Abstract
We have previously characterized lipocalin 2 (Lcn2) as a new adipokine having a critical role in energy and lipid metabolism in male mice. Previous studies by others have suggested that Lcn2 is a putative target gene of estrogens. In this study, we reported the effect of Lcn2 deficiency on estradiol biosynthesis and estrogen receptor signaling in female Lcn2-deficient (Lcn2−/−) mice. We found that Lcn2 expression in white adipose tissue is gender, depot, and age dependent. In female mice, Lcn2 is predominantly expressed in inguinal adipose tissue but at relatively very low levels in perigonadal depot and ovary. After 22 wk of high-fat diet (HFD) feeding or at old age, Lcn2−/− female mice had significantly reduced levels of serum 17β-estradiol and down-regulated expression of estrogen receptor α in multiple metabolic tissues. Consistently, the expression of estrogen-regulated genes involved in cholesterol homeostasis, such as liver X receptor β and low-density lipoprotein receptor was also down-regulated in the adipose tissue of Lcn2−/− mice. These changes were in line with the development of atherogenic dyslipidemia in response to HFD feeding; female Lcn2−/− mice had significantly elevated levels of total cholesterol and low-density lipoprotein cholesterol, whereas reduced high-density lipoprotein cholesterol levels compared with wild-type female mice. Interestingly, when compared with wild-type controls, HFD-fed female Lcn2−/− mice had significantly reduced expression levels of aromatase, a key enzyme regulating estradiol biosynthesis, in adipose tissue. Moreover, Lcn2 deficiency markedly blunted age-related increase in adipose aromatase expression but had no significant impact on age-related reduction in ovarian aromatase expression. Our findings suggest that Lcn2 has a tissue-specific role in adipose estradiol biosynthesis, which may link Lcn2 to obesity- and age-related estradiol production and metabolic complications in females.
Adipose tissue plays a pivotal role as a fat-buffering system as well as a secretory organ in the pathogenesis of obesity, insulin resistance, and related metabolic disorders (1, 2). Upon various stimulations, including dietary fat, sex hormone, and genetic mutations, a variety of altered adipokines/cytokines and dysregulated lipid metabolism interplay in adipose tissue, resulting in the development of systemic insulin resistance and metabolic dysregulation (3, 4). Although a number of adipocytes-secreted adipokines and cytokines have been known to function in the modulation of a wide variety of pathophysiological responses, including inflammation, blood pressure, angiogenesis, and reproductive function, their roles in the regulation of adiposity, insulin sensitivity, and metabolic homeostasis are far from fully understood.
Lipocalin 2 (Lcn2), also referred to as neutrophil gelatinase-associated lipocalin or 24p3, is a novel adipose-derived cytokine belonging to the lipocalin subfamily of small secreted proteins that bind hydrophobic molecules, including retinoids, fatty acids, and various steroids. Lcn2 deficiency resulted in an increased susceptibility to bacterial infection in mice (5, 6), which is associated with the role of Lcn2 as an iron transport protein in regulation of iron uptake by sequestrating iron from iron-laden bacterial siderophores (6). We have previously identified Lcn2 as adipose-derived cytokine, the expression of which is up-regulated in adipose tissue of obese rodents (7). We further characterized Lcn2 as a critical regulator of energy metabolism, glucose and lipid homeostasis, and insulin resistance in Lcn2-deficient male mice (8, 9). In the study, male Lcn2-deficient mice were cold intolerant and developed significantly increased body fat mass, exacerbated adipocyte hypertrophy, dyslipidemia, fatty liver, and insulin resistance upon a high-fat diet (HFD) feeding. Although the role of Lcn2 in obesity and insulin resistance is still controversial mainly due to the discrepancy of the results between ours and other two groups (10, 11), our group additionally discovered that Lcn2 deficiency significantly affects energy metabolism, leading to the impaired thermoregulation in mice. This defect makes Lcn2−/− mice more sensitive to the differences in ambient temperature and diets as discussed by Jun et al. (11).
A number of studies have shown that Lcn2 gene promoter region contains multiple transcription factor binding sites and nuclear receptor response elements, including nuclear factor κ-light-chain-enhancer of activated B cells and CCAAT-enhancer-binding proteins, retinoic acid response element (12), and glucocorticoid response element (13). Additionally, an estrogen response element has also been identified in the promoter region of both human and mouse Lcn2 gene (14), suggesting that Lcn2 is a putative direct target gene of estrogen receptor (ER). On one hand, estrogens regulate Lcn2 expression in uterus, and the uterine expression of Lcn2 fluctuates during the estrous cycle in female mice (15). In ovariectomized mice, estrogen supplementation attenuates lipopolysaccharide-induced Lcn2 gene expression in aorta (16). On the other hand, Lcn2 modulates the gene expression of ERα (Esr1) in breast cancer cells and reduces the cell responses to estrogen treatment (17, 18). All the above information suggests a close relationship between Lcn2 and estrogens. However, it is completely unknown whether and how Lcn2 interferes with estrogen action in adiposity and metabolism.
It is well documented that estrogens and ER signaling play a critical role in the regulation of adiposity and lipid homeostasis (19–21), which is the major contributor to the gender difference in the risk of developing obesity-related cardiovascular diseases (22, 23). Decreased endogenous estrogens have been linked to postmenopausal obesity and increased cardiovascular risk in postmenopausal women (24, 25). Moreover, estrogens play important roles in cholesterol metabolism through ERα signaling in obesity (26). Hence, in this study, we investigated the role of Lcn2 in estradiol production and ER signaling in female mice.
We demonstrate that Lcn2 expression is gender, fat depot, and age dependent. Lcn2 deficiency results in decreased production and action of estrogens and enhances HFD-induced fat mass increase and atherogenic dyslipidemia in female mice. We also found that the protein expression of aromatase, a key enzyme regulating estradiol biosynthesis, was significantly decreased in adipose tissue of HFD-fed or aged female Lcn2−/− mice. Thus, our findings suggest that Lcn2 is important for the regulation of estradiol biosynthesis and ER signaling in adipose tissue in female mice.
Materials and Methods
Animals and experimental design
Animals were housed in a specific pathogen-free facility at the University of Minnesota. All animal studies were conducted with the approval of the University of Minnesota Animal Care and Use Committee. All experiments conformed to the National Institutes of Health guidelines of laboratory animal care.
Lcn2-deficient mice were generated by gene targeting and backcrossed onto C57BL/6 background mice (The Jackson Laboratory, Bar Harbor, ME) for 10 generations as previously described (8). Five-week-old Lcn2-deficient female mice were housed with a 12-h light, 12-h dark cycle and fed with either a regular chow diet (RCD) or a HFD (fat calories, 60%, Bio-Serv F3282; Bio-Serv, New Brunswick, NJ) for 22 wk. Age-matched wild-type (WT) female mice served as controls. Body weight was monitored every week until euthanization and tissue collection. The inguinal, perigonadal, and retroperitoneal white adipose tissue, as well as brown adipose tissue and lean mass, were harvested, weighed, and frozen for use.
Metabolic analyses
Glucose and insulin tolerance tests were conducted in the same set of mice after 22 and 24 wk of diet regimen, respectively. After overnight fasting (for glucose tolerance test) or fasting for 6 h (for insulin tolerance test), mice were subjected to ip injection of glucose (1 mg/g body weight) or insulin (0.75 U/kg), followed by glucose measurement with blood collection via the tail vein at 0, 15, 30, 60, 90, and 120 min using an Ascensia glucometer (Bayer, Tarrytown, NY).
Serum analyses
Serum triglyceride and cholesterol were determined using enzymatic assay kits (Stanbio Laboratory, Boerne, TX). Serum-free fatty acids were assessed using a commercially available kit (BioVision, Inc., Mountain View, CA). Serum levels of 17β-estradiol were determined using an ELISA kit (Cayman Chemical, Ann Arbor, MI).
Triglyceride and cholesterol content measurement in tissues
Lipid extraction was performed using the Bligh-Dyer method (27). Briefly, frozen liver or perigonadal adipose tissue (100 mg) was homogenized in 1 ml of water. Lipid was extracted using chloroform:methanol (2:1). An aliquot of the organic phase was collected and dried with nitrogen and then dissolved in isopropanol alcohol containing 1% Triton. Triglyceride content in liver and total cholesterol content in the perigonadal adipose tissue were determined using commercially available kits (Stanbio Laboratory).
Primary mouse adipocytes and stromal-vascular (S-V) cells isolation and 17β-estradiol treatment
Adipose cells and S-V cells were isolated from white adipose tissue of WT and Lcn2-deficient mice as previously described (28). After mincing, perigonadal fat pads were digested with collagenase type 1 (2 mg/ml; Worthington Biochemical Corp., Lakewood, NJ) in digestion vials containing Krebs-Ringer bicarbonate HEPES buffer (pH 7.4), 200 nmol/liter adenosine, and 3.5% BSA. After a 2-h digestion, adipose cells were separated from S-V cells by centrifugation at 1200 rpm for 1 min. Both fractions were washed twice with Krebs-Ringer bicarbonate HEPES buffer and then PBS for collection and further treatment.
RNA isolation and relative quantitative RT-PCR
Total RNA was prepared from frozen tissues with TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from deoxyribonuclease-treated total RNA (1 μg) using a Superscript II reverse transcriptase kit (Invitrogen). Quantitative amplification by PCR was carried out using SYBR Green qPCR Master Mix (SABiosciences, Frederick, MD) by a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA). For quantification, β-actin mRNA served as an endogenous control. The ΔΔCt method was used to calculate the results. The primer sequences for amplifying the target genes and the GenBank accession no. are summarized in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Western blot analysis
The isolated primary adipocytes and S-V cells, as well as the perigonadal adipose tissue, were homogenized in a lysis buffer before subjecting to Western blot analysis with specific antibodies. The primary antibodies included goat polyclonal antibody to Lcn2 (R&D Systems, Minneapolis, MN) and rabbit monoclonal antibodies to β-actin, ERα, and peroxisome proliferator-activated receptor (PPAR)γ (Cell Signaling Technology, Danvers, MA), and liver X receptor (LXR)β and low-density lipoprotein (LDL) receptor (LDL-R) (Novus Biologicals, Littleton, CO). Densitometry results were reported as a ratio to the actin signal.
Assay of ERα transcription activity
The nuclear transcriptional activity of ERα was determined using an ER transcription factor assay kit (Active Motif, Carlsbad, CA) according to the manufacturers' protocols. In brief, nuclear protein was extracted using a Nuclear Extract kit (Active Motif). Equal amount of nuclear protein (5 μg) was added to a 96-well plate precoated with oligonucleotides that contains an ERα consensus binding site (5′-GGTCACAGTGACC-3′). After incubation and washing, the primary antibody to ERα was added to the plate to bind to ERα protein upon DNA binding. After an addition of a secondary horseradish peroxidase-conjugated antibody and developing solution, a sensitive colorimetric readout was quantified by absorbance on a spectrophotometer at 450 nm. The sensitivity of the assay was 0.6 μg of nuclear extracts per well for ER activation, which is 5-fold more sensitive than EMSA.
Statistical analysis
A test of normal distribution of the variants was performed, showing that the variants were normally distributed before ANOVA and Student's t test were used for the data analysis. Results were expressed as means ± se. Differences in parameters of body weight, and serum 17β-estradiol as well as lipid profiles between groups, were evaluated by ANOVA. Differences in parameters of blood glucose, and the gene and protein expression in adipose tissue between WT and Lcn2-deficient mice, were evaluated using the two-tailed Student's t test. A P value less than 0.05 was considered significant.
Results
Adipose tissue Lcn2 expression is depot, gender, and age dependent
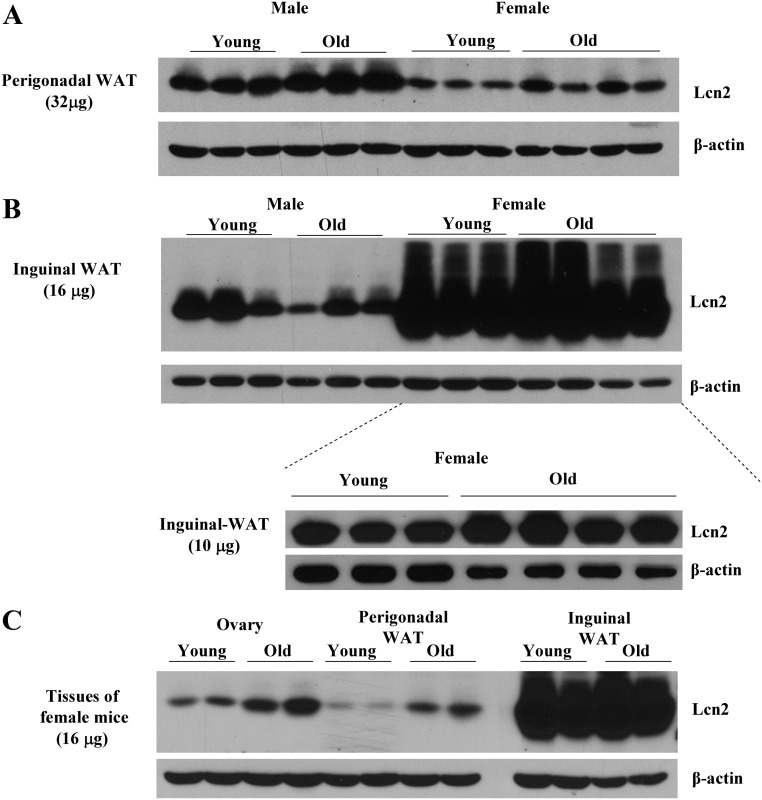
WT male and female mice fed a regular chow diet were killed at the age of 2–3 and 8–9 months; the expression of Lcn2 protein in adipose tissue was determined by Western blotting. As illustrated in Fig. 1, A and B, the expression levels of Lcn2 protein were significantly higher in inguinal than in perigonadal adipose tissue in both male and female mice. This depot difference in the expression of Lcn2 protein was much more dramatic in female mice; female inguinal depot expressed tremendously higher levels of Lcn2 proteins than perigonadal depot. More interestingly, Lcn2 protein expression in adipose tissue also showed age-related difference; the levels of Lcn2 protein expression in both perigonadal (Fig. 1A) and inguinal (Fig. 1B) adipose tissue were significantly increased in old female mice with 8–9 months of age compared with young female mice at 2–3 months of age. However, the Lcn2 protein levels were increased in perigonadal adipose tissue but decreased in inguinal adipose tissue in old compared with those in young male mice. We also observed the increased Lcn2 expression in the ovary of old WT mice (Fig. 1C). However, when compared with the expression of Lcn2 in the inguinal adipose tissue, the ovarian Lcn2 expression appeared to be at a relatively very low level.
Fig. 1.
Depot, gender, and age-dependent expression of Lcn2 in adipose tissue and ovary. A and B, Lcn2 expression in the perigonadal (A) and inguinal (B) adipose tissue of male and female mice (n = 3–4) at young and old age, respectively. C, Lcn2 expression in the ovary at young and old age, comparing with the same amount of total protein from perigonadal, and inguinal adipose tissue. WAT, White adipose tissue.
Lcn2 deficiency increases adiposity and reduces estradiol production and ERα signaling
Similar to those observed in male Lcn2−/− mice, Lcn2−/− female mice gained significantly more body weight than WT mice on a HFD (Supplemental Fig. 1A). The body fat mass, including inguinal, perigonadal, and retroperitoneal white adipose tissue as well as brown adipose tissue, was also markedly increased in Lcn2−/− female mice on HFD when compared with WT mice (Supplemental Fig. 1B). Decreased biosynthesis of estrogens with aging has been linked to increased risk for developing obesity-related insulin resistance, diabetes, and atherosclerosis. Given that more predominant expression of Lcn2 in sc adipose tissue of female mice than that of male mice, we tested whether Lcn2 has a role in obesity- or age-related reduction in estrogen levels in females by examining the estradiol production and action in Lcn2−/− female mice.
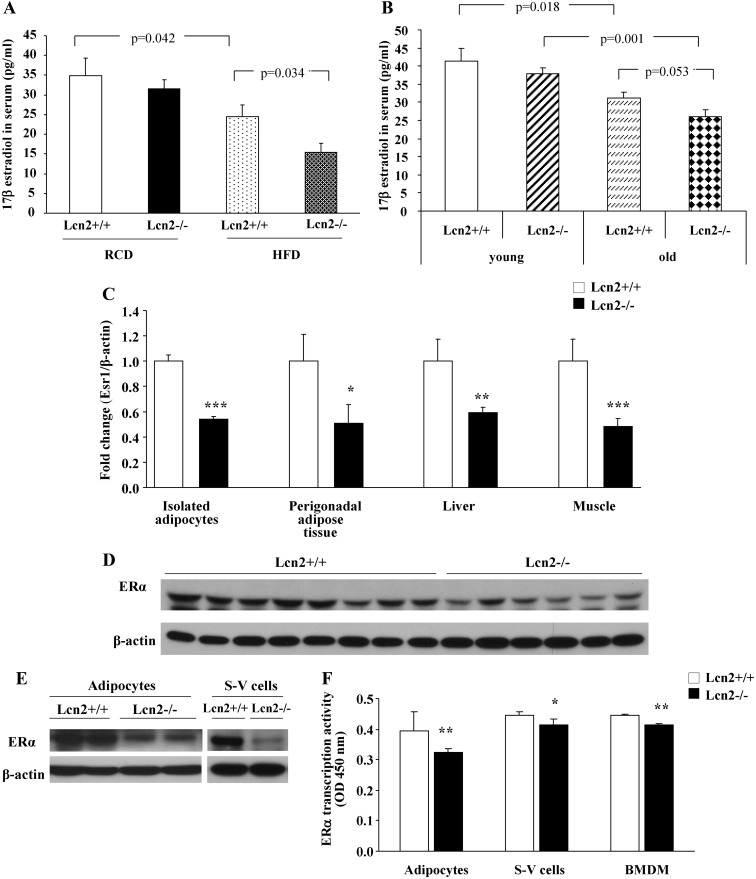
In female mice on RCD, there was a trend toward a decrease in serum levels of 17β-estradiol, in Lcn2−/− mice compared with WT controls; but the difference failed to reach any statistical significance. Intriguingly, Lcn2−/− mice had significantly lower levels of serum 17β-estradiol than WT controls when fed HFD (Fig. 2A). Moreover, the levels of serum 17β-estradiol were significantly reduced in old WT and Lcn2−/− mice when compared with those in young mice (Fig. 2B). This age-related effect was more significant in Lcn2−/− mice; old Lcn2−/− mice had much lower levels of 17β-estradiol than old WT mice (Fig. 2B). Accordingly, female Lcn2−/− mice on HFD displayed markedly down-regulated mRNA expression of Esr1 in the perigonadal adipose tissue, liver, and muscle as well as isolated primary adipocytes from the perigonadal adipose tissue in comparison with WT mice (Fig. 2C). Lcn2−/− mice also exhibited decreased Esr1 expression in the perigonadal adipose tissue (Fig. 2D), isolated primary adipocytes, and S-V cells (Fig. 2E) at the protein level as compared with WT controls. Moreover, the examination of the nuclear transcriptional activation of ERα using DNA-binding ELISA showed that the DNA binding of activated ERα was also reduced in isolated adipocytes and S-V cells as well as bone marrow-derived macrophages (BMDM) of Lcn2−/− mice (Fig. 2F).
Fig. 2.
Serum levels of 17β-estradiol and ERα signaling in Lcn2−/− mice. A, Serum levels of 17β-estradiol in Lcn2−/− mice (n = 6–8) on RCD and HFD. B, Serum levels of 17β-estradiol in young (n = 8–10) and old (n = 8–10) Lcn2−/− mice. C, Esr1 gene expression in multiple metabolic tissues of Lcn2−/− mice (n = 6–8) fed a HFD. D and E, Protein expression of ERα in perigonadal adipose tissue (D) and primary adipocytes and SV cells (E) in Lcn2−/− female mice. F, Nuclear transcriptional activity of ERα in isolated primary adipocytes, S-V cells, and BMDM of Lcn2−/− mice. The results are presented as mean ± sem; *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. WT mice.
Lcn2 deficiency alters lipid metabolism in adipose tissue in female mice
We further assessed the metabolic consequences of Lcn2 deficiency in female mice. Consistent with the significantly increased liver weights, the measurement of liver triglyceride content showed that there was 50–60% increase in triglyceride accumulation in liver of female Lcn2−/− mice fed a HFD compared with WT mice (Table 1). Serum lipid profiles are summarized in Table 1. Compared with WT mice, female Lcn2−/− mice on RCD displayed a trend toward an increase in serum levels of free fatty acid, triglyceride, total cholesterol, and LDL cholesterol, whereas there was no significant difference in serum levels of high-density lipoprotein (HDL) cholesterol between female Lcn2−/− and WT mice. However, female Lcn2−/− mice fed a HFD had significantly elevated levels of total and LDL cholesterol while reduced levels of HDL cholesterol in serum when compared with WT mice. Female Lcn2−/− mice on HFD also showed elevated cholesterol levels in the perigonadal adipose tissue.
Table 1.
Lipid profiles in wild-type and Lcn2-null mice
| RCD |
HFD |
|||
|---|---|---|---|---|
| Lcn+/+ (n = 5) | Lcn2−/− (n = 6) | Lcn+/+ (n = 8) | Lcn2−/− (n = 6) | |
| Serum | ||||
| FFA (mmol/liter) | 0.52 ± 0.16 | 0.82 ± 0.11 | 1.09 ± 0.14a | 0.97 ± 0.10 |
| Triglycerides (mg/dl) | 80.5 ± 10.1 | 91.1 ± 7.5 | 83.3 ± 8.2 | 63.2 ± 2.5d |
| Total cholesterol (mg/dl) | 96.1 ± 10.3 | 116.7 ± 1.1 | 145.2 ± 5.3b | 175.3 ± 11.0d |
| LDL cholesterol (mg/dl) | 28.0 ± 2.0 | 32.4 ± 7.6 | 87.5 ± 5.8c | 128.6 ± 11.7d |
| HDL cholesterol (mg/dl) | 58.8 ± 5.4 | 60.5 ± 9.9 | 47.0 ± 2.7a | 34.8 ± 4.7d |
| Liver | ||||
| Triglycerides (mg/g) | 34.9 ± 4.1 | 34.2 ± 11.6 | 79.5 ± 13.0 | 180.9 ± 14.2e |
| Total cholesterol (mg/g) | 11.2 ± 0.7 | 10.7 ± 0.5 | 27.3 ± 1.9b | 30.1 ± 2.5 |
| Perigonadal adipose tissue | ||||
| Total cholesterol (mg/g) | 15.1 ± 0.4 | 15.8 ± 1.0 | 42.4 ± 0.9 | 46.3 ± 1.0d |
FFA, Free fatty acid.
P < 0.05.
P < 0.01.
P < 0.001 vs. WT on RCD.
P < 0.05.
P < 0.001 vs. WT on HFD.
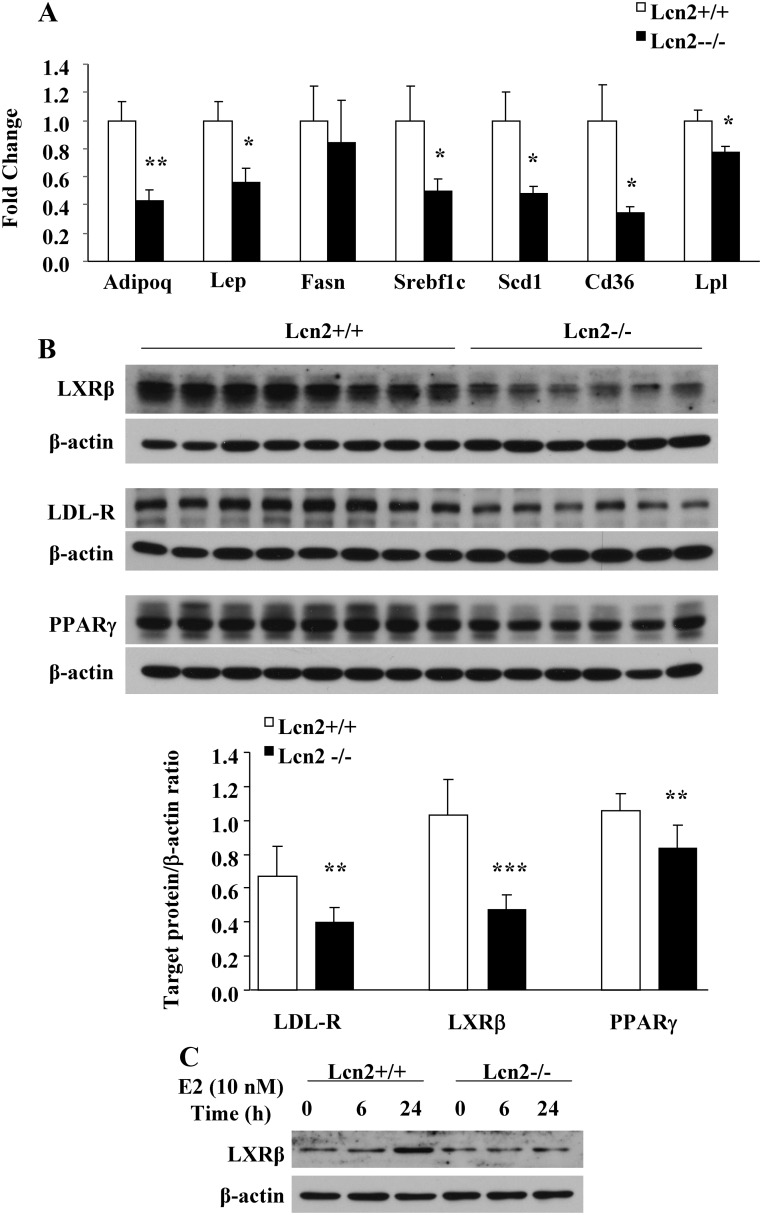
We next examined the expression of adipokines and genes involved in adipogenesis/lipogenesis in adipose tissue. In Lcn2−/− mice on HFD, the expression of adiponectin (adipoq), leptin (lep), sterol regulatory element-binding transcription factor 1c (Srebf1c), stearoyl-coenzyme A desaturase 1 (Scd1), CD36 antigen (Cd36), and lipoprotein lipase (Lpl) was significantly down-regulated in adipose tissue compared with WT mice (Fig. 3A). Moreover, the expression of LDL-R, LXRβ, and PPARγ was all markedly reduced at protein levels (Fig. 3B) in the perigonadal adipose tissues of Lcn2−/− mice fed with HFD in comparison with WT controls (P = 0.003, P < 0.001, and P = 0.009, respectively). To further determine whether Lcn2 deficiency also affects ERα signaling activation, which may additionally contribute to reduced estrogen action in female Lcn2−/− mice, 17β-estradiol stimulation of LXRβ expression was examined in primary adipocytes and BMDM isolated from female WT and Lcn2−/− mice. After incubation with exogenous 17β-estradiol for 24 h, the expression of LXRβ was significantly up-regulated in BMDM from WT mice but not in that from Lcn2−/− mice (Fig. 3C). The failure of 17β-estradiol stimulation on LXRβ expression in Lcn2−/− cells suggests that Lcn2 modulates the action of estrogens in BMDM. However, no significant change in LXRβ expression was observed in primary adipocytes of WT and Lcn2−/− mice after a 6-h incubation with 17β-estradiol (data not shown).
Fig. 3.
Effects of Lcn2 deficiency on lipid metabolism in adipose tissue. A, Adipokines and genes involved in lipid metabolism in perigonadal adipose tissue of WT (n = 8) and Lcn2−/− mice (n = 6) on HFD. B, The protein expression of LDL-R, LXRβ, and PPARγ in perigonadal adipose tissue of WT and Lcn2−/− mice on HFD. C, 17β-Estradiol regulation of LXRβ protein expression in BMDM from of WT and Lcn2−/− mice. The results are presented as mean ± sem; *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. WT mice.
Lcn2 deficiency reduces the expression of aromatase, a key enzyme for estradiol biosynthesis
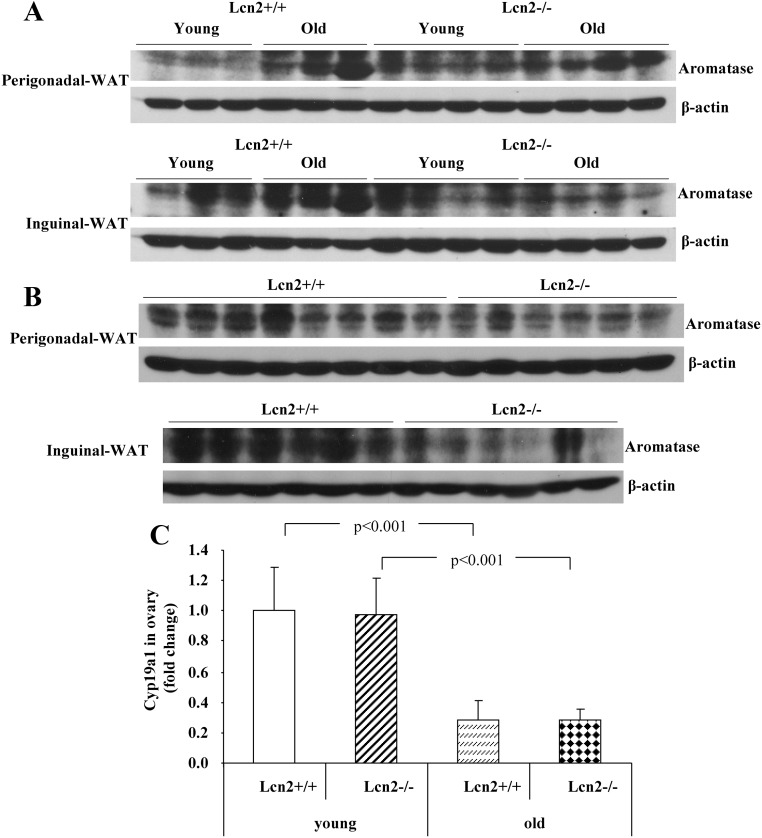
Numerous studies have demonstrated that the expression of aromatase and the efficiency of local production of estrogens in adipose tissue are increased with aging (4, 6, 7, 29–31). As shown in the Results section, Lcn2 protein expression in perigonadal and inguinal depots was increased with age in female mice. Consistent with this observation, the aromatase protein contents in both fat depots were also up-regulated in old (9–10 months) compared with young (4 months) female WT mice (Fig. 4A). However, this age-induced increase in the protein content of aromatase was significantly blunted in old female Lcn2−/− mice (Fig. 4A). We further examined how Lcn2 deficiency affects the expression of aromatase in adipose tissue in female mice under the HFD feeding condition. The protein contents of aromatase were significantly lower in both perigonadal and inguinal adipose tissue of Lcn2−/− mice compared with that in WT mice (Fig. 4B). Because ovary is the major site for the biosynthesis of estrogens, contributing to the circulating estrogens in young females, it is of importance to determine whether ovarian estrogen biosynthesis is also affected, which may additionally contribute to the decreased 17β-estradiol levels in Lcn2−/− mice. We examined and compared the difference in the expression levels of aromatase in the ovary between WT and Lcn2−/− female mice at the young (19 wk) and old (45 wk) age, respectively. The expression of aromatase gene in the ovary of old WT and Lcn2−/− mice was significantly down-regulated when compared with their young controls, whereas the expression of ovarian aromatase was not significantly different between WT and Lcn2−/− mice at either young or old age (Fig. 4C).
Fig. 4.
The expression of aromatase in adipose tissue and ovary of Lcn2−/− mice. A and B, The aromatase protein contents in gonadal and inguinal adipose tissue of WT and Lcn2−/− female mice on RCD at young (4 months, n = 3) and old (9–10 months, n = 3) age (A), and on HFD at 26 wk of age (B). C, The gene expression of aromatase in the ovary of young and old mice on RCD. WAT, White adipose tissue.
Discussion
We have previously demonstrated that the molecular disruption of Lcn2 significantly affects thermogenesis and metabolic homeostasis in male mice (8). In this study, we showed that Lcn2 expression is gender, fat depot, and age dependent. Most importantly, we discovered that Lcn2 has an important regulatory role in obesity- and age-related estradiol biosynthesis. Lcn2 deficiency leads to a reduction in 17β-estradiol concentration in sera and ERα signaling, which may contribute at least in part to the metabolic disruptions by Lcn2 deficiency in female mice when challenged with a HFD or with age. We also demonstrate that Lcn2-deficient mice exhibited decreased expression of estrogen-regulated LXRβ and LDL-R in the perigonadal adipose tissue under HFD feeding condition. These findings identify Lcn2 as an important modulator of estradiol biosynthesis and action in lipid metabolism in the pathogenesis of obesity in female mice.
Previous reports by others have demonstrated a regulatory relationship between Lcn2 and estrogen/ER signaling, as evidenced by the observations that Lcn2 is not only a direct target gene of estrogens (14) but a modulator of ERα activity in breast cancer cells (17, 18). In male mice, Lcn2 deficiency has a significant impact on energy metabolism, leading to increased adiposity, exacerbated adipocyte hypertrophy, dyslipidemia, fatty liver, and insulin resistance in response to a HFD feeding (8). This information has indicated that Lcn2 and estrogens have overlapping functions in adiposity and lipid metabolism. However, the functional interrelationship between Lcn2 and estrogens has not been previously studied. It is well documented that estrogens and ER signaling play a critical role in the regulation of adiposity and lipid homeostasis, which mainly contributes to the gender difference in body fat distribution and development of obesity-related metabolic syndrome (20, 21, 25). Estrogens have been known as a major regulator of adipose tissue development and fat deposition and distribution in females. Decreased endogenous estrogens have been linked to postmenopausal obesity; increased adiposity, particularly adipose tissue redistribution from sc to abdominal region, is more closely correlated with increased risk of developing obesity-related metabolic disorders in women after menopause (22, 23). Estrogens exert their actions through the classic nuclear ER (ERα and ERβ) and alternative extranuclear pathways (21, 25). ERα is the major form in adipose tissue having a role in the regulation of energy balance and glucose and lipid metabolism. The disruption of ERα or aromatase, the key enzyme controlling the biosynthesis of estrogens, resulted in the development of obesity and insulin resistance in mice (20, 32, 33).
In the present study, we showed that female Lcn2−/− mice developed significantly increased adiposity, fatty liver, and HFD-induced atherogenic dyslipidemia, such as elevated serum levels of total and LDL cholesterol compared with WT mice. We found that Lcn2 shows an inguinal depot-specific expression, and this depot difference is much more significant in female mice than in male mice. More interestingly, Lcn2 expression in adipose tissue is age dependent. All these data suggest a connection of Lcn2 to sex hormones, and this led us to speculate and examine whether estrogens or estrogen action was altered in female Lcn2−/− mice. Intriguingly, we discovered that there was a significant reduction in serum estradiol levels in HFD-fed Lcn2−/− mice. Moreover, the expression of ERα in adipose tissue was significantly reduced at both gene and protein levels, so was the nuclear transcriptional activity of ERα in multiple cells isolated from HFD-fed Lcn2−/− mice. These findings strongly suggest that the estradiol production and action is disrupted, which may contribute in part to the exacerbated metabolic deterioration by HFD in female Lcn2−/− mice. However, further investigations are needed to find out how much the reduced estradiol production contributes to the HFD-induced metabolic deterioration in female Lcn2−/− mice compared with other estrogen-independent mechanism.
The nuclear hormone receptors LXR, including LXRα and LXRβ, PPAR, and LDL-R, which regulate lipid metabolism, especially cholesterol homeostasis, are the important target genes of estrogens (34). LXRα is highly expressed in the liver and at lower levels in the adrenal glands, adipose tissue, and macrophages, whereas LXRβ is more extensively expressed in many tissues (35). Estrogens have been indicated to directly control the expression of Srebf1c gene, because Srebf1c contains an estrogen response element in its promoter region (36). PPARγ, highly expressed in white adipose tissue, is one of the key transcriptional factors governing adipogenesis via directly activating many genes involved in adipocyte lipid storage (37), including fatty acid transporter/CD36 (38). It is also reported that PPARγ activation may promote foam-cell formation and control the initial steps of the reverse cholesterol-transport pathway through its action on CD36 antigen (Cd36) and ATP-binding cassette, subfamily A, member 1 (Abca1) (39, 40). Estrogens have been reported to regulate PPARγ expression in astrocytes (41). Therefore, we further examined and linked the changes in the expression of these estrogen-regulated genes to the lower estradiol levels and the deterioration of lipid metabolism in female Lcn2−/− mice. We observed that the expression of LXRβ, Srebf1c, and PPARγ was down-regulated in adipose tissue of HFD-fed Lcn2−/− mice. We also found that 17β-estradiol treatment for 24 h significantly stimulated the expression of LXRβ in BMDM from WT mice, but this stimulation was markedly attenuated in Lcn2−/− BMDM. This indicates that the action of estrogens in BMDM is reduced in the absence of Lcn2.
LDL-R is another estrogen-regulated gene that controls cholesterol hoemostasis; LDL-R deficiency is associated with increased atherosclerosis in mice (42). Additionally, estrogens stimulate LDL-R expression in the liver, leading to an increase in the uptake of LDL and the lowering of circulating cholesterol (43). Interestingly, we showed that the expression of LDL-R was decreased in the perigonadal adipose tissue of HFD-fed Lcn2−/− mice, which is consistent with the reduced serum 17β-estradiol and impaired ERα signaling and the development of atherogenic dyslipidemia. The decreased LDL-R in adipose tissue may lead to the reduced binding and internalizing cholesterol transported by LDL, further interfering with intracellular lipid metabolism. Taken together, the above data suggest that Lcn2 is an important regulator of estradiol biosynthesis and ER signaling.
In young females, ovary is the major site for the biosynthesis of estrogens, contributing to the circulating estrogens. Estradiol can also be converted from androstenedione and testosterone in extragonadal tissues, such as adipose tissue, bone, liver, and adrenal glands (44, 45). In postmenopausal women, most of estradiol is formed by extragonadal conversion of testosterone. Estrogens produced in extragonadal tissues mostly act in a paracrine or intracrine fashion, and this local estradiol biosynthesis plays a vital role in maintaining normal action of estrogens in postmenopausal women. Estrogen therapy has been beneficial for reducing the risk for the development of estrogen-related metabolic complications, such as type 2 diabetes, arteriosclerosis, and osteoporosis in females during aging. Physiologically, the level of estradiol biosynthesis in extragonadal tissues increases as a function of age and body weight in females (46), which is of paramount importance in reducing the risk of age-related metabolic deterioration. In this study, we found that female Lcn2−/− mice at the age of 24–26 wk (during premeopausal period) had a trend toward a decrease in 17β-estradiol levels when fed RCD. This decreasing trend of serum 17β-estradiol levels became more significant in Lcn2−/− mice with age or when challenged with a HFD. These results have clearly linked Lcn2 to obesity- and age-related estradiol reduction. Moreover, the preferential expression of Lcn2 in inguinal adipose tissue suggests that Lcn2 may have a tissue-specific role in adipose estradiol production in female mice. To further test this hypothesis and to elucidate the mechanism underlying the reduced estradiol levels by metabolic stress of HFD feeding and aging in female Lcn2−/− mice, we investigated the efficiency of estradiol biosynthesis in adipose tissue of Lcn2−/− mice. The expression of aromatase, a key enzyme for estradiol biosynthesis in adipose tissue, was examined and compared between WT and Lcn2−/− mice fed HFD and at old age. Our results showed that the expression levels of aromatase protein were significantly reduced in both perigonadal and inguinal adipose tissues of Lcn2−/− mice fed HFD. Moreover, age-induced increase in aromatase expression in white adipose tissue was markedly blunted in Lcn2−/− mice, suggesting a more direct regulatory role of Lcn2 in aromatase expression. However, the age-induced reduction in aromatase expression was not significantly different in ovary between WT and Lcn2−/− mice. All the data together lead us to believe that Lcn2 deficiency has a profound impact on peripheral estradiol production especially in sc adipose tissue in HFD-induced obese and aged female mice but has a minimal effect on ovarian estradiol biosynthesis in female mice.
A number of regulators, including hormones (e.g. estrogens and androgens), cytokines, and growth factors, have been shown to be involved in the regulation of aromatase expression (47). Retinoic acid has been reported to be efficient inducer of aromatase expression and estradiol synthesis (48). Lcn2 structurally possesses a unique hydrophobic ligand-binding pocket that confers to Lcn2 the ability to bind and transport hydrophobic molecules, including lipids, retinoids, and steroids (49). It is conceivable that Lcn2 deficiency may alter the metabolism of lipids, retinoids, and steroids, which subsequently affects the expression of aromatase and estradiol biosynthesis. The evidence from previous studies demonstrated that estrogens produced by aromatization could act as a positive feedback regulator via ERα to further stimulate Cyp19 promoter activity and the production of estrogens in certain tissues (50, 51). Alternatively, the decreased aromatase in Lcn2−/− mice may not be the primary cause but rather be the result of the decreased ERα expression. For instance, LCN2 deficiency exacerbates HFD- or age-induced lipid dysregualtion, leading to a more significant reduction in ERα expression and subsequently decreased aromatase expression and estradiol biosynthesis in adipose tissue of Lcn2−/− female mice. However, a full understanding of the mechanisms underlying the role of Lcn2 in regulation of aromatase expression and estradiol production in adipose tissue requires further investigation.
In summary, we demonstrate for the first time that Lcn2 displays a significant difference in depot, gender, and age-related expression in white adipose tissue. Lcn2 deficiency leads to increased adiposity, fatty liver, and atherogenic dyslipidemia induced by HFD in female mice. More intriguingly, we discovered that Lcn2 deficiency affects estradiol biosynthesis; Lcn2 deficiency results in decreased estradiol production and ER signaling, and this phenomenon is reinforced under the condition of HFD feeding and with age. Our findings emphasize the novel role of Lcn2 as a regulator of estradiol biosynthesis in adipose tissue, suggesting a novel therapeutic target for preventing estrogen-related obesity, insulin resistance, and atherogenic dyslipidemia.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK080743 (to X.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMDM
- Bone marrow-derived macrophage
- ER
- estrogen receptor
- HDL
- high-density lipoprotein
- HFD
- high-fat diet
- Lcn2
- lipocalin 2
- LDL
- low-density lipoprotein
- LDL-R
- LDL receptor
- LXR
- liver X receptor
- PPAR
- peroxisome proliferator-activated receptor
- RCD
- regular chow diet
- Srebf1c
- sterol regulatory element binding transcription factor 1c
- S-V
- stromal-vascular
- WT
- wild type.
References
- 1. Savage DB, Petersen KF, Shulman GI. 2007. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 4. Rosen ED, Spiegelman BM. 2006. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444:847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. 2006. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA 103:1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921 [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. 2008. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol 22:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. 2010. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin D, Guo H, Bu SY, Zhang Y, Hannaford J, Mashek DG, Chen X. 2011. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-γ activation and function in lipid homeostasis and energy expenditure. FASEB J 25:754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. 2010. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jun LS, Siddall CP, Rosen ED. 2011. A minor role for lipocalin 2 in high fat diet-induced glucose intolerance. Am J Physiol Endocrinol Metab 301:E825–E835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen F, Hu Z, Goswami J, Gaffen SL. 2006. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem 281:24138–24148 [DOI] [PubMed] [Google Scholar]
- 13. Garay-Rojas E, Harper M, Hraba-Renevey S, Kress M. 1996. An apparent autocrine mechanism amplifies the dexamethasone- and retinoic acid-induced expression of mouse lipocalin-encoding gene 24p3. Gene 170:173–180 [DOI] [PubMed] [Google Scholar]
- 14. Seth P, Porter D, Lahti-Domenici J, Geng Y, Richardson A, Polyak K. 2002. Cellular and molecular targets of estrogen in normal human breast tissue. Cancer Res 62:4540–4544 [PubMed] [Google Scholar]
- 15. Huang HL, Chu ST, Chen YH. 1999. Ovarian steroids regulate 24p3 expression in mouse uterus during the natural estrous cycle and the preimplantation period. J Endocrinol 162:11–19 [DOI] [PubMed] [Google Scholar]
- 16. Gao H, Liang M, Bergdahl A, Hamrén A, Lindholm MW, Dahlman-Wright K, Nilsson BO. 2006. Estrogen attenuates vascular expression of inflammation associated genes and adhesion of monocytes to endothelial cells. Inflamm Res 55:349–353 [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Moses MA. 2009. Lipocalin 2: a multifaceted modulator of human cancer. Cell Cycle 8:2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA. 2009. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA 106:3913–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. 2009. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150:2109–2117 [DOI] [PubMed] [Google Scholar]
- 20. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathieu P, Lemieux I, Després JP. 2010. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87:407–416 [DOI] [PubMed] [Google Scholar]
- 23. Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, Buchan I, Day N, Khaw KT. 2007. Body fat distribution and risk of coronary heart disease in men and women in the European prospective investigation into cancer and nutrition in Norfolk cohort: a population-based prospective study. Circulation 116:2933–2943 [DOI] [PubMed] [Google Scholar]
- 24. Carr MC. 2003. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 25. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M, Women's Health Initiative Investigators 2003. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349:523–534 [DOI] [PubMed] [Google Scholar]
- 26. Sertic J, Juricic L, Ljubic H, Bozina T, Lovric J, Markeljevic J, Jelakovic B, Merkler M, Reiner Z. 2009. Variants of ESR1, APOE, LPL and IL-6 loci in young healthy subjects: association with lipid status and obesity. BMC Res Notes 2:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- 28. Xiang CC, Wu YJ, Ma L, Ding L, Lisinski I, Brownstein MJ, Cushman SW, Chen X. 2007. Characterisation of insulin-resistant phenotype of cultured rat primary adipose cells. Diabetologia 50:1070–1079 [DOI] [PubMed] [Google Scholar]
- 29. Cowland JB, Muta T, Borregaard N. 2006. IL-1β-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IκB-ζ. J Immunol 176:5559–5566 [DOI] [PubMed] [Google Scholar]
- 30. Cowland JB, Sørensen OE, Sehested M, Borregaard N. 2003. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol 171:6630–6639 [DOI] [PubMed] [Google Scholar]
- 31. Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Salvador J, Frühbeck G. 2009. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J Mol Med 87:803–813 [DOI] [PubMed] [Google Scholar]
- 32. Takeda K, Toda K, Saibara T, Nakagawa M, Saika K, Onishi T, Sugiura T, Shizuta Y. 2003. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol 176:237–246 [DOI] [PubMed] [Google Scholar]
- 33. Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. 2006. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49:588–597 [DOI] [PubMed] [Google Scholar]
- 34. Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. 2006. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev 27:575–605 [DOI] [PubMed] [Google Scholar]
- 35. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383:728–731 [DOI] [PubMed] [Google Scholar]
- 36. Bajic VB, Tan SL, Chong A, Tang S, Ström A, Gustafsson JA, Lin CY, Liu ET. 2003. Dragon ERE Finder version 2: a tool for accurate detection and analysis of estrogen response elements in vertebrate genomes. Nucleic Acids Res 31:3605–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Auwerx J. 1999. PPARγ, the ultimate thrifty gene. Diabetologia 42:1033–1049 [DOI] [PubMed] [Google Scholar]
- 38. Larsen TM, Toubro S, Astrup A. 2003. PPARγ agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord 27:147–161 [DOI] [PubMed] [Google Scholar]
- 39. Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. 2001. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 7:53–58 [DOI] [PubMed] [Google Scholar]
- 40. Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. 1998. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93:241–252 [DOI] [PubMed] [Google Scholar]
- 41. Valles SL, Dolz-Gaiton P, Gambini J, Borras C, Lloret A, Pallardo FV, Viña J. 2010. Estradiol or genistein prevent Alzheimer's disease-associated inflammation correlating with an increase PPARγ expression in cultured astrocytes. Brain Res 1312:138–144 [DOI] [PubMed] [Google Scholar]
- 42. Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. 2002. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab 282:E207–E214 [DOI] [PubMed] [Google Scholar]
- 43. Kovanen PT. 1987. Regulation of plasma cholesterol by hepatic low-density lipoprotein receptors. Am Heart J 113:464–469 [DOI] [PubMed] [Google Scholar]
- 44. Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. 2002. Production and actions of estrogens. N Engl J Med 346:340–352 [DOI] [PubMed] [Google Scholar]
- 45. Simpson E, Rubin G, Clyne C, Robertson K, O'Donnell L, Davis S, Jones M. 1999. Local estrogen biosynthesis in males and females. Endocr Relat Cancer 6:131–137 [DOI] [PubMed] [Google Scholar]
- 46. Bulun SE, Simpson ER. 1994. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab 78:428–432 [DOI] [PubMed] [Google Scholar]
- 47. Simpson ER. 2004. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med 22:11–23 [DOI] [PubMed] [Google Scholar]
- 48. Munetsuna E, Hojo Y, Hattori M, Ishii H, Kawato S, Ishida A, Kominami SA, Yamazaki T. 2009. Retinoic acid stimulates 17β-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology 150:4260–4269 [DOI] [PubMed] [Google Scholar]
- 49. Flower DR. 1996. The lipocalin protein family: structure and function. Biochem J 318(Pt 1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bukulmez O, Hardy DB, Carr BR, Auchus RJ, Toloubeydokhti T, Word RA, Mendelson CR. 2008. Androstenedione up-regulation of endometrial aromatase expression via local conversion to estrogen: potential relevance to the pathogenesis of endometriosis. J Clin Endocrinol Metab 93:3471–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumar P, Kamat A, Mendelson CR. 2009. Estrogen receptor α (ERα) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol Endocrinol 23:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.