Abstract
Patients bearing mutations in TAC3 and TACR3 (which encode neurokinin B and its receptor, respectively) have sexual infantilism and infertility due to GnRH deficiency. In contrast, Tacr3−/− mice have previously been reported to be fertile. Because of this apparent phenotypic discordance between mice and men bearing disabling mutations in Tacr3/TACR3, Tacr3 null mice were phenotyped with close attention to pubertal development, estrous cyclicity, and fertility. Tacr3−/− mice demonstrated normal timing of preputial separation and day of first estrus, markers of sexual maturation. However, at postnatal d 60, Tacr3−/− males had significantly smaller testes and lower FSH levels than their wild-type littermates. Tacr3−/− females had lower uterine weights and abnormal estrous cyclicity. Approximately half of Tacr3−/− females had no detectable corpora lutea on ovarian histology at postnatal d 60. Despite this apparent ovulatory defect, all Tacr3−/− females achieved fertility when mated. However, Tacr3−/− females were subfertile, having both reduced numbers of litters and pups per litter. The subfertility of these animals was not due to a primary ovarian defect, because they demonstrated a robust response to exogenous gonadotropins. Thus, although capable of fertility, Tacr3-deficient mice have central reproductive defects. The remarkable ability of acyclic female Tacr3 null mice to achieve fertility is reminiscent of the reversal of hypogonadotropic hypogonadism seen in a high proportion of human patients bearing mutations in TACR3. Tacr3 mice are a useful model to examine the mechanisms by which neurokinin B signaling modulates GnRH release.
GnRH neurons regulate the timing of puberty and fertility in all mammalian species. In the past 15 yr, several gene defects have been identified in patients with abnormal pubertal development and hypogonadotropic hypogonadism. These genes affect either the migration of GnRH neurons from the nasal placode into the brain during embryonic development or the synthesis/secretion of GnRH hormone (1–6).
Loss-of-function mutations in the genes encoding neurokinin B (TAC3) and its receptor (TACR3) were recently described as leading to abnormal pubertal development and hypogonadotropic hypogonadism in humans (7–12). Mutation-bearing patients have high rates of microphallus, indicating androgen deficiency in utero. However, they also have paradoxically high rates of reversal of their hypogonadotropism, such that their hypothalamic-pituitary-gonadal axes are able to recover spontaneous function, as attested to by normal sex-steroid production, menstrual cycling, and/or fertility (10).
However, years before the genetic discoveries in humans that put the neurokinin B pathway into the spotlight for the hypothalamic control of reproduction, mice with targeted deletion of the neurokinin B receptor had already been genetically engineered and used in studies examining cognitive performance and anxiety (13–15). Although these studies were not focused on the reproductive phenotype per se, Tacr3−/− mice were described as fertile. The reported fertility of these Tacr3−/− mice stood in sharp contrast to the phenotypes of other mice with targeted deletion of genes associated with GnRH deficiency [i.e. GNRH1/Gnrh1 (16), KISS1/Kiss1 (17, 18), KISS1R/Kiss1r (19, 20), FGFR1/Fgfr1 (21), PROK2/Prokr2 (22, 23), and CHD7 (24, 25)] who have hypogonadotropic hypogonadism and reproductive incompetence. Because of this human-mouse phenotypic discrepancy for TACR3/Tacr3, the present study set out to explore the reproductive neuroendocrinology, estrous cyclicity, and gonadal function of Tacr3-deficient mice in detail. Although Tacr3−/− mice are capable of fertility, they also have multiple reproductive abnormalities, making them closer in phenotype to their human counterparts than previously appreciated.
Materials and Methods
Animals
Mice with heterozygous mutations of Tacr3 on a C57Bl/6 /129Sv background strain (backcrossed two generations on C57Bl/6) were generated by Deltagen (San Mateo, CA) as previously described (13). Briefly, exon 1 of Tacr3 was replaced by an IRES-lacZ-neo cassette via homologous recombination. Heterozygous males and females were crossed to generate Tacr3−/−, Tacr3+/−, and wild-type (WT) littermates. Mice were group housed (three to five per cage) at the Massachusetts General Hospital Center for Comparative Medicine in a temperature- and light-controlled environment with lights on from 0600–1800 h and food and water provided ad libitum. All procedures were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital.
Phenotyping
Day of birth was marked at postnatal day (P)0, and litter size was recorded. Penile width of male pups was measured at P20 using calipers (n = 15–25). Pups were weaned between P20 and P24, and DNA samples from the tail or ear were used for genotyping. Mice were examined daily from P24 to P36 for evidence of sexual maturation: females, day of vaginal opening (VO) (n = 17–20); males, day of preputial separation (n = 12–22) (26). Body weight was recorded from P20–P60 mice (n = 7–15). Adult P60 mice were killed between 0900 and 1200 h. At killing, mice were weighed and blood was collected via submandibular bleed and/or cardiac puncture. Estrous stage of females was determined by vaginal lavage (27). Body length and anogenital distance were measured in males (n = 7–17). Gonads were collected and fixed in Bouin's solution until ready for further processing. Ovarian and uterine weights were collected from females in diestrus (n = 7–15). Brains of adult males were fixed in 4% paraformaldehyde for approximately 5 d at 4 C followed by cryoprotection in 15 and 30% of sucrose/0.1 m phosphate buffer (pH 7.4).
For the estrous cycle study, a separate cohort of P70–P80 females was used (n = 5–10). Vaginal lavages were collected daily between 1500 and 1700 h for 31 continuous days. Bedding soiled with male urine was placed into the female cages every 3 d to prevent females from entering anestrus (28, 29).
Tissue preparation and analysis
Gonads were paraffin embedded, sectioned (10 μm), and stained with hematoxylin and eosin (H&E) stain (Dana-Farber/Harvard Cancer Center Pathology Core). Ovaries (n = 9–13) were examined for presence or absences of corpora lutea, and testes (n = 3–4) were examined for presence of spermatogenesis. Coronal brain sections were cut in serial sections (30 μm) using a Leica (Heerbrugg, Switzerland) cryostat from the level of the septum through the lateral hypothalamic area corresponding to the mouse brain atlas at approximately 1.94 and −2.30 mm bregma, respectively (30). Six serial series were generated for each animal, and the first and fourth series were stained and counted.
Immunohistochemistry
Brain sections (n = 4) were treated with 3% hydrogen peroxide for 10 min to quench endogenous peroxidase activity and then washed in PBS. Sections were then blocked in PBS containing 5% normal goat serum and 0.3% Triton X-100 (PBST) for 1 h, washed several times in PBS, and incubated for 48 h at 4 C in a polyclonal rabbit anti-GnRH antibody (1:8000 in PBS containing 5% normal goat serum, PA-1-120; Thermo Scientific, Rockford, IL). After several washes in PBS, sections were incubated in biotinylated antirabbit immunoglobulins (1:500 in PBST; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. After subsequent washes in PBS, sections were incubated in avidin-biotin complex solution (1:1500 in PBST, ABC Elite Vectastain kit; Vector Laboratories) for 1 h. Peroxidase labeling was visualized with nickel-diaminobenzidine tetrahydrochloride subtract (∼10 min of incubation, DAB Substrate kit; Vector Laboratories).
GnRH neurons counts
Immunolabeled GnRH cells were counted at ×40 using brightfield microscopy. For each animal, the total cell number was estimated by multiplying the average number of cells counted from the first and fourth series by 6.
Fertility testing
Fertility was examined by pairing Tacr3−/− females (n = 7) with heterozygous or WT males and Tacr3−/− males (n = 6) with heterozygous or WT females. WT littermate females paired with WT males (n = 9) served as the control breeding paradigm. Mean number of litters and number of pups per litter over a 12-wk period were determined.
Superovulation
Adult (P30–P95) Tacr3−/− (n = 6), and control Tacr3+/− (n = 2) and WT females (n = 2), were injected with pregnant mare serum gonadotropin 5 IU ip followed by human chorionic gonadotropin 5 IU ip 48 h later, then paired with a stud male. Females were killed 40–50 h after human chorionic gonadotropin injection and the genital tract excised. Oviducts were flushed with PBS, and oocytes were counted two to three times and averaged per oviduct using a dissecting microscope at ×2–4 magnification.
Hormone assay
Serum LH, FSH, and T levels were measured as singlets (n = 6–16) by RIA performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA). The measurable range was 0.04–37.4 ng/ml for LH, 3.4–75.0 ng/ml for FSH, and 10.9–872.9 ng/dl for testosterone. Intra- and interassay coefficients of variation were 3.1 and 9.0% for LH, 5.5 and 8.0% for FSH, and 3.6 and 7.0% for testosterone, respectively. Standards used were mouse LH reference prep AFP5306A and mouse FSH reference prep AFP5308D.
Statistical analysis
Data are expressed as mean ± sem unless stated otherwise. Statistical analysis was done using GraphPad Prism 4 (GraphPad, San Diego, CA). Differences between Tacr3−/−, Tacr3+/−, and WT were analyzed using one-way ANOVA followed by Tukey's post hoc test or two-way ANOVA. Differences between Tacr3−/− and WT in the GnRH count and fertility experiments were analyzed using unpaired t tests. Fisher's exact test was used to analyze the dichotomous variable of presence or absence of corpora lutea in Tacr3−/− vs. WT ovaries. P < 0.05 was considered statistically significant.
Results
Tacr3 breeding
Mice deficient for Tacr3 were viable and developed normally. Among 222 pups born from 11 Tacr3+/− × Tacr3+/− breeding pairs, the sex ratio was 112 males vs. 110 females and the genotype distribution was 56 WT vs. 100 Tacr3+/− vs. 66 Tacr3−/−. There were no differences in body weight between Tacr3−/−, Tacr3+/−, and WT males from P20 to P60 (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The body weight of Tacr3−/− females did not differ from that of WT mice from P20 to P50 (Supplemental Fig. 1B). However, on P60, Tacr3−/− (19.4 ± 0.4 g, n = 11) and Tacr3+/− (20.3 ± 0.6 g, n = 10) females weighed significantly less than WT females (22.8 ± 0.5 g, n = 9; two-way ANOVA, P < 0.001).
Males. Sexual maturation and testicular size
Because IHH patients with loss-of-function mutations in neurokinin B signaling have a high propensity for microphallus (10), penile width was examined at P20 as a biomarker of in utero androgen exposure. The penile width of Tacr3−/− mice was not different from that of Tacr3+/− and WT mice (Table 1). Similarly, in Tacr3−/− males, the timing of preputial separation, a marker of sexual maturation, was not different from that of Tacr3+/− and WT mice (Table 1). Body length and anogenital distance at P60, a marker of androgen exposure across sexual maturation, also did not differ between the genotypes (Table 1).
Table 1.
Male reproductive and endocrine phenotype
| WT | n | Tacr3+/− | n | Tacr3−/− | n | |
|---|---|---|---|---|---|---|
| Penile width (mm) at P20 | 2.48 ± 0.09 | 25 | 2.41 ± 0.10 | 15 | 2.52 ± 0.06 | 20 |
| Day of preputial separation | 30.25 ± 0.65 | 12 | 30.0 ± 0.95 | 12 | 30.64 ± 0.70 | 22 |
| Adult (P60–P80) | ||||||
| Anogenital distance (cm) | 1.94 ± 0.03 | 8 | 1.93 ± 0.04 | 15 | 1.89 ± 0.05 | 17 |
| Body length (cm) | 9.8 ± 0.10 | 7 | 9.9 ± 0.1 | 15 | 9.8 ± 0.1 | 17 |
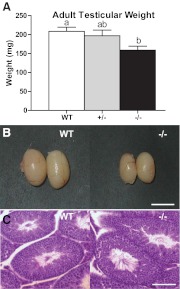
| Testicular weight (mg) | 208.4 ± 11.0 | 10 | 197.0 ± 14.7 | 7 | 158.8 ± 10.6a | 11 |
| Testosterone (ng/dl) | 125.2 ± 61.3 | 7 | 318.3 ± 99.9 | 6 | 173.5 ± 63.6 | 8 |
| LH (ng/ml) | 0.37 ± 0.12 | 9 | 0.39 ± 0.11 | 9 | 0.48 ± 0.17 | 9 |
| FSH (ng/ml) | 28.9 ± 2.0 | 9 | 30.1 ± 2.9 | 9 | 9.9 ± 0.7b | 9 |
P < 0.01.
P < 0.0001 (ANOVA with Tukey post hoc test).
However, at P60, Tacr3−/− males (159 ± 11 mg) had smaller testicles compared with WT males (208 ± 11 mg; P < 0.01) (Table 1 and Fig. 1, A and B). Histology revealed no differences between knockout and WT males with respect to the gross testicular architecture of the seminiferous tubules and Sertoli cells. The lumens of the seminiferous tubules were open and filled with mature spermatazoa (Fig. 1C), indicating that all stages of spermatogenesis were present.
Fig. 1.
Tacr3−/− male mice have lower testicular weight but apparently normal spermatogenesis. A, Mean ± sem of testicular weight of WT, Tacr3+/−, and Tacr3−/− males. Different letters above error bars represent groups that are statistically different, P < 0.05, one-way ANOVA. B, Testicles from WT and Tacr3−/− males. Scale bar, 0.5 cm. C, Histology of testicular cross-sections stained with H&E at ×10 magnification. Scale bar, 0.1 mm. Note the open lumen of seminiferous tubules and tail of mature spermatozoa.
Serum FSH levels were significantly lower in Tacr3−/− males (9.9 ± 0.7 ng/ml) compared with Tacr3+/− (30.1 ± 2.9 ng/ml) and WT males (28.9 ± 2.0 ng/ml; P < 0.0001) (Table 1). There were no significant differences between serum testosterone and LH levels between Tacr3−/−, Tacr3+/−, and WT males (Table 1).
Tacr3−/− males and GnRH neuron number
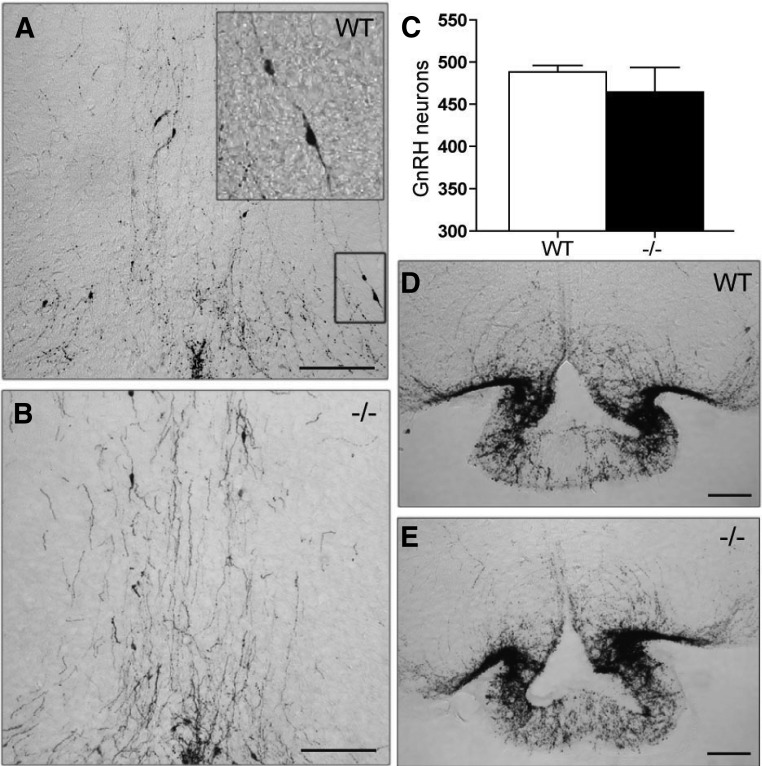
The reduction in FSH levels suggested a deficiency in GnRH secretion. To determine whether this was due to loss of GnRH neurons, the number of GnRH neurons in the preoptic area was quantified. The distribution pattern of GnRH neurons in the preoptic area was not different between Tacr3−/− and WT mice (Fig. 2, A and B). Moreover, the total number of GnRH cell bodies in the Tacr3−/− mice (77.7 ± 5 cells per series; ≈464 ± 29 cells per animal, n = 4) was not different from that observed in WT adult mice (81.4 ± 1.2 cells per series; ≈488 ± 8 cells per animal, n = 4) (Fig. 2C). In addition, the density of GnRH fibers present in the median eminence was qualitatively similar between the two genotypes (Fig. 2, D and E).
Fig. 2.
Deletion of Tacr3 does not affect GnRH neuronal number. A–E, Coronal brain sections through the preoptic area (A and B) and median eminence (D and E) from WT (A and D) and Tacr3−/− (B and E) adult males stained with an antibody against GnRH. Mean ± sem of GnRH counted from the preoptic area (POA) (C). Scale bar, 0.1 mm. Note the normal distribution pattern and density of GnRH fibers in the POA and median eminence.
Tacr3−/− females. Sexual maturation, gonadal size, and estrous cyclicity
The timing of sexual maturation was also examined in females. The average day of VO of Tacr3−/− mice was similar to that of Tacr3+/− and WT mice (Table 2). In addition, the average day of first estrus of Tacr3−/− females was statistically indistinguishable from that of Tacr3+/− and WT mice (Table 2) and occurred approximately 5–6 d after VO.
Table 2.
Female reproductive and endocrine phenotype
| WT | n | Tacr3+/− | n | Tacr3−/− | n | |
|---|---|---|---|---|---|---|
| Day of VO | 28.8 ± 0.6 | 20 | 29.9 ± 0.6 | 17 | 29.6 ± 0.9 | 19 |
| Day of first estrus | 35.4 ± 1.1 | 10 | 33.9 ± 0.3 | 11 | 34.3 ± 1.2 | 10 |
| Uterine weight (% BW) | 0.32 ± 0.03 | 7 | 0.26 ± 0.04 | 7 | 0.06 ± 0.02a | 7 |
| Uterine weight (mg) | 68.6 ± 6.4 | 7 | 54.1 ± 7.7 | 7 | 33.4 ± 3.5b | 9 |
| Ovarian weight (% BW) | 0.08 ± 0.02 | 5 | 0.06 ± 0.01 | 6 | 0.01 ± 0.13 | 7 |
| Ovarian weight (mg) | 13.8 ± 2.1 | 11 | 13.7 ± 1.1 | 12 | 10.2 ± 1.6 | 15 |
| LH (ng/ml) | 0.29 ± 0.07 | 12 | 0.25 ± 0.04 | 16 | 0.35 ± 0.07 | 13 |
| FSH (ng/ml) | 10.36 ± 1.60 | 8 | 7.38 ± 1.59 | 12 | 7.45 ± 1.29 | 13 |
BW, Body weight.
P < 0.05.
P < 0.001 (ANOVA with Tukey post hoc test).
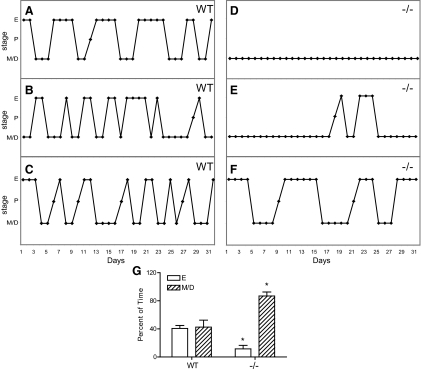
Daily vaginal lavage was performed for 31 d in adult mice (P70–P80) (Fig. 3). Eighty percent of WT females (n = 5) had normal 4- to 5-d estrous cycles (Fig. 3, A–C) with an average cycle length of 5.1 ± 0.4 d (40.6 ± 4.2% estrus, 52.5 + 4.1% metestrus/diestrus). In contrast, all Tacr3−/− females (n = 10) were acyclic or had irregular cycles (Fig. 3, D–F). Four Tacr3−/− females were in constant diestrus (Fig. 3D), five had 2–4 d of estrus with prolonged periods of diestrus (Fig. 3E), and one female had prolonged cycles with an average cycle length of 10.5 d (Fig. 3F). Tacr3−/− females spent less time in estrus compared with WT females (11.5 ± 5.0 vs. 40.6 ± 4.2%; t test, P < 0.01) and more time in diestrus (86.8 ± 5.7 vs. 52.5 ± 4.1%; t test, P < 0.01).
Fig. 3.
Tacr3−/− females have abnormal estrous cycles. A–F, Cycling profiles of WT (A–C) and Tacr3−/− (D–F) females during a 31-d period. G, Percent of time spent females spent in estrus (E) and metestrus/diestrus (M/D). *, P < 0.05 compared with WT, t test.
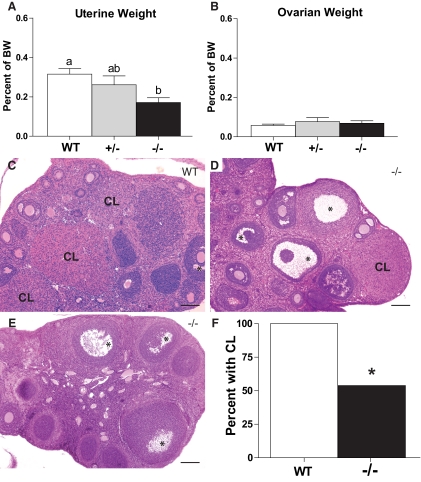
Uterine weight (as a percent of body weight) of Tacr3−/− diestrous females (0.06 ± 0.02%, n = 7) (Fig. 4A and Table 2) was significantly less than Tacr3+/− (0.26 ± 0.04%, n = 7; P < 0.05) and WT females (0.32 ± 0.03%, n = 7; P < 0.05). The ovarian weights (as a percent of body weight) of Tacr3−/−, Tacr3+/−, and WT mice were comparable (Fig. 4B and Table 2). Tacr3−/− and WT mice had follicles at all stages of maturation, including primordial, primary, and mature preovulatory follicles (Fig 4, C–E). All WT females (n = 9) had ovulated as evidenced by the presence of corpora lutea in the ovaries, whereas only 53.8% of Tacr3−/− females (n = 13) had corpora lutea (P < 0.05, Fisher's exact test) (Fig. 4, D–F).
Fig. 4.
Tacr3−/− females have smaller uterine weight and abnormal ovarian histology. Mean ± sem of uterine weight (A) and ovarian weight (B) as percent of body weight (BW) of WT, Tacr3+/−, and Tacr3−/− females. Different letters above error bars represent groups that are statistically different. P < 0.05, one-way ANOVA. C–E, Photomicrograph of ovarian cross-sections stained with H&E at ×5 magnification. Scale bar, 0.1 mm. *, Antra of mature follicles; CL, corpus luteum. F, Percent of WT and Tacr3−/− females with CL. *, P < 0.05, Fisher's exact test.
Gonadotropin levels were assessed in P60 females. Because there was no difference in the morning gonadotropin levels of estrous and diestrous females (data not shown), the values were combined. Serum LH and FSH levels of Tacr3−/− mice were not different from Tacr3+/− and WT females (Table 2).
Fertility
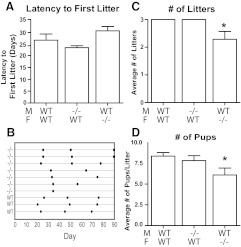
Fertility was assessed by pairing Tacr3−/− males with control females and pairing Tacr3−/− females with control males of proven fertility. Matings between WT males and WT females produced 3.0 ± 0.0 litters (n = 9) and 8.4 ± 0.4 pups/litter (n = 9) over the first 12 wk. Matings between Tacr3−/− males and control females produced similar numbers of litters (3.0 ± 0.0 litters, n = 6) and pups/litter (7.8 ± 0.6 pups, n = 6) over the same time interval. However, subfertility was observed when the Tacr3−/− females (n = 7) were paired with control males. Although the average latency to the first litter for Tacr3−/− females (30.4 ± 4.9 d) was not statistically different from the latency to first litter of WT (26.5 ± 7.3 d) (Fig. 5A), Tacr3−/− females failed to sustain reproductive robustness (Fig. 5B). They demonstrated subfertility having significantly fewer litters (2.3 ± 0.3, n = 7) and fewer pups/litter (6.1 ± 0.8, n = 7) overall compared with WT females (3.0 ± 0.0, n = 9, P < 0.01 and 8.4 ± 0.4, n = 9, P < 0.05, respectively) (Fig. 5, C and D).
Fig. 5.
Fertility is impaired in Tacr3−/− females. A, Mean ± sem of latency (days) until first litter. B, Schematic representation of female reproductive history over a 90-d (12 wk) period. Filled circles and squares indicate new litters from Tacr3−/− and WT females, respectively. Mean ± sem of number of litters (C) and pups/litter (D). *, P < 0.05 compared with WT, t test. M, Males; F, females.
Notably, the four Tacr3−/− females with constant diestrus described earlier, when partnered with Tacr3−/− males, were also able to produce litters (1.5 ± 0.29 litters, n = 4; 5.38 ± 0.37 pups/litter, n = 4; and 41.5 ± 8.39 d to first, n = 4), resulting in the same subfertility observed in pairing of Tacr3−/− females with control males.
Superovulation
To determine whether the subfertility in Tacr3−/− females was due to an ovarian defect, null animals were treated with exogenous gonadotropins. Tacr3−/− females ovulated an average of 14.6 ± 2.9 oocytes (n = 6) in response to superovulation compared with control females who ovulated 14.3 ± 3.6 oocytes (n = 4).
Discussion
Humans with disabling mutations of TACR3 (encoding neurokinin B receptor) have abnormal pubertal development and hypogonadotropic hypogonadism that can be successfully treated with exogenous GnRH, providing compelling evidence that the neurokinin B signaling pathway has a stimulatory influence on GnRH secretion (7–11). However, Tacr3−/− mice were originally reported to be fertile (13), creating a striking human-mouse phenotypic discordance. The data assembled here demonstrate that Tacr3−/− animals have numerous reproductive defects that appear to be due to a central, as opposed to a gonadal, etiology. Tacr3−/− males have decreased FSH levels and smaller testes than WT males; Tacr3−/− females have abnormal estrous cycles, reduced presence of corpora lutea, decreased uterine weights, and subfertility compared with WT and heterozygous females. Thus, despite normal timing of sexual maturation and ability to achieve fertility, Tacr3−/− mice have several reproductive defects, demonstrating that they are more similar to the human patients with TAC3/TACR3 mutations than previously appreciated and confirming that neurokinin B signaling is an important regulator of gonadotropin secretion in rodents as it is in humans.
All Tacr3−/− females had abnormal estrous cycles despite being exposed to male pheromones from male-soiled bedding. Although there was no gross abnormality in the olfactory bulb of these mice, their olfactory capability was not formally tested, and it is theoretically possible that the animals were simply unable to detect pheromones in the male-soiled bedding. However, all five Tacr3−/− females that showed irregular cycles had litters after being paired with males, suggesting that the physical appearance of the male or the act of mating itself helped to trigger ovulation. This latter hypothesis flies in the face of conventional wisdom, because mice have been traditionally classified as spontaneous (as opposed to reflex) ovulators. However, reflex ovulation has been observed in hpg mice (lacking the Gnrh1 gene) that received GnRH neuronal grafts and were then mated with normal males (31). These animals, initially acyclic, demonstrated a rapid rise in LH within 10 min of ejaculation and a subsequent significant pregnancy rate (40%), all consistent with reflex ovulation (32). In addition, vaginocervical stimulation of mating has been shown to activate GnRH neurons in rats (33) and LH secretion in voles and mice (31). Thus, it is possible that copulatory behavior plays a positive role in stimulating ovulatory surges and that these pathways become more apparent in the absence of neurokinin B receptor signaling. Stated alternatively, there appear to be two pathways to ovulation: spontaneous ovulation that requires neurokinin B signaling and reflex ovulation that does not. The improvement in reproductive function in female Tacr3−/− mice may be the rodent “homolog” to the reversible hypogonadotropism of humans with mutations in the neurokinin B pathway (10), in which patients are inexplicably able to recover normal functioning of the hypothalamic-pituitary-gonadal axis in the absence of hormonal medications. Although the mechanisms underlying these observations (in both mice and men) may be distinct and species specific, these intriguing similarities suggest commonality between mice and humans.
Despite this phenotypic concordance between Tacr3 null mice and TACR3 mutation-bearing patients, there are still considerable differences between these two models. Patients with mutations in TACR3 have high rates of microphallus and abnormal pubertal development (9, 10). In contrast, Tacr3−/− male mice do not have smaller penile width at P20, demonstrating that Tacr3 is not involved in the in utero development of the sex organ in mice. Moreover, Tacr3 null mice have normal sexual maturation, because the timing of VO, first estrus, and preputial separation are similar between WT and Tacr3−/− animals. Therefore, although both mice and men with mutations in the neurokinin B signaling pathway have reproductive defects in adult life, only humans have abnormalities in utero and during sexual maturation. As demonstrated by the Gnr23−/− mouse, relatively few GnRH neurons are necessary to initiate sexual maturation in the mouse, raising the possibility that there are different thresholds for the numbers of GnRH neurons required for puberty, as well as other reproductive functions, in mice vs. humans (34). In the case of Tacr3−/− mice, because GnRH neuronal number was not different from WT mice, it appears that the function of GnRH neurons was impaired, and thus there may also be different thresholds for GnRH neuronal function (as opposed to number). In addition, it has been well established that there are fundamental differences in the gating mechanisms for sexual maturation between rodents and primates; for instance, rodents have gonadal suppression of GnRH secretion before sexual maturation, whereas primates do not (35). In totality, these differences between rodents and primates may explain why patients with mutations in the neurokinin B pathway mutations are infertile unless they reverse their hypogonadotropism (10), whereas mutant mice are fertile.
Tacr3−/− females are clearly subfertile, an observation concordant with a pharmacologic model in which a neurokinin B receptor antagonist reduced reproductive success and litter size in rats (36). Although they were able to achieve fertility, Tacr3−/− females were unable to sustain full reproductive capacity. Approximately 50% of Tacr3−/− females did not have corpora lutea in their ovaries. Although neurokinin B receptor is expressed in the ovary (37, 38), and specifically in the corpora lutea (39), the ovulatory defect of Tacr3−/− animals does not appear to be primarily gonadal in origin, because Tacr3−/− females responded as robustly as WT females when given exogenous gonadotropins. Lower uterine weight, an indication of reduced estradiol levels, may also have contributed to the subfertility, although direct effects of neurokinin B receptor on the uterus cannot be excluded (40–42).
Another difference between the human and mouse models is the pattern of peripheral gonadotropin levels. Low or inappropriately normal gonadotropins in the setting of low sex steroids are the biochemical hallmarks of GnRH-deficient states across mammalian species. FSH levels were significantly lower in Tacr3−/− males compared with WT, but LH values were indistinguishable between the two genotypes. This pattern (low FSH, normal LH) has been observed in several other GnRH-deficient mouse models (i.e. Kiss1−/−, Kiss1r−/−, Gnr23−/−, and Prok2−/−) (18, 22, 34). In contrast, patients with mutations in the gene encoding neurokinin B (TAC3) have higher FSH:LH ratios than other patients with GnRH deficiency who carry mutations in nonneurokinin B pathway genes (9, 12). Although the explanation underlying this gonadotropin pattern has not yet been elucidated, it is possible that abnormalities in the neurokinin B signaling pathway lead to reductions in the quantity of GnRH released and/or reduced frequency of GnRH pulses (9, 12), the latter favoring FSH secretion (43–46). Further studies will be required to explore these possibilities in both rodent and human models.
Although Tacr3−/− males have reduced FSH levels, the number of GnRH neurons is indistinguishable between Tacr3−/− and WT mice, further supporting the concept that the neuroendocrine defect in these animals is due to impaired GnRH release and not impaired GnRH neuronal migration. The reduced FSH levels in Tacr3−/− mice add to physiologic models, supporting the importance of neurokinin B signaling in modulating gonadotropin release. For example, administration of the neurokinin B receptor agonist, senktide, has been shown to stimulate LH secretion in rodents and monkeys, although this response appears to be highly dependent on the prevailing sex-steroid milieu (47–50). Second, the stimulatory effect of senktide on gonadotropin secretion is dependent upon GnRH signaling, because preadministration of a GnRH receptor antagonist blocks these effects (47). Neurokinin B signaling appears to lie physiologically upstream of kisspeptin, a powerful stimulus for GnRH release (19, 51–56), because desensitization of the kisspeptin receptor blocks the stimulatory effect of senktide in monkeys (49). In fact, because neurokinin B receptors have been found lie on kisspeptin/neurokinin B expressing neurons themselves, it has been proposed that neurokinin B participates in an auto-feedback loop, shaping the pulsatile pattern of kisspeptin secretion and, by extension, GnRH release (57). Thus, the significant reduction of FSH observed in Tacr3 null males supports the importance of neurokinin B signaling in modulating gonadotropin release, either directly or through complex interrelationships with other neuropeptides in the brain.
These complex interrelationships may extend not only to other peptide families but may also operate within the tachykinin superfamily itself. The partial nature of the reproductive defects in Tacr3 null mice could be due to promiscuity among the tachykinins and their receptors (58, 59). In the absence of Tacr3, neurokinin B may activate other tachykinin receptors to partially stimulate GnRH release. In fact, blocking all three neurokinin receptors lowered castration-induced LH and testosterone secretion in male rats, but blockade of the neurokinin B receptor alone did not, further demonstrating that other tachykinin pathways are important in regulating GnRH function (60).
In conclusion, targeted deletion of Tacr3 has a significant deleterious impact on reproductive function. As in humans, Tacr3 null mice are able to overcome their reproductive defects. Thus, Tacr3 null mice will be useful for further examining the mechanisms by which neurokinin B receptor signaling modulates GnRH release and reproductive function and may serve as a unique model for the human phenomenon of reversal of hypogonadotropic hypogonadism.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant U54 HD028138, NIH Grant T32 HD07396 (to J.Y.Y. and C.S.C.), and a Charles A. King Trust Postdoctoral Fellowship and a Children's Hospital Boston Career Development Award (Y.-M.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- H&E
- Hematoxylin and eosin
- P
- postnatal day
- PBST
- PBS containing 0.3% Triton X-100
- VO
- vaginal opening
- WT
- wild type.
References
- 1. Karges B, de Roux N. 2005. Molecular genetics of isolated hypogonadotropic hypogonadism and Kallmann syndrome. Endocr Dev 8:67–80 [DOI] [PubMed] [Google Scholar]
- 2. Hardelin JP, Dodé C. 2008. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev 2:181–193 [DOI] [PubMed] [Google Scholar]
- 3. Bianco SD, Kaiser UB. 2009. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Semple RK, Topaloglu AK. 2010. The recent genetics of hypogonadotrophic hypogonadism - novel insights and new questions. Clin Endocrinol 72:427–435 [DOI] [PubMed] [Google Scholar]
- 5. Balasubramanian R, Crowley WF., Jr 2011. Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Mol Cell Endocrinol 346:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowley WF. 2011. The developmental biology of the GnRH neurons. Mol Cell Endocrinol 346:1–3 [DOI] [PubMed] [Google Scholar]
- 7. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. 2009. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. 2010. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 10. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. 2010. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukami M, Maruyama T, Dateki S, Sato N, Yoshimura Y, Ogata T. 2010. Hypothalamic dysfunction in a female with isolated hypogonadotropic hypogonadism and compound heterozygous TACR3 mutations and clinical manifestation in her heterozygous mother. Horm Res Paediatr 73:477–481 [DOI] [PubMed] [Google Scholar]
- 12. Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, Meduri G, Brailly-Tabard S, Chanson P, Lecomte P, Guiochon-Mantel A, Young J. 2011. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS One 6:e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siuciak JA, McCarthy SA, Martin AN, Chapin DS, Stock J, Nadeau DM, Kantesaria S, Bryce-Pritt D, McLean S. 2007. Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology 194:185–195 [DOI] [PubMed] [Google Scholar]
- 14. Nordquist RE, Delenclos M, Ballard TM, Savignac H, Pauly-Evers M, Ozmen L, Spooren W. 2008. Cognitive performance in neurokinin 3 receptor knockout mice. Psychopharmacology 198:211–220 [DOI] [PubMed] [Google Scholar]
- 15. Nordquist RE, Savignac H, Pauly-Evers M, Walker G, Knoflach F, Borroni E, Glaentzlin P, Bohrmann B, Messer J, Ozmen L, Albientz A, Spooren W. 2008. Characterization of behavioral response to amphetamine, tyrosine hydroxylase levels, and dopamine receptor levels in neurokinin 3 receptor knockout mice. Behav Pharmacol 19:518–529 [DOI] [PubMed] [Google Scholar]
- 16. Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. 1977. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- 17. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 19. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 20. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. 2003. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 21. Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI. 2005. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol 19:225–236 [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. 2006. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA 103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr 2007. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jongmans MC, Admiraal RJ, van der Donk KP, Vissers LE, Baas AF, Kapusta L, van Hagen JM, Donnai D, de Ravel TJ, Veltman JA, Geurts van Kessel A, De Vries BB, Brunner HG, Hoefsloot LH, van Ravenswaaij CM. 2006. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet 43:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergman JE, Bosman EA, van Ravenswaaij-Arts CM, Steel KP. 2010. Study of smell and reproductive organs in a mouse model for CHARGE syndrome. Eur J Hum Genet 18:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korenbrot CC, Huhtaniemi IT, Weiner RI. 1977. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod 17:298–303 [DOI] [PubMed] [Google Scholar]
- 27. Caligioni CS. 2009. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4:Appendix 4I [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whitten WK. 1959. Occurrence of anoestrus in mice caged in groups. J Endocrinol 18:102–107 [DOI] [PubMed] [Google Scholar]
- 29. Whitten WK, Bronson FH, Greenstein JA. 1968. Estrus-inducing pheromone of male mice: transport by movement of air. Science 161:584–585 [DOI] [PubMed] [Google Scholar]
- 30. Paxinos G, Franklin KBJ. 2001. The mouse brain in stereotaxic coordinates. 2nd ed San Diego: Academic Press [Google Scholar]
- 31. Gibson MJ, Moscovitz HC, Kokoris GJ, Silverman AJ. 1987. Plasma LH rises rapidly following mating in hypogonadal female mice with preoptic area (POA) brain grafts. Brain Res 424:133–138 [DOI] [PubMed] [Google Scholar]
- 32. Gibson MJ, Charlton HM, Perlow MJ, Zimmerman EA, Davies TF, Krieger DT. 1984. Preoptic area brain grafts in hypogonadal (hpg) female mice abolish effects of congenital hypothalamic gonadotropin-releasing hormone (GnRH) deficiency. Endocrinology 114:1938–1940 [DOI] [PubMed] [Google Scholar]
- 33. Pfaus JG, Jakob A, Kleopoulos SP, Gibbs RB, Pfaff DW. 1994. Sexual stimulation induces Fos immunoreactivity within GnRH neurons of the female rat preoptic area: interaction with steroid hormones. Neuroendocrinology 60:283–290 [DOI] [PubMed] [Google Scholar]
- 34. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. 2008. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plant TM. 2006. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol Cell Endocrinol 254- 255:97–102 [DOI] [PubMed] [Google Scholar]
- 36. Pintado CO, Pinto FM, Pennefather JN, Hidalgo A, Baamonde A, Sanchez T, Candenas ML. 2003. A role for tachykinins in female mouse and rat reproductive function. Biol Reprod 69:940–946 [DOI] [PubMed] [Google Scholar]
- 37. Pinto FM, Almeida TA, Hernandez M, Devillier P, Advenier C, Candenas ML. 2004. mRNA expression of tachykinins and tachykinin receptors in different human tissues. Eur J Pharmacol 494:233–239 [DOI] [PubMed] [Google Scholar]
- 38. Löffler S, Schulz A, Brylla E, Nieber K, Spanel-Borowski K. 2004. Transcripts of neurokinin B and neurokinin 3 receptor in superovulated rat ovaries and increased number of corpora lutea as a non-specific effect of intraperitoneal agonist application. Regul Pept 122:131–137 [DOI] [PubMed] [Google Scholar]
- 39. Brylla E, Aust G, Geyer M, Uckermann O, Löffler S, Spanel-Borowski K. 2005. Coexpression of preprotachykinin A and B transcripts in the bovine corpus luteum and evidence for functional neurokinin receptor activity in luteal endothelial cells and ovarian macrophages. Regul Pept 125:125–133 [DOI] [PubMed] [Google Scholar]
- 40. Patak E, Pinto FM, Story ME, Pintado CO, Fleming A, Page NM, Pennefather JN, Candenas ML. 2005. Functional and molecular characterization of tachykinins and tachykinin receptors in the mouse uterus. Biol Reprod 72:1125–1133 [DOI] [PubMed] [Google Scholar]
- 41. Page NM, Dakour J, Morrish DW. 2006. Gene regulation of neurokinin B and its receptor NK3 in late pregnancy and pre-eclampsia. Mol Hum Reprod 12:427–433 [DOI] [PubMed] [Google Scholar]
- 42. Page NM. 2010. Neurokinin B and pre-eclampsia: a decade of discovery. Reprod Biol Endocrinol 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- 44. Savoy-Moore RT, Swartz KH. 1987. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Adv Exp Med Biol 219:641–645 [DOI] [PubMed] [Google Scholar]
- 45. Haisenleder DJ, Ortolano GA, Dalkin AC, Ellis TR, Paul SJ, Marshall JC. 1990. Differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone pulse amplitude in female rats. Endocrinology 127:2869–2875 [DOI] [PubMed] [Google Scholar]
- 46. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. 1997. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- 47. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramaswamy S, Seminara SB, Plant TM. 2011. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. 2011. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 52. Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. 2004. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- 53. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 54. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. 2005. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436 [DOI] [PubMed] [Google Scholar]
- 56. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Munekata E. 1991. Neurokinin A and B. Comp Biochem Physiol C 98:171–179 [PubMed] [Google Scholar]
- 59. Beaujouan JC, Saffroy M, Torrens Y, Glowinski J. 1997. Potency and selectivity of the tachykinin NK3 receptor antagonist SR 142801. Eur J Pharmacol 319:307–316 [DOI] [PubMed] [Google Scholar]
- 60. Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. 2011. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev 57:409–415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.