Abstract
Pancreatic islet α-cell glucagon secretion is critically dependent on pancreatic islet β-cell insulin secretion. Normally, a decrease in the plasma glucose concentration causes a decrease in β-cell insulin secretion that signals an increase in α-cell glucagon secretion during hypoglycemia. In contrast, an increase in the plasma glucose concentration, among other stimuli, causes an increase in β-cell insulin secretion that signals a decrease, or at least no change, in α-cell glucagon secretion after a meal. In absolute endogenous insulin deficiency (i.e. in type 1 diabetes and in advanced type 2 diabetes), however, β-cell failure results in no decrease in β-cell insulin secretion and thus no increase in α-cell glucagon secretion during hypoglycemia and no increase in β-cell insulin secretion and thus an increase in α-cell glucagon secretion after a meal. In type 1 diabetes and advanced type 2 diabetes, the absence of an increment in glucagon secretion, in the setting of an absent decrement in insulin secretion and an attenuated increment in sympathoadrenal activity, in response to falling plasma glucose concentrations plays a key role in the pathogenesis of iatrogenic hypoglycemia. In addition, there is increasing evidence that, in the aggregate, suggests that relative hyperglucagonemia, in the setting of deficient insulin secretion, plays a role in the pathogenesis of hyperglycemia in diabetes. If so, abnormal glucagon secretion is involved in the pathogenesis of both hypoglycemia and hyperglycemia in diabetes.
Discovered as a contaminant of pancreatic insulin extracts as early as in 1921, glucagon is a 29-amino-acid, 3485-Da peptide cleaved by prohormone convertase 2 from the proglucagon molecule in pancreatic islet α-cells. From there the hormone is secreted into the hepatic portal vein from which it acts on G protein-coupled receptors in the liver to stimulate glucose production (1–3). By itself, glucagon largely stimulates hepatic glycogenolysis. However, in concert with other glucose counterregulatory (plasma glucose raising) hormones, such as epinephrine, that mobilize gluconeogenic precursors (lactate, amino acids, and glycerol) to the liver, glucagon also stimulates hepatic gluconeogenesis (2). Glucagon stimulates hepatic fatty acid oxidation and ketogenesis (4). Although there is evidence that the hormone regulates hepatic lipoprotein particle metabolism (5), stimulation of lipolysis does not appear to be part of the physiological action profile of glucagon (6–8).
Renewed interest in the biology of glucagon has ranged from studies of the fundamental molecular and cellular aspects of glucagon secretion and action to those extending the concepts of the physiology and pathophysiology of the hormone from experimental animals to humans. The latter is the focus of this brief review. At this point, two conclusions seem appropriate. First, in type 1 diabetes and advanced type 2 diabetes, the absence of an increment in glucagon secretion, in the setting of an absent decrement in insulin secretion and an attenuated increment in sympathoadrenal activity, in response to falling plasma glucose concentrations plays a key role in the pathogenesis of iatrogenic (therapeutic hyperinsulinemia induced) hypoglycemia (9–15). Second, there is increasing evidence that, in the aggregate, suggests that relative hyperglucagonemia, in the setting of deficient insulin secretion, plays a role in the pathogenesis of hyperglycemia in diabetes (16–24).
Regulation of Glucagon Secretion by Insulin
Regulatory redundancy and hierarchy are principles of critical physiology. For example, multiple mechanisms are involved in the normal defense against falling plasma glucose concentrations, but some are more important than others (12). There are also redundant mechanisms involved in the regulation of glucagon secretion (25–34). The view that restraint of glucagon secretion by insulin stands high in the hierarchy of those mechanisms is developed in the paragraphs that follow.
The regulation of α-cell glucagon secretion by nutrients, hormones, neurotransmitters, and drugs is complex (25–34). It involves direct signaling of α-cells (25) and indirect signaling of α-cells by β-cell (26–29) and δ-cell (30) secretory products, the autonomic nervous system (31, 32), and gut incretins (33) as well as an array of autocrine signals (34). Perfusion of the brain with glucose in dogs (35) and ganglionic blockade with trimethaphan in humans (36) have been reported to decrease, but not eliminate, the plasma glucagon response to marked hypoglycemia. However, the plasma glucagon response to hypoglycemia is normal in humans with no sympathoadrenal response because of cervical spinal cord transection (37) or preganglionic sympathectomy (38). Furthermore, in response to low glucose concentrations, quantitatively normal glucagon secretion occurs from the denervated (transplanted) human pancreas (39) and the denervated dog pancreas (40), and qualitatively normal glucagon secretion occurs from the perfused rodent pancreas (41) and perifused rodent and human islets (25). Therefore, islet innervation and gut factors do not appear to be critical to the glucagon secretory response to hypoglycemia, and loss of these extrapancreatic factors cannot explain the complete loss of the glucagon secretory response to hypoglycemia that occurs in insulin-deficient diabetes (13–15). Among the intraislet mechanisms, a decrease in δ-cell somatostatin secretion could signal an increase in glucagon secretion during hypoglycemia (30). Indeed, administration of a somatostatin receptor antagonist has been reported to increase the glucagon response to hypoglycemia in streptozotocin diabetic rats (42). Nonetheless, selective destruction of β-cell insulin secretion with streptozotocin (43) results in both diabetes and loss of the α-cell glucagon response to hypoglycemia in rats (44). Furthermore, although glucose suppressed glucagon release and stimulated somatostatin release from perifused human islets, inclusion of a somatostatin receptor antagonist did not prevent glucose-induced suppression of glucagon release (25). Therefore, the focus here is on reciprocal regulation of α-cell glucagon secretion by β-cell insulin.
There are several debated issues that are of considerable interest but are not discussed here because they are not critical to the interpretation of the data that follow. 1) The microcirculation does (45) or does not (46) flow from β-cells to α-cells to δ-cells in human islets. That is relevant to the extent to which effects of β-cell insulin secretion on α-cell glucagon secretion are via the intraislet or the systemic circulation. 2) Glucose acts directly on α-cells to suppress (25) or stimulate (29) glucagon secretion. 3) The β-cell secretory product that inhibits α-cell glucagon secretion is insulin (47) or zinc (44). That insulin is at least one such secretory product is discussed.
There is considerable evidence that insulin is a β-cell secretory product that reciprocally regulates α-cell glucagon secretion in experimental animals (48). First, administration of insulin suppresses plasma glucagon concentrations in several species (49). Second, perfusion of the rat (27) and the human (50) pancreas (and incubation of rat islets) (51) with an antibody to insulin increases glucagon release. Third, small interfering RNA-mediated knockdown of insulin receptors prevents the effect of low glucose concentrations to increase glucagon release from isolated mouse islets (52), and blockade of insulin signaling with the phosphatidylinositol 3-kinase inhibitor wortmannin prevents the effect of high glucose concentrations to decrease glucagon release from isolated islets (53). Fourth, α-cell-specific insulin receptor knockout mice display hyperglucagonemia, glucose intolerance with hyperglycemia in the fed state, and an enhanced glucagon response to hypoglycemia (54).
The evidence that indirect, reciprocal β-cell insulin signaling of α-cells normally predominates over direct α-cell signaling in the regulation of glucagon secretion has now been extended to humans (47, 55–59). In humans, 1) intraislet hyperinsulinemia prevents the increment in circulating glucagon in response to hypoglycemia (55); 2) reduction of the decrement in intraislet insulin reduces the increment in circulating glucagon during hypoglycemia (56, 57); 3) enhancement of the decrement in intraislet insulin increases the increment in circulating glucagon during hypoglycemia (58); 4) a mixed meal (or the sulfonylurea glimepiride) suppresses plasma glucagon levels in individuals with normal endogenous insulin secretion but increases plasma glucagon levels in individuals who cannot increase insulin secretion (i.e. those with type 1 diabetes) (59); and 5) an increase in systemic, and thus intraislet, zinc-free insulin suppresses glucagon secretion, and a sharp decrease in systemic, and thus intraislet, zinc-free insulin causes an increment in glucagon secretion during hypoglycemia in people with type 1 diabetes who have no glucagon response to hypoglycemia in the absence of a decrease in circulating insulin or to a decrease in insulin in the absence of hypoglycemia (47). Thus, both an indirect signal to α-cells, a decrease in β-cell insulin secretion, and a direct signal to α-cells, a low plasma glucose concentration, are required for a positive glucagon secretory response to hypoglycemia.
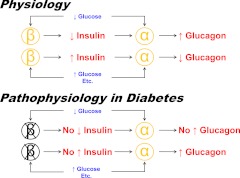
Based on these data, the physiological construct is as follows (47): 1) β-cell insulin, perhaps among other β-cell secretory products, tonically restrains α-cell glucagon secretion during postabsorptive euglycemia; 2) a decrease in β-cell insulin secretion, in concert with a low plasma glucose concentration, signals an increase in α-cell glucagon secretion during hypoglycemia; and 3) an increase in β-cell insulin secretion negates direct α-cell stimulation and thus results in no change in, or even suppression of, α-cell glucagon secretion after a mixed meal. Clearly, insulin is a β-cell secretory product that, in concert with glucose and among other signals, reciprocally regulates α-cell glucagon secretion in humans. The intimate relationship between β-cell insulin secretion and α-cell glucagon secretion is illustrated in Fig. 1.
Fig. 1.
Physiology (in nondiabetes) and pathophysiology (in absolute endogenous insulin-deficient type 1 diabetes and advanced type 2 diabetes) of the intimate relationship between the inhibitory effect of pancreatic β-cell insulin secretion on pancreatic islet α-cell glucagon secretion in humans. Normally a decrease in plasma glucose causes a decrease in β-cell insulin secretion that signals an increase in α-cell glucagon secretion during hypoglycemia. An increase in plasma glucose, among other nutrients, causes an increase in β-cell insulin secretion that prevents an increase in α-cell glucagon secretion in response to those nutrients after a mixed meal. On the other hand, in the setting of β-cell failure in type 1 diabetes and advanced type 2 diabetes, a decrease in plasma glucose cannot cause a decrease in β-cell insulin secretion, and the absence of that signal results in no increase in pancreatic α-cell glucagon secretion during hypoglycemia. Conversely, in the setting of β-cell failure, an increase in plasma glucose, among other nutrients, cannot cause an increase in β-cell insulin secretion, and the absence of that restraining signal results in an increase in pancreatic α-cell glucagon secretion after a mixed meal.
Relevance of Glucagon to Hypoglycemia in Diabetes
Glucose is an obligate metabolic fuel for the brain under physiological conditions (15). The brain cannot synthesize glucose, and it cannot store more than a few minutes supply as glycogen. Thus, survival of the brain, and therefore the individual, requires a virtually continuous supply of glucose from the circulation to the brain. Blood-to-brain glucose transport is a direct function of the arterial plasma glucose concentration. Therefore, it is not surprising that mechanisms that normally very effectively prevent or rapidly correct hypoglycemia have evolved (15). The physiological defenses against falling plasma glucose concentrations in humans are 1) a decrease in β-cell insulin secretion, 2) an increase in α-cell glucagon secretion, and 3) absent the latter, an increase in adrenomedullary epinephrine secretion (9–12). [Epinephrine is not critical if insulin and glucagon secretion are intact (12).] The behavioral defense is carbohydrate ingestion prompted by symptoms, which are largely sympathoadrenal in origin (12, 60), that cause the individual to recognize hypoglycemia (9–12).
Because of the effectiveness of these defenses, hypoglycemia is a distinctly uncommon clinical event except in people with diabetes who use medications, such as a sulfonylurea, a glinide, or insulin, that raise circulating insulin concentrations (regardless of the plasma glucose concentration) to lower their plasma glucose levels (15). In that setting, hypoglycemia is common. Indeed, hypoglycemia is the limiting factor in the glycemic management of diabetes (15). It causes recurrent morbidity in most people with type 1 diabetes and many with advanced (absolute endogenous insulin-deficient) type 2 diabetes and is sometimes fatal. It generally precludes maintenance of euglycemia over a lifetime of diabetes and, thus, full realization of the vascular benefits of glycemic control. It impairs defenses against subsequent falling plasma glucose concentrations and, thus, causes a vicious cycle of recurrent hypoglycemia.
Hypoglycemia in diabetes is fundamentally iatrogenic, the result of relative or absolute therapeutic hyperinsulinemia that causes the plasma glucose concentration to decline. However, that alone seldom results in hypoglycemia. Rather, hypoglycemia is typically the result of the interplay of therapeutic hyperinsulinemia and compromised defenses against falling plasma glucose concentrations (13–15). The compromised defenses include loss of the decrease in insulin and loss of the increase in glucagon as plasma glucose concentrations fall (13–15). [They also include attenuated adrenomedullary and sympathetic neural responses to falling plasma glucose concentrations resulting in the clinical syndromes of defective glucose counterregulation and hypoglycemia unawareness, respectively, collectively termed hypoglycemia-associated autonomic failure in diabetes (15), but defective glucose counterregulation, with its markedly increased risk for severe hypoglycemia, develops only in the setting of absent insulin and glucagon responses (13–15).]
The mechanism of the loss of the decrease in insulin secretion as plasma glucose concentrations fall in response to therapeutic hyperinsulinemia in type 1 diabetes and advanced type 2 diabetes is straightforward. It is β-cell failure (13–15). Given the evidence that β-cell insulin reciprocally regulates α-cell glucagon secretion not only in experimental animals (27, 48–54) but also in humans (47, 48, 55–59), it follows that loss of the increase in glucagon as plasma glucose concentrations fall in type 1 diabetes and advanced type 2 diabetes is also plausibly attributable to β-cell failure, specifically, the absence of a decrease in insulin secretion to signal an increase in glucagon secretion during hypoglycemia (13–15). That construct is supported by the findings that 1) an increase in glucagon secretion can be triggered by a decrease in (exogenous) insulin during hypoglycemia in people with type 1 diabetes (47), 2) the degree of loss of glucagon secretion is associated with the degree of loss of insulin secretion in diabetes (61), and 3) the normal inverse relationship between pulses of insulin and glucagon secretion, with insulin possibly driving glucagon, is lost in diabetes (62). Thus, the pathophysiology of glucose counterregulation is the same in type 1 and type 2 diabetes, although it develops at different rates (13–15). Loss of the decrement in insulin secretion and loss of the increment in glucagon secretion as plasma glucose concentrations fall develops early, and recurrent hypoglycemia becomes a major clinical problem early, in type 1 diabetes. In contrast, absolute loss of insulin secretion, and the resulting loss of the decrement in insulin secretion and loss of the increment in glucagon secretion as plasma glucose concentrations fall, develops slowly, and recurrent hypoglycemia becomes a major clinical problem later, in type 2 diabetes. This mechanism is illustrated in Fig. 1.
As mentioned earlier, α-cell glucagon secretion is unchanged or even suppressed after a mixed meal in nondiabetic individuals, a pattern attributable to negation of direct α-cell stimulation by nutrients (e.g. amino acids) by β-cell insulin secretion (59). In contrast, in the absence of β-cell insulin secretion, in people with type 1 diabetes, α-cell glucagon secretion increases after a mixed meal (59, 63, 64). Given that pattern, it is conceivable that progressively reduced early insulin secretion might underlie the progressive failure of postprandial suppression of glucagon secretion as individuals pass from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes (65). However, an initial experiment failed to support that construct. Partial inhibition of insulin secretion, with the KATP channel agonist (opener) diazoxide, in nondiabetic individuals produced an increase in plasma glucose concentrations but not hyperglucagonemia after a mixed meal (66).
In summary, the data support the conclusion that in type 1 diabetes and advanced type 2 diabetes, the absence of an increment in glucagon secretion, in the setting of an absent decrement in insulin secretion and an attenuated increment in sympathoadrenal activity, in response to falling plasma glucose concentrations plays a key role in the pathogenesis of iatrogenic hypoglycemia (9–15). Furthermore, the data suggest that loss of the increment in glucagon secretion, like the loss of the decrement in insulin secretion, during hypoglycemia is the result of β-cell failure in type 1 diabetes and advanced type 2 diabetes (13–15) (Fig. 1).
Glucagon Supports the Plasma Glucose Concentration
There is considerable evidence that glucagon supports the plasma glucose concentration in nondiabetic humans (67–73). That includes studies of the effect of infusion of somatostatin (or of the somatostatin analog octreotide) to suppress insulin and glucagon secretion and infusion of somatostatin (or octreotide) with insulin replacement to define the effect of isolated glucagon deficiency. Those studies demonstrated substantial decrements in plasma glucose concentrations (or glucose production rates) during infusion of somatostatin with insulin (67–71). However, the finding that insulin infusion alone in doses smaller than the putative replacement doses used in the earlier somatostatin studies lowered plasma glucose concentrations to subphysiological levels (72), and of the failure of a replacement dose of glucagon to completely reverse the effect of octreotide plus an even lower dose of insulin on plasma glucose concentrations (71), both in nondiabetic humans, raised the possibility of excessive insulin replacement in the earlier studies. Obviously, insulin over-replacement would exaggerate the apparent effect of glucagon lack on plasma glucose concentrations. However, that concern was obviated by a study based on the premise that postabsorptive people with type 1 diabetes receiving iv insulin in an individualized dose that maintains euglycemia over time are receiving biologically optimal insulin replacement. In such patients, suppression of glucagon secretion with octreotide caused a progressive fall in plasma glucose concentrations that was prevented by low-dose glucagon replacement (73).
Additional evidence that glucagon supports the plasma glucose concentration, largely in experimental animals, includes studies with neutralizing glucagon antibodies (74), glucagon antagonists (75–79), and glucagon receptor antisense oligonucleotides (80–82), those in glucagon receptor-null (23, 83, 84) and α-cell-deleted (85) mice, and those of the effect of leptin (86–89). Administration of a neutralizing glucagon antibody has been shown to lower plasma glucose concentrations in several species including nondiabetic and diabetic rabbits (75). Glucagon antagonists have been reported to lower plasma glucose concentrations in ob/ob mice (76), reduce the blood glucose response to glucagon administration in mice and rhesus monkeys, and lower blood glucose concentrations in mice fed a high-fat diet (77), block the effect of administered glucagon to increase hepatic glucose production in dogs (78), and lower fasting plasma glucose concentrations in mice fed a high-fat diet (79). One glucagon antagonist was shown to reduce the plasma glucose response to administered glucagon in humans (80). Administration of glucagon receptor antisense oligonucleotides has been found to reduce blood glucose concentrations in diabetic rodent models (81, 82). Indeed, administration of a glucagon receptor antisense nucleotide to nondiabetic humans over 6 wk has been reported to blunt the glucagon-induced increase in glucose production and plasma glucose (83); interestingly, hypoglycemia did not occur in treated individuals. Glucagon receptor-null mice were found to have lower fasting and fed plasma glucose concentrations (84, 85). Notably, in contrast to mice with intact glucagon receptors, glucagon receptor-null mice did not develop diabetes after streptozotocin administration that reduced islet β-cell volumes and plasma insulin concentrations by 90% (23). In addition, leptin administration suppressed glucagon gene expression in vitro (86) and plasma glucagon and glucose concentrations in vivo in nonobese diabetic mice (87). However, compared with placebo, administration of recombinant methionyl human leptin, in doses that raised plasma leptin concentrations 3-fold and 150-fold, over 14 d to obese humans with newly diagnosed type 2 diabetes had no effect on insulin-mediated suppression of glucose, glycerol, or palmitate rates of appearance and did not increase insulin-mediated stimulation of glucose disposal (88) and metreleptin and administered over 16 wk did not reduce A1C levels substantially in patients with type 2 diabetes (89). Those findings are consistent with earlier reports that leptin administration did not cause a greater reduction in plasma glucose concentrations than caloric restriction alone in obese subjects (90, 91). Finally, a patient with a glucagon receptor mutation and marked hyperglucagonemia, but not well documented hypoglycemia, has been reported (92, 93).
Given the convincing evidence that glucagon supports the plasma glucose concentration in humans (67–73, 79, 82, 92, 93) as well as in experimental animals (23, 74–78, 80, 81, 83–86), just discussed, what is the evidence that glucagon, in the setting of deficient insulin secretion, plays a distinct role in the pathogenesis of hyperglycemia in human diabetes?
Relevance of Glucagon to Hyperglycemia in Human Diabetes
Fasting plasma glucagon concentrations are not consistently elevated in type 1 diabetes (59, 94) or in type 2 diabetes (16, 94), although significant elevations have been found with serial sampling in both type 1 diabetes (95) and type 2 diabetes (95, 96). Thus, the general notion that glucagon plays a role in the pathogenesis of hyperglycemia in diabetes rests, at least in part, on the concept of relative hyperglucagonemia, plasma glucagon concentrations that are inappropriately high in the setting of hyperglycemia that would be expected to suppress glucagon secretion (16). However, that concept requires two assumptions. First, it assumes a long-term suppressive effect of hyperglycemia on glucagon secretion that is similar to the short-term effect of acute hyperglycemia typically associated with hyperinsulinemia. Second, it assumes that despite its transient glycemic effect in the short term (16), hyperglucagonemia continues to stimulate glucose production in the long term. With respect to the latter, glucagon infusion for 4 wk has been reported to persistently raise blood glucose concentrations in mice, an effect prevented by coadministration of a glucagon antagonist (97).
Early evidence that relative hyperglucagonemia, in the setting of deficient insulin secretion, plays a role in the pathogenesis of hyperglycemia in human diabetes (16–24) came from the studies of Gerich and his colleagues (17, 18). In people with type 1 diabetes, they found that infusion of somatostatin 1) delayed the development of hyperglycemia and ketosis after withdrawal of insulin therapy (17) and 2) lowered plasma glucose concentrations in insulin-withdrawn, hyperglycemic patients (18). Because those type 1 diabetic individuals almost assuredly lacked appreciable endogenous insulin secretion, it is quite reasonable to attribute the plasma glucose-lowering actions of somatostatin to its suppression of glucagon secretion. Thus, a somatostatin-responsive factor, presumably glucagon, is involved in the pathogenesis of hyperglycemia in type 1 diabetes. However, the data do not document that glucagon supports the plasma glucose concentration to a greater extent in people with type 1 diabetes than in nondiabetic individuals. The interpretation of the findings of Baron and colleagues (98) that infusion of somatostatin reduced endogenous glucose production and plasma glucose concentrations in postabsorptive people with type 2 diabetes and nondiabetic controls is also challenging, as the authors acknowledged. The initial decrements in glucose production and plasma glucose concentrations during somatostatin infusions were similar in those with type 2 diabetes and in nondiabetic controls. That does not indicate a disproportionate plasma glucose-raising effect of glucagon in diabetes.
Administration of a long-acting somatostatin analog to people with type 1 diabetes over days to weeks has been reported to reduce glycemia in some (99) but not other (100, 101) studies. However, it increases sensitivity to insulin (102, 103), perhaps because of suppression of glucagon secretion.
Clearly, higher plasma glucagon concentrations can result in higher rates of glucose production and higher plasma glucose concentrations after a meal. For example, that occurred in people with type 1 diabetes given glucagon infusions to maintain postprandial plasma glucagon concentrations compared with when such glucagon infusions were delayed for 2 h (104).
Reduced suppression of plasma glucagon concentrations and higher rates of glucose production and plasma glucose concentrations after a meal are features of diabetes (20, 21, 56, 105–108). Aside from the temporal relationships, what is the evidence that the higher postprandial glucagon levels, as opposed to the lower insulin action alone, is causative of the higher rates of glucose production and plasma glucose concentrations (19)? In somatostatin-infused nondiabetic individuals given prandial glucose infusions with insulin infused to produce a diabetic profile, Shah et al. (20) found higher plasma glucose concentrations when glucagon was infused to maintain basal levels compared with when glucagon was allowed to fall over 2 h. Notably, when a nondiabetic insulin profile was provided, the differences in plasma glucose were small whether plasma glucagon concentrations were held constant or allowed to decline. Interestingly, when plasma glucagon concentrations were suppressed, plasma glucose concentrations differed only minimally during the nondiabetic and diabetic insulin profiles. Thus, these data indicate that a lack of postprandial suppression of plasma glucagon concentrations can cause greater postprandial hyperglycemia when insulin availability is limited, as it is in diabetes. A similar study in people with type 2 diabetes (21) led the authors to the same conclusions. This concept is also illustrated in Fig. 1.
Finally, the finding of Ahrén (24) that women who developed impaired glucose tolerance, compared with those who maintained normal glucose tolerance, exhibited defective suppression of plasma glucagon concentrations during earlier testing when they were still glucose tolerant provides another clue that glucagon contributes to the pathogenesis of hyperglycemia in diabetes in humans.
Although antagonism of the action of glucagon might seem an attractive treatment of diabetes, there are some concerns about the safety of that approach (109). Glucagon receptor-null mice have islet hyperplasia and markedly elevated plasma glucagon concentrations (109) and the long-term outcomes of those in humans are unknown. There is also a risk of liver injury (109). The contention that glucagon receptor-null mice display increased glucose counterregulation (109) is not supported by appropriate in vivo data (84). It is conceivable that blockade of glucagon action or secretion might increase the risk of iatrogenic hypoglycemia in the setting of endogenous insulin deficiency, but the glucagon response to falling plasma glucose concentrations is typically lost in individuals with absolute endogenous insulin deficiency as discussed earlier (15).
In summary, there is increasing evidence that, in the aggregate, suggests that relative hyperglucagonemia, in the setting of deficient insulin secretion, plays a role in the pathogenesis of hyperglycemia in diabetes (16–24) (Fig. 1). That appears to have been documented in humans. In two randomized, placebo-controlled trials, administration of glucagon receptor antagonists lowered fasting and postprandial plasma glucose concentrations and A1C levels, albeit with low-density lipoprotein cholesterol and transaminase elevations, in people with type 2 diabetes (110, 111).
Acknowledgments
Ms. Janet Dedeke, the author's assistant, prepared this manuscript.
The author's original work cited was supported, in part, by National Institutes of Health Grants R37 DK27085, MO1 RR00036 (now UL1 RR24992), P60 DK20579, and T32 DK07120 and by a fellowship award from the American Diabetes Association.
Disclosure Summary: The author has served as a consultant to Merck & Co., Bristol-Meyers Squibb/AstraZeneca, MannKind Corp., Marcadia Biotech, and Novo Nordisk in recent years. He does not receive research funds from, hold stock in, or speak for any pharmaceutical or device firm.
References
- 1. Cherrington AD. 2001. Control of glucose production in vivo by insulin and glucagon. In: Jefferson LS, Cherrington AD, eds. Handbook of physiology. Section 7: the endocrine system. Vol II: the endocrine pancreas and regulation of metabolism. New York: Oxford University Press; 759–785 [Google Scholar]
- 2. Gustavson SM, Chu CA, Nishizawa M, Farmer B, Neal D, Yang Y, Vaughan S, Donahue EP, Flakoll P, Cherrington AD. 2003. Glucagon's actions are modified by the combination of epinephrine and gluconeogenic precursor infusion. Am J Physiol Endocrinol Metab 285:E534–E544 [DOI] [PubMed] [Google Scholar]
- 3. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. 2011. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 13(Suppl 1):118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGarry JD, Foster DW. 1980. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 49:395–420 [DOI] [PubMed] [Google Scholar]
- 5. Xiao C, Pavlic M, Szeto L, Patterson BW, Lewis GF. 2011. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ranganath L, Schaper F, Gama R, Morgan L. 2001. Does glucagon have a lipolytic effect? Clin Endocrinol (Oxf) 54:125–126 [DOI] [PubMed] [Google Scholar]
- 7. Bertin E, Arner P, Bolinder J, Hagström-Toft E. 2001. Action of glucagon and glucagon-like peptide-1-(7–36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J Clin Endocrinol Metab 86:1229–1234 [DOI] [PubMed] [Google Scholar]
- 8. Gravholt CH, Møller N, Jensen MD, Christiansen JS, Schmitz O. 2001. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J Clin Endocrinol Metab 86:2085–2089 [DOI] [PubMed] [Google Scholar]
- 9. Garber AJ, Cryer PE, Santiago JV, Haymond MW, Pagliara AS, Kipnis DM. 1976. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest 58:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerich J, Davis J, Lorenzi M, Rizza R, Bohannon N, Karam J, Lewis S, Kaplan R, Schultz T, Cryer P. 1979. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol 236:E380–E385 [DOI] [PubMed] [Google Scholar]
- 11. Rizza RA, Cryer PE, Gerich JE. 1979. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined α- and β-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest 64:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cryer PE. 2001. The prevention and correction of hypoglycemia. In: Jefferson LS, Cherrington AD, eds. Handbook of physiology. Section 7: the endocrine system. Vol II: the endocrine pancreas and regulation of metabolism. New York: Oxford University Press; 1057–1092 [Google Scholar]
- 13. Dagogo-Jack SE, Craft S, Cryer PE. 1993. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus: recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Segel SA, Paramore DS, Cryer PE. 2002. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes 51:724–733 [DOI] [PubMed] [Google Scholar]
- 15. Cryer PE. 2009. Hypoglycemia in diabetes: pathophysiology, prevalence and prevention. Alexandria, VA: American Diabetes Association; 45–95 [Google Scholar]
- 16. Dunning BE, Gerich JE. 2007. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 28:253–283 [DOI] [PubMed] [Google Scholar]
- 17. Gerich JE, Lorenzi M, Bier DM, Schneider V, Tsalikian E, Karam JH, Forsham PH. 1975. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med 292:985–989 [DOI] [PubMed] [Google Scholar]
- 18. Gerich JE, Schneider VS, Lorenzi M, Tsalikian E, Karam JH, Bier DM, Forsham PH. 1976. Role of glucagon in human diabetic ketoacidosis: studies using somatostatin. Clin Endocrinol (Oxf) 5(Suppl):299S–305S [DOI] [PubMed] [Google Scholar]
- 19. Rizza RA. 2010. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59:2697–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah P, Basu A, Basu R, Rizza R. 1999. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 277(2 Pt 1):E283–E290 [DOI] [PubMed] [Google Scholar]
- 21. Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. 2000. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85:4053–4059 [DOI] [PubMed] [Google Scholar]
- 22. Unger RH, Orci L. 2010. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. 2011. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahrén B. 2009. β- and α-cell dysfunction in subjects developing impaired glucose tolerance. Diabetes 58:726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker JN, Ramracheya R, Zhang Q, Johnson PRV, Braun M, Rorsman P. 2011. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab 13(Suppl 1):95–105 [DOI] [PubMed] [Google Scholar]
- 26. Samols E, Tyler J, Marks V. 1972. Glucagon-insulin interrelationships. In: Lefebvre P, Unger RH, eds. Glucagon: molecular physiology, clinical and therapeutic implications. Elmsford, NY: Pergamon Press; 151–174 [Google Scholar]
- 27. Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. 1984. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC. 2006. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion. Diabetes 55:1051–1056 [DOI] [PubMed] [Google Scholar]
- 29. Gromada J, Franklin I, Wollheim CB. 2007. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84–116 [DOI] [PubMed] [Google Scholar]
- 30. Braun M, Ramracheya R, Amisten S, Bengtsson M, Moritoh Y, Zhang Q, Johnson PR, Rorsman P. 2009. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic δ-cells. Diabetologia 52:1566–1578 [DOI] [PubMed] [Google Scholar]
- 31. Taborsky GJ, Jr, Ahrén B, Havel PJ. 1998. Autonomic mediation of glucagon secretion during hypoglycemia. Diabetes 47:995–1005 [DOI] [PubMed] [Google Scholar]
- 32. Marty N, Dallaporta M, Thorens B. 2007. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 22:241–251 [DOI] [PubMed] [Google Scholar]
- 33. Holst JJ. 2007. The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 34. Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Köhler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, Berggren PO. 2008. Glutamate is a positive autocrine signal for glucagon release. Cell Metab 7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Frizzell RT. 1989. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes 38:7–16 [DOI] [PubMed] [Google Scholar]
- 36. Havel PJ, Ahren B. 1997. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced hypoglycemia in women. Diabetes 46:801–807 [DOI] [PubMed] [Google Scholar]
- 37. Palmer JP, Henry DP, Benson JW, Johnson DG, Ensinck JW. 1976. Glucagon response to hypoglycemia in sympathectomized man. J Clin Invest 57:522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frier BM, Corrall RJ, Ratcliffe JG, Ashby JP, McClemont EJ. 1981. Autonomic neural control mechanisms of substrate and hormonal responses to acute hypoglycaemia in man. Clin Endocrinol (Oxf) 14:425–433 [DOI] [PubMed] [Google Scholar]
- 39. Diem P, Redmon JB, Abid M, Moran A, Sutherland DE, Halter JB, Robertson RP. 1990. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest 86:2008–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sherck SM, Shiota M, Saccomando J, Cardin S, Allen EJ, Hastings JR, Neal DW, Williams PE, Cherrington AD. 2001. Pancreatic response to mild non-insulin induced hypoglycemia does not involve extrinsic neural input. Diabetes 50:2487–2496 [DOI] [PubMed] [Google Scholar]
- 41. Gerich JE, Charles MA, Grodsky GM. 1974. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 54:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yue JTY, Burdett E, Coy DH, Efendic S, Vranic M. 2009. Normalization of the glucagon response to hypoglycemia in STZ-diabetic rats via somatostatin type 2 receptor antagonism. Diabetes 58:A35 (Abstract) [Google Scholar]
- 43. Dufrane D, Maillart JF, Aouassar N, Goebbels RM, Guiot Y, Gianello P. 2009. Native pancreatic α-cell adaptation in streptozotocin-induced diabetic primates: importance for pig islet xenotransplantation. Xenotransplantation 16:152–163 [DOI] [PubMed] [Google Scholar]
- 44. Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. 2007. Zinc, not insulin, regulates the rat α-cell response to hypoglycemia in vivo. Diabetes 56:1107–1112 [DOI] [PubMed] [Google Scholar]
- 45. Stagner JI, Samols E. 1992. The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41:93–97 [DOI] [PubMed] [Google Scholar]
- 46. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. 2006. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooperberg BA, Cryer PE. 2010. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 59:2936–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bansal P, Wang Q. 2008. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab 295:E751–E761 [DOI] [PubMed] [Google Scholar]
- 49. Galassetti P, Davis SN. 2000. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci (Lond) 99:351–362 [PubMed] [Google Scholar]
- 50. Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, Gingerich R. 2001. Immunoneutralization of somatostatin, insulin and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 23:302–308 [DOI] [PubMed] [Google Scholar]
- 51. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. 2005. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 52. Diao J, Asghar Z, Chan CB, Wheeler MB. 2005. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic α-cells. J Biol Chem 280:33487–33496 [DOI] [PubMed] [Google Scholar]
- 53. Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. 2006. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3:47–58 [DOI] [PubMed] [Google Scholar]
- 54. Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. 2009. Insulin signaling in α-cells modulates glucagon secretion in vivo. Cell Metab 9:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banarer S, McGregor VP, Cryer PE. 2002. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 51:958–965 [DOI] [PubMed] [Google Scholar]
- 56. Raju B, Cryer PE. 2005. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes. Diabetes 54:757–764 [DOI] [PubMed] [Google Scholar]
- 57. Gosmanov NR, Szoke E, Israelian Z, Smith T, Cryer PE, Gerich JE, Meyer C. 2005. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care 28:1124–1131 [DOI] [PubMed] [Google Scholar]
- 58. Israelian Z, Gosmanov NR, Szoke E, Schorr M, Bokhari S, Cryer PE, Gerich JE, Meyer C. 2005. Increasing the decrement in insulin secretion improves glucagon responses to hypoglycemia in advanced type 2 diabetes. Diabetes Care 28:2691–2696 [DOI] [PubMed] [Google Scholar]
- 59. Cooperberg BA, Cryer PE. 2009. β-Cell-mediated signaling predominates over direct α-cell signaling in the regulation of glucagon secretion in humans. Diabetes Care 32:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Towler DA, Havlin CE, Craft S, Cryer P. 1993. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 42:1791–1798 [DOI] [PubMed] [Google Scholar]
- 61. Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y, Shima K. 1988. Correlation between minimal secretory capacity of pancreatic β-cells and stability of diabetic control. Diabetes 37:81–88 [DOI] [PubMed] [Google Scholar]
- 62. Menge BA, Grüber L, Jørgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ. 2011. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes 60:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown RJ, Sinaii N, Rother KI. 2008. Too much glucagon, too little insulin. Diabetes Care 31:1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pörksen S, Nielsen LB, Kaas A, Kocova M, Chiarelli F, Orskov C, Holst JJ, Ploug KB, Hougaard P, Hansen L, Mortensen HB; Hvidøre Study Group on Childhood Diabetes 2007. Meal-stimulated glucagon release is associated with postprandial blood glucose level and does not interfere with glycemic control in children and adolescents with new-onset type 1 diabetes. J Clin Endocrinol Metab 92:2910–2916 [DOI] [PubMed] [Google Scholar]
- 65. Abdul-Ghani M, DeFronzo RA. 2007. Fasting hyperglycemia impairs glucose- but not insulin-mediated suppression of glucagon secretion. J Clin Endocrinol Metab 92:1778–1784 [DOI] [PubMed] [Google Scholar]
- 66. Ramanathan RP, Arbeláez AM, Cryer PE. 2011. Partial inhibition of insulin secretion results in glucose intolerance but not hyperglucagonemia. Diabetes 60:1324–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lins PE, Efendić S. 1976. Hyperglycemia induced by somatostatin in normal subjects. Horm Metab Res 8:497–498 [DOI] [PubMed] [Google Scholar]
- 68. Liljenquist JE, Mueller GL, Cherrington AD, Keller U, Chiasson J-L, Perry JM, Lacy WW, Rabinowitz D. 1977. Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest 59:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sherwin RS, Hendler R, DeFronzo R, Wahren J, Felic P. 1977. Glucose homeostasis during prolonged suppression of glucagon and insulin secretion by somatostatin. Proc Natl Acad Sci USA 74:348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosen SG, Clutter WE, Berk MA, Shah SD, Cryer PE. 1984. Epinephrine supports the postabsorptive plasma glucose concentration and prevents hypoglycemia when glucagon secretion is deficient in man. J Clin Invest 73:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Breckenridge SM, Cooperberg BA, Arbelaez AM, Patterson BW, Cryer PE. 2007. Glucagon, in concert with insulin, supports the postabsorptive plasma glucose concentration in humans. Diabetes 56:2442–2448 [DOI] [PubMed] [Google Scholar]
- 72. Breckenridge SM, Raju B, Arbelaez AM, Patterson BW, Cooperberg BA, Cryer PE. 2007. Basal insulin, glucagon, and growth hormone replacement. Am J Physiol Endocrinol Metab 293:E1303–E1310 [DOI] [PubMed] [Google Scholar]
- 73. Cooperberg BA, Cryer PE. 2010. Glucagon supports postabsorptive plasma glucose concentrations in humans with biologically optimal insulin levels. Diabetes 59:2941–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brand CL, Jørgensen PN, Svendsen I, Holst JJ. 1996. Evidence for a major role for glucagon in regulation of plasma glucose in conscious, nondiabetic, and alloxan-induced diabetic rabbits. Diabetes 45:1076–1083 [DOI] [PubMed] [Google Scholar]
- 75. Lau YY, Ma P, Gibiansky L, Komorowski R, Wang J, Wang G, Yan H, Véniant MM, Kakkar T. 2009. Pharmacokinetic and pharmacodynamic modeling of a monoclonal antibody antagonist of glucagon receptor in male ob/ob mice. AAPS J 11:700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim RM, Chang J, Lins AR, Brady E, Candelore MR, Dallas-Yang Q, Ding V, Dragovic J, Iliff S, Jiang G, Mock S, Qureshi S, Saperstein R, Szalkowski D, Tamvakopoulos C, Tota L, Wright M, Yang X, Tata JR, Chapman K, Zhang BB, Parmee ER. 2008. Discovery of potent, orally active benzimidazole glucagon receptor antagonists. Bioorg Med Chem Lett 18:3701–3705 [DOI] [PubMed] [Google Scholar]
- 77. Rivera N, Everett-Grueter CA, Edgerton DS, Rodewald T, Neal DW, Nishimura E, Larsen MO, Jacobsen LO, Kristensen K, Brand CL, Cherrington AD. 2007. A novel glucagon receptor antagonist, NNC 25-0926, blunts hepatic glucose production in the conscious dog. J Pharmacol Exp Ther 321:743–752 [DOI] [PubMed] [Google Scholar]
- 78. Winzell MS, Brand CL, Wierup N, Sidelmann UG, Sundler F, Nishimura E, Ahrén B. 2007. Glucagon receptor antagonism improves islet function in mice with insulin resistance induced by a high-fat diet. Diabetologia 50:1453–1462 [DOI] [PubMed] [Google Scholar]
- 79. Petersen KF, Sullivan JT. 2001. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia 44:2018–2024 [DOI] [PubMed] [Google Scholar]
- 80. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. 2004. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53:410–417 [DOI] [PubMed] [Google Scholar]
- 81. Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD, Brenner MB, Raap A, Gromada J, Berridge BR, Monteith DK, Porksen N, McKay RA, Monia BP, Bhanot S, Watts LM, Michael MD. 2004. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 113:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morgan ES, Brandt TA, Van Dongen MGJ, Geerts BF, Burggraaf J, Romijn JA, Cohen AF, Watanabe TA, Geary RS, Bhanot S. 2010. First proof of pharmacology of a novel glucagon receptor antisense drug in humans. Diabetes 59:79 (Abstract) [DOI] [PubMed] [Google Scholar]
- 83. Parker JC, Andrews KM, Allen MR, Stock JL, McNeish JD. 2002. Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun 290:839–843 [DOI] [PubMed] [Google Scholar]
- 84. Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. 2003. Lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hancock AS, Du A, Liu J, Miller M, May CL. 2010. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol 24:1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marroquí L, Vieira E, Gonzalez A, Nadal A, Quesada I. 2011. Leptin downregulates expression of the gene encoding glucagon in alphaTC1–9 cells and mouse islets. Diabetologia 54:843–851 [DOI] [PubMed] [Google Scholar]
- 87. Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH. 2010. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA 107:4813–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. 2011. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes 60:1474–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, Mylvaganam GH, Abanni L, Carbone F, Williams CJ, De Paoli AM, Schneider BE, Mantzoros CS. 2011. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes 60:1647–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. 2002. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord 26:504–509 [DOI] [PubMed] [Google Scholar]
- 91. Zelissen PM, Stenlof K, Lean ME, Fogteloo J, Keulen ET, Wilding J, Finer N, Rössner S, Lawrence E, Fletcher C, McCamish M; Author Group 2005. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab 7:755–761 [DOI] [PubMed] [Google Scholar]
- 92. Yu R, Nissen NN, Dhall D, Heaney AP. 2008. Nesidioblastosis and hyperplasia of α-cells, microglucagonoma, and nonfunctioning islet cell tumor of the pancreas. Pancreas 36:428–431 [DOI] [PubMed] [Google Scholar]
- 93. Zhou C, Dhall D, Nissen NN, Chen CR, Yu R. 2009. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, α-cell hyperplasia, and islet cell tumor. Pancreas 38:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. 1970. Studies of pancreatic α-cell function in normal and diabetic subjects. J Clin Invest 49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Raskin P, Unger RH. 1978. Effect of insulin therapy on the profiles of plasma immunoreactive glucagon in juvenile-type and adult-type diabetics. Diabetes 27:411–419 [DOI] [PubMed] [Google Scholar]
- 96. Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. 1987. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64:106–110 [DOI] [PubMed] [Google Scholar]
- 97. Li XC, Liao TD, Zhuo JL. 2008. Long-term hyperglucagonaemia induces early metabolic and renal phenotypes of type 2 diabetes in mice. Clin Sci (Lond) 114:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Baron AD, Schaeffer L, Shragg P, Kolterman OG. 1987. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36:274–283 [DOI] [PubMed] [Google Scholar]
- 99. Dimitriadis G, Gerich J. 1985. Effect of twice daily subcutaneous administration of a long-acting somatostatin analog on 24-hour plasma glucose profiles in patients with insulin-dependent diabetes mellitus. Horm Metab Res 17:510–511 [DOI] [PubMed] [Google Scholar]
- 100. Grossman LD, Shumak SL, George SR, Singer W, Zinman B. 1989. The effects of SMS 201–995 (Sandostatin) on metabolic profiles in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 68:63–67 [DOI] [PubMed] [Google Scholar]
- 101. Osei K, O'Dorisio TM, Malarkey WB, Craig EL, Cataland S. 1989. Metabolic effects of long-acting somatostatin analogue (Sandostatin) in type I diabetic patients on conventional therapy. Diabetes 38:704–709 [DOI] [PubMed] [Google Scholar]
- 102. Orskov L, Møller N, Bak JF, Pørksen N, Schmitz O. 1996. Effects of the somatostatin analog, octreotide, on glucose metabolism and insulin sensitivity in insulin-dependent diabetes mellitus. Metabolism 45:211–217 [DOI] [PubMed] [Google Scholar]
- 103. Bruttomesso D, Fongher C, Silvestri B, Barberio S, Marescotti MC, Iori E, Valerio A, Crazzolara D, Pianta A, Tiengo A, Del Prato S. 2001. Combination of continuous subcutaneous infusion of insulin and octreotide in type 1 diabetic patients. Diabetes Res Clin Pract 51:97–105 [DOI] [PubMed] [Google Scholar]
- 104. Dinneen S, Alzaid A, Turk D, Rizza R. 1995. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia 38:337–343 [DOI] [PubMed] [Google Scholar]
- 105. Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. 1970. Abnormal α-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283:109–115 [DOI] [PubMed] [Google Scholar]
- 106. Mitrakou A, Kelley D, Veneman T, Jenssen T, Pangburn T, Reilly J, Gerich J. 1990. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes 39:1381–1390 [DOI] [PubMed] [Google Scholar]
- 107. Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. 1992. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 326:22–29 [DOI] [PubMed] [Google Scholar]
- 108. Woerle HJ, Szoke E, Meyer C, Dostou JM, Wittlin SD, Gosmanov NR, Welle SL, Gerich JE. 2006. Mechanisms for abnormal postprandial glucose metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 290:E67–E77 [DOI] [PubMed] [Google Scholar]
- 109. Ali S, Drucker DJ. 2009. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab 296:E415–E421 [DOI] [PubMed] [Google Scholar]
- 110. Engel SS, Xu L, Andryuk PJ, Davies MJ, Amatruda J, Kaufman K, Goldstein BJ. 2011. Efficacy and tolerability of MK-0893, a glucagon receptor antagonist, in patients with type 2 diabetes. Diabetes 60:A85 (Abstract) [Google Scholar]
- 111. Kelly RP, Garhyan P, Abu-Raddad EJ, Fu H, Lim CN, Prince MJ, Pinaire JA, Loh MT, Deeg MA. 2011. Short-term treatment with glucagon receptor antagonist LY2409021 effectively reduces fasting blood glucose and HbA1c in patients with type 2 diabetes mellitus. Diabetes 60:A84 (Abstract) [DOI] [PubMed] [Google Scholar]