Abstract
Glucagon is traditionally thought of as an antihypoglycemic hormone, for example in response to starvation. However, it actually increases energy expenditure and has other actions not in line with protection from hypoglycemia. Furthermore, it is often found to be elevated when glucose is also raised, for example in circumstances of psychological and metabolic stress. These findings seem more in keeping with glucagon having some role as a hormone enhancing the response to stress.
The conventional view puts glucagon as a regulator of glucose homeostasis, acting in response to hypoglycemia to elevate blood glucose concentration and prevent tissue glycopenia (1). Indeed, the hormone was named for this property, after Kimball and Murlin (2) identified a compound contaminating pancreatic extracts that had the opposite effect to the insulin they were seeking to purify. Recently, there has been a resurgence in interest in the finding that glucagon increases energy expenditure (3). This observation produces a conundrum. Why does a hormone that counteracts hypoglycemia, a state of energy deficiency, also increase energy expenditure? This could be explained by a more wide-ranging role that includes aiding in physiological responses to stress. In this review, we will describe how acute physical stress leads to glucagon secretion as well as how the metabolic responses mediated by the hormone fit within the adaptive stress response. We will also survey the evidence for its role in energy balance in general and how this could be exploited therapeutically.
Glucagon as a Stress Hormone
The physiological and behavioral responses to acute stress result from the activation of an array of neural and hormonal pathways in reaction to the presence of a variety of different adverse stimuli, such as combat, threat from predators, injury, and infection. Such physical stressors, although dissimilar, pose a threat to the homeostasis of the organism and elicit a common series of adaptive physiological responses. Glucocorticoids and catecholamines are the best-known stress hormones, because they are released in the context of stress and mediate elements of an adaptive response to it. However, as will be discussed, glucagon also fits with this definition, in addition to its role in protection against hypoglycemia, another important form of stress.
Glucagon release in stress
Evidence for glucagon release in a wide variety of stressful situations began to accumulate after improvements in glucagon assays made accurate measurement possible in the early 1970s. In animal models, large elevations in plasma glucagon are observed immediately after acutely stressful stimuli (4, 5). Hyperglucagonemia is also well recognized in patients under a range of physiological stress states, including trauma (6), burns (7, 8), surgery (9), sepsis (10), hemorrhage (11), acute myocardial infarction (12), cardiac arrest (13), and hypoxia (14) including in neonates (15). Very high plasma glucagon concentrations are seen in diabetic ketoacidosis (16) and contribute to hyperglycemia in this setting. In all of these pathological scenarios, hypoglycemia is not a primary driver for glucagon secretion, and instead, other provoking factors must be sought.
Mechanism of glucagon release in stress
Both α1- and β-adrenergic receptors are present on pancreatic α-cells (17) and, when stimulated, induce glucagon secretion (18). Importantly, this effect occurs at plasma glucose concentrations that would ordinarily inhibit glucagon release (19). This pathway could be activated during stress either via direct islet sympathetic innervation or through a systemic catecholamine response. Several pieces of indirect evidence infer neural regulation may be responsible. Direct projections exist between stress-sensing nuclei in the hypothalamus and pancreas, including from the paraventricular nucleus as demonstrated by viral tracer studies (20), and experimental manipulation of various points along these pathways supports a plausible role in glucagon release during stress. For example, hyperglucagonemia results from splanchnic nerve stimulation in adrenalectomized calves (21, 22) and this phenomenon is significantly attenuated by selective denervation of the pancreas (23). Furthermore, stimulating the stress-responsive hypothalamic ventromedial nucleus leads to release of glucagon (24), whereas lesions of the same area inhibit it (25), and the effect persists after adrenalectomy (26). In isolated rat islets, a paradoxical inhibitory effect of epinephrine at concentrations found in the systemic circulation was noted on glucagon release, whereas at higher concentrations akin to those produced locally by direct neurotransmitter release, glucagon secretion was enhanced (27). The implication is that the islet sympathetic innervation is the primary controlling influence for glucagon release. However, despite the presence of this anatomical and physiological basis for autonomic stimulation of glucagon release through generalized sympathetic neural activation, few direct mechanistic studies under conditions of nonhypoglycemic stress have been performed to test this hypothesis. In dogs, pancreatic autonomic nerves were shown to be responsible for glucagon release in neuroglycopenic stress but not hypotensive or hypoxic stress (28). On the other hand, exercise-induced stress led to persistence of glucagon secretion in adrenalectomized but not sympathectomized rats (29). Thus, it is not clear whether neural or systemic mechanisms are primarily responsible for glucagon release in acute stress.
Glucagon in relation to other stress hormones
As well as catecholamine-induced glucagon secretion, glucagon potently stimulates catecholamine release (30). Similarly, glucagon's ability to stimulate ACTH-induced cortisol release is well known and forms the basis of the glucagon stimulation test to assess ACTH and GH reserve (31). Thus, irrespective of any direct effects, glucagon is able to indirectly augment the acute stress response via other stress hormones. However, the wide expression of the glucagon receptor, including in liver, kidney, adipose tissue, pancreas, heart, brain, gastrointestinal tract, and adrenal glands (32, 33), suggests the hormone is more than an intermediary in a catecholamine-dominated response. We discuss some of the evidence indicating a specific, adaptive role for glucagon in the stress response below.
Glucagon increases substrate availability
The best-characterized effect of glucagon is that of increasing hepatic glucose output through stimulation of glycogenolysis, which is critical for its role in protecting against hypoglycemia. Increasing glucose availability is also an adaptive response to situations when strenuous exertion is required, e.g. to fight or to flee. The cellular mechanism involves binding of glucagon to its G protein-coupled receptor in hepatocytes resulting in activation of adenylate cyclase and inhibition of cAMP phosphodiesterase, producing a rise in intracellular cAMP and subsequently an increase in glycogen phosphorylase activity via activation of protein kinase A (34, 35). That a rise in glucagon per se during exercise is most important, rather than a fall in insulin, has been demonstrated by the use of pancreatic clamp techniques in which the effect of individual pancreatic hormone infusions are assessed while endogenous hormone production is clamped by somatostatin (36). The hyperglycemic effect of glucagon is independent of that of other stress hormones, because during a combination stress hormone infusion of glucagon, epinephrine, norepinephrine, and cortisol, discontinuation of glucagon results in a drop in hepatic glucose output (37). This study also demonstrated the primary role of glucagon-responsive glycogenolysis in increasing hepatic glucose output during exercise rather than gluconeogenesis. Additional evidence for the primary role of glycogenolysis was provided by correlating rate of magnetic resonance imaging-derived hepatic glycogen removal with rate of glucose production measured by tracer (38). Synergy with other stress hormones is nevertheless likely to be important, and glucagon at a low dose may still potentiate hepatic glucose output in response to epinephrine (39, 40) and similarly facilitates an increase in cortisol-induced gluconeogenesis (41).
Cardiac effects of glucagon support a fight-or-flight response
That glucagon has positive inotropic and chronotropic effects on the myocardium has long been recognized (42). The effect is noted within a few minutes of administration of glucagon, a time course compatible with a role in a hyperacute stress response. This effect is not abolished by autonomic blockade (43), implying a specific action of glucagon on the heart independent of the sympathetic drive induced by the hormone. The glucagon receptor is expressed in cardiac myocytes, where it facilitates an increase in contractility, primarily through increased cAMP production and subsequent Ca2+ release (44). This mechanism has been harnessed therapeutically, where high doses of glucagon are used to treat symptomatic β-blocker overdose. The high dose required has been used to argue that the cardiac effects of glucagon could be pharmacological rather than physiological. However, acute stress can produce peripheral plasma glucagon concentrations considerably in excess of those experienced during fasting (45) and approximate to those used experimentally to test the effect of glucagon on isolated cardiac myocytes or perfused hearts, e.g. 500 pmol/liter (4) and 1000 pmol/liter (46). Physiological levels of glucagon at about 100 pmol/liter are also known to facilitate myocardial glucose uptake, arguing that glucagon has a key role in regulating cardiac function (47).
Glucagon in Energy Homeostasis
Recent interest in nonhypoglycemic glucagon release centers on its role in energy homeostasis. As will be reviewed, the hormone generates a net negative energy balance through a reduction in food intake and an increase in energy expenditure. Such responses would appear to be maladaptive if the primary role of glucagon is to raise blood glucose. On the other hand, these responses could be seen as adaptive within the context of certain stressful situations such as fleeing from a predator, when stopping to eat may be disadvantageous, or extreme cold or infection, in which case heat generation would be beneficial. Although speculative, this concept attempts to explain an otherwise unexpected property of glucagon out of keeping with a predominant role in hypoglycemia.
Glucagon reduces food intake
Glucagon was first shown to inhibit food intake in man in 1957 (48). The nausea associated with pharmacological doses of glucagon is well recognized (49), but glucagon can have an anorectic effect without significant nausea in humans (50) and rats (51). This appears to be due primarily to a satiety effect of the hormone, because subjects finish eating earlier, but other aspects of meal behavior including meal frequency and rate of ingestion are unchanged (52). Critically, a reduction in food intake has been demonstrated in man in response to glucagon infusion at a dose that achieves plasma glucagon concentrations of 50–100 pmol/liter, comparable to those seen in the portal circulation (50). The mechanism by which glucagon reduces food intake is as yet unclear. One effect is to delay gastric emptying (53). It may also result from vagal relay of a peripherally sensed glucagon signal to the hypothalamic nuclei that regulate food intake, as determined by the observation that the anorectic effect of glucagon is abolished after selective vagotomy (54, 55). On the other hand, a centrally mediated mechanism has also been suggested by the observation that intraventricular infusion of glucagon at physiological concentration also causes a reduction in food intake (56) and that central administration is more potent that peripheral (57).
Glucagon is thermogenic and increases energy expenditure
Glucagon secretion is provoked by cold acclimatization (58). An adaptive role for glucagon in this setting is suggested by repeated observations that glucagon is thermogenic and increases oxygen consumption when administered exogenously. For example, this was initially shown in adrenal demedullated rats (59) in which confounding by a systemic catecholamine response was blocked, and also during glucagon infusion in man with contributions of other pancreatic hormones minimized by a somatostatin clamp (60). The physiological processes that lead to this change have not been conclusively established, but two possible candidates include 1) an increase in thermogenesis by brown adipose tissue (BAT) and 2) futile substrate cycling. Evidence supporting the former includes increases in BAT temperature (61) and blood flow (62) in the context of a measured increase in metabolic rate after glucagon administration. Critically, this effect persists at physiological doses (63). Glucagon receptors are present in BAT (64), and when administered to BAT in vitro, glucagon causes an increase in oxygen consumption (65), although supraphysiological doses were used in these studies. In contrast, other mechanistic data suggest the observed effect of glucagon is mediated indirectly via catecholamines, because it is blunted after BAT denervation (66), β-blockade (67), and sympathectomy (68). Futile substrate cycling provides a means to increasing the metabolic rate through stimulation of energy-consuming cyclical metabolic pathways with no net change in product formation in the liver and other tissues. In addition to heat production, such cycling is potentially of benefit in preparing the cell for periods of high metabolic demand, because small changes in enzyme activity away from the equilibrium will rapidly lead to large increases in flux (69). Tracer studies have indicated a significant effect on glucose cycling in response to glucagon infusion (70), an effect that occurs predominantly at low insulin levels and is abolished with high-dose insulin infusion (71). In the latter study, however, the increase in substrate cycling was not the major factor determining basal metabolic rate. The physiology underpinning glucagon-induced thermogenesis is thus as yet unproven, but the phenomenon presents an exciting opportunity for therapeutic intervention.
Therapeutic potential of glucagon's effect on energy homeostasis
Whatever the mechanism, glucagon's apparent ability to increase energy expenditure and reduce food intake has generated considerable pharmaceutical interest because these two effects could clearly combine to cause significant weight loss. Weight loss due to chronic glucagon administration has been noted in man (48) and rats (72) but at the expense of a degree of hyperglycemia. Interest in this approach has resurfaced with the finding that dual agonists which stimulate both the glucagon and glucagon-like peptide-1 (GLP-1) receptors can not only reverse diet-induced obesity in animal models but also induce marked improvements in glucose tolerance (73, 74). This effect is similar to that achieved by administration of the naturally occurring glucagon/GLP-1 dual agonist oxyntomodulin to humans (75, 76). These observations, therefore, suggest that GLP-1 is able to counter the hyperglycemic effect of glucagon and pave the way for the future development of novel agents to tackle obesity and diabetes.
Conclusions
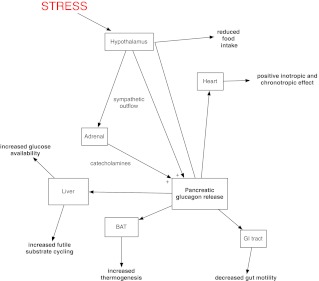
Glucagon receptors are widely expressed in a number of tissues, and it is therefore not surprising that glucagon has a number of physiological effects beyond the one that has dominated the majority of research into the hormone over the past 40 yr, i.e. protection of the organism from hypoglycemia. The available evidence shows that there exist a wide range of glucagon-stimulating factors, and glucagon-stimulated responses (Fig. 1), beyond those involved in glucose homeostasis. Glucagon release has been demonstrated to be elevated in many forms of physiological stress in which hypoglycemia is not a typical feature. Hyperglucagonemia leads to physiological and behavioral responses, such as increased substrate availability and improved cardiovascular performance, which are key features of the stress response. Undoubtedly, it performs this role alongside the classical stress hormones, but numerous physiological studies indicate its effects are to a degree mediated independently. Similarly, the antihypoglycemic role of glucagon does not occur in isolation, with catecholamines, glucocorticoids, and GH playing an important role here too. Indeed, neither glucagon receptor knockout mice (77) nor pancreatectomized humans (78) usually die of hypoglycemia, despite an almost total lack of glucagon. Whether effects on energy homeostasis fit within a remit as a stress hormone can be debated, but they do not sit easily with a role in hypoglycemia. The mechanism underpinning its effect on food intake and energy expenditure are yet to be fully elucidated and require further detailed work to be undertaken. Nevertheless, the ability of the hormone to generate a net energy deficit is an appealing prospect in the context of the current paucity of effective pharmacological therapies to aid weight loss in the face of an ever-increasing prevalence of obesity.
Fig. 1.
Summary of glucagon effects which may play a role in the response to stress.
Acknowledgments
This work was supported by the Medical Research Council, Biotechnology and Biological Sciences Research Council, National Institute for Health Research (NIHR), an Integrative Mammalian Biology Capacity Building Award, an FP7-HEALTH-2009-241592 EuroCHIP Grant and is supported by the NIHR Imperial Biomedical Research Centre Funding Scheme.
Disclosure Summary: The authors have no competing interests to disclose.
Footnotes
- BAT
- Brown adipose tissue
- GLP-1
- glucagon-like peptide-1.
References
- 1. Jiang G, Zhang BB. 2003. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 284:E671–E678 [DOI] [PubMed] [Google Scholar]
- 2. Kimball C, Murlin JR. 1923. Aqueous extracts of pancreas. J Biol Chem 58:337–348 [Google Scholar]
- 3. Heppner KM, Habegger KM, Day J, Pfluger PT, Perez-Tilve D, Ward B, Gelfanov V, Woods SC, DiMarchi R, Tschöp M. 2010. Glucagon regulation of energy metabolism. Physiol Behav 100:545–548 [DOI] [PubMed] [Google Scholar]
- 4. Bloom SR, Daniel PM, Johnston DI, Ogawa O, Pratt OE. 1973. Release of glucagon, induced by stress. Q J Exp Physiol Cogn Med Sci 58:99–108 [DOI] [PubMed] [Google Scholar]
- 5. Freeman BM. 1975. Physiological basis of stress. Proc R Soc Med 68:427–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brockman RP, Manns JG. 1976. Effect of trauma on plasma glucagon and insulin concentrations in sheep. Can J Comp Med 40:5–8 [PMC free article] [PubMed] [Google Scholar]
- 7. Orton CI, Segal AW, Bloom SR, Clarke J. 1975. Hypersecretion of glucagon and gastrin in severely burnt patients. Br Med J 2:170–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venter M, Rode H, Sive A, Visser M. 2007. Enteral resuscitation and early enteral feeding in children with major burns: effect on McFarlane response to stress. Burns 33:464–471 [DOI] [PubMed] [Google Scholar]
- 9. Russell RC, Walker CJ, Bloom SR. 1975. Hyperglucagonaemia in the surgical patient. Br Med J 1:10–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rocha DM, Santeusanio F, Faloona GR, Unger RH. 1973. Abnormal pancreatic α-cell function in bacterial infections. N Engl J Med 288:700–703 [DOI] [PubMed] [Google Scholar]
- 11. Russel RC, Pardy BJ, Carruthers ME, Bloom SR. 1977. Plasma glucagon levels in haemorrhagic shock. Br J Surg 64:285–289 [DOI] [PubMed] [Google Scholar]
- 12. Segal P, Esrig B. 1973. The role of glucagon hypersecretion in the pathogenesis of hyperglycemia following acute myocardial infarction. Circulation 48:797–800 [Google Scholar]
- 13. Oshima C, Kaneko T, Tsuruta R, Oda Y, Miyauchi T, Fujita M, Kawamura Y, Kasaoka S, Maekawa T. 2010. Increase in plasma glucagon, a factor in hyperglycemia, is related to neurological outcome in postcardiac-arrest patients. Resuscitation 81:187–192 [DOI] [PubMed] [Google Scholar]
- 14. Bloom SR, Edwards AV, Hardy RN. 1978. The role of the autonomic nervous system in the control of glucagon, insulin and pancreatic polypeptide release from the pancreas. J Physiol (Lond) 280:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnston DI, Bloom SR. 1973. Plasma glucagon levels in the term human infant and effect of hypoxia. Arch Dis Child 48:451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston DI, Bloom SR, O'Brien D. 1975. Proceedings: Pancreatic glucagon in diabetic ketoacidosis. Arch Dis Child 50:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuit FC, Pipeleers DG. 1986. Differences in adrenergic recognition by pancreatic A and B cells. Science 232:875–877 [DOI] [PubMed] [Google Scholar]
- 18. Vieira E, Liu YJ, Gylfe E. 2004. Involvement of α1 and β-adrenoceptors in adrenaline stimulation of the glucagon-secreting mouse α-cell. Naunyn Schmiedebergs Arch Pharmacol 369:179–183 [DOI] [PubMed] [Google Scholar]
- 19. Iversen J. 1973. Adrenergic receptors and the secretion of glucagon and insulin from the isolated, perfused canine pancreas. J Clin Invest 52:2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jansen AS, Hoffman JL, Loewy AD. 1997. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res 766:29–38 [DOI] [PubMed] [Google Scholar]
- 21. Bloom SR, Edwards AV. 1975. The release of pancreatic glucagon and inhibition of insulin in response to stimulation of the sympathetic innervation. J Physiol (Lond) 253:157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bloom SR, Edwards AV. 1980. Pancreatic endocrine responses to stimulation of the peripheral ends of the splanchnic nerves in the conscious adrenalectomized calf. J Physiol (Lond) 308:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Havel PJ, Mundinger TO, Taborsky GJ., Jr 1996. Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs. Am J Physiol 270:E20–E26 [DOI] [PubMed] [Google Scholar]
- 24. Shimazu T, Ishikawa K. 1981. Modulation by the hypothalamus of glucagon and insulin secretion in rabbits: studies with electrical and chemical stimulations. Endocrinology 108:605–611 [DOI] [PubMed] [Google Scholar]
- 25. Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. 1994. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frohman LA, Bernardis LL. 1971. Effect of hypothalamic stimulation on plasma glucose, insulin, and glucagon levels. Am J Physiol 221:1596–1603 [DOI] [PubMed] [Google Scholar]
- 27. De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, Habib AM, Moritoh Y, Zhang E, Reimann F, Rosengren AH, Shibasaki T, Gribble F, Renström E, Seino S, Eliasson L, Rorsman P. 2010. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab 11:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Havel PJ, Veith RC, Dunning BE, Taborsky GJ., Jr 1988. Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog. Evidence for stress-specific and regionally selective activation of the sympathetic nervous system. J Clin Invest 82:1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luyckx AS, Dresse A, Cession-Fossion A, Lefebvre PJ. 1975. Catecholamines and exercise-induced glucagon and fatty acid mobilization in the rat. Am J Physiol 229:376–383 [DOI] [PubMed] [Google Scholar]
- 30. Lawrence AM. 1967. Glucagon provocative test for pheochromocytoma. Ann Intern Med 66:1091–1096 [DOI] [PubMed] [Google Scholar]
- 31. Berg C, Meinel T, Lahner H, Yuece A, Mann K, Petersenn S. 2010. Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Eur J Endocrinol 162:477–482 [DOI] [PubMed] [Google Scholar]
- 32. Svoboda M, Tastenoy M, Vertongen P, Robberecht P. 1994. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol Cell Endocrinol 105:131–137 [DOI] [PubMed] [Google Scholar]
- 33. Christophe J. 1996. Glucagon and its receptor in various tissues. Ann NY Acad Sci 805:31–42; discussion 42–43 [DOI] [PubMed] [Google Scholar]
- 34. Pohl SL, Birnbaumer L, Rodbell M. 1969. Glucagon-sensitive adenyl cylase in plasma membrane of hepatic parenchymal cells. Science 164:566–567 [DOI] [PubMed] [Google Scholar]
- 35. Robles-Flores M, Allende G, Piña E, García-Sáinz JA. 1995. Cross-talk between glucagon- and adenosine-mediated signalling systems in rat hepatocytes: effects on cyclic AMP-phosphodiesterase activity. Biochem J 312(Pt 3):763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wasserman DH, Spalding JA, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD. 1989. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol 257:E108–E117 [DOI] [PubMed] [Google Scholar]
- 37. McGuinness OP, Murrell S, Moran C, Bracy D, Cherrington AD. 1994. The effect of acute glucagon removal on the metabolic response to stress hormone infusion in the conscious dog. Metabolism 43:1310–1317 [DOI] [PubMed] [Google Scholar]
- 38. Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. 1995. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44:185–189 [DOI] [PubMed] [Google Scholar]
- 39. Morgan NG, Charest R, Blackmore PF, Exton JH. 1984. Potentiation of α1-adrenergic responses in rat liver by a cAMP-dependent mechanism. Proc Natl Acad Sci USA 81:4208–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shamoon H, Hendler R, Sherwin RS. 1981. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab 52:1235–1241 [DOI] [PubMed] [Google Scholar]
- 41. Lecavalier L, Bolli G, Gerich J. 1990. Glucagon-cortisol interactions on glucose turnover and lactate gluconeogenesis in normal humans. Am J Physiol 258:E569–E575 [DOI] [PubMed] [Google Scholar]
- 42. Regan TJ, Lehan PH, Henneman DH, Behar A, Hellems HK. 1964. Myocardial, metabolic and contractile response to glucagon and epinephrine. J Lab Clin Med 63:638–647 [PubMed] [Google Scholar]
- 43. Stuesse SL, Levy MN, Zieske H. 1982. Effects of glucagon on cardiac chronotropic response to vagal stimulation in the dog. Am J Physiol 242:H7–H12 [DOI] [PubMed] [Google Scholar]
- 44. Méry PF, Brechler V, Pavoine C, Pecker F, Fischmeister R. 1990. Glucagon stimulates the cardiac Ca2+ current by activation of adenylyl cyclase and inhibition of phosphodiesterase. Nature 345:158–161 [DOI] [PubMed] [Google Scholar]
- 45. Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF., Jr 1970. Glucagon levels and metabolic effects in fasting man. J Clin Invest 49:2256–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodgers RL, MacLeod KM, McNeill JH. 1981. Responses of rat and guinea pig hearts to glucagon. Lack of evidence for a dissociation between changes in myocardial cyclic 3′,5′-adenosine monophosphate and contractility. Circ Res 49:216–225 [DOI] [PubMed] [Google Scholar]
- 47. Harney JA, Rodgers RL. 2008. Insulin-like stimulation of cardiac fuel metabolism by physiological levels of glucagon: involvement of PI3K but not cAMP. Am J Physiol Endocrinol Metab 295:E155–E161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schulman JL, Carleton JL, Whiteney G, Whitehorn JC. 1957. Effect of glucagon on food intake and body weight in man. J Appl Physiol 11:419–421 [DOI] [PubMed] [Google Scholar]
- 49. Waldhäusl W, Haydl H, Nowotny P. 1976. ACTH and cortisol responses to glucagon stimulation. J Clin Endocrinol Metab 43:675–678 [DOI] [PubMed] [Google Scholar]
- 50. Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. 1992. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol 262:R975–R980 [DOI] [PubMed] [Google Scholar]
- 51. Martin JR, Novin D. 1977. Decreased feeding in rats following hepatic-portal infusion of glucagon. Physiol Behav 19:461–466 [DOI] [PubMed] [Google Scholar]
- 52. Le Sauter J, Geary N. 1991. Hepatic portal glucagon infusion decreases spontaneous meal size in rats. Am J Physiol 261:R154–R161 [DOI] [PubMed] [Google Scholar]
- 53. Chernish SM, Brunelle RR, Rosenak BD, Ahmadzai S. 1978. Comparison of the effects of glucagon and atropine sulfate on gastric emptying. Am J Gastroenterol 70:581–586 [PubMed] [Google Scholar]
- 54. Geary N, Smith GP. 1983. Selective hepatic vagotomy blocks pancreatic glucagon's satiety effect. Physiol Behav 31:391–394 [DOI] [PubMed] [Google Scholar]
- 55. Martin JR, Novin D, Vanderweele DA. 1978. Loss of glucagon suppression of feeding after vagotomy in rats. Am J Physiol 234:E314–E318 [DOI] [PubMed] [Google Scholar]
- 56. Honda K, Kamisoyama H, Saito N, Kurose Y, Sugahara K, Hasegawa S. 2007. Central administration of glucagon suppresses food intake in chicks. Neurosci Lett 416:198–201 [DOI] [PubMed] [Google Scholar]
- 57. Inokuchi A, Oomura Y, Nishimura H. 1984. Effect of intracerebroventricularly infused glucagon on feeding behavior. Physiol Behav 33:397–400 [DOI] [PubMed] [Google Scholar]
- 58. Edwards CI, Howland RJ. 1986. Adaptive changes in insulin and glucagon secretion during cold acclimation in the rat. Am J Physiol 250:E669–E676 [DOI] [PubMed] [Google Scholar]
- 59. Davidson I, Salter JM, Best CH. 1960. The effect of glucagon on the metabolic rate of rats. Am J Clin Nutr 8:540–546 [Google Scholar]
- 60. Nair KS. 1987. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab 64:896–901 [DOI] [PubMed] [Google Scholar]
- 61. Cockburn F, Hull D, Walton I. 1967. The effect of lipolytic hormones and theophylline on heat production in brown adipose tissue in vivo. Br J Pharmacol Chemother 31:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yahata T, Kuroshima A. 1982. Influence of endocrine and chemical factors on glucagon induced thermogenesis in brown adipocytes. Jpn J Physiol 32:303–307 [DOI] [PubMed] [Google Scholar]
- 63. Billington CJ, Briggs JE, Link JG, Levine AS. 1991. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am J Physiol 261:R501–R507 [DOI] [PubMed] [Google Scholar]
- 64. Morales A, Lachuer J, Duchamp C, Vera N, Georges B, Cohen-Adad F, Moulin C, Barré H. 1998. Tissue-specific modulation of rat glucagon receptor mRNA by thyroid status. Mol Cell Endocrinol 144:71–81 [DOI] [PubMed] [Google Scholar]
- 65. Joel CD. 1966. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J Biol Chem 241:814–821 [PubMed] [Google Scholar]
- 66. Billington CJ, Bartness TJ, Briggs J, Levine AS, Morley JE. 1987. Glucagon stimulation of brown adipose tissue growth and thermogenesis. Am J Physiol 252:R160–R165 [DOI] [PubMed] [Google Scholar]
- 67. Dicker A, Zhao J, Cannon B, Nedergaard J. 1998. Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Am J Physiol 275:R1674–R1682 [DOI] [PubMed] [Google Scholar]
- 68. Filali-Zegzouti Y, Abdelmelek H, Rouanet JL, Cottet-Emard JM, Pequignot JM, Barré H. 2005. Role of catecholamines in glucagon-induced thermogenesis. J Neural Transm 112:481–489 [DOI] [PubMed] [Google Scholar]
- 69. Samoilov M, Plyasunov S, Arkin AP. 2005. Stochastic amplification and signaling in enzymatic futile cycles through noise-induced bistability with oscillations. Proc Natl Acad Sci USA 102:2310–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miyoshi H, Shulman GI, Peters EJ, Wolfe MH, Elahi D, Wolfe RR. 1988. Hormonal control of substrate cycling in humans. J Clin Invest 81:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Calles-Escandón J. 1994. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. Metabolism 43:1000–1005 [DOI] [PubMed] [Google Scholar]
- 72. de Castro JM, Paullin SK, DeLugas GM. 1978. Insulin and glucagon as determinants of body weight set point and microregulation in rats. J Comp Physiol Psych 92:571–579 [DOI] [PubMed] [Google Scholar]
- 73. Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, Holland J, Hembree J, Abplanalp W, Grant E, Ruehl J, Wilson H, Kirchner H, Lockie SH, Hofmann S, Woods SC, Nogueiras R, Pfluger PT, Perez-Tilve D, DiMarchi R, Tschöp MH. 2009. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 5:749–757 [DOI] [PubMed] [Google Scholar]
- 74. Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, Liu F, Miller C, Tota LM, Zhou G, Zhang X, Sountis MM, Santoprete A, Capito' E, Chicchi GG, Thornberry N, Bianchi E, Pessi A, Marsh DJ, SinhaRoy R. 2009. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 58:2258–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. 2003. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 88:4696–4701 [DOI] [PubMed] [Google Scholar]
- 76. Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR. 2005. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 54:2390–2395 [DOI] [PubMed] [Google Scholar]
- 77. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. 2007. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated β-cell loss and hyperglycaemia. Diabetologia 50:142–150 [DOI] [PubMed] [Google Scholar]
- 78. Polonsky KS, Herold KC, Gilden JL, Bergenstal RM, Fang VS, Moossa AR, Jaspan JB. 1984. Glucose counterregulation in patients after pancreatectomy. Comparison with other clinical forms of diabetes. Diabetes 33:1112–1119 [DOI] [PubMed] [Google Scholar]