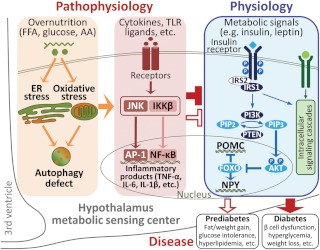
Type 2 diabetes (T2D) is a devastating disease that arises from prediabetic conditions including glucose intolerance, insulin resistance, hyperlipidemia, and obesity, which, together with a few others, collectively is termed metabolic syndrome. Due to the absence of severe hyperglycemia and its chronic complications, prediabetes offers an early therapeutic window to reverse glucose and insulin abnormalities to prevent the development of T2D. To this end, understanding the pathogenic mechanisms underlying the progression from prediabetes to diabetes represents an area of keen research. In this issue, Burgos-Ramos et al. (1) provide evidence demonstrating that the development of diabetes from prediabetes in a mouse model may involve a central nervous system mechanism in relation with hypothalamic inflammation (Fig. 1).
Fig. 1.
Hypothalamic inflammation links central insulin resistance to diabetes. Hypothalamic regulation of feeding, body weight, and glucose homeostasis is mediated by multiple signaling pathways including insulin singling. The hypothalamic insulin signaling cascade is directed by activation of insulin receptor, IRS proteins, and downstream phosphatidylinositol-3-OH kinase (PI3K), which control AKT and FOXO to regulate gene expression of neuropeptides such as proopiomelanocortin (POMC) and neuropeptide Y (NPY). In IRS2-deficient mice, some of them can develop diabetes, but others present only prediabetic syndrome, likely due to the differential compensatory effects by IRS1. The compromised compensation of hypothalamic IRS1 signaling in diabetic IRS2-deficient mice is related to the certain pattern and perhaps increased magnitude of hypothalamic inflammation. Such hypothalamic changes in IRS2-deficient mice align with recent literature showing the central mechanisms of obesity and T2D involve IκB kinase β (IKKβ)/NF-κB- and JNK-mediated hypothalamic inflammation, a central pathogenic event caused by overnutrition-induced endoplasmic reticulum (ER) stress, oxidative stress, autophagy defects, and cytokine activation. AA, Amino acids; AP1, activator protein-1; FFA, free fatty acid; FOXO, forkhead box O; PIP2, phosphatidylinositol 4,5-bisphosphate; PTEN, phosphate and tensin homolog.
This study from Burgos-Ramos et al. (1) was based on mice that are genetically deficient of insulin receptor substrate 2 (IRS2), an animal model that resembles T2D (2, 3). IRS proteins are intracellular docking molecules that bind to activated insulin receptors in response to insulin and undergo rapid activation through tyrosine phosphorylation by insulin receptors. Activated IRS proteins bind SH2 domain-containing proteins such as phosphatidylinositol-3-OH kinase to relay intracellular signaling of insulin stimulation. IRS2 protein was originally cloned based on sequence resemblance to IRS1 (4). IRS2 is widely expressed throughout the body including the arcuate nucleus and paraventricular nucleus of the hypothalamus (5), two critical hypothalamic regions that regulate whole-body energy and glucose homeostasis (6–12). IRS2 was found to play nonredundant roles with IRS1 in regulating peripheral glucose metabolism, β-cell function, and energy homeostasis (2, 3, 5, 13). IRS2-deficient mice often develop diabetic symptoms including hyperglycemia and β-cell dysfunction (2, 3, 5, 13). Interestingly, magnitude of glucose disorders in IRS2-deficient mice can be variable, with certain congenic strains only presenting prediabetic changes (14). This phenotypic divergence can offer an experimental tool to study pathogenic factors that may differentially modulate central insulin signaling pathway leading to two different outcomes: prediabetes or overt diabetes.
T2D and related metabolic syndrome belong to systemic endocrine problems, and recent research has interestingly pointed to a pathogenic root in brain immune dysregulation (15–28). Systemic glucose homeostasis, which is a physiological state of balanced glucose disposal and production, is achieved through the coordinated actions of multiple organs including the liver, pancreas, adipose tissue, skeletal muscle, and the brain as well. The hypothalamus in the brain exerts essential roles in this process by sensing circulating metabolic signals (e.g. insulin and leptin) and instructing various neuroendocrine and neural pathways to control peripheral glucose metabolism (9–11). However, under pathological conditions such as overnutrition and obesity, hypothalamic neurons are interrupted due to the induction of metabolic inflammation, mediated by proinflammatory pathways such as IκB kinase β (IKKβ) and downstream nuclear factor-κB (NF-κB) (15–21) or c-Jun N-terminal kinase (JNK) (22–24) (Fig. 1). Although many probably still remain unidentified, hypothalamic regulatory changes caused by inflammation/stress essentially include the reduction of insulin and leptin signaling in the hypothalamus (15–27, 29). Among these findings, hypothalamic inflammation was shown to not only cause feeding and body weight changes but also employ body weight-independent manners to cause systemic glucose intolerance. The molecular mechanisms can involve changes in hypothalamic insulin signaling, because hypothalamic action of insulin is indeed crucial for control of body weight and also body weight-independent peripheral glucose metabolism. In the study by Burgos-Ramos et al. (1), IRS2-deficient mice were divided into two phenotypic groups, one having diabetes and the other only prediabetes. By profiling the compensatory reaction of IRS1 signaling and the relationship with hypothalamic inflammation, the study suggested that differential activation of hypothalamic inflammatory pathways seems to be a pathogenic factor for variable sensitivities of hypothalamic insulin signaling and correlated onset of diseases (Fig. 1).
In the first set of studies, the authors comparatively profiled feeding, body weight, and glucose homeostasis between diabetic and prediabetic IRS2-deficient mice. Both groups have elevated blood glucose and insulin levels, indicative of a common systemic insulin resistance. In prediabetic IRS2-deficient mice, their body weight significantly increased and hyperleptinemia developed even when maintained on a normal chow, a finding consistent with the obesogenic action of hypothalamic insulin resistance. However, these mice did not develop diabetes, which might be related to compensatory action of IRS1 in the form of increased IRS1 protein level and enhanced IRS1-induced Janus kinase 2 and Akt activation. Compared with prediabetic IRS2-deficient mice, diabetic IRS2-deficient mice displayed overeating and impaired energy expenditure, but body weight decreased due to chronic malnutrition, all of which belong to diabetic symptoms. The development of diabetes in these mice correlated with the observation that IRS1 did not respond to compensate for the genetic deficiency of IRS2. Altogether, when IRS1 could provide certain compensation, IRS2-deficient mice were only prediabetic, characterized by overweight/obesity and systemic insulin resistance. In contrast, when IRS2 was deficient but without compensatory effects of IRS1, mice developed diabetes. Overall, levels of compensation from hypothalamic IRS1 signaling may underlie the differential diabetic phenotypes among IRS2-deficient mice.
Subsequently, the authors found that the development of diabetes in IRS2-deficient mice was related to a more complete induction of hypothalamic inflammation, and on the other hand, only a partial induction of hypothalamic inflammation was detected in prediabetic IRS2-deficient mice. Regarding the potential underlying pathways, both NF-κB and JNK were significantly activated in diabetic IRS2-deficient mice, agreeing with recent findings showing that NF-κB- and JNK-mediated hypothalamic inflammation cause peripheral insulin resistance and systemic glucose intolerance (20, 22–24). Furthermore, several classical NF-κB products were examined, such as TNF-α, suppressor of cytokine signaling 3 (SOCS3), and IL-6. Results showed that TNF-α mRNA increased in the hypothalamus of the diabetic group, SOCS3 mRNA increased in the hypothalamus of the prediabetic group, and IL-6 mRNA increased in both groups. Thus, a certain format of hypothalamic inflammation, presumably due to activation of both NF-κB and JNK, was associated with the absent compensatory action of hypothalamic IRS1 signaling in the diabetic IRS2-deficient mice. This finding aligns with the established role of these inflammatory pathways in inhibiting insulin signaling (15, 30, 31), and the effects of inhibiting the hypothalamic NF-κB or JNK pathway in counteracting glucose metabolic disorders in peripheral tissues (17–23, 25). On the other hand, despite these understandings, it remains to be experimentally proved whether the pattern of hypothalamic inflammation revealed in this study could indeed mediate the transition from prediabetes to diabetes in the group of IRS2-deficient mice. Further research is needed to examine the predicted causal role of hypothalamic inflammation in the development of diabetes in this genetic model and perhaps other models as well.
In summary, taking advantage of the divergent metabolic phenotypes of IRS2-deficient mice, this study suggests that central insulin resistance can cause both prediabetic syndrome and diabetes, and different formats of hypothalamic inflammation could represent a pathogenic factor that determines prediabetic vs. diabetic outcomes. It can also suggest that proinflammatory NF-κB and JNK pathways are significantly involved in this potential hypothalamic mechanism, an interesting concept that will anticipate experimental assessments in near-future research.
Acknowledgments
I sincerely thank all laboratory personnel for their work in relevant research projects.
D.C.'s laboratory is supported by the Albert Einstein College of Medicine start-up funds and NIH R01 DK078750 and R01 AG031774 (all to D.C.) for related research projects.
Disclosure Summary: The author has nothing to disclose.
For article see page 1129
- IRS2
- Insulin receptor substrate 2
- JNK
- c-Jun N-terminal kinase
- NF-κB
- nuclear factor-κB
- T2D
- type 2 diabetes.
References
- 1. Burgos-Ramos E, González-Rodríguez Á, Canelles S, Baquedano E, Frago LM, Revuelta-Cervantes J, Gómez-Ambrosi J, Frühbeck G, Chowen JA, Argente J, Valverde ÁM, Barrios V. 2012. Differential insulin receptor substrate-1 (IRS1)-related modulation of neuropeptide Y and proopiomelanocortin expression in nondiabetic and diabetic IRS2−/− mice. Endocrinology 153:1129–1140 [DOI] [PubMed] [Google Scholar]
- 2. Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- 3. Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. 1999. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 23:32–40 [DOI] [PubMed] [Google Scholar]
- 4. Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG, Jr, Glasheen E, Lane WS, Pierce JH, White MF. 1995. Role of IRS-2 in insulin and cytokine signalling. Nature 377:173–177 [DOI] [PubMed] [Google Scholar]
- 5. Taguchi A, Wartschow LM, White MF. 2007. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317:369–372 [DOI] [PubMed] [Google Scholar]
- 6. Flier JS. 2006. Neuroscience. Regulating energy balance: the substrate strikes back. Science 312:861–864 [DOI] [PubMed] [Google Scholar]
- 7. Coll AP, Farooqi IS, O'Rahilly S. 2007. The hormonal control of food intake. Cell 129:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- 9. Obici S, Zhang BB, Karkanias G, Rossetti L. 2002. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 10. Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. 2005. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71 [DOI] [PubMed] [Google Scholar]
- 11. Morton GJ. 2007. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol 583:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D. 2011. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69:523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF. 2000. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407:377–382 [DOI] [PubMed] [Google Scholar]
- 14. Hashimoto H. 2011. Study on establishment of congenic strains and screening of characteristics in IRS-2 deficient mice to support translational research on type 2 diabetes. Exp Anim 60:21–32 [DOI] [PubMed] [Google Scholar]
- 15. Cai D. 2009. NFκB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle 8:2542–2548 [DOI] [PubMed] [Google Scholar]
- 16. Lumeng CN, Saltiel AR. 2011. Inflammatory links between obesity and metabolic disease. J Clin Invest 121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. 2008. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. 2009. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296:E1003–E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Brüning JC. 2009. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purkayastha S, Zhang H, Zhang G, Ahmed Z, Wang Y, Cai D. 2011. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci USA 108:2939–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meng Q, Cai D. 2011. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase β (IKKβ)/NF-κB pathway. J Biol Chem 286:32324–32332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, Davis RJ. 2010. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev 24:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Brönneke HS, Brodesser S, Hampel B, Schauss AC, Brüning JC. 2010. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA 107:6028–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Unger EK, Piper ML, Olofsson LE, Xu AW. 2010. Functional role of c-Jun-N-terminal kinase in feeding regulation. Endocrinology 151:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. 2006. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4:123–132 [DOI] [PubMed] [Google Scholar]
- 26. Yi CX, Habegger KM, Chowen JA, Stern J, Tschöp MH. 2011. A role for astrocytes in the central control of metabolism. Neuroendocrinology 93:143–149 [DOI] [PubMed] [Google Scholar]
- 27. Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG, Jr, Xu AW. 2010. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 59:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Purkayastha S, Zhang G, Cai D. 2011. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med 17:883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. 2009. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9:35–51 [DOI] [PubMed] [Google Scholar]
- 30. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 31. Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]