Abstract
Background
Many believe that variation in vascular practice may affect limb salvage rates in patients with severe PAD. However, the extent of variation in procedural vascular care obtained by patients with critical limb ischemia (CLI) remains unknown.
Methods and Results
Using Medicare 2003–2006, we identified all patients with CLI who underwent major lower extremity amputation in the 306 hospital referral regions (HRRs) described in the Dartmouth Atlas of Healthcare. For each patient, we studied the use of lower extremity vascular procedures (open surgery or endovascular intervention) in the year prior to amputation. Our main outcome measure was the intensity of vascular care, defined as the proportion of patients in the HRR undergoing vascular procedure in the year before amputation. Overall, 20,464 patients with CLI underwent major lower extremity amputations during the study period, and collectively underwent 25,800 vascular procedures in the year prior to undergoing amputation. However, these procedures were not distributed evenly − 54% of patients had no vascular procedures performed in the year prior to amputation, 14% underwent 1 vascular procedure, and 21% underwent more than one vascular procedure. In the regions in the lowest quintile of vascular intensity, vascular procedures were performed in 32% of patients. Conversely, in the regions in the highest quintile of vascular intensity, revascularization was performed in 58% of patients in the year prior to amputation (p<0.0001). In analyses accounting for differences in age, sex, race, and comorbidities, patients in high intensity regions were 2.4 times as likely to undergo revascularization in the year prior to amputation than patients in low intensity regions (adjusted OR=2.4, 95% CI 2.1–2.6, p<0.001).
Conclusions
Significant variation exists in the intensity of vascular care provided to patients in the year prior to major amputation. In some regions, patients receive intensive care, while in other regions, far less vascular care is provided. Future work is needed to determine the association between intensity of vascular care and limb salvage.
Keywords: epidemiology, outcomes research, peripheral vascular disease, treatment disparities, vascular disease
Introduction
Lower extremity peripheral arterial disease (PAD) manifests in its most severe form, with limb-threatening rest pain and ulceration, as “critical limb ischemia” (CLI) in nearly 1 million patients1–7. Estimates of the economic burden of CLI patients alone exceed 3.1 billion dollars annually in Medicare, because of the high incidence of limb loss and need for major amputation8. These costs are driven, at least in part, by the rise in the use of catheter-based endovascular interventions as a major component of vascular care in recent years9–11.
Despite the increase in health care resources dedicated to PAD, major lower extremity amputation remains common, and the incidence of major amputation varies according to several factors, such as patient age, race, and socioeconomic status, and local health care environment12–16. For example, the major amputation rate in Corpus Christi, Texas (4.4 amputations per 1,000) is ten times higher than the rate of amputation in Grand Junction, Colorado (0.4 amputations per 1,000). Research in the Dartmouth Atlas of Health Care has attributed some of these regional differences in amputation to patient-level factors, such as five-fold higher amputation rates among blacks and those of low socioeconomic status (SES)12. However, because patient-level differences do not fully explain the variation in amputation rates across regions, we hypothesized that the intensity of vascular care may vary across regions as well.
For this reason, we sought to characterize the variation in invasive treatments provided to patients with CLI across the United States. Because the extent of PAD affects the threshold for use of invasive vascular procedures, we chose to specifically focus on patients with PAD who had undergone major limb amputation. We chose this group because, by definition, they had PAD which can definitively classified as CLI in the year prior to their amputation. Therefore, in this group of patients with a similar extent of PAD, we sought to examine the presence and extent of variation in procedural vascular care.
Methods
Databases
We used the Medicare Physician/Supplier file and the Medicare Denominator file for these analyses, for the years between 2003 and 2006. The Physician/Supplier file contains all claims submitted by physicians for performance of procedures under the Medicare Part B program, including CPT codes17, ICD-9 diagnosis codes, date of procedure, and the age, gender, and race of the beneficiary receiving the procedure. The denominator file contains information about eligibility by year for Part B and information about age, gender, and race of eligible beneficiaries. We excluded patients under age 65. Records with missing values for HRR, gender, age, and race strata were also removed from the analysis.
Establishing a Cohort of Patients with Severe PAD
In this project, we studied the utilization of lower extremity revascularization for CLI. Although distinct ICD-9 codes exist for claudication and CLI, many patients are assigned other non-specific PAD codes. Preliminary work using a linked clinical-claims dataset suggested that up to 40% of patients with PAD receive nonspecific PAD codes, making delineation of the extent of PAD difficult using administrative claims alone. Therefore, to establish a cohort of patients with severe CLI, wherein all patients have a similar extent of disease, we studied the vascular care provided in patients in the 12 months prior to major lower extremity amputation (below or above-knee). To create this cohort, we first identified all patients with peripheral arterial disease who underwent major lower extremity amputation (below or above-knee) using Medicare Part B claims between 2003 and 2006. To ensure these amputations were not due to etiologies other than PAD, such as malignancy or trauma, we omitted any patients without ICD-9 diagnosis codes for PAD. After we identified a cohort of patients with diagnosis codes for lower extremity PAD who had undergone major amputation, we “looked back” 12 months prior to the date of amputation to determine if these patients had undergone lower extremity vascular procedures during that time period.
Unit of Analysis
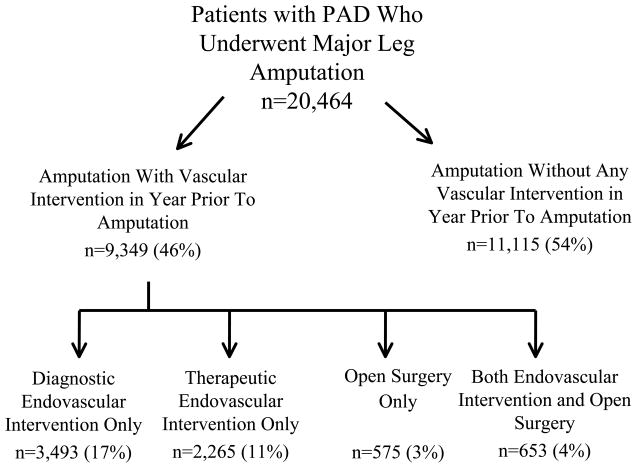
Our unit of analysis was the patient, and we used indicator variables to study the first and any subsequent procedures each individual patient underwent in the year prior to amputation. Because patients may have been treated with more than one type (open or endovascular) of vascular procedure during the time interval, we categorized vascular procedures in four ways: (1) diagnostic endovascular intervention only (2) therapeutic endovascular intervention, (3) open surgical treatment, and (4) both open and endovascular revascularization (Figure 1). We recorded patient characteristics, such as age, sex, race, and comorbidites (both individually and using the Charlson index18). Further, while we collected the details of each individual CPT code performed, we collapsed the open bypass surgery and endovascular procedures into inflow (above the inguinal ligament) and outflow procedures (distal to the inguinal ligament) for purposes of presentation.
Figure 1.
Number of patients in the cohort, by revascularization status and type.
Comparison of Vascular Procedure Rates Across Regions
Vascular procedure rates were defined as the number of patients undergoing vascular procedures(either bypass surgery or endovascular interventions), divided by the number of patients undergoing amputation secondary to PAD. To examine geographic variation in vascular procedure rates, we examined the rates of bypass surgery and endovascular interventions during this time period within each of the 306 hospital referral regions (HRRs) in the United States.
HRRs, as described by the Dartmouth Atlas of Healthcare19, represent distinct tertiary medical care markets (as defined by where cardiovascular and neurosurgical care is provided). Each HRR has at least one tertiary care center and several smaller centers. After defining crude rates of bypass surgery and endovascular intervention within each HRR, regions were aggregated into evenly sized quintiles, according to the use of vascular procedures, ranging from highest to lowest. HRR level rates of utilization for vascular procedures were calculated, between 2003 and 2006.
We used t-tests to compare rates between regions, and p values <0.05 were considered significant. Univariate associations with a p value <0.20 were entered into a multivariable logistic regression model used to predict the likelihood of undergoing a vascular procedure in the year prior to amputation. Model performance was assessed using receiver operating curves. All analyses were performed using SAS (SAS Institute, Cary, NC), and STATA 10 (College Station, TX).
Results
Characteristics of the Cohort of Patients Undergoing Amputation
Overall, we identified 20,464 Medicare patients who underwent major lower extremity amputation between 2003 and 2006. These amputations consisted of 42% below-knee amputations and 58% above knee amputations. The ten most common ICD-9 diagnostic codes for PAD in these patients are shown in Table 1. Gangrene was seen most commonly, followed by nonspecific peripheral arterial disease and ulcerations. No patients were included in the cohort based on diagnosis codes for non limb-threatening diagnoses such as claudication.
Table 1.
Ten most common ICD9 diagnosis codes found in cohort of patients undergoing major leg amputation for PAD
| ICD9 code | Description | N | percent of all patients |
|---|---|---|---|
| 785.4 | Gangrene | 7321 | 35.7 |
| 440.24 | Atherosclerosis, extensive, native artery, with gangrene | 4736 | 23.1 |
| 443.9 | Peripheral vascular disease, not otherwise specified | 1146 | 5.6 |
| 440.23 | Atherosclerosis, extensive, native artery, with ulceration | 713 | 3.4 |
| 459.9 | Circulatory disease, not otherwise specified | 423 | 2.1 |
| 444.22 | Lower extremity embolism | 393 | 1.9 |
| 440.22 | Atherosclerosis, extensive, native artery, with rest pain | 385 | 1.8 |
| 250.7 | Diabetes with circulatory disorder | 381 | 1.8 |
| 707.1 | Chronic ulcer of leg | 256 | 1.3 |
| 707.15 | Ulcer, other part of foot. | 232 | 1.1 |
Patient characteristics of those in the cohort are shown in Table 2. Overall, patients were elderly (mean age=78 years), had a history of diabetes (49%), coronary artery disease (14%), and congestive heart failure (35%). While patients undergoing revascularization were younger than those not undergoing revascularization (77 versus 79 years, p<0.001), patients undergoing revascularization had a slightly higher incidence of comorbidities such as myocardial infarction, congestive heart failure, and renal failure.
Table 2.
Demographics of the cohort of patients undergoing major amputation for PAD
| Overall (n=20,464) | Underwent Vascular Procedure In Year Prior to Amputation (n=9,349) | Did Not Undergo Vascular Procedure In Year Prior to Amputation (n=11,115) | ||
|---|---|---|---|---|
| Characteristic | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | p value |
| Age | 78.4 (78.3–78.5) | 77.0 (76.9–77.2) | 79.2 (79.0–79.3) | <0.001 |
| Female Gender (%) | 49.3 (48.6–50.0) | 46.1 (45.0–47.3) | 51.0 (50.2–51.9) | <0.001 |
| Black Race (%) | 27.7 (27.0–28.3) | 24.8 (23.8–25.8) | 29.3 (28.5–30.0) | <0.001 |
| Diabetes (%) | 49.3 (48.6–49.9) | 55.2 (54.0–56.3) | 45.9 (45.1–48.8) | <0.001 |
| Coronary Artery Disease (%) | 13.5 (12.9–13.9) | 16.5 (15.7–17.4) | 11.7 (11.1–12.2) | <0.001 |
| Congestive Heart Failure (%) | 35.1 (34.5–35.8) | 38.3 (37.1–39.2) | 33.4 (32.6–34.2) | <0.001 |
| COPD (%) | 22.8 (22.2–23.4) | 26.7 (25.7–27.7) | 20.6 (19.9–21.3) | <0.001 |
| Cerebrovascular Disease (%) | 11.3 (10.8–11.7) | 11.3 (10.6–12.0) | 11.3 (10.7–11.8) | 0.548 |
| Renal Failure (%) | 17.4 (16.8–17.9) | 22.0 (21.1–22.9) | 14.7 (14.1–15.4) | <0.001 |
| Charlson Comorbidity Score | 2.9 (2.8–2.9) | 3.3 (3.3–3.4) | 2.6 (2.6–2.7) | <0.001 |
Utilization of Vascular Procedures in the Year Prior to Amputation
Overall, we found that 54% of patients (11,115) did not undergo any vascular procedure in the year prior to amputation, whereas 46.0% of patients (9,349/20,464) underwent a vascular procedure in the year prior to amputation (Figure 1). For example, a diagnostic endovascular intervention alone, such as “catheter placement into the aorta from the groin or arm” was performed in 3,791 patients (14.7% of the entire cohort). Similarly, therapeutic endovascular interventions such as percutaneous femoral-popliteal angioplasty (n=2,015, 7.8% of patients) or open surgical procedures (such femoral-popliteal bypass, n=854, 3.3% of patients) were performed on patients during the year prior to amputation.
Endovascular diagnostic procedures were used most commonly, followed by endovascular therapeutic and open surgical procedures. The ten most commonly used endovascular and open lower extremity vascular procedures are shown in Table 3. In terms of the anatomic location in which revascularization procedures were performed, outflow arteries (such as the femoro-popliteal segment or the tibial arteries) were more commonly treated than inflow arteries (such as the iliac arteries). For example, endovascular interventions were more commonly used in outflow arteries rather than inflow arteries (83% outflow arteries, 17% inflow arteries). This pattern was similar in open revascularization (93% outflow arteries, 7% inflow arteries).
Table 3.
Ten most common open and endovascular CPT codes performed in the year prior to amputation
| Endovascular Interventions
| |||
|---|---|---|---|
| CPT Code | Description | n* | percent of all CPTs (n=25,800) |
| 36200 | Catheter placement aorta, from groin or arm | 3791 | 14.7 |
| 36246 | Abdominal, pelvic, or lower extremity arteriography, 2nd order selective | 3469 | 13.5 |
| 36247 | Abdominal, pelvic, or lower extremity arteriography, 3nd order selective | 3271 | 12.7 |
| 36245 | Abdominal, pelvic, or lower extremity arteriography, 1st order selective | 2055 | 8.0 |
| 35474 | Transluminal balloon angioplasty, percutaneous;femoral-popliteal | 2015 | 7.8 |
| 37205 | Transcatheter placement of an intravascular stent(s), (except coronary, carotid, and vertebral vessel), percutaneous; initial vessel | 1850 | 7.2 |
| 35470 | Transluminal balloon angioplasty, percutaneous; tibioperoneal trunk or branches, each vessel | 894 | 3.5 |
| 35473 | Transluminal balloon angioplasty, percutaneous;iliac | 532 | 2.1 |
| 37206 | Transcatheter placement of an intravascular stent(s), (except coronary, carotid, and vertebral vessel), percutaneous;each additional vessel | 473 | 1.8 |
| 36248 | Abdominal, pelvic, or lower extremity arteriography, Additional order selective | 444 | 1.7 |
|
| |||
| Open Surgical Procedures | |||
|
| |||
| CPT Code | Description | n* | percent of all CPTs |
|
| |||
| 35656 | Bypass graft, with other than vein;femoral-popliteal | 854 | 3.3 |
| 35566 | Bypass graft, with vein;femoral-anterior tibial, posterior tibial, peroneal artery | 762 | 3.0 |
| 35666 | Bypass graft, with other than vein;femoral-anterior tibial, posterior tibial, or peroneal artery | 526 | 2.0 |
| 35571 | Bypass graft, with vein;popliteal-tibial, -peroneal artery or other distal vessels | 454 | 1.8 |
| 35585 | In-situ vein bypass;femoral-anterior tibial, posterior tibial, or peroneal artery | 443 | 1.7 |
| 35371 | Thromboendarterectomy, including patch graft, if performed;common femoral | 408 | 1.6 |
| 35556 | Bypass graft, with vein;femoral-popliteal | 405 | 1.6 |
| 35372 | Thromboendarterectomy, including patch graft, if performed;deep (profunda) femoral | 232 | 0.9 |
| 35661 | Bypass graft, with other than vein;femoral-femoral | 196 | 0.8 |
| 35681 | Bypass graft; composite, prosthetic and vein | 180 | 0.7 |
The “n” represented here is the total number of procedures performed, which is greater than the total number of unique patients undergoing vascular procedures (n=9,349). Further details regarding patients who underwent more than one (or more than one type) of vascular procedure number are shown in Figures 1 and 2.
Use of Multiple Revascularization Procedures In the Year Prior to Amputation
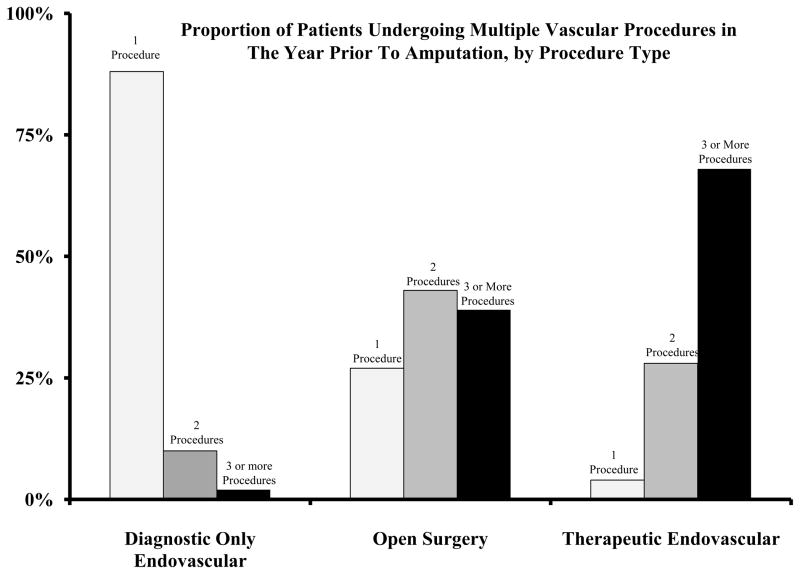
Many patients underwent more than one vascular procedure in the year prior to amputation. Across 9,349 patients, 25,800 vascular procedures were performed. While 14.3% of patients underwent one vascular procedure, 13.2% underwent 2–3 vascular procedures, and 8.4% underwent more than three vascular procedures. These findings remained similar, even when we analyzed our results exclusive of diagnostic angiography.
In Figure 2, we compared, by procedure type, the proportion of patients who received one, two, and three or more vascular procedures during the year prior to amputation. We found that patients undergoing therapeutic endovascular interventions were likely to undergo 3 or more procedures more commonly than patients treated with open surgery (68% versus 39%, p<0.001). Therefore, treatment with endovascular interventions made it more likely that a patient would experience a subsequent revascularization.
Figure 2.
Proportion of patients undergoing 1, 2, and 3 or more vascular procedures in the year prior to amputation, by procedure type.
Overall, 653 patients (3.1%) underwent both types (therapeutic endovascular intervention and open surgical revascularization) in the year prior to amputation. Patients who underwent open surgery had a slightly higher risk of undergoing additional endovascular intervention (31%), as compared to the risk of endovascular intervention patients undergoing additional open surgery (27%) (risk ratio = 1.15, 95% CI 1.09–1.19, p=0.04).
Variation in the Use of Vascular Procedures, by Patient Characteristics
We examined differences in age and race between patients selected to undergo vascular procedures and those who did not undergo a vascular procedure in the year prior to amputation. We found that older patients and black patients were less likely to undergo revascularization procedures in the year prior to amputation. For example, patients over age 90 were less likely to undergo a vascular procedure than patients under age 70 (21% versus 41%, p<0.001). Black patients were less likely to undergo any revascularization procedure in the year prior to amputation than white patients (32% versus 37%, p=0.001). These findings were similar in both open surgical and therapeutic endovascular interventions.
Variation in the Use of Vascular Procedures, by Hospital Referral Region
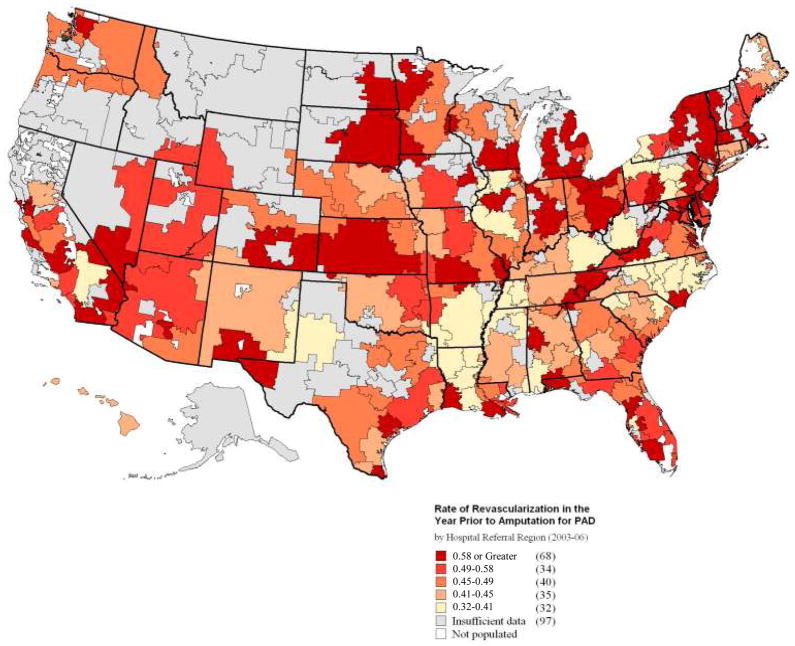
To examine regional differences in practice patterns, we studied the use of invasive vascular procedures across the 306 HRRs in the United States. In the most “intensive” HRRs, such as Elyria, Ohio, Munster, Indiana, and Santa Cruz, California, between 71–80% of patients underwent a vascular procedure in the year prior to amputation. However, in the least aggressive regions, such as Sayre, Pennsylvania, Billings, Montana, and Bryan, Texas, fewer than 12 % of patients underwent a vascular procedure in the year prior to amputation.
A national map of regional utilization rates (Figure 3) demonstrates that regions of high intensity of care (shown in dark red) are not concentrated in any one area of the US. Rather, these regions are widely distributed across the US. Further, when we examined the specific use of open surgery or therapeutic endovascular interventions (Figure 4), we found broad variation in the use of all types of revascularization procedures, including diagnostic and therapeutic endovascular intervention as well as open surgical revascularization.
Figure 3.
Map or revascularization rates, by HRR, 2003–2006.
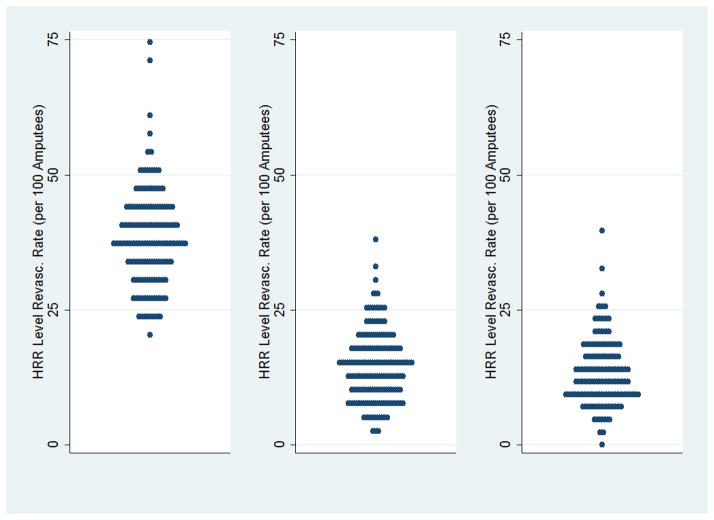
Figure 4.
Revascularization rates, by hospital referral region. Each blue dot represents an individual hospital referral region.
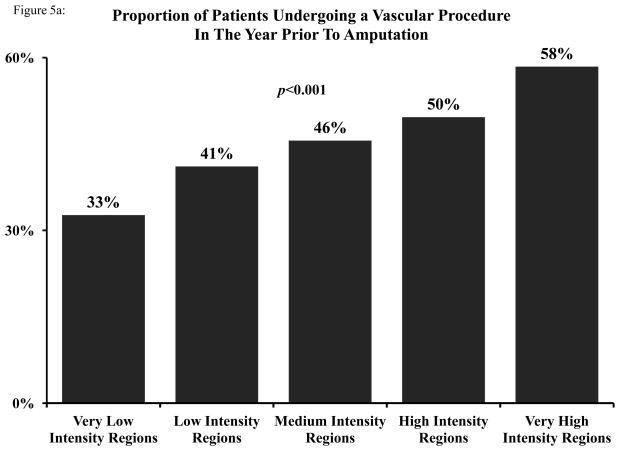
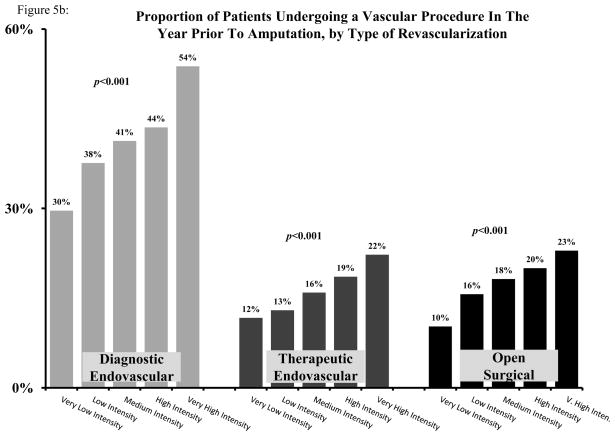
To allow for comparison across regions, we categorized all HRRs into five evenly sized groups, ranging from very low intensity (Quintile 1) to very high intensity (Quintile 5). While overall rates of revascularization were low in the very low intensity group (32.6%), the rate of revascularization was significantly higher in the very high intensity group (58.4%) (p<0.001) (Figure 5a). Variation in procedural vascular care by intensity was again evident in diagnostic endovascular, therapeutic endovascular, and open surgical revascularization (Figure 5b).
Figure 5.
A: Proportion of patients undergoing any vascular procedure in the year prior to amputation, by quintile of vascular intensity of care. B: Proportion of patients undergoing a diagnostic endovascular, therapeutic endovascular, or open surgical vascular procedure in the year prior to amputation, by quintile of vascular intensity of care.
Variation in intensity was not directly explained by differences in patient age or race. For example, the differences in revascularization across very high and very low intensity regions existed in elderly patients (32.3% in very low intensity regions, 57.9% in very high intensity regions) as well as black patients (33.1% in very low intensity regions, 58.1% in very high intensity regions).
Multivariate analysis to predict the likelihood of undergoing revascularization
To examine interactions between patient characteristics and regional patterns in utilization, we developed a multivariable logistic model to identify variables associated with the use of vascular procedures. Even when adjusting for the effect of age, sex, race, and comorbidities, patients in very high intensity regions were more than twice as likely to undergo revascularization in the year prior to amputation (OR for very high intensity versus very low intensity = 2.4, 95% CI 2.1–2.6, p<0.0001) (Table 4). Patient age and race also were independently associated with differences in the use of vascular procedures in the year prior to amputation. These differences were consistent across different definitions of procedural vascular care (endovascular diagnostic, endovascular therapeutic, or open procedure, or as any of its individual components, as shown in Appendix 2). Our model had moderate predictive ability with an AUC = 0.64.
Table 4.
Multivariable model used to predict the likelihood of undergoing revascularization in the year prior to amputation
| Odds Ratio | 95% Confidence | Interval | p value | |
|---|---|---|---|---|
| Intensity of Vascular Care | ||||
| Very Low Intensity | Referent | “---” | “---” | “---” |
| Low Intensity | 1.4 | 1.2 | 1.6 | <0.001 |
| Medium Intensity | 1.6 | 1.4 | 1.8 | <0.001 |
| High Intensity | 1.9 | 1.7 | 2.1 | <0.001 |
| Very High Intensity | 2.4 | 2.1 | 2.6 | <0.001 |
| Age | ||||
| Age 65–75 | Referent | “---” | “---” | “---” |
| Age 76–80 | 1.0 | 0.9 | 1.1 | 0.731 |
| Age 81–85 | 1.0 | 0.9 | 1.1 | 0.39 |
| Age 86–90 | 0.8 | 0.7 | 0.9 | <0.001 |
| Age 91–95 | 0.6 | 0.6 | 0.7 | <0.001 |
| Age >95 | 0.4 | 0.3 | 0.4 | <0.001 |
| Male Gender | 1.0 | 0.9 | 1.0 | 0.462 |
| Black Race | 0.8 | 0.8 | 0.9 | <0.001 |
| Congestive Heart Failure | 1.0 | 0.9 | 1.0 | 0.366 |
| COPD | 1.3 | 1.2 | 1.5 | <0.001 |
| Myocardial Infarction | 1.3 | 1.1 | 1.4 | <0.001 |
| Diabetes | 1.3 | 1.2 | 1.4 | <0.001 |
| End Stage Renal Disease | 1.3 | 1.2 | 1.4 | <0.001 |
Discussion
The treatment of lower extremity PAD and its consequences are among the most costly and morbid challenges faced by elderly Medicare patients. Therefore, efforts to limit major amputation secondary to PAD are a priority recognized by several societies and leaders in vascular care7, 20. And while prior research suggests that broad expansion in the use of vascular care has occurred in recent years11, our analyses demonstrate that aggressive vascular care for patients at risk for amputation have been unevenly applied across the US. In many regions of the United States, a majority of patients with severe PAD undergo amputation without even a diagnostic arteriogram performed in the year prior to amputation. However, in other regions, patients with a similar extent of PAD undergo a multitude of vascular procedures, especially therapeutic endovascular interventions. These decisions are unlikely to be driven solely by insurance access, as all patients in our analysis are over age 65 and thus insured by Medicare. Therefore, if the variation in vascular care is not entirely explained by patient-level factors, this variation must be due to differences in regional practice patterns.
Physicians who care for patients with vascular disease will attest that treatment decisions in patients with CLI can, at times, be straightforward. Patients with poor functional status, such as living in a nursing home or being unable to ambulate, have been shown to have very poor results in limb salvage attempts21, 22, and most agree that these patients should undergo primary amputation without attempts at limb salvage23. Conversely, patients with good functional status, favorable anatomic characteristics, and few comorbidities have consistently good outcomes following lower extremity revascularization, either in an open or endovascular fashion24, 25. However, decision-making in lower extremity revascularization is often not so clear-cut, and physicians who care for patients with CLI have many treatment options. In settings wherein clinical equipoise meets with multiple treatment options, the occurrence of health care variation has been well documented26, and prior work has shown that the treatment of vascular disease is no exception 12, 27. Accordingly, our present study indicates that in some regions, patients are treated with intensive revascularization strategies, while in other regions patients with a similar extent of CLI commonly undergo primary amputation.
Why does regional vascular practice vary, and what is the impact of this variation? Our future work aims to address these questions. First, in terms of variation, prior research in cardiovascular disease has demonstrated that the structural characteristics of hospitals (size, teaching status, financial status) and surgeons (volume, specialty, use of endovascular procedures) may explain this variation28. And while our current dataset is limited in its ability to describe these variables, our future efforts will study the effect of these covariates on variation in the intensity of vascular care. And second, it remains uncertain if treatment intensity is related to outcome in vascular care (i.e., “is more better”). Too little treatment intensity, we hypothesize, may be associated with inappropriately low rates of limb salvage. However, we also suspect that thresholds will exist where treatment intensity reaches the “flat of the curve”, and more vascular procedures may not provide added value. Accordingly, our future work will study the relationships between intensity of vascular care and limb salvage.
Our study has limitations. First, many will argue that administrative data lacks adequate anatomic, hemodynamic, and physiologic information to study patients with CLI29. However, our combining a known event (major amputation) and established diagnosis codes for PAD makes it unlikely that these amputations occurred in the absence of significant vascular disease, and our prior work has validated this approach in similar clinical-claims datasets27, 30. Second, the sided-ness of vascular procedures is not recorded in claims data, and we cannot be sure that vascular interventions described in our CPT codes were not contralateral to the limb that was eventually amputated. However, in our regional dataset in the VSGNE, we found that among over 4,000 patients with CLI, this event in fewer than 7% of all amputations, and was usually bilateral in nature (not contralateral). Third, while we studied vascular care in the 12 months prior to amputation, other studies have used longer intervals, without significant differences in the direction or size of their effect16. Finally, our model had an AUC of 0.64, which indicates that our model has a “moderate” ability to discriminate between those likely to undergo revascularization and those who are unlikely to undergo revascularization. Achieving better model discrimination will likely require more granular detail, in terms of as provider and hospital-level differences in care. Our future work will aim to use clinical and claims datasets to explore these determinants of treatment intensity for procedural vascular care, as well as analytic strategies such as instrumental variables to deal with unmeasured cofounding. Especially in observational analyses such as the work described herein, the role of unmeasured confounding must be acknowledged and considered, especially when determining the impact of our findings on future health policy decisions.
In conclusion, in many regions of the United States, a majority of Medicare patients with CLI undergo amputation with little procedural vascular care in the year prior to their amputation, while in other regions patients in with a similar extent of PAD undergo a variety of diagnostic, endovascular, and open interventions. Further, these treatment decisions are not driven solely by patient characteristics. Rather, these differences in treatment appear to be explained, at least in part, by regional differences in the intensity of vascular care. Our future work aims to explore the determinants of intensity of vascular care, as well as characterize the effectiveness of intensive and non-intensive treatment strategies. It is our ultimate goal to use this information to design algorithms that allow delivery of the most effective vascular care at the lowest treatment intensity for patients with CLI.
Supplementary Material
What is Known
Major lower extremity amputation occurs commonly, especially among black patients, those of low socioeconomic status, and among those residing in certain regions of the United States.
What This Study Adds
There is broad variation in the “intensity” of vascular care provided to Medicare patients in the year prior to amputation; in some regions patients receive extensive invasive care, while in other regions, little vascular care is provided.
This variation is evident in traditional leg bypass surgery, as well as in newer diagnostic and therapeutic endovascular procedures.
Patients who are treated only with newer, less-invasive therapeutic endovascular procedures are likely to undergo several procedures in the year prior to amputation.
Acknowledgments
Funding Sources: Dr. Goodney was supported by a Career Development Award from the NHLBI (1K08HL05676-01), and a Society for Vascular Surgery Foundation/American College of Surgeons Supplemental Funding Award.
Footnotes
Presented at the Society for Vascular Surgery’s Annual Meeting, June 15th, 2011.
Conflict of Interest Disclosures: None
References
- 1.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. [see comment] JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 2.O’Hare AM, Newman AB, Katz R, Fried LF, Stehman-Breen CO, Seliger SL, Siscovick DS, Shlipak MG. Cystatin c and incident peripheral arterial disease events in the elderly: Results from the cardiovascular health study. Archives of Internal Medicine. 2005;165:2666–2670. doi: 10.1001/archinte.165.22.2666. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states: Results from the natinoal health and nutrition examination survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM. The magnitude of the problem of peripheral arterial disease: Epidemiology and clinical significance. Cleveland Clinic Journal of Medicine. 2006;73 (Suppl 4):S2–7. doi: 10.3949/ccjm.73.suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- 5.Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. International Journal of Epidemiology. 1996;25:1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH, Langer RD, Fronek A, Feigelson HS. Coronary disease and stroke in patients with large-vessel peripheral arterial disease. Drugs. 1991;42 (Suppl 5):16–21. doi: 10.2165/00003495-199100425-00005. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AT. Treatment of peripheral arterial disease--extending “intervention” to “therapeutic choice”.[see comment][comment] New England Journal of Medicine. 2006;354:1944–1947. doi: 10.1056/NEJMe068037. [DOI] [PubMed] [Google Scholar]
- 8.Peacock JM, Keo HH, Yu X, Oldeberg N, Duval S, Henry TD, Jaff MR, Baumgartner I, Hirsch AT. Abstract 5788: The incidence and health economic burden of critical limb ischemia and ischemic amputation in minnesota: 2005–2007. Circulation. 2009;120:S1148. [Google Scholar]
- 9.Anderson PL, Gelijns A, Moskowitz A, Arons R, Gupta L, Weinberg A, Faries PL, Nowygrod R, Kent KC. Understanding trends in inpatient surgical volume: Vascular interventions, 1980–2000. Journal of Vascular Surgery. 2004;39:1200–1208. doi: 10.1016/j.jvs.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 10.White CJ, Gray WA. Endovascular therapies for peripheral arterial disease: An evidence-based review. Circulation. 2007;116:2203–2215. doi: 10.1161/CIRCULATIONAHA.106.621391. [DOI] [PubMed] [Google Scholar]
- 11.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. Journal of Vascular Surgery. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Dartmouth atlas cardiovascular and thoracic healthcare health care. Manning Selvage & Lee; 1998. [Google Scholar]
- 13.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in medicare spending. Part 1: The content, quality, and accessibility of care.[see comment][summary for patients in ann intern med. 2003 feb 18;138(4):I36; pmid: 12585853] Annals of Internal Medicine. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in medicare spending. Part 2: Health outcomes and satisfaction with care.[see comment][summary for patients in ann intern med. 2003 feb 18;138(4):I49; pmid: 12585852] Annals of Internal Medicine. 2003;138:288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 15.Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspectives in Biology & Medicine. 2003;46:69–79. doi: 10.1353/pbm.2003.0004. [DOI] [PubMed] [Google Scholar]
- 16.Holman KH, Henke P, Dimick JB, Birkmeyer JD. Racial disparities in end-of-limb care among medicare amputees. Journal of Vascular Surgery. 2011;54:420–426. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. [Date of Access = September 9th, 2011];Cpt schedule. Accessed at https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp.
- 18.Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the charlson comorbidity index derived from medical record data and administrative billing data. Journal of Clinical Epidemiology. 1999;52:137–142. doi: 10.1016/s0895-4356(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 19.Dartmouth atlas of healthcare. 2007 October 1st; www.dartmouthatlas.org.
- 20.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, Nehler MR, Powell RJ, Sidawy AN. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–1473. e1461–1463. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 21.Simons JP, Goodney PP, Nolan BW, Cronenwett JL, Messina LM, Schanzer A. Failure to achieve clinical improvement despite graft patency in patients undergoing infrainguinal lower extremity bypass for critical limb ischemia. J Vasc Surg. 2010;51:1419–1424. doi: 10.1016/j.jvs.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Bertges DJ, Stanley AC, Stone DH, Walsh DB, Powell RJ, Likosky DS, Cronenwett JL. Factors associated with amputation or graft occlusion one year after lower extremity bypass in northern new england. Ann Vasc Surg. 2010;24:57–68. doi: 10.1016/j.avsg.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SM, Cull DL, Kalbaugh CA, Cass AL, Harmon SA, Langan EM, 3rd, Youkey JR. Critical analysis of clinical success after surgical bypass for lower-extremity ischemic tissue loss using a standardized definition combining multiple parameters: A new paradigm of outcomes assessment. Journal of the American College of Surgeons. 2007;204:831–838. doi: 10.1016/j.jamcollsurg.2007.01.037. discussion 838–839. [DOI] [PubMed] [Google Scholar]
- 24.Goodney PP, Schanzer A, Demartino RR, Nolan BW, Hevelone ND, Conte MS, Powell RJ, Cronenwett JL. Validation of the society for vascular surgery’s objective performance goals for critical limb ischemia in everyday vascular surgery practice. J Vasc Surg. 2011 doi: 10.1016/j.jvs.2010.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schanzer A, Goodney PP, Li Y, Eslami M, Cronenwett J, Messina L, Conte MS. Validation of the piii cli risk score for the prediction of amputation-free survival in patients undergoing infrainguinal autogenous vein bypass for critical limb ischemia. J Vasc Surg. 2009;50:769–775. doi: 10.1016/j.jvs.2009.05.055. discussion 775. [DOI] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Sharp SM, Finlayson SR, Fisher ES, Wennberg JE. Variation profiles of common surgical procedures. Surgery. 1998;124:917–923. [PubMed] [Google Scholar]
- 27.Goodney PP, Travis LL, Malenka D, Bronner KK, Lucas FL, Cronenwett JL, Goodman DC, Fisher ES. Regional variation in carotid artery stenting and endarterectomy in the Medicare population. Circ Cardiovasc Qual Outcomes. 2010;3:15–24. doi: 10.1161/CIRCOUTCOMES.109.864736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nallamothu BK, Rogers MAM, Chernew ME, Krumholz HM, Eagle KA, Birkmeyer JD. Opening of specialty cardiac hospitals and use of coronary revascularization in medicare beneficiaries.[see comment] JAMA. 2007;297:962–968. doi: 10.1001/jama.297.9.962. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JB, Bubolz T, Paul JE, Pashos CL, Escarce JJ, Muhlbaier LH, Wiesman JM, Young WW, Epstein RS, Javitt JC. Using medicare claims for outcomes research. Med Care. 1994;32:JS38–51. [PubMed] [Google Scholar]
- 30.Goodney PP, Likosky DS, Cronenwett JL Vascular Study Group of Northern New E. Factors associated with stroke or death after carotid endarterectomy in northern new england. Journal of Vascular Surgery. 2008;48:1139–1145. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.