry Neurosci. 2006;31:263–270. [PMC free article] [PubMed] [Google Scholar]
- 26.Gross-Isseroff R, Luca-Haimovici K, Sasson Y, Kindler S, Kotler M, Zohar J. Olfactory sensitivity in major depressive disorder and obsessive compulsive disorder. Biol Psychiatry. 1994;35:798–802. doi: 10.1016/0006-3223(94)91142-8. [DOI] [PubMed] [Google Scholar]
- 27.Scinska A, Wrobel E, Korkosz A, et al. Depressive symptoms and olfactory function in older adults. Psychiatry Clin Neurosci. 2008;62:450–456. doi: 10.1111/j.1440-1819.2008.01824.x. [DOI] [PubMed] [Google Scholar]
- 28.Lombion-Pouthier S, Vandel P, Nezelof S, Haffen E, Millot JL. Odor perception in patients with mood disorders. J Affect Disord. 2006;90:187–91. doi: 10.1016/j.jad.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Pause BM, Miranda A, Goder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271–277. doi: 10.1016/s0022-3956(01)00029-2. [DOI] [PubMed] [Google Scholar]
- 30.Postolache TT, Doty RL, Wehr TA, et al. Monorhinal odor identification and depression scores in patients with seasonal affective disorder. J Affect Disord. 1999;56:27–35. doi: 10.1016/s0165-0327(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 31.Postolache TT, Wehr TA, Doty RL, Sher L, Turner EH, Bartko JJ, et al. Patients with seasonal affective disorder have lower odor detection thresholds than control subjects. Arch Gen Psychiatry. 2002;59:1119–1122. doi: 10.1001/archpsyc.59.12.1119. [DOI] [PubMed] [Google Scholar]
- 32.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 33.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 34.White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997;30:263–274. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- 35.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 36.Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 37.Heimberg RG, Horner KJ, Juster HR, et al. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- 38.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 39.Wechsler D. WAIS-III administration and scoring manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 40.Wechsler D. Wechsler Memory Scale, Revised (WMS-R) San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 41.Reitan R. Validity of the Trail Making Test as an indication of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- 42.Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 2002. [Google Scholar]
- 43.Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 44.Pini S, Maser JD, Dell’Osso L, et al. Social anxiety disorder comorbidity in patients with bipolar disorder: a clinical replication. J Anxiety Disord. 2006;20:1148–1157. doi: 10.1016/j.janxdis.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Simon NM, Otto MW, Fischmann D, et al. Panic disorder and bipolar disorder: anxiety sensitivity as a potential mediator of panic during manic states. J Affect Disord. 2005;87:101–105. doi: 10.1016/j.jad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Pollatos O, Albrecht J, Kopietz R, et al. Reduced olfactory sensitivity in subjects with depressive symptoms. J Affect Disord. 2007;102:101–108. doi: 10.1016/j.jad.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Negoias S, Croy I, Gerber J, et al. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–421. doi: 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Hirschfeld RM. Screening for bipolar disorder. Am J Manag Care. 2007;13:S164–169. [PubMed] [Google Scholar]
- 49.Atanasova B, El-Hage W, Chabanet C, Gaillard P, Belzung C, Camus V. Olfactory anhedonia and negative olfactory alliesthesia in depressed patients. Psychiatry Res. 2010;176:190–196. doi: 10.1016/j.psychres.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Malaspina D, Goetz R, Ketter A, et al. Olfactory processing, sex effects and heterogeneity in schizophrenia. Schizophr Res. 2011 doi: 10.1016/j.schres.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malaspina D, Wray AD, Friedman JH, et al. Odor discrimination deficits in schizophrenia: association with eye movement dysfunction. J Neuropsychiatry Clin Neurosci. 1994;6:273–278. doi: 10.1176/jnp.6.3.273. [DOI] [PubMed] [Google Scholar]
- 52.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Versace A, Thompson WK, Zhou D, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev. 2008;32:1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 58.Dias VV, Brissos S, Frey BN, Andreazza AC, Cardoso C, Kapczinski F. Cognitive function and serum levels of brain-derived neurotrophic factor in patients with bipolar disorder. Bipolar Disord. 2009;11:663–671. doi: 10.1111/j.1399-5618.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 59.Kapczinski F, Frey BN, Kauer-Sant’Anna M, Grassi-Oliveira R. Brain-derived neurotrophic factor and neuroplasticity in bipolar disorder. Expert Rev Neurother. 2008;8:1101–1113. doi: 10.1586/14737175.8.7.1101. [DOI] [PubMed] [Google Scholar]
- 60.Ship JA, Weiffenbach JM. Age, gender, medical treatment, and medication effects on smell identification. J Gerontol. 1993;48:M26–32. doi: 10.1093/geronj/48.1.m26. [DOI] [PubMed] [Google Scholar]
- 61.Coleman E, Goetz RR, Leitman D, Yale S, Stanford A, Gorman JM, Malaspina D. Odor identification impairments in schizophrenia: relationship with demographic measures, clinical variables, and diagnostic subtypes. CNS Spectr. 2002;7:43–48. doi: 10.1017/s1092852900022252. [DOI] [PubMed] [Google Scholar]
- 62.Corcoran C, Whitaker A, Coleman E, Fried J, Feldman J, Goudsmit N, Malaspina D. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80:283–293. doi: 10.1016/j.schres.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 64.Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166:226–233. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]