Abstract
Exposures to sex steroids during fetal development are thought to contribute to the unique urogenital anatomy and social dominance of the female spotted hyena: overt phenotypes not shared by other hyenids (i.e. striped hyena, brown hyena, and aardwolf). Because both androgens and estrogens influence development of genitalia and behavior, and because plasma SHBG regulates their access to tissues, we compared the Shbg gene sequences, structures, and steroid-binding properties in the four extant hyenids. We found the hyenid Shbg genes (>95% identical) and mature protein sequences (98% identical) are highly conserved. As in other mammals, the hyenid SHBG all bind 5α-dihydrotestosterone with high affinity (Kd = 0.62–1.47 nm), but they also bind estrone and dehydroepiandrosterone with similarly high affinity, and this unusual property was attributed to specific amino acids within their SHBG steroid-binding sites. Phylogenetic comparisons also indicated that the spotted hyena SHBG precursor uniquely lacks two leucine residues and has a L15W substitution within its secretion signal polypeptide, the reduced size and hydrophobicity of which markedly decreases the production of SHBG and may therefore explain why serum SHBG concentrations in male and female spotted hyenas are approximately five times lower than in other hyenids. This is important because low plasma SHBG concentrations in spotted hyenas will increase exposure to biologically active androgens and estrogen as well as to their precursors (dehydroepiandrosterone and estrone), which may contribute to the masculinized external genitalia of female spotted hyenas and to female social dominance over males.
Female spotted hyena (Crocuta crocuta) external genitalia comprise a pseudoscrotum and an erectile peniform clitoris, through which the female urinates, copulates, and gives birth, instead of a vagina (1). Masculinization of female spotted hyenas also increases their aggressiveness and physical fitness, but it comes with a reproductive cost to primiparous mothers and their neonates due primarily to dystocia, or birthing difficulties, caused by the physical limitations of the first delivery through a narrow peniform clitoris (2, 3). Although there is substantial evidence that excessive prenatal androgen exposures influence genital development of the female spotted hyena fetus in utero (4, 5), the formation, differentiation, and development of the external genitalia in this species may not follow a simple androgenic route (6, 7). It has been proposed therefore that other endocrine factors or pathways may influence this process, including for instance the actions of estrogens (8). In this context, estrogen receptors are present in the developing genitalia of both male (9) and female (10) human fetuses and could therefore mediate the effects of estrogens either directly or after local metabolism of their precursors that are abundant in the fetal circulation.
In addition to effects on development of the external genitalia, androgens and estrogens could have effects on the central nervous system of spotted hyenas. They may operate as organizing agents during fetal life, or activating agents during various stages of postnatal life, and might attenuate or reverse traditional male-biased sexual dimorphism in various regions of the brain and spinal cord (11–13). Indeed, a recent report linking androgen concentrations in pregnant spotted hyenas to frequencies of aggression and mounting by their cubs (14) offers strong support for an effect of fetal androgen exposure on their social behavior.
The access of biologically active sex steroids (androgens and estrogens) to their target tissues is controlled by a plasma glycoprotein, known as SHBG, which is produced primarily by the liver (15). Expression of the Shbg gene first occurs in the liver during fetal life in all mammals (16, 17), including rodents, even though they do not express the gene in the liver after birth (18). Because the presence of SHBG in fetal blood must influence the access of sex steroids to developing genitalia, as well as to structures in the central nervous system, we set out to characterize the Shbg gene and its protein products in spotted hyenas and the three other extant members of Hyaenidae, i.e. the striped hyena (Hyaena hyaena), brown hyena (Parahyaena brunnea), and aardwolf (Proteles cristatus). This phylogenetic comparison revealed that hyenid Shbg gene sequences are highly conserved, and all encode SHBG with unusually high affinities for some immediate sex steroid precursors as well as the main biologically active androgens and their immediate metabolites. More importantly, it also revealed a major difference in the spotted hyena SHBG secretion signal polypeptide that compromises SHBG production, which may explain the much lower serum SHBG concentrations in spotted hyenas when compared with other hyenid species.
Materials and Methods
Animals
Blood and tissue samples were obtained from spotted hyenas maintained at the Field Station for the Study of Behavior, Ecology, and Reproduction of the University of California at Berkeley with approval of the Animal Care and Use Committee of the University of California at Berkeley. Blood samples were obtained from a wild population of striped hyenas in Laikipia District Kenya with approval from Montana State University's Institutional Animal Care and Use Committee and the Kenya Wildlife Service (19). Blood samples were also obtained from a wild population of brown hyenas inhabiting the coastal Namib Desert, in southern Namibia. The animals were captured using foot-snaring techniques perfected for the safe and routine capture of carnivores (20), which permitted sedation blood sampling in accordance with the U.S. Fish and Wildlife Endangered Species Act and by procedures approved by the Animal Care and Use Committee of Duke University, Durham, NC. Blood samples were also obtained from striped hyenas and aardwolves during annual health checkups at The Living Desert Zoo and Gardens (Palm Desert, CA). In all cases, samples were taken from sexually mature animals, and none of the female animals were pregnant at the time of blood sampling, apart from three spotted hyenas.
Cloning and analysis of hyena Shbg genes
Genomic DNA was isolated from spotted hyena liver, and blood cells were used as the source for genomic DNA of striped hyena, brown hyena, and aardwolf (21). These genomic DNA were used as template for PCR using oligonucleotides corresponding to 5′ and 3′ noncoding sequences and internal sequences of the spotted hyena SHBG cDNA sequence (GenBank accession no. DQ285473). This allowed amplification of overlapping regions of the 3.6-kb Shbg genes in all four hyena species, and the products were cloned into pCR-Blunt II-Topo vector (Invitrogen, Carlsbad, CA) and sequenced.
Steroid-binding assays
The concentrations of human or hyena SHBG in serum or of recombinant spotted hyena SHBG in tissue culture medium were determined by saturation analysis using [3H]5α-dihydrotestosterone (DHT) (PerkinElmer Life Sciences, Boston, MA, or Amersham Biosciences, Baie d'Urfé, Quebec, Canada) as labeled ligand and dextran-coated charcoal to separate bound and free steroid (22). The same method was used to determine the steroid-binding properties of SHBG, as described previously (22), using data analysis and graphing software from OriginLab Corp. (Northampton, MA). For Scatchard analysis, data were plotted and subjected to linear regression analysis to obtain the slope of the line that defines the affinity constant (Kd), while the intercept on the x-axis that defines the concentration of binding sites (nanomolar). In the competitive steroid-binding assays, increasing amounts of unlabeled steroid ligands were used to displace [3H]DHT from the SHBG steroid-binding sites, and the data were plotted using a curve-fitting program (OriginLab) to obtain relative binding affinity (RBA) values, which were calculated by comparing the concentrations of ligands that result in a 50% reduction in [3H]DHT binding relative to that obtained for unlabeled DHT and expressed as a percentage.
Production of human and spotted hyena SHBG mutants
A cDNA encoding the human SHBG precursor was used as a template for site-directed mutagenesis using oligonucleotide primer sequences (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The mutated cDNA were subcloned into pRc/CMV (Invitrogen) and sequenced to confirm that only the targeted mutations had occurred, as described previously (23). Additionally, the wild-type spotted hyena SHBG cDNA sequence was inserted into the pRc/CMV expression vector and mutated using the QuikChange II XL mutagenesis kit from Stratagene (La Jolla, CA) and mutagenic oligonucleotides (Supplemental Table 1). The resulting plasmids were used for transfection of Chinese hamster ovary cells (CHO) using Lipofectamine reagent (Invitrogen), and stably transfected cells were grown to near confluence in HyQ PF-CHO LS (HyClone, Logan, UT) media for the production of recombinant proteins.
To test the effect of sequence variations in the secretion signal polypeptide of hyena SHBG on the secretion of the protein, it was also necessary to determine the spotted hyena SHBG mRNA levels in CHO cells transfected with the modified expression constructs. Briefly, total RNA was extracted from the stably transfected CHO cells using TRIzol reagent (Invitrogen). Reverse transcription was performed at 42 C for 50 min using 2–3 μg total RNA and 200 U Superscript II together with an oligo-deoxythymidine primer and reagents provided by Invitrogen. An aliquot of the reverse transcription product was subjected to PCR using spotted hyena SHBG-specific oligonucleotides (forward, 5′-TCACGCGGAATTCAGTCTCCAAGA-3′; reverse, 5′-CATGCAGAGGACAGCACCACTTT-3′) in a quantitative PCR in which CHO cell GAPDH mRNA levels were also measured as an internal control using specific oligonucleotides (forward primer, 5′-TCGCCGAGTATGTTGTGGAATCTACTG-3′; reverse primer, 5′-TGGTGGTGCAGGACGCATTG-3′). The PCR were performed using a Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), and products were measured in a real-time PCR system (Applied Biosystems).
Results
Phylogenetic comparisons of hyena SHBG sequences
The spotted hyena Shbg gene was cloned using combinations of specific oligonucleotides corresponding to 5′ and 3′ noncoding sequences and internal sequences of the spotted hyena SHBG cDNA sequence (DQ285473). This revealed the coding sequence for the spotted hyena SHBG precursor polypeptide (Supplemental Fig. 1). It should be noted that this sequence deviates from the cDNA sequence in that it has an additional Leu codon within the secretion signal polypeptide sequence. We therefore used RT-PCR to amplify SHBG cDNA from liver RNA extracts from three individual spotted hyenas, and sequences of the PCR products all conformed to the genomic coding sequence (Supplemental Fig. 1).
When aligned against the human SHBG sequence (Supplemental Fig. 1), the spotted hyena SHBG sequence shares several structurally and functionally conserved features. In particular, it comprises two laminin G-like (LG) domains that contain conserved cysteine residues that form disulfide bridges and has two N-linked glycosylation sites close to the carboxy terminus. Also present are highly conserved residues (i.e. serine and aspartic acid) that hydrogen bond with the functional groups at C3 and C17 of steroid ligands within the human SHBG steroid-binding site (24).
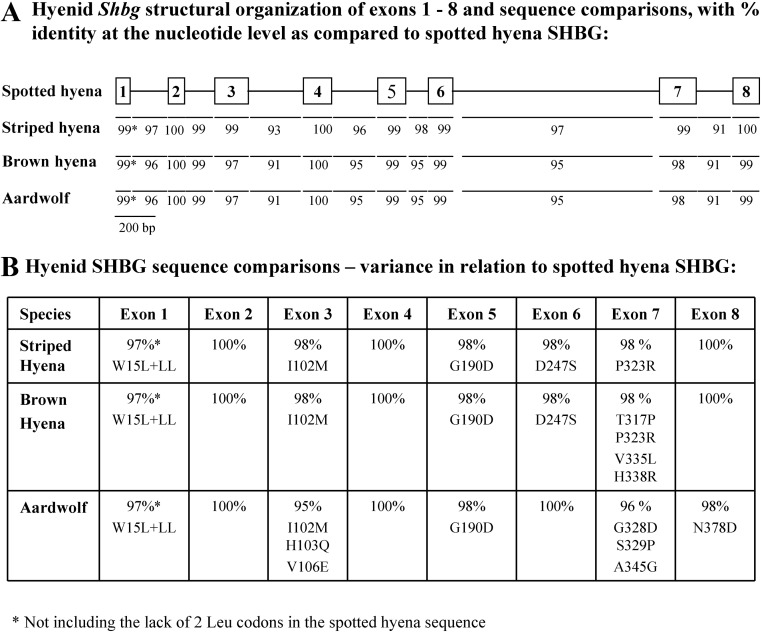
The striped hyena (GenBank accession no. DQ304534), brown hyena (GenBank accession no. GQ265893), and aardwolf (GenBank accession no. DQ304535) Shbg genes were cloned by PCR amplification using oligonucleotides corresponding to the spotted hyena Shbg gene sequence (GenBank accession no. DQ302141). When these sequences were compared (Fig. 1), it was evident that the Shbg genes in these four hyenid species are highly conserved. Their transcription units span 3.6 kb and contain eight exons with boundaries conforming to consensus sequences for 5′-donor and 3′-acceptor splice sites.
Fig. 1.
Comparison of Shbg genes and mature SHBG polypeptide sequences from spotted hyena, striped hyena, brown hyena, and aardwolf. A, Structural organization and comparison of nucleotide sequences of the four hyenid Shbg genes. Exons are shown in relation to their sizes as numbered boxes with intron sizes depicted by lines. Percentages of identity at the nucleotide level for intron and exon sequences are shown for striped hyena, brown hyena, and aardwolf with respect to the corresponding spotted hyena sequences. B, Comparison of the mature SHBG polypeptide sequences encoded between exons 2–8 of the striped hyena, brown hyena, and aardwolf Shbg genes in relation to the spotted hyena SHBG sequence, indicating the percentages of amino acid sequence identity. Amino acid substitutions or addition (+) for the striped hyena, brown hyena, and aardwolf SHBG are indicated in relation to the corresponding residues in the spotted hyena SHBG sequence.
The striped hyena, brown hyena, and aardwolf Shbg gene sequences allowed us to deduce their SHBG precursor polypeptide sequences. When exons 2–8 that encode the different hyenid mature SHBG polypeptides are compared, they exhibit an overall 99% sequence identity at the nucleotide level (Fig. 1A). Moreover, within the region (exons 2–5) encoding the amino-terminal LG domain that contains the steroid-binding site (24), there are only two amino acid differences among the spotted hyena, brown hyena, and striped hyena SHBG sequences (Fig. 1B) and only two additional amino acid differences within the aardwolf SHBG amino-terminal LG domain (Fig. 1B). However, the most striking difference between the SHBG sequences of these four hyenid species is that the spotted hyena SHBG secretion signal polypeptide, which is removed during processing of the precursor polypeptide, is unique in that it lacks two leucines and has a tryptophan instead of a leucine at residue 15 (Fig. 1B).
Unusual steroid-binding properties of hyena SHBG
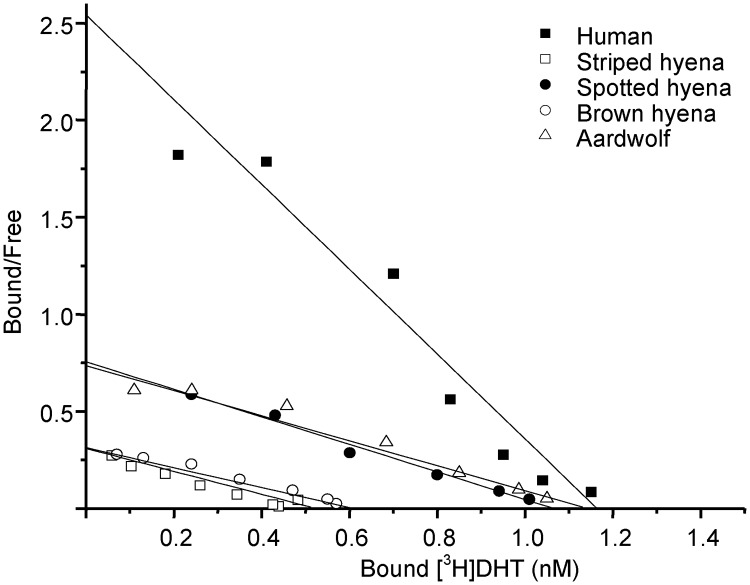
The steroid-binding affinities of SHBG in spotted hyena, striped hyena, brown hyena, aardwolf, and human SHBG blood samples were first assessed by Scatchard analysis (23, 25) using [3H]DHT as the labeled ligand (Fig. 2). This demonstrated that the affinities of the spotted hyena (Kd = 1.40 nm), brown hyena (Kd = 1.95 nm), striped hyena (Kd = 1.69 nm), and aardwolf (Kd = 1.55 nm) SHBG for [3H]DHT are similar and 2- to 4-fold lower than that of human SHBG (Kd = 0.47 nm).
Fig. 2.
Steroid-binding affinities of hyena SHBG compared with human SHBG. Scatchard analysis of the binding of [3H]DHT to human SHBG (■), striped hyena SHBG (□), spotted hyena SHBG (●), brown hyena (○) and aardwolf SHBG (▵) in serum samples. Samples were diluted for analysis as follows: nonpregnant female striped hyena (1:200), nonpregnant brown hyena (1:200), nonpregnant aardwolf (1:400), pregnant spotted hyena (1:2500). Data were plotted and subjected to linear regression analysis using data analysis and graphing software from OriginLab; the slope of the line defines the dissociation constant (Kd), and intercept on the x-axis defines the concentration of binding sites (nanomolar).
The steroid-binding specificities of hyenid SHBG were also examined in conventional competitive binding assays using [3H]DHT as the labeled ligand (23). Our initial analysis of spotted hyena SHBG revealed that it has a remarkably high affinity for estrone and dehydroepiandrosterone (DHEA), when compared with human SHBG (Table 1). We therefore compared the steroid-binding specificities of SHBG from two other hyenid species (striped hyena and aardwolf) with those of the spotted hyena, using human SHBG as the reference (Table 1), and this analysis indicated that the unusual steroid-binding specificity of spotted hyena SHBG was shared by the SHBG from other hyenids. Interestingly, although the affinity of spotted hyena SHBG for estrone was higher than in the other hyenid species, it was also more variable between animals. Because the amino-terminal LG domain contains the steroid-binding site, our attention focused on residues within this region that are unique to the hyena SHBG compared with other mammalian SHBG sequences, and especially to those in close proximity to steroid ligands (24). These residues included F56, W84, and M139 in the human SHBG sequence, and we converted them separately and in various combinations into the corresponding residues found in hyena SHBG, i.e. F56L, W84V, and M139V, within the context of a human SHBG expression vector.
Table 1.
Comparison of the RBA of SHBG in human pregnancy serum (HPS) and spotted hyena, striped hyena, or aardwolf serum samples for various steroids
| Steroid | RBA (DHT = 100%) |
|||
|---|---|---|---|---|
| HPS | Spotted | Striped | Aardwolf | |
| Testosterone | 27.8 ± 6.9 | 35.7 ± 3.8 | 41.7 ± 6.9a | 55.6 ± 9.3a |
| DHEA | <0.4 | 11.6 ± 2.7a | 8.6 ± 1.9a | 8.6 ± 1.3a |
| 5αAndrostane-3α,17β-diol | 18.5 ± 5.5 | 16.7 ± 1.7 | 7.5 ± 1.2a | 7.0 ± 0.9a |
| 5αAndrostane-3β,17β-diol | 31.3 ± 4.9 | 9.4 ± 1.5a | 13 ± 0.8a | 13.2 ± 2.4a |
| 4-Androsten-3,17-dione | 0.9 ± 0.1 | 1.3 ± 0.1a | 1.3 ± 0.2a | 0.8 ± 0.1 |
| 5-Androsten-3β,17β-diol | 6.1 ± 0.2 | 3.4 ± 0.5a | 3.7 ± 0.2a | 4.4 ± 1.0 |
| Estradiol | 6.0 ± 1.0 | 0.9 ± 0.1a | 0.7 ± 0.1a | 0.5 ± 0.1a |
| Estriol | <0.1 | <0.1 | <0.1 | <0.1 |
| Estrone | 1.5 ± 0.1 | 100.0 ± 20.0a | 15.2 ± 4.1a | 20.8 ± 2.6a |
| 2-Methoxyestradiol | 14.5 ± 5.7 | 1.4 ± 0.0a | 0.7 ± 0.0a | 1.1 ± 0.1a |
| Progesterone | <0.1 | 1.0 ± 0.2a | 0.5 ± 0.1a | 0.6 ± 0.1a |
| Cortisol | <0.1 | <0.1 | <0.1 | <0.1 |
| Corticosterone | <0.1 | <0.1 | <0.1 | <0.1 |
RBA values were calculated by comparing the concentrations of ligands that result in a 50% reduction in [3H]DHT binding relative to that obtained for unlabeled DHT, and expressed as a percentage (means ± sd of 3–4 independent analyses).
Significantly different from human serum (P < 0.05).
When the steroid-binding properties of these human SHBG variants were examined, those containing M139V substitutions displayed a 2- to 3-fold reduced affinity for DHT (data not shown), but when introduced alone, this substitution had little influence on the RBA for estrone or DHEA (Table 2). By contrast, the F56L substitution resulted in a marked (5.5 times) increase in the RBA for estrone but no change in the RBA for DHEA, whereas the W84V substitution increased the RBA for both estrone and DHEA by 7.2 and 10.5 times, respectively. When these two substitutions (F56L and W84V) were introduced together, there was a more than 5-fold augmentation in the RBA for estrone over that seen with either of the single substitutions, with no further increase in affinity for DHEA. Interestingly, when all three substitutions (F56L, W84V, and M139V) were introduced together in the human SHBG, these changes recapitulate the very high affinities of spotted hyena SHBG for both estrone and DHEA (Table 2).
Table 2.
RBA of wild-type human SHBG vs. human SHBG mutants containing residues substituted with those specifically found in hyena SHBG sequences for estrone and DHEA
| Human SHBG | RBA (DHT = 100%) |
|
|---|---|---|
| Estrone | DHEA | |
| SHBG | 2.1 | 0.4 |
| SHBG +G184 | 1.5 | 1.2 |
| SHBG M139V | 1.5 | 0.9 |
| SHBG F56L | 11.6 (↑5.5×) | 0.2 |
| SHBG W84V | 15.1 (↑7.2×) | 4.2 (↑10.5×) |
| SHBG F56L + W84V | 77.3 (↑36.8×) | 1.6 (↑4.0×) |
| SHBG F56L + W84V + M139V | 74.4 (↑35.4×) | 24.0 (↑60.0×) |
RBA values were calculated by comparing the concentrations of estrone or DHEA that result in a 50% reduction in [3H]DHT binding relative to that obtained for unlabeled DHT and expressed as a percentage. Fold increases (↑) are shown for human SHBG mutants when compared with corresponding wild-type human SHBG.
Another major difference between hyenid SHBG sequences and those of most mammalian SHBG, including human SHBG (Supplemental Fig. 1) is an additional Gly residue between the linked cysteine residues in the amino-terminal LG domains of the hyenid SHBG, but addition of this residue to the human SHBG sequence did not have an effect on its affinity for estrone and produced only a small increase in its affinity for DHEA (Table 2).
Sequence variation in the secretion signal polypeptide of spotted hyena SHBG limits its production
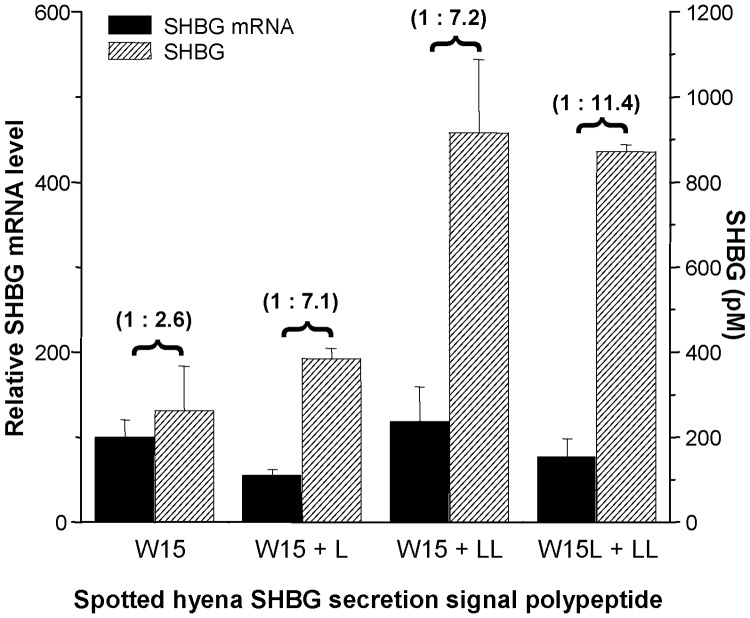
To assess how the unique sequence of the spotted hyena SHBG secretion signal polypeptide (MEGRGPLATSPRRRWLLLLLLLLPHS) influences its production, we compared the levels of spotted hyena SHBG secreted by CHO cells stably transfected with a wild-type spotted hyena SHBG cDNA or mutant spotted hyena SHBG cDNA. The latter cDNA included either one or two additional leucine codons or two additional leucine codons plus a mutation of the codon for W15 (TGG) to a leucine (TTG) codon within the secretion signal sequence, as found in the corresponding SHBG secretion signal polypeptides of other hyenid species (i.e. MEGRGPLATSPRRRLLLLLLLLLLLPHS). These cDNA were expressed in CHO cells under the control of the same CMV promoter, and the amounts of SHBG in the culture medium were determined in relation to the corresponding amounts of spotted hyena SHBG mRNA in the same transfected cell populations. This demonstrated that the addition of either one or two extra Leu residues to the wild-type spotted hyena secretion signal polypeptide resulted in a more than 2.5-fold increase in the production of SHBG by CHO cells, and a more than 4-fold increase when the W15 was also converted to a Leu residue, as found in the secretion signal polypeptides of SHBG in other hyena species (Fig. 3).
Fig. 3.
The addition of leucine residues in the spotted hyena SHBG secretion polypeptide increases it production/secretion. The production of wild-type and mutant spotted hyena SHBG by CHO cells was determined by the ratio of SHBG concentration in the culture medium to SHBG mRNA in cells (shown above the horizontal brackets). The SHBG concentration in the medium was determined by a steroid-binding capacity assay, and the mRNA levels were determined by quantitative RT-PCR (see Materials and Methods for details). Data represent the means ± sd of triplicate cell cultures.
Serum concentrations of SHBG are lower in spotted hyenas than in other hyena species
There is no obvious sexual dimorphism in the serum concentrations of SHBG in sexually mature male and nonpregnant female spotted hyenas (Table 3). By contrast, the serum concentrations of SHBG in striped hyenas, brown hyenas, and aardwolves exceeded the male or female spotted hyena reference ranges by at least 4- to 5-fold (Table 3). Serum SHBG concentrations in female striped hyenas, brown hyenas, and aardwolves were all remarkably high, and in brown hyenas we were able to study sufficient numbers of male samples to confirm they were sexually dimorphic (Table 3). Interestingly, serum SHBG concentrations (mean ± sd) in three female spotted hyenas increased by 50-fold by late gestation (2246 ± 213 nm), when compared with early gestation values (42 ± 17 nm) measured in the same animals.
Table 3.
Serum SHBG concentrations in different Hyaenidae
| Species | n | SHBG (nm) (means ± sd) |
|---|---|---|
| Spotted hyena | ||
| Male | 6 | 37 ± 7 |
| Female | 9 | 53 ± 32 |
| Striped hyena | ||
| Male | 1 | 120 |
| Female | 8 | 283 ± 55 |
| Brown hyena | ||
| Male | 5 | 154 ± 31 |
| Female | 6 | 321 ± 59 |
| Aardwolf | ||
| Female | 2 | 800 |
Discussion
A plasma protein with high affinity for androgens was first described in spotted hyenas as a function of age, sex, and reproductive status (26). Although that study revealed a correlation between plasma steroid-binding capacity measurements and ovarian steroid concentrations in female spotted hyenas, the authors failed to detect the protein in males, and no firm conclusions were drawn regarding its identity. We therefore set out to define the molecular and biochemical properties of SHBG in the spotted hyena and related members of Hyaenidae.
Cloning of the spotted hyena Shbg gene revealed the spotted hyena SHBG precursor coding sequence, which was confirmed by subsequent cDNA cloning. Comparison of the coding sequences for SHBG in other mammalian species listed in public databases revealed that the spotted hyena SHBG is most closely related (81% sequence identity) to cat SHBG, consistent with the phylogenetic assignment of hyenas within the Feliforma suborder of Carnivora. The hyena SHBG sequences have an unusual additional residue between the linked Cys residues within their amino-terminal LG4 domains, but this does not appear to alter the way these proteins bind steroids. However, several structurally important regions of mammalian SHBG are conserved in the spotted hyena SHBG; these include the paired cysteines that form intramolecular disulfide bridges (27), the N-glycosylation sites within the carboxyl-terminal region (25), and key residues (corresponding to Ser42 and Asp65 in human SHBG) in the steroid-binding pocket that hydrogen bond with sex steroids (24, 28).
The size and structural organization of the spotted hyena Shbg gene resembles the corresponding gene sequences in other mammals (25). Moreover, the Shbg gene sequences from other extant Hyaenidae family members exhibit a remarkable degree of similarity at the nucleotide level within both exons and introns, with greater sequence similarities between spotted and striped hyenas. The amino-terminal LG4 domain of SHBG contains the steroid-binding site, and within this region, there is only one conserved amino acid substitution among spotted, striped, and brown hyenas. The carboxyl-terminal LG domain sequences encoded by exon 7 are the least conserved, especially in brown hyenas and aardwolves, but the overall identity of the mature SHBG sequences among various Hyaenidae family members exceeds 98%.
As in most other mammals (29), the SHBG in all four Hyaenidae family members bind androgens with high affinity, and like SHBG in most subprimate mammalian species, they bind estradiol and 2-methoxyestradiol with relatively low affinities (29). However, the exceptionally high affinities of the hyena SHBG for estrone and DHEA sets them apart from SHBG in other mammals (29), and the serum concentrations of these sex steroid precursors are expected to be relatively high in female spotted hyenas because their binding to SHBG will limit their metabolic clearance (30).
The key amino acids that interact with ligands within the human SHBG steroid-binding site (i.e. Ser42, Asp65, and Asn82 in the human SHBG sequence) are highly conserved across all vertebrate species and are present in the hyena SHBG sequences. These residues are all encoded by exon 3 sequences, and there is one residue (Leu) within this sequence that is unique to the hyena SHBG sequences when compared with SHBG in other vertebrate species. This leucine substitutes for a phenylalanine (Phe56) located within the human SHBG steroid-binding pocket (24, 28). It was therefore of interest that the affinity of human SHBG for estrone increased when its Phe56 was substituted with a Leu. The only other striking difference in the hyena SHBG sequences encoded by exon 3 is the residue that corresponds to Trp84 in human SHBG (28). In the hyena SHBG sequences, a valine is present in this position, and when we substituted Trp84 with Val, in the context of human SHBG, the relative binding affinities for both estrone and DHEA increased. Moreover, this effect was accentuated by the additional introductions of F56L and M139V substitutions into the human SHBG sequence, and a human SHBG variant (W84L, F56L, and M139V) containing the three hyena-specific residues binds estrone and DHEA with affinities similar to those of spotted hyena SHBG. Thus, these less bulky residues appear to allow estrone and DHEA to be accommodated within the SHBG steroid-binding site.
The only remarkable difference in the SHBG precursor polypeptide sequences among the four hyenid species is that the spotted hyena SHBG signal polypeptide uniquely lacks two leucine residues. In addition, the leucine at position 15 in striped hyena, brown hyena, and aardwolf SHBG sequences is replaced in spotted hyenas by tryptophan. Because the hydrophobic nature of secretion signal polypeptides, and especially the number of leucine residues, plays a role in directing nascent polypeptides across the endoplasmic reticulum for secretion (31), we explored whether the differences in the spotted hyena SHBG secretion signal polypeptide might influence the production of SHBG and account for the much lower plasma concentrations of SHBG in spotted hyenas when compared with the other hyenids. In accordance with observations made using the preprolactin precursor polypeptide (31), our experiments demonstrated that the addition of one or two extra leucines to the spotted hyena SHBG signal polypeptide (which provides a stretch of nine leucine residues) increases the amounts of SHBG produced by more than 2.5 times. Moreover, this increase in SHBG production was further enhanced by substituting Trp15 in the spotted hyena SHBG secretion signal polypeptide with leucine to yield a stretch of 11 leucine residues, as found in all three other hyena species.
The much lower plasma concentrations of SHBG in spotted hyenas, when compared with other hyena species, will almost certainly increase the metabolic clearance of SHBG ligands in spotted hyenas (30). The plasma concentrations of testosterone would therefore be expected to be lower in female spotted hyenas when compared, for instance, to female striped hyenas, but this does not appear to be the case (32). Therefore, given that the percentages of free testosterone in female spotted hyenas must be much higher than in other hyenid species, the absolute amounts of free testosterone in female spotted hyenas should be correspondingly elevated.
As in humans, the serum concentrations of SHBG in spotted hyenas increase during pregnancy, presumably because of induction of hepatic Shbg gene expression by estrogens (29). The physiological relevance of this pregnancy-associated increase in plasma SHBG levels is not well understood. However, it does not appear to protect the fetus from exposure to maternal sex steroids, and androgens in particular, because the female offspring of a highly androgenized woman with barely detectable levels of serum SHBG were born without evidence of in utero androgenization or virilization (33). Nevertheless, maintaining appropriate levels of plasma SHBG in the fetus may be especially important during stages of development when the feedback control mechanisms that regulate steroidogenesis in mature animals are not in play.
Although recent studies have shown that the initial masculinization of the female spotted hyena external genitalia occurs before the onset of ovarian steroidogenesis, and may therefore not involve androgens (7, 34), other endocrine factors including androgen precursors of maternal or adrenal origin could provide a source of active androgens, including ligands of hyena SHBG such as DHEA. Moreover, the very high affinity of hyena SHBG for estrone, a direct precursor of the biologically active estrogen, estradiol, is also intriguing because estrogens appear to influence formation and development of spotted hyena external genitalia during fetal life, whereas androgens appear to play a key role in the eventual morphological differences between the male penis and female peniform clitoris (7, 8, 34).
Although we have not been able to obtain blood from fetal spotted hyenas at the time when the female genitalia exhibit the first signs of masculinization, i.e. at 28 d of a 110-d gestation, we have detected a very low concentration of SHBG (4 nm) in a blood sample from a female spotted hyena fetus at 45 d gestation, when sex differences in urogenital anatomy are consolidated (7). Thus, the fetal liver is therefore at least capable of producing SHBG by 45 d of gestation, and the amounts of SHBG in the blood may influence tissue exposures to steroids that originate from the mother or placenta, even at this early stage. Given that the hepatic production of SHBG by the spotted hyena fetus is also probably much less efficient than in other hyena species, spotted hyena fetuses are likely exposed to greater amounts of sex steroids and their immediate precursors.
In conclusion, the SHBG in hyenas is characterized by an unusual affinity for estrone and DHEA, and residues within the steroid-binding domain that are likely responsible for this have been identified. The high affinity of SHBG for these sex steroid precursors should alter their metabolic clearance rates, as well as their access to tissues, where they may undergo metabolic conversion to active androgens and/or estrogen. However, these steroid-binding properties are shared among hyenid species and therefore cannot account for the unusual phenotypes of the spotted hyena. By contrast, the unique difference in the spotted hyena SHBG precursor polypeptide that limits the secretion of SHBG may play a role in this regard. This genetic difference is quite remarkable given the otherwise very high conservation between Shbg gene sequences in the different Hyaenidae. Thus, we propose that limited hepatic production of plasma SHBG in spotted hyenas increases the exposures of reproductive tissues to androgens and estrogen at critical stages of development, when compared with other hyenids, and contributes to the unusual urogenital phenotype and reproductive costs experienced by female spotted hyenas (3). However, benefits might also accrue from enhanced sex steroid hormone effects on structures in the central nervous system associated for instance with aggression (13), which enables female spotted hyenas to displace males during competitive feeding at a kill and to obtain the calories essential for the prolonged period of nursing observed in this species (35). Such marked female dominance does not occur in other hyenids (36) and may be explained by the fact that serum concentrations of SHBG in female spotted hyenas are very low and approach those seen in males. Consequently, it can be expected that the female spotted hyena central nervous system is exposed to elevated levels of testosterone and precursor steroids that can be converted into active androgens and estrogens within the brain (37, 38).
Supplementary Material
Acknowledgments
We thank Dr. Kevin Leiske, Amy Roberts, and Peter Siminski of The Living Desert Zoo and Gardens for providing the striped hyena and aardwolf samples; Dairen Simpson and Dr. Ingrid Wiesel for help in obtaining the brown hyena samples; Mary Weldele for technical assistance; Dr. George Avvakumov for his advice and assistance; and Nathalie Pilkington for editorial assistance.
This work was supported by the National Institute of Mental Health (MH-39917) and the National Science Foundation (NSF-0809914 and NSF-0845121). G.L.H. holds a Tier 1 Canada Research Chair in Reproductive Health and is supported by the Canadian Institutes of Health Research.
The following nucleotide sequences have been submitted to the GenBank database: spotted hyena SHBG cDNA (accession no. DQ285473), spotted hyena Shbg gene (accession no. DQ302141), striped hyena Shbg gene (accession no. DQ304534), brown hyena Shbg gene (accession no. GQ265893), and aardwolf Shbg gene (accession no. DQ304535).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DHEA
- Dehydroepiandrosterone
- DHT
- 5α-dihydrotestosterone
- LG
- laminin G-like
- RBA
- relative binding affinity.
References
- 1. Frank L, Glickman S, Powch I. 1990. Sexual dimorphism in the spotted hyaena (Crocuta crocuta). J Zool Lond 221:308–313 [Google Scholar]
- 2. Frank LG, Weldele ML, Glickman SE. 1995. Masculinization costs in hyaenas. Nature 377:584–585 [DOI] [PubMed] [Google Scholar]
- 3. Drea CM, Place NJ, Weldele ML, Coscia EM, Licht P, Glickman SE. 2002. Exposure to naturally circulating androgens during foetal life incurs direct reproductive costs in female spotted hyenas, but is prerequisite for male mating. Proc Biol Sci 269:1981–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Licht P, Frank LG, Pavgi S, Yalcinkaya TM, Siiteri PK, Glickman SE. 1992. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 2. Maternal and fetal steroids. J Reprod Fertil 95:463–474 [DOI] [PubMed] [Google Scholar]
- 5. Yalcinkaya TM, Siiteri PK, Vigne JL, Licht P, Pavgi S, Frank LG, Glickman SE. 1993. A mechanism for virilization of female spotted hyenas in utero. Science 260:1929–1931 [DOI] [PubMed] [Google Scholar]
- 6. Drea CM, Weldele ML, Forger NG, Coscia EM, Frank LG, Licht P, Glickman SE. 1998. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. J Reprod Fertil 113:117–127 [DOI] [PubMed] [Google Scholar]
- 7. Cunha GR, Place NJ, Baskin L, Conley A, Weldele M, Cunha TJ, Wang YZ, Cao M, Glickman SE. 2005. The ontogeny of the urogenital system of the spotted hyena (Crocuta crocuta Erxleben). Biol Reprod 73:554–564 [DOI] [PubMed] [Google Scholar]
- 8. Place NJ, Glickman SE. 2004. Masculinization of female mammals: lessons from nature. Adv Exp Med Biol 545:243–253 [DOI] [PubMed] [Google Scholar]
- 9. Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, Mancina R, Muratori M, Forti G, Serio M, Luconi M. 2003. Expression of functional estrogen receptors in human fetal male external genitalia. J Clin Endocrinol Metab 88:1815–1824 [DOI] [PubMed] [Google Scholar]
- 10. Kalloo NB, Gearhart JP, Barrack ER. 1993. Sexually dimorphic expression of estrogen receptors, but not of androgen receptors in human fetal external genitalia. J Clin Endocrinol Metab 77:692–698 [DOI] [PubMed] [Google Scholar]
- 11. Forger NG, Frank LG, Breedlove SM, Glickman SE. 1996. Sexual dimorphism of perineal muscles and motoneurons in spotted hyenas. J Comp Neurol 375:333–343 [DOI] [PubMed] [Google Scholar]
- 12. Fenstemaker SB, Zup SL, Frank LG, Glickman SE, Forger NG. 1999. A sex difference in the hypothalamus of the spotted hyena. Nat Neurosci 2:943–945 [DOI] [PubMed] [Google Scholar]
- 13. Rosen GJ, De Vries GJ, Villalba C, Weldele ML, Place NJ, Coscia EM, Glickman SE, Forger NG. 2006. Distribution of vasopressin in the forebrain of spotted hyenas. J Comp Neurol 498:80–92 [DOI] [PubMed] [Google Scholar]
- 14. Dloniak SM, French JA, Holekamp KE. 2006. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature 440:1190–1193 [DOI] [PubMed] [Google Scholar]
- 15. Hammond GL. 1990. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr Rev 11:65–79 [DOI] [PubMed] [Google Scholar]
- 16. Jänne M, Deol HK, Power SG, Yee SP, Hammond GL. 1998. Human sex hormone-binding globulin gene expression in transgenic mice. Mol Endocrinol 12:123–136 [DOI] [PubMed] [Google Scholar]
- 17. Sullivan PM, Petrusz P, Szpirer C, Joseph DR. 1991. Alternative processing of androgen-binding protein RNA transcripts in fetal rat liver. Identification of a transcript formed by trans splicing. J Biol Chem 266:143–154 [PubMed] [Google Scholar]
- 18. Jänne M, Hogeveen KN, Deol HK, Hammond GL. 1999. Expression and regulation of human sex hormone-binding globulin transgenes in mice during development. Endocrinology 140:4166–4174 [DOI] [PubMed] [Google Scholar]
- 19. Wagner AP, Creel S, Frank LG, Kalinowski ST. 2007. Patterns of relatedness and parentage in an asocial, polyandrous striped hyena population. Mol Ecol 16:4356–4369 [DOI] [PubMed] [Google Scholar]
- 20. Frank L, Simpson D, Woodroffe R. 2003. Foot Snares: An Effective Method for Capturing African Lions. Wildl Soc Bull 31:309–314 [Google Scholar]
- 21. Miller SA, Dykes DD, Polesky HF. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammond GL, Lähteenmäki PL. 1983. A versatile method for the determination of serum cortisol binding globulin and sex hormone binding globulin binding capacities. Clin Chim Acta 132:101–110 [DOI] [PubMed] [Google Scholar]
- 23. Bocchinfuso WP, Warmels-Rodenhiser S, Hammond GL. 1991. Expression and differential glycosylation of human sex hormone-binding globulin by mammalian cell lines. Mol Endocrinol 5:1723–1729 [DOI] [PubMed] [Google Scholar]
- 24. Grishkovskaya I, Avvakumov GV, Sklenar G, Dales D, Hammond GL, Muller YA. 2000. Crystal structure of human sex hormone-binding globulin: steroid transport by a laminin G-like domain. EMBO J 19:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammond GL, Bocchinfuso WP. 1996. Sex hormone-binding globulin: gene organization and structure/function analyses. Horm Res 45:197–201 [DOI] [PubMed] [Google Scholar]
- 26. van Jaarsveld AS. 1992. A multivariate approach to socio-ecological development and endocrine variance in the spotted hyaena, Crocuta crocuta Erxleben. Experientia 48:774–778 [DOI] [PubMed] [Google Scholar]
- 27. Walsh KA, Titani K, Takio K, Kumar S, Hayes R, Petra PH. 1986. Amino acid sequence of the sex steroid binding protein of human blood plasma. Biochemistry 25:7584–7590 [DOI] [PubMed] [Google Scholar]
- 28. Grishkovskaya I, Avvakumov GV, Hammond GL, Catalano MG, Muller YA. 2002. Steroid ligands bind human sex hormone-binding globulin in specific orientations and produce distinct changes in protein conformation. J Biol Chem 277:32086–32093 [DOI] [PubMed] [Google Scholar]
- 29. Westphal U. 1986. Steroid-protein interactions II. Monogr Endocrinol 27:1–603 [PubMed] [Google Scholar]
- 30. Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. 1982. The serum transport of steroid hormones. Recent Prog Horm Res 38:457–510 [DOI] [PubMed] [Google Scholar]
- 31. Hatsuzawa K, Tagaya M, Mizushima S. 1997. The hydrophobic region of signal peptides is a determinant for SRP recognition and protein translocation across the ER membrane. J Biochem 121:270–277 [DOI] [PubMed] [Google Scholar]
- 32. Wagner AP, Frank LG, Creel S, Coscia EM. 2007. Transient genital abnormalities in striped hyenas (Hyaena hyaena). Horm Behav 51:626–632 [DOI] [PubMed] [Google Scholar]
- 33. Hogeveen KN, Cousin P, Pugeat M, Dewailly D, Soudan B, Hammond GL. 2002. Human sex hormone-binding globulin variants associated with hyperandrogenism and ovarian dysfunction. J Clin Invest 109:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Browne P, Place NJ, Vidal JD, Moore IT, Cunha GR, Glickman SE, Conley AJ. 2006. Endocrine differentiation of fetal ovaries and testes of the spotted hyena (Crocuta crocuta): timing of androgen-independent versus androgen-driven genital development. Reproduction 132:649–659 [DOI] [PubMed] [Google Scholar]
- 35. Hofer H, East ML. 1993. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. III. Attendance and maternal care. Anim Behav 46:575–589 [Google Scholar]
- 36. Watts HE, Holekamp KE. 2007. Hyena societies. Curr Biol 17:R657–R60 [DOI] [PubMed] [Google Scholar]
- 37. Majewska MD. 1995. Neuronal actions of dehydroepiandrosterone. Possible roles in brain development, aging, memory, and affect. Ann NY Acad Sci 774:111–120 [DOI] [PubMed] [Google Scholar]
- 38. Steckelbroeck S, Watzka M, Reissinger A, Wegener-Toper P, Bidlingmaier F, Bliesener N, Hans VH, Clusmann H, Ludwig M, Siekmann L, Klingmüller D. 2003. Characterisation of estrogenic 17β-hydroxysteroid dehydrogenase (17β-HSD) activity in the human brain. J Steroid Biochem Mol Biol 86:79–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.