Abstract
More than 80% of thymocytes are CD4+CD8+ double positive (DP) cells which subject to positive/negative selection. The lifespan of DP thymocytes is critical in shaping the peripheral T cell repertoire essential for mounting immune responses against foreign, but not self, antigens. During T cell maturation, if the first round of T cell receptor (TCR) α chain rearrangement fails to generate a productive T cell receptor, DP cells start another round of α chain rearrangement until positive selection or cell death intervenes. Thus, the lifespan of DP cells determines how many rounds of α chain rearrangement can be carried out, and influences the likelihood of completing positive selection. The anti-apoptotic protein Bcl-xL is the ultimate effector regulating DP cell survival, and several transcription factors critical for T cell development, such as TCF-1, E proteins, c-Myb, and RORγt, regulate DP survival via a Bcl-xL-dependent pathway. However, the relationship between these transcription factors in this process is largely unclear. Recent results are revealing an interactive network among these critical factors during regulation of DP thymocyte survival. This review will discuss how these transcription factors potentially work together to control DP thymocyte survival that is critical for successful completion of T cell development.
T cell development
T cells are important players in adaptive immunity against infection and parasites. Similar to other developmental processes, T cell development in the thymus, which is believed to be the only organ where T cell development occurs, is highly-organized and involves a network of multiple players. The goal of T cell development is to arm T cells with all necessary machineries to, upon activation of T cells in the periphery, launch responses to the insult by either direct killing (CD8+ T cells) or helping cells in the battle against the insult (CD4+ T cells). However, during T cell development, T cells must be educated to target only “non-self” antigens, and this is accomplished by purging self-responsive T cells. Both acquisition of the armature to fight insults and the ability to recognize “non-self” antigens as targets are obtained during T cell development in the thymus, and underscore how critical T cell development is in T cell-mediated cellular immunity.
T cell development is usually divided into three stages:1 double negative (DN), double positive (DP), and single positive (SP). At the DN stage, thymocytes express neither CD4 nor CD8, and based on their expression of CD25 and CD44, they are further divided into the DN1 (CD25− CD44+), DN2 (CD25+CD44+), DN3 (CD25+CD44−) and DN4 (CD25−CD44−) subsets. Strictly, only DN cells at stage DN3 or later should be called thymocytes, since starting from early T cell precursors, which are newly immigrated into the thymus from the bone marrow, to the DN2 stage, cells gradually lose the potential to differentiate into other non-T cell lineages, such as NK cells, monocytes and dendritic cells. Once cells reach the DN3 stage, they are committed to the T cell lineage and can no longer develop into alternative cell types.2 At the DN3 stage, thymocytes start rearranging T cell receptor (TCR) β chain locus to produce TCRβ chain, and only those thymocytes that have a successfully rearranged TCRβ chain are able to survive and move on in 4 the process of T cell development. A successfully rearranged TCRβ chain, combined with the invariant pre-TCRα chain, form a pre-TCR, which is critical for proliferation of post-β thymocytes and the transition from the DN to DP stage. DN4 thymocytes proliferate extensively to prepare for the stringent DP selection process. Although more than 80% of total thymocytes are DP, only 5% are positively selected and mature into either CD4+ or CD8+ SP cells if a DP thymocyte bears a TCR that interacts with an MHC-self peptide complex with sufficiently low affinity. The other cells are either negatively selected and eliminated through apoptosis if the TCR is recognized by an MHC-self peptide with high affinity, or die by neglect if the TCR cannot be recognized at all.3 Once thymocytes mature into SP cells, they migrate out of the thymus and into peripheral lymphoid organs, such as lymph nodes and spleens, to take effect in adaptive immunity.
A network of transcription factors regulates DP thymocyte survival
DP thymocytes are able to initiate multiple rounds of TCRα chain locus rearrangement until they are positively selected or die due to limited lifespan. Thus, the duration of the window for positive/negative selection determines the productivity of the selection process.4 A longer lifespan for DP cells leads to greater opportunity for the thymocytes to eventually generate a TCR that responds to foreign, but not self, antigens. Given the importance of DP thymocyte survival, it is of great interest to exploit the mechanisms that prevent DP thymocytes from undergoing pre-mature apoptosis. Several factors that regulate DP thymocyte survival have been implicated, including the Wnt/β-catenin/TCF-1 pathway,5, 6 RORγt (Retinoid-related orphan nuclear factor γt),7 c-myb (myeloblastosis oncogene),8 and HEB (also called TCF-12, transcription factor 12).9 In this review, we summarize recent findings on these factors that play important roles in extending DP thymocyte survival and attempt to weave these “dots” into “strings” of signaling pathways, aiming to eventually reveal the molecular network that supports DP thymocyte survival.
Role of RORγt in DP thymocyte survival
RORγt is a transcription factor that belongs to the steroid nuclear receptor superfamily. Similar to other members of this family, RORγt contains a conserved DNA binding domain and a ligand binding domain. Upon binding to its ligand, which has yet to be identified, RORγt is thought to be activated, at which point it stimulates target gene expression. RORγt was initially identified by expression cloning to screen for molecules that regulate activation-induced cell death.10 We created RORγt knockout mice and demonstrated that RORγt is required for DP thymocyte survival and lymph node genesis,11 which was also confirmed with an independently generated RORγt knockout mouse strain.12 RORγt−/− DP thymocytes undergo accelerated apoptosis accompanied by greatly reduced Bcl-xL levels, and overexpression of Bcl-xL rescues RORγt−/− thymocyte apoptosis, demonstrating that RORγt enhances DP cell survival by up-regulating Bcl-xL expression.11 We further demonstrated that the recruitment of the steroid receptor coactivator (SRC) through the motif of activation function 2 (AF2) of RORγt is essential for RORγt to support thymocyte survival. With both the RORγt downstream effector, Bcl-xL, and interacting partner, SRC, being identified, the upstream signaling pathways that regulate RORγt during thymocyte survival, however, remained unknown.
Role of the Wnt/β-catenin/TCF-1 signaling pathway in thymocyte development
The Wnt pathway includes the canonical Wnt pathway mediated by β-catenin/TCF-1 complexes, the non-canonical Wnt pathway, which involves intracellular calcium mobilization, and the planar cell polarity pathway mediated by activation of the small GTPase RHOA (RAS homologue gene-family member A). Although non-canonical Wnt signaling has been implicated in immunological process such as regulation of αβ-lineage thymocyte survival,13 we will mainly focus on canonical Wnt signaling because it is the most significant and extensively studied among all Wnt pathways.
Activation of the canonical Wnt signaling pathway is initiated by binding of ligands, one of the 19 different Wnts, to the cell membrane-localized receptor complex composed of Frizzled and LDL receptor-related protein 5 (LRP5) or LRP6.1 Ligand binding initiates a signaling cascade that dissociates the anchor protein AXIN1 of the destruction complex, which contains GSK3β. GSK3β phosphorylates and degrades β-catenin in the absence of Wnt signals. Dissociation of AXIN1 prevents phosphorylation and degradation of β-catenin, leading to its accumulation in the cytoplasm. The ultimate effector of the canonical Wnt pathway is TCF-1/LEF-1. In the absence of Wnt signals, TCF-1 is bound by co-repressors such as Groucho/Transducin-like enhancer (GRG/TLE) and turns off target gene expression. Once Wnt initiates the signaling pathway, the accumulated β-catenin translocates into the nucleus, replaces the GRG/TLE repressor, and binds TCF-1 as a co-activator, leading to transcriptional activation of the target genes.
The Wnt pathway regulates multiple basic developmental processes, such as cell-fate specification, progenitor cell proliferation, establishment of the dorsal axis, and control of asymmetric cell division.14 In addition, Wnt signaling has been linked to pathogenesis of diseases such as colon carcinoma15 and leukemia.16 Wnt signaling is also required for hematopoietic stem cell self-renewal and the proliferation of progenitor cells. In addition, it appears that Wnt signals can confer a more undifferentiated phenotype on lineage-committed progenitors, inhibiting them from differentiating into various lineages.17
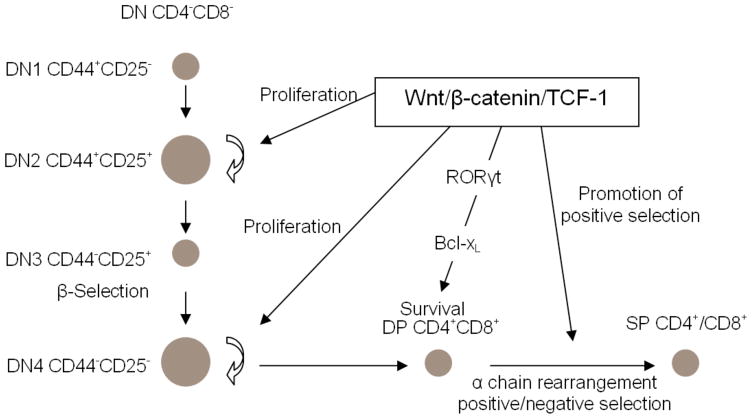
Wnt signaling is also important at multiple stages of thymocyte development (Fig. 1). The role of Wnt signaling pathway in T cell development was first evaluated through fetal thymic organ cultures (FTOC) grown in the presence of soluble Frizzled receptors as decoys for WNT proteins.18 in vitro thymocyte differentiation was inhibited by the soluble Frizzled receptors, suggesting an essential role for the Wnt pathway in thymocyte development. In addition, the inhibition primarily negatively affected thymocyte proliferation, which was confirmed by several other studies. For instance, Schilham et al.19 used TCF-1−/− mice, which lack exon 7 of TCF-1, to study the potential role of the effector of the Wnt signaling pathway, TCF-1. They found very few actively cycling DN2 and DN4 thymocytes, which should have been extensively proliferating to prepare for β-selection and positive/negative selection, respectively. DN4 thymocyte proliferation was also negatively affected in a β-catenin-deficient mouse line.20 In accordance with the role of TCF-1 in regulating thymocyte proliferation, the thymi of mice deficient in both Wnt1 and Wnt4 had low cellularity.21
Figure 1.
Wnt/β-catenin/TCF-1 regulates thymocyte development at multiple stages. At the DN stage, particularly for DN2 and DN4, β-catenin/TCF-1 promotes thymocyte proliferation to expand the thymocyte pool extensively in order to prepare for β-selection and positive/negative selection. DP thymocyte survival requires β-catenin/TCF-1, which acts upstream of RORγt in the upregulation of Bcl-xL. β-catenin/TCF-1 also promotes positive selection.
In addition to promoting proliferation of DN thymocytes, Wnt signaling has been proposed to be important for the DN to DP transition. Work by Verbeek et al., showed that differentiation of thymocytes from TCF-1−/− mice was blocked at the ISP (CD8+ Immature Single positive) to DP transition.22 This work was further supported by later studies. For instance, when ICAT, the inhibitor of β-catenin and TCF, which blocks the binding of β-catenin to TCF-1 and LEF-1, was over-expressed through retroviral transduction, thymocyte development was blocked at the DN to DP transition in FTOC assays,23 indicating an essential role for β-catenin/TCF-1 in the DN to DP transition during thymocyte development.
Wnt signaling has also been implicated in thymocyte development at the DP stage. DP thymocytes from TCF-1−/− mice undergo rapid apoptosis during in vitro culture, and thymocyte survival can be restored by a transgene that expresses full length TCF-1 but not truncated TCF-1 that lacks the domain mediating the interaction with β-catenin, suggesting that the Wnt signaling pathway mediated by β-catenin is required to support DP thymocyte survival.6 To further establish the importance of Wnt signaling pathway in DP thymocyte survival, we have established a β-catenin transgenic mouse strain (β-catTg) that overexpresses constitutively active β-catenin under the control of a CD4 promoter.5 The β-catenin transgene is not expressed until the DP stage, which ensures that thymocyte development at DN or earlier stages is not affected. As expected, the four DN subsets have normal distribution and cell numbers in these mice. In contrast, both the frequency and numbers of thymocytes at the DP stage are significantly greater in β-catTg mice compared to wild-type (WT). In addition, when DP thymocytes from β-catTg mice are grown in vitro, they undergo much slower apoptosis than WT counterparts in both spontaneous and glucocorticoid-induced apoptosis. Furthermore, the promotion of DP thymocyte survival by the transgene of β-catenin is mediated by up-regulation of Bcl-xL, a key anti-apoptosis factor that supports DP thymocyte survival. These data demonstrated that β-catenin/TCF-1 extends DP thymocyte survival by upregulating Bcl-xL. However, whether Wnt signaling mediated by β-catenin directly targets Bcl-xL or acts through factors downstream of the β-catenin/TCF-1 complex remained unknown. Our recent work has shed light on this question by showing that enhancement of DP thymocyte survival by β-catenin/TCF-1 is mediated by RORγt. More than 20 genes have been reported to be direct targets of the canonical Wnt/β-catenin/TCF signaling pathway,24 including Drosophila Ultrabithorax (Ubx), the Xenopus homeobox genes siamois, twinned, and engrailed-2, PPARδ (a member of the nuclear receptor superfamily), c-Jun and Fra-1 (two components of the AP-1 transcription complex), and E-cadherin. However, in spite of the apparent significance of the canonical Wnt signaling pathway in the hematopoietic and immunological system, the target genes of the pathway in these systems remain largely unknown. RORγt seems to be one of the target genes of β-catenin/TCF-1 pathway during thymocyte development because TCF-1-deficient thymocytes express strikingly lower level of RORγt whereas β-catenin transgenic thymocytes have significantly higher expression of RORγt compared to WT counterparts (unpublished data). In addition, forced expression of RORγt rescued survival of TCF-1-deficient DP thymocytes in an in vitro thymocyte differentiation system (unpublished data). To explore on the molecular mechanism, we tested the capability of β-catenin to stimulate a reporter construct harboring RORγt promoter region, and found that β-catenin/TCF-1 complex is able to activate RORγt promote. And furthermore, an antibody specific for β-catenin was able to pull down a fragment of RORγt promoter region contained by the reporter construct in chromatin immuno-precipitation assays (ChIPs) (unpublished data). Therefore, it appears that β-catenin/TCF-1 is able to activate RORγt transcription by directly targeting its regulatory region in thymocytes. Although more investigations on identifying TCF-1-responsive elements within RORγt regulatory region is needed to ultimately confirm the assignment of RORγt as a target of β-catenin/TCF-1 pathway, our studies demonstrated for the first time that during DP stage of thymocyte development, β-catenin/TCF-1 supports thymocyte survival by activating RORγt expression.
The window for DP thymocytes to complete positive and negative selection must be sufficiently long that thymocytes can have a reasonable lifespan, but not so long that detrimental effects such as promoting the accumulation of “unwanted” thymocytes occur. Guo et al. reported that in transgenic mice that overexpressed constitutively active β-catenin due to deletion of β-catenin exon 3, thymocyte development was blocked at the DP to SP transition. In addition, these mice developed T cell lymphomas that contained mainly DP thymocytes,25 suggesting that Wnt signaling activity at the DP stage must be controlled tightly to enable thymocytes to finish positive and negative selection properly. Furthermore, these findings indicated that inappropriate activation of Wnt signals may lead to tumorigenesis.
In addition to enhancing DP thymocyte survival, the Wnt signaling pathway has also been implicated in regulating positive and negative selection of DP thymocytes prior to their eventual maturation into either CD4 or CD8 SP thymocytes. Through use of a β-catenin transgenic mouse strain that expressed an Lck promoter-driven stabilized β-catenin (due to deletion of the N terminal 87 amino acids), Yu et al. showed that positive selection was promoted, and that the transgene expression accelerated the generation of CD8 SP thymocytes, which usually occurs later than the generation of CD4 SP thymocytes, such that the generation of both types of thymocytes took place with the same kinetics.26 These data suggest that β-catenin/TCF-1 also plays important roles in positive selection, and maybe in CD4/CD8 lineage differentiation as well.
Given the versatile roles of β-catenin/TCF-1 in other developmental processes, it is not surprising that this pathway is required for thymocyte development at multiple stages. Starting from the commitment of earliest stage of ETPs (early T cell progenitors) to T cell lineage commitment, positive selection and CD4 versus CD8 lineage decision, β-catenin/TCF-1 could be regulated/influenced by different upstream events such as preTCR signals and the binding of Wnt ligands to their receptors; and it might also regulate distinct downstream factors to meet the developmental requirement at different stages. So far, the roles of β-catenin/TCF-1 at each individual stage has been emerging; however, the more important questions to answer are that how thymocytes dynamically regulate their β-catenin/TCF-1 signaling, and also how β-catenin/TCF-1 differentially regulates its downstream factors at different stage of thymocyte development. To answer these questions, approaches that allow in vivo tracing of preTCR or β-catenin/TCF-1 activity at different thymocyte development stages could be of great help. In addition, a systematic analysis on β-catenin/TCF-1 target genes at different thymocyte developmental stages would also reveal important information as to how β-catenin/TCF-1 shifts the targets from a specific stage to the next during thymocyte development.
c-Myb in DP thymocyte survival
A recent paper by Yuan et al. identified another transcription factor, c-Myb, encoded by the proto-oncogene Myb, as an important factor for regulating DP thymocyte survival.8 Previously, using conditional mutagenesis at the Myb locus,27 the same group showed that c-Myb is crucial at multiple stages of thymocyte development, including transition from the DN to DP stage, survival of preselection DP thymocytes and differentiation of CD4 SP thymocytes. In their recent work, more efficient deletion of c-Myb starting from DP stage was achieved by crossing the Mybf/f and cd4/cre mouse strains. Deficiency of c-Myb led to premature DP thymocyte apoptosis due to decreased expression of Bcl-xL. More specifically, due to enhanced dependence on Bcl-xL for survival, small pre-selection DP thymocytes underwent faster premature apoptosis than large pre-selection and post-selection DP thymocytes. Forced expression of Bcl-xL rescued thymocyte survival, and re-introduction of c-Myb restored both Bcl-xL expression and the small preselection DP compartment. The defective DP thymocyte survival due to reduced expression of Bcl-xL exhibited by c-Myb-deficient mice is reminiscent of what has been observed in TCF-1−/− and RORγt−/− mice. However, the authors proposed that the transcriptional regulation of Bcl-xL by c-Myb is independent of both TCF-1 and RORγt, since c-Myb expression in both TCF-1- and RORγt-deficient thymocytes was comparable to that in WT thymocytes, indicating that multiple pro-survival pathways could synergize to ensure proper survival of DP thymocytes.
HEB is required for DP thymocyte survival
The E protein family of transcription factors regulates lymphocyte differentiation in multiple processes, including controlling the expression of genes essential to lineage commitment, targeting antigen-receptor gene rearrangement and enforcing key developmental checkpoints.28, 29 Mammals have four E proteins (E2A, HEB, E12 and E2-2), of which E2A and HEB promote TCR rearrangement and control the developmental progression, survival and proliferation of developing T cells, thereby accounting for the majority of E protein function in the thymus.29 E2A is involved in regulating the pre-TCR and Notch signaling pathway at the earliest stages of thymocyte development, restraining DN to DP progression until rearrangement of a productive TCRβ chain.28, 30, 31 E2A and HEB have been thought to compensate for each other during lymphocyte development. For instance, HEB-deficient thymocyte development are blocked at the DN to DP transition, but this can be partially overcome by the compensatory activity of E2A.32, 33 Recently, HEB was found to have a distinctive role in promoting DP thymocyte survival.9 Thymocytes from T lineage-specific HEB-deleted mice undergo rapid apoptosis and have reduced Bcl-xL expression. As observed in c-Myb or RORγt-deficient thymocytes, forced expression of Bcl-xL rescued DP thymocyte survival, indicating that HEB is another transcription factor that functions upstream of Bcl-xL to promote DP thymocyte survival. In contrast to the independence of RORγt and TCF-1 in c-Myb-mediated DP thymocyte survival regulation, HEB regulates RORγt expression by binding to the two E-box sites present in the RORγt promoter and stimulates its transcription, which suggests that HEB could act upstream of RORγt in the same pathway to promote DP thymocyte survival.
Regulation of Bcl-xL expression in DP thymocytes
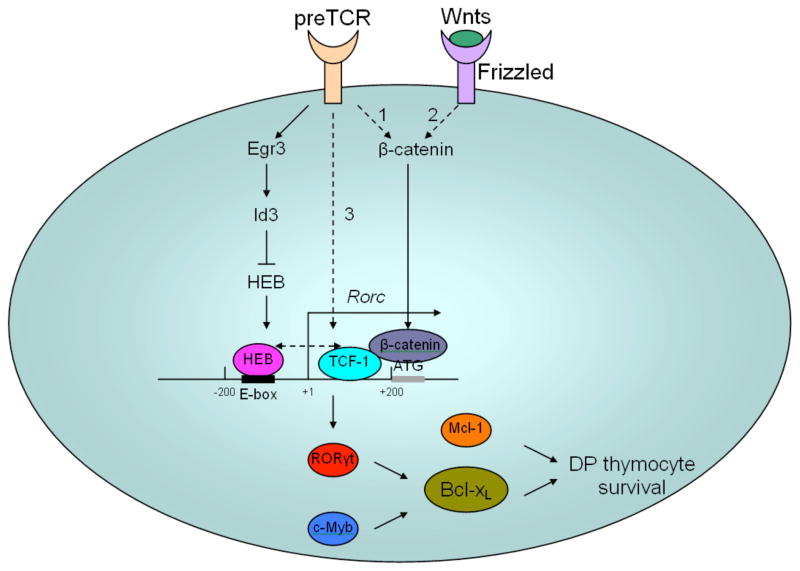
All four factors discussed to this point promote DP thymocyte survival by upregulating Bcl-xL, and transgenic expression of Bcl-xL rescued thymocyte survival when each of the factors was deficient, indicating that Bcl-xL is the main anti-apoptotic factor protecting thymocytes from apoptosis. Although another pro-survival Bcl-2 family member, Mcl-1, has also been implicated in the regulation of DP thymocyte survival, it is believed to only compensate for the loss of Bcl-xL when later is deficient.34 Both RORγt and c-Myb can regulate the transcription of Bcl-xL, indicative of possible synergy between these two transcription factors in stimulating Bcl-xL transcription. Whether RORγt and/or c-Myb can bind to the Bcl-xL promoter and thereby promote its transcription needs further investigation. We have shown that β-catenin/TCF-1 is able to target the RORγt promoter to activate its activity (unpublished data). Interestingly, HEB is also able to bind to the two E-box sites located approximately 200 bp upstream of RORγt transcription starting site, activating RORγt transcription. Therefore, it appears that TCF-1 and HEB could bind close to each other on the RORγt promoter, suggesting that these two factors might interact with each other and cooperate to stimulate the RORγt promoter (Figure 2).
Figure 2.
Model for possible mechanisms of regulation of DP thymocytes by multiple factors. Activation of RORγt transcription requires cooperation of the transcription factors TCF-1 and HEB. There are three possible pathways responsible for activation of TCF-1. (1) preTCR signals might activate β-catenin, which translocates into the nucleus and switches TCF-1 from a repressor to an activator, eventually leading to induction of RORγt transcription. (2) Activation of β-catenin could also result from the binding of Wnt ligands to the Frizzled receptor in the canonical Wnt signaling pathway. (3) preTCR signals might induce TCF-1 transcription independently of β-catenin. These three possible pathways may be non-exclusive, and could have synergy that leads to maximal activation of TCF-1 at the DN4 and DP stages. Compared to TCF-1, which upon activation functions to stimulate RORγt transcription, HEB seems to mediate fine-tuning of RORγt transcription. At the DN to DP transition, preTCR signals upregulate Egr3 transiently. Egr3-induced Id3 sequesters E proteins such as HEB from binding to the RORγt promoter. Because both HEB and TCF-1 are required for substantial induction of RORγt, the absence of HEB leads to reduction of RORγt expression. When thymocytes differentiate to the DP stage, Egr3 subsides and Id3 is removed, which leads to recovery of HEB activity. HEB then binds to the RORγt promoter, together with already activated TCF-1, and leads to stimulation of maximal RORγt transcription. HEB binds to two E-box sites 200 bp upstream of the RORγt transcription starting site, whereas TCF-1 targets the 200 bp fragment between the RORγt transcription starting site and translation starting site, as determined based on our ChIP assay results. Whether HEB and TCF-1 interact with and influence each other’s activity remains unknown. RORγt and c-Myb cooperate in upregulating Bcl-xL, which eventually leads to protection of DP thymocytes from apoptosis. Mcl-1 could also enhance DP thymocyte survival; however, it is not essential and may only partially compensate for lack of Bcl-xL. Dashed lines indicate pathways and interactions that have not yet been proven.
preTCR signals and the transcription factor network regulating DP thymocyte survival
preTCR signals are major components of the signaling network in immature T cell development, and are key to survival and proliferation of DN cells.35, 36 Whether there is any relationship between preTCR/TCR and the β-catenin/TCF-1 signaling pathway remains controversial. β-catenin has been suggested as a target of both preTCR and TCR signaling.20 Stimulation of isolated thymocytes or splenic CD4+ T cells with either phorbol 12-myristate 13-acetate or CD3 monoclonal antibody caused accumulation of more β-catenin in the nucleus than in nuclei of untreated samples, suggesting that both preTCR and TCR signaling could activate β-catenin. This idea was corroborated by a recent observation that, in primary human T cells, TCR signaling led to stabilization of β-catenin.37 However, in another study, the non-phosphorylated, active form of β-catenin was readily detected in Rag-1-deficient thymocytes which lack preTCR due to impaired TCRβ chain locus rearrangement, and, furthermore, when Rag-1-deficient mice were injected with anti-CD3 antibodies to induce preTCR signaling, neither total nor active β-catenin increased. Interestingly the authors found that TCF-1 levels were significantly increased after preTCR induction through anti-CD3 antibody injection, indicating that preTCR signals might induce TCF-1 independently of Wnt-induced β-catenin.38 However, because TCF-1 is usually repressed by Groucho, unless Groucho is replaced by the activated β-catenin, it is hard to explain how an increase in the absolute quantity of TCF-1 alone would lead to activation of target genes. Therefore, we believe that if TCF-1 is regulated by preTCR signals, it could be mediated by both activation of β-catenin, which “turns on” TCF-1, and induction of TCF-1 transcription.
Interestingly, preTCR signals have also been shown to induce a delayed elevation in RORγt expression, which is thought to allow for a period of proliferation to occur after preTCR signaling.39 Based on these findings, Xi et al. proposed a molecular model to explain the mechanisms that regulate the delayed induction of RORγt by preTCR signals.40 preTCR signals initially upregulate both RORγt and early response genes-3 (Egr3); however, the upregulated RORγt is kept at moderate level due to the absence of E proteins (E2A/HEB), which are sequestered by Id3, the E protein inhibitor, induced by Egr3. Expression of Egr3 is transient, and once Egr3 subsides, E proteins are released from Id3-mediated inhibition, bind to the E box sites located in the RORγt promoter region and stimulate its transcription. The binding of E proteins to the RORγt promoter eventually leads to upregulation of RORγt to its maximal levels. However, two questions remain to be answered: 1) how preTCR signals initially induce RORγt, and 2) why overexpression of Egr3 in Egr3 transgenic mice could not completely abolish expression of RORγt. To answer these two questions, we propose that the initial moderate induction of RORγt by preTCR signals could be mediated by TCF-1. When E proteins are absent during the earliest period of preTCR signals, due to the transient up-regulation of Egr3, TCF-1 is only able to stimulate RORγt transcription to a moderate level. When E proteins are relieved of Id3-mediated inhibition due to the reduction in Egr3 levels, synergy between TCF-1 and E proteins would induce a maximal level of RORγt transcription. Lack of E proteins at the RORγt promoter due to overexpressed Egr3 would lead to a substantial reduction in RORγt; however, TCF-1 alone might still be able to induce some expression of RORγt, which could explain the incomplete abolishment of RORγt expression in Egr3 transgenic mice. Taking into account the model proposed by Xi et al. and our own findings that TCF-1 regulates DP thymocyte survival through a RORγt pathway, we propose a new model for the molecular mechanism regulating DP thymocyte survival (Figure 2). At the DN4 to DP transition, preTCR signals might initially lead to up-regulation of Egr3 and activation of TCF-1; subsidence of Egr3 relieves E proteins from inhibition by Id3, which, together with already activated TCF-1, induces the maximal level of RORγt expression. Then RORγt, joined by c-Myb, upregulate Bcl-xL, which eventually promotes DP thymocyte survival.
Some transcription factors discussed above somehow have a similar function in mature T cells in addition as in immature thymocytes. Stabilized β-catenin is able to extend regulatory T cell (Treg) survival through up-regulating Bcl-xL,41 suggesting that T cells at different development stages may utilize the same pathway to regulate their survival. On the other hand, we also showed that in conventional T cells β-catenin is able to promote FasL-mediated activation-induced cell death,42 suggesting that β-catenin pathway can also be used to promote cell death. TCF-1 is shown to be essential for differentiation and persistency of memory CD8+ T cells. TCF-1-deficient memory CD8+ T cells express diminished survival molecule Bcl-2, part of the mechanism whereby TCF-1 regulates memory CD8+ T cell differentiation thus could be through promoting the survival,43 In this case, TCF-1 regulates memory T cell survival via Bcl-2 instead of Bcl-xL as in DP thymocyte. The same transcription factor are also involved in promoting survival and proliferation in other cell types. For example, c-myb has been shown to regulate B cell development likely via affecting IL-7-dependent survival pathway.44 Both c-myb and TCF-1 promote tumorigenesis and self-renewal of hematopoietic stem cells by stimulating proliferation.14, 45–49 Similarly, Heb has been associated with lymphoma and expansion of neuron tissues.47, 50 Taken together, the same transcription factors regulating DP thymocyte survival may also affect survival pathways in other types of cells. However, RORγt is unique for T cells, since it is only expressed in developing thymocytes and Th17 cells. Therefore, the same transcription factors although regulate survival in different cell types but likely via stimulating different target genes.
Conclusion
Life and death are the central theme of T cell development, which maintains survival of T cells that recognize foreign antigens presented by self-MHC, but induces death of self-reactive T cells. A critical event in DP thymocytes, α chain rearrangement, allows for the generation of a repertoire of T cells subject to positive and negative selection. The lifespan of DP cells determines the rate of successful selection, and thereby limits the size of the peripheral T cell repertoire. Previous single gene or multiple gene studies with traditional molecular biology techniques have revealed that many transcription factors critical for T cell development regulate the survival of DP cells. Instead of working alone, these transcription factors regulate or cooperate with each other to form a network to ensure the survival of DP thymocytes critical for completion of T cell development. Our proposed model (Figure 2) is surely oversimplified since it is based on results generated mostly from one gene-one function analysis. However, with the development of microarray and deep-sequencing technology, it is now possible to perform genome-wide analysis to reveal a global network responsible for regulating thymocyte survival. Genome-wide analysis is also expected to reveal novel critical genes that otherwise would be difficult to identify. Detailed mechanisms responsible for these candidate genes that regulate thymocyte survival in turns require study by traditional molecular biology. Future work therefore requires a combination of genome-wide and traditional single/multi-gene analyses to define the complete picture of regulation of DP thymocyte survival.
Acknowledgments
Our work was supported by grants from NIH R01-AI053147, NIH R56-AI072554, Nesvig lymphoma Fellowship and Research Fund and City of Hope.
References
- 1.Xie H, Huang Z, Wang R, Sun Z. Regulation of thymocyte survival by transcriptional coactivators. Crit Rev Immunol. 2006;26(6):475–86. doi: 10.1615/critrevimmunol.v26.i6.10. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8(1):9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402(6759):255–62. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 4.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He YW. Regulation of the TCRalpha repertoire by the survival window of CD4(+)CD8(+) thymocytes. Nat Immunol. 2002;3(5):469–76. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 5.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175(12):7981–8. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 6.Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin--TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2(8):691–7. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J, Crittenden RB, Bender TP. c-Myb promotes the survival of CD4+CD8+ double-positive thymocytes through upregulation of Bcl-xL. J Immunol. 2010;184(6):2793–804. doi: 10.4049/jimmunol.0902846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11(3):240–9. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9(6):797–806. doi: 10.1016/s1074-7613(00)80645-7. Epub 1999/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 12.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U S A. 2000;97(18):10132–7. doi: 10.1073/pnas.97.18.10132. Epub 2000/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H, Coles AH, Zhu Z, Zayas J, Jurecic R, Kang J, Jones SN. Noncanonical Wnt signaling promotes apoptosis in thymocyte development. J Exp Med. 2007;204(13):3077–84. doi: 10.1084/jem.20062692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018. Epub 2006/11/04. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5(12):997–1014. doi: 10.1038/nrd2154. Epub 2006/12/02. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 17.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8(8):581–93. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 18.Staal FJ, Meeldijk J, Moerer P, Jay P, van de Weerdt BC, Vainio S, Nolan GP, Clevers H. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31(1):285–93. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161(8):3984–91. [PubMed] [Google Scholar]
- 20.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4(12):1177–82. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 21.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32(4):967–71. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374(6517):70–4. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 23.Pongracz JE, Parnell SM, Jones T, Anderson G, Jenkinson EJ. Overexpression of ICAT highlights a role for catenin-mediated canonical Wnt signalling in early T cell development. Eur J Immunol. 2006;36(9):2376–83. doi: 10.1002/eji.200535721. [DOI] [PubMed] [Google Scholar]
- 24.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296(5573):1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 25.Guo Z, Dose M, Kovalovsky D, Chang R, O’Neil J, Look AT, von Boehmer H, Khazaie K, Gounari F. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109(12):5463–72. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q, Sen JM. Beta-catenin regulates positive selection of thymocytes but not lineage commitment. J Immunol. 2007;178(8):5028–34. doi: 10.4049/jimmunol.178.8.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K. Critical functions for c-Myb at three checkpoints during thymocyte development. Nat Immunol. 2004;5(7):721–9. doi: 10.1038/ni1085. [DOI] [PubMed] [Google Scholar]
- 28.Engel I, Johns C, Bain G, Rivera RR, Murre C. Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med. 2001;194(6):733–45. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6(11):1079–86. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 30.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. Embo J. 2004;23(1):202–11. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med. 2006;203(5):1329–42. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barndt R, Dai MF, Zhuang Y. A novel role for HEB downstream or parallel to the pre-TCR signaling pathway during alpha beta thymopoiesis. J Immunol. 1999;163(6):3331–43. [PubMed] [Google Scholar]
- 33.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20(18):6677–85. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181(1):521–8. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375(6534):795–8. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 36.von Boehmer H, Aifantis I, Feinberg J, Lechner O, Saint-Ruf C, Walter U, Buer J, Azogui O. Pleiotropic changes controlled by the pre-T-cell receptor. Curr Opin Immunol. 1999;11(2):135–42. doi: 10.1016/s0952-7915(99)80024-7. [DOI] [PubMed] [Google Scholar]
- 37.Lovatt M, Bijlmakers MJ. Stabilisation of beta-catenin downstream of T cell receptor signalling. PLoS One. 5(9) doi: 10.1371/journal.pone.0012794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goux D, Coudert JD, Maurice D, Scarpellino L, Jeannet G, Piccolo S, Weston K, Huelsken J, Held W. Cooperating pre-T-cell receptor and TCF-1-dependent signals ensure thymocyte survival. Blood. 2005;106(5):1726–33. doi: 10.1182/blood-2005-01-0337. [DOI] [PubMed] [Google Scholar]
- 39.Xi H, Kersh GJ. Sustained early growth response gene 3 expression inhibits the survival of CD4/CD8 double-positive thymocytes. J Immunol. 2004;173(1):340–8. doi: 10.4049/jimmunol.173.1.340. [DOI] [PubMed] [Google Scholar]
- 40.Xi H, Schwartz R, Engel I, Murre C, Kersh GJ. Interplay between RORgammat, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity. 2006;24(6):813–26. doi: 10.1016/j.immuni.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14(2):162–9. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 42.Huang Z, Wang R, Xie H, Shang W, Manicassamy S, Sun Z. Stabilized beta-catenin potentiates Fas-mediated T cell apoptosis. J Immunol. 2008;180(10):6586–92. doi: 10.4049/jimmunol.180.10.6586. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 33(2):229–40. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greig KT, de Graaf CA, Murphy JM, Carpinelli MR, Pang SH, Frampton J, Kile BT, Hilton DJ, Nutt SL. Critical roles for c-Myb in lymphoid priming and early B-cell development. Blood. 2010;115(14):2796–805. doi: 10.1182/blood-2009-08-239210. Epub 2010/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieu YK, Reddy EP. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci U S A. 2009;106(51):21689–94. doi: 10.1073/pnas.0907623106. Epub 2009/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciznadija D, Tothill R, Waterman ML, Zhao L, Huynh D, Yu RM, Ernst M, Ishii S, Mantamadiotis T, Gonda TJ, Ramsay RG, Malaterre J. Intestinal adenoma formation and MYC activation are regulated by cooperation between MYB and Wnt signaling. Cell Death Differ. 2009;16(11):1530–8. doi: 10.1038/cdd.2009.94. Epub 2009/07/18. [DOI] [PubMed] [Google Scholar]
- 47.Uittenbogaard M, Chiaramello A. Expression of the bHLH transcription factor Tcf12 (ME1) gene is linked to the expansion of precursor cell populations during neurogenesis. Brain Res Gene Expr Patterns. 2002;1(2):115–21. doi: 10.1016/s1567-133x(01)00022-9. Epub 2004/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28(1):53–7. doi: 10.1038/ng0501-53. Epub 2001/04/28. [DOI] [PubMed] [Google Scholar]
- 49.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50. doi: 10.1038/nature03319. Epub 2005/04/15. [DOI] [PubMed] [Google Scholar]
- 50.O’Neil J, Shank J, Cusson N, Murre C, Kelliher M. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell. 2004;5(6):587–96. doi: 10.1016/j.ccr.2004.05.023. Epub 2004/06/15. [DOI] [PubMed] [Google Scholar]