Some mechanisms of epithelial-mesenchymal transition (EMT) in normal development also facilitate disease progression (e.g., fibrosis, cancer).
SUMMARY
Epithelial-mesenchymal transition (EMT) is a physiological process in which epithelial cells acquire the motile and invasive characteristics of mesenchymal cells. Although EMT in embryonic development is a coordinated, organized process involving interaction between many different cells and tissue types, aspects of the EMT program can be inappropriately activated in response to microenvironmental alterations and aberrant stimuli, and this can contribute to disease conditions including tissue fibrosis and cancer progression. Here we will outline how EMT functions in normal development, how it could be activated in pathologic conditions—especially by matrix metalloproteinases—and how it may be targeted for therapeutic benefit.
1. INTRODUCTION
Epithelial-mesenchymal transition (EMT) is a process integral to the formation of many tissues and organs during development (Shook and Keller 2003; Radisky 2005; Hugo et al. 2007; Thiery et al. 2009). Activation of developmental EMT has been found to follow a defined sequence of events (Fig. 1) (Shook and Keller 2003). First, the region of the tissue where the EMT events will occur must be specified through temporal and spatial patterning of the cells that will undergo EMT, as well as morphogenic rearrangement of the epithelial tissue so as to move those cells to the site of EMT. Second, there must be disruption of the interaction between epithelial cells and the basement membrane (BM), a specialized form of the extracellular matrix (ECM) that underlies epithelial tissue. This can occur through release of cell-BM contacts or through proteolytic degradation of the BM. Third, the transitioning cells must detach from the epithelial sheet through processes that minimize loss of epithelial integrity; this generally involves actomyosin-based rearrangements of cell shape for the transitioning cells in combination with crawling of the retained epithelial cells to close the gap. Finally, the ingressed cells must differentiate into the mesenchymal phenotype, altering cell-ECM interactions, cytoskeletal organization, and fundamental aspects of cellular metabolism. Thus, developmental EMT is more than just the acquisition of motility but is a process involving the entire epithelial tissue. It is organized through a combination of cell–cell and cell-ECM interactions and many soluble molecular factors. During disease, the coordination of these processes is lost and activation of the EMT process can occur in an uncoordinated and cell-autonomous fashion, leading to disruption of epithelial integrity and disorganization of epithelial tissue as well as production of new mesenchymal cells, which can perpetuate the disease process. Here, we will discuss the basic features of developmental EMT at the tissue and cellular level, how these processes may be activated during disease, and how pathological EMT may become a possible target for therapy.
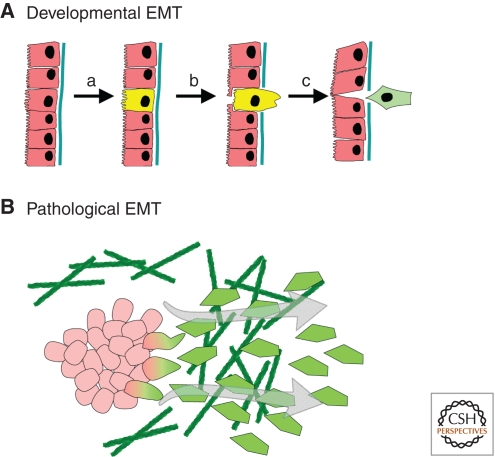
Figure 1.
Developmental versus pathological EMT. (A) Developmental EMT is a process that involves the entire epithelial tissue. (a) Specification of cells that will undergo EMT occurs through coordination of cell–cell, cell-ECM, and soluble signals. (b) Degradation or disruption of the basement membrane is followed by cell ingression and morphogenesis of the retained epithelial cells to close the gap. (c) The fully detached cell undergoes phenotypic mesenchymal shift. (B) Activation of the EMT program in pathological conditions can occur in a disorganized and more cell-autonomous fashion.
2. GENERAL PRINCIPLES AND KEY PROCESSES
EMT plays a critical role in early developmental processes, such as gastrulation, leading to formation of mesoderm. Investigation of gastrulation in Drosophila, Xenopus, birds, and mammals, led to definition of the convergent extension process, through which the morphogenic movements of the endoderm are coordinated with the delamination and invasion of the early mesodermal cells. EMT activation occurring during neural crest formation is responsible for the release of mesenchymal cells that migrate through the body, generate the vertebrate head and a wide variety of tissue types, including glial and neuronal cells, adrenal glandular tissues, melanocytes, and skeletal and connective tissues (Shook and Keller 2003; Duband 2006). EMT of embryonic endocardial cells into the endocardial cushion creates precursors of the valvular and septal structures (Runyan et al. 2005). Significantly, EMT in embryonic development generally occurs in an immunologically privileged setting with reduced inflammatory responses. In the adult organism, plastic transition between epithelial and mesenchymal cell types occurs during wound healing and remodeling of tissues that develop postnatally, such as the mammary gland, and in the pathological context, such as inflammation, fibrosis, or tumor progression (Lopez-Novoa and Nieto 2009; Nieto 2010).
At the cellular level, the process of EMT is defined by the conversion of epithelial cells into a mesenchymal phenotype (Fig. 2). Epithelial cells are interconnected by cell–cell junctions, and whereas some cell shape rearrangement is possible, movement of epithelial cells is largely constrained to the confines of the epithelial sheet. Epithelial function and integrity depends on intercellular junctions. Key components of epithelial junctions include E-cadherin, a transmembrane molecule that connects adjacent cells, and β-catenin, part of the protein complex that connects cadherins to the actin cytoskeleton at the adherens junctions (AJ). Proper regulation of E-cadherin and β-catenin is crucial for control of the epithelial phenotype, as these molecules not only maintain intercellular adhesion but also coordinate cellular organization, transmit information from the microenvironment to the cells, and control phenotypic transitions of epithelial cells (Perez-Moreno and Fuchs 2006; LaBarge et al. 2009; Chanson et al. 2011).
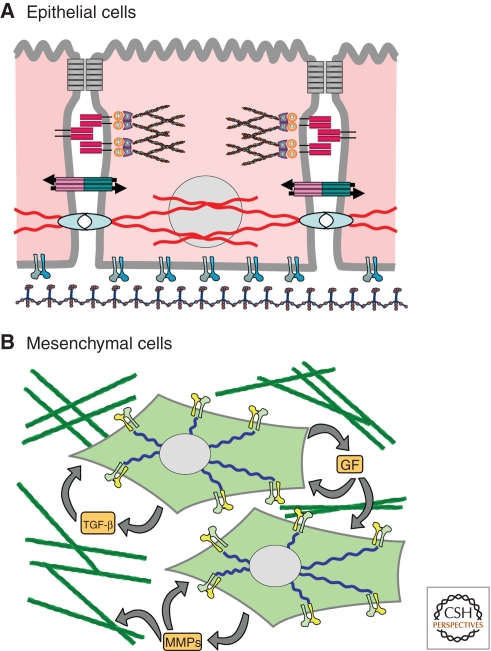
Figure 2.
Characteristics of epithelial and mesenchymal cells. (A) Epithelial cells are interconnected through tight junctions (gray), E-cadherin-based junctions (red), which are connected to the actin cytoskeleton, gap junctions (red/blue), and hemidesmosomes (cyan), which are connected to the cytokeratin-based intermediate filament cytoskeleton. Epithelial cells also have specialized cell-ECM interactions for adhesion to the laminin-rich basement membrane. (B) Mesenchymal cells show a shift to a vimentin-based intermediate filament cytoskeleton and altered composition of cell-ECM interactions optimized for adhesion to the collagen-rich interstitial matrix. Mesenchymal cells also produce abundant TGFb, growth factors (GF), and matrix metalloproteinases (MMPs), as well as components of the extracellular matrix.
In contrast, mesenchymal cells can move in three dimensions, traveling along and within collagen networks, and although cell-ECM contacts are still critical for mesenchymal cells, these contacts become specialized for interaction with the components of the interstitial ECM (Cukierman et al. 2001). During the EMT process many major cell structures and functions are affected. The loss of cell–cell adhesion, the down-regulation of E-cadherin and other epithelial genes, components of the tight junctions, including members of the claudin family and cytokeratins, are the principal characteristic of EMT. These changes can occur in concert with the up-regulation of key transcription factors and with the expression of associated mesenchymal genes, such as vimentin and fibronectin (Yang and Weinberg 2008). EMT in adult tissues is regulated by the composition and structure of the ECM components and ECM-remodeling matrix metalloproteinases (MMPs) (Lochter et al. 1997a,b; Radisky and Radisky 2010), as well as soluble growth factors or cytokines, including epidermal growth factor (EGF), hepatocyte growth factor (HGF), fibroblast growth factors (FGFs), and transforming growth factor (TGF)-β (Shirakihara et al. 2011).
TGF-β is a potent activator of EMT; during gastrulation, EMT induced by members of the TGF-β family leads to the formation of the three multipotent germ layers. In organogenesis TGF-β is involved in EMT processes in the kidney, endocardial cushion, and subsequent atrioventricular valve formation (Acloque et al. 2009). Virtually all cell types are responsive to TGF-β and it is produced by many immune and nonimmune cells (Sanjabi et al. 2009). TGF-β also regulates proliferation, migration, differentiation, and survival processes, and in cancer, can act either to suppress tumor growth or to activate tumor progression, depending on cellular context (Sieweke et al. 1989, 1990; Massague 2008). Although TGF-β is key to normal inflammatory responses, its sustained expression during chronic inflammation stimulates fibrogenic processes and tumor promotion. The cooperation between TGF-β1 and the proinflammatory cytokines produced in a chronically inflamed microenvironment activates autoregulatory loops, which further reinforce the EMT program (Yang et al. 2010). Induction of EMT by TGF-β involves a combination of Smad-dependent and Smad-independent events on cell junction complexes. The activation of Smad transcriptionally regulates key EMT-associated factors, including Snail, Snail2 (Slug), Twist, and HMGA2 (high-mobility group A2) (Fuxe et al. 2010). Smad-independent activity of TGF-β leads to phosphorylation of Par6 with a subsequent disorganization of the Par complex, leading to loss of polarity (Ozdamar et al. 2005).
Several transcription factors have been identified in EMT, involved either in transcriptional inactivation of epithelial genes or activation of mesenchymal genes (Yang and Weinberg 2008). Investigation using mouse models has revealed that among these, Snail is a central regulator of both developmental and pathological EMT (Barrallo-Gimeno and Nieto 2005). Snail is critical for gastrulation in normal development of mice; homozygous knockout of Snail is lethal as embryos fail to produce mesoderm (Carver et al. 2001). Snail has also been associated with pathological conditions, such as fibrosis and cancer, in which its detrimental role is determined by its ability to induce EMT-like processes (Thiery et al. 2009). It directly suppresses E-cadherin, claudins, and other epithelial cell–cell adhesion molecules, and promotes expression of mesenchymal proteins, such as fibronectin and MMP-9 (Fuxe et al. 2010). Snail is found to be expressed in neoplastic epithelial cells and in fibroblasts associated with damaged or neoplastic tissues (Rowe et al. 2009), and its increased expression is commonly observed in cultured cells treated with agents that stimulate the EMT program, for example, by treatment with TGF-β, interleukin 6, growth factors such as EGF or FGF, or by exposure to MMPs (Przybylo and Radisky 2007; de Herreros et al. 2010). Expression of Snail is further regulated by an integrated and complex signaling network at the transcriptional and posttranscriptional levels that includes integrin-linked kinase (ILK), phosphatidylinositol 3-kinase (P13-K), mitogen-activated protein kinases (MAPKs), glycogen synthase kinase 3-β (GSK-3β), and NFκB pathways. The increase of the transcription of Snail comes about through the direct binding of NFκB to the Snail promoter (Franco et al. 2010), which can be potentiated by activation of NFκB by GSK-3β inhibition (de Herreros et al. 2010; Fuxe et al. 2010).
3. ALTERNATIVE SPLICING IN THE EMT PROGRAM
Although EMT has largely been studied at the level of altered gene transcription or posttranslational modifications, it is becoming increasingly clear that alternative splicing (AS) processes provide an additional layer of gene regulation that is critical in shaping the EMT process, particularly in cancer progression (Tavanez and Valcarcel 2010). Epithelial splicing regulatory proteins 1 and 2 (ESRP1 and ESRP2), regulators of epithelial cell specificity through regulation of genes, have been implicated as central coordinators of AS networks in EMT (Warzecha et al. 2010). ESRP target genes are involved in key EMT processes, such as regulating cell polarity, motility, cell-cell and cell-matrix adhesion (Tavanez and Valcarcel 2010), and ESRP-regulated expression of epithelial versus mesenchymal splice isoforms differentially promotes cell adhesion versus cell migration. This is the case for the ESRP-regulated gene hMena, which along with VASP (vasodilator-stimulated phosphoprotein) and Evl (Ena/VASP-like), comprises the Ena/VASP family of actin regulatory proteins that control cell movement, shape, and adhesion (Gertler and Condeelis 2011). The epithelial-specific hMena11a splice isoform belongs to a cluster of genes regulated by ESRP1/2 that are involved in the actin cytoskeleton organization, cell adhesion, and cell motility (Warzecha et al. 2010). Ectopic expression of ESRP1 is associated with an up-regulation of hMena11a, a dramatic reorganization of the actin cytoskeleton, changes in cell morphology from spindle-shaped to cobblestone appearance, and suppressed tumor cell invasion, associated with a mesenchymal-to-epithelial transition (MET) (P Nisticò and MJ Bissell, in prep.). In vivo, the hMena11a isoform is expressed in epithelial but not in mesenchymal cell lines (Di Modugno et al. 2007; Pino et al. 2008). In human breast cancer cells, hMena11a is phosphorylated downstream from HER2 and EGFR following EGF and NRG1 treatment, and this in turn influences the mitogenic signals of these receptors in luminal breast cancer cell lines (Di Modugno et al. 2007). Conversely, the lack of hMena11a expression is generally accompanied by increased expression of the alternatively spliced hMenaΔv6, absence of E-cadherin, and presence of N-cadherin and vimentin in invasive tumor cell lines, indicating that hMena AS is associated with the EMT process (P Nisticò and MJ Bissell, in prep.).
Similarly, AS of CD44 with a switch from a variant isoform (CD44v) to a standard isoform (CD44s) is regulated by ESRP1 and is strongly related to the EMT process (Brown et al. 2011). CD44s expression is up-regulated in breast tumors and correlates with levels of the mesenchymal marker N-cadherin and breast cancer progression. Down-regulation of ESRP1 expression, using small hairpin RNA (shRNA), led to a switch from CD44v to CD44s. Conversely, overexpression of ESRP1 increases CD44v expression and prevents cells from undergoing EMT (Brown et al. 2011).
4. EMT IN MAMMARY GLAND DEVELOPMENT
Physiological EMT has largely been studied in the developing embryo, whereas pathologic EMT is commonly viewed as a phenomenon that occurs in the adult organism. However, the majority of mammary gland development occurs postnatally, providing a unique opportunity to investigate functional EMT processes. During puberty, the rudimentary mammary gland develops into the fat pad through extension and branching morphogenesis of the ductal tree (Wiseman and Werb 2002; Fata et al. 2004; Sternlicht et al. 2006). Ductal extension in the developing gland occurs at endbuds, invasive structures that express high levels of EMT-associated transcription factors, including Snail and Twist (Kouros-Mehr and Werb 2006), as well as MMP-2 and MT1-MMP (Wiseman et al. 2003). Ramification of the mammary tree through branching morphogenesis occurs by two separate mechanisms: primary branching or endbud bifurcation, and secondary branching, in which differentiated, ductal epithelium dedifferentiates, detaches from the adjacent epithelial cells, penetrates the BM, and invades into the surrounding tissue (Fig. 3). MMP-3 is a key mediator of secondary branch formation, as transgenic mice lacking MMP-3 expression have significantly reduced secondary branching, whereas the whey acidic protein (WAP)-MMP-3 mice have increased secondary branching and ductal complexity (Sympson et al. 1994; Wiseman et al. 2003). Inhibition of retinoic acid signaling pathways during mammary development increases MMP-3 expression and also increases side branching (Wang et al. 2005). The mechanism by which MMP-3 induces branching morphogenesis has been investigated in 3D collagen I cell culture models. These studies have shown that epimorphin induces expression of MMP-3, and that this is both necessary and sufficient for cell invasion into the ECM (Hirai et al. 1998, 2001; Radisky et al. 2003, 2009; Chen et al. 2009). Activation of the fibroblast growth receptor signaling pathway, which has also been implicated in mammary branching morphogenesis, also induced MMP-3 expression and branch initiation in mammary epithelial cells grown in 3D collagen (Xian et al. 2005), a process reminiscent of secondary branch initiation in vivo.
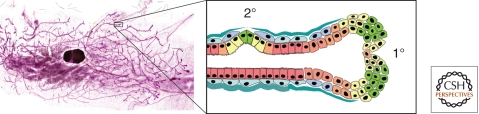
Figure 3.
Primary and secondary branching morphogenesis of the mammary gland. (Left) Whole mount of mouse mammary gland. (Inset, right) Primary (1°) branching occurs through bifurcation of the endbuds, whereas secondary (2°) branching involves many aspects of the EMT program, including breakdown of epithelial tissue structure, degradation of basement membrane, and acquisition of invasive characteristics, processes dependent on MMP-3, which is produced in response to morphogenic signals by the surrounding stromal cells.
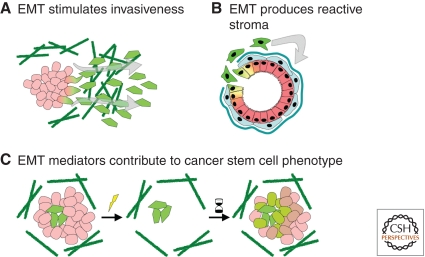
Figure 4.
EMT facilitates tumor progression. (A) EMT stimulates proinvasive and antiapoptotic processes that facilitate metastasis. (B) EMT can produce reactive stromal cells, which drive tumor initiation and progression through disruption of the surrounding ECM and production of soluble tumorigenic factors. (C) EMT mediators contribute to the cancer stem cell phenotype, which confers resistance to radiation or chemotherapeutic agents and increased ability of tumor regrowth.
5. EMT AND BREAST CANCER
Activation of the EMT program in cancer cells facilitates tumor progression through several distinct mechanisms: (1) EMT in the tumor cells can trigger invasive and antiapoptotic mechanisms that drive cancer metastasis, (2) EMT can generate activated stromal cells that drive cancer progression through biochemical and structural alterations of the tumor microenvironment, and (3) EMT mediators can stimulate the increased malignancy associated with the cancer stem cell phenotype. Examples of many of these phenomena are seen in response to increased levels of MMPs (Sternlicht and Werb 2001; Page-McCaw et al. 2007). MMP-dependent activation of the EMT program has been observed in a variety of epithelial cell types, including kidney (Cheng and Lovett 2003; Cheng et al. 2006; Zheng et al. 2009; Tan et al. 2010), ovary (Cowden Dahl et al. 2008), lens (West-Mays and Pino 2007), lung (Illman et al. 2006), and prostate (Cao et al. 2008), although MMP-induced EMT has been best characterized in mammary epithelial cells. Transgenic mice that expressed MMP-3 under control of the WAP promoter developed tissue fibrosis, followed by spontaneous formation of mammary tumors (Sympson et al. 1995; Sternlicht et al. 1999, 2000); experiments using cultured mammary epithelial cells revealed that MMP-3 directly stimulates EMT (Lochter et al. 1997a,b). MMP-3 activates the EMT program through induction of the alternative splice isoform Rac1b (Radisky et al. 2005), a constitutively activated variant of Rac1 initially identified in breast and colorectal cancer cells (Jordan et al. 1999; Schnelzer et al. 2000; Matos et al. 2003; Fiegen et al. 2004; Singh et al. 2004). Rac1b in turn stimulates EMT through increasing levels of cellular reactive oxygen species (Radisky et al. 2005; Nelson et al. 2008). Although the process by which expression of MMP-3 leads to AS of Rac1b and the consequent downstream effects has not been completely defined, it should be noted that a key substrate of MMP-3 is the cell–cell junction protein E-cadherin, and that loss of E-cadherin is sufficient to induce EMT in many cell types (Lochter et al. 1997a; Noe et al. 2001; Onder et al. 2008). Although targeting MMPs as a cancer therapeutic strategy has proven difficult (Coussens et al. 2002), identification of specific cancer-associated processes induced by MMPs, such as MMP3-induced expression of Rac1b, offers the potential for development of new and more effective inhibitors of MMP-induced EMT.
6. EMT-INDUCED MYOFIBROBLAST FORMATION AND FIBROSIS
Myofibroblasts play a key function in the repair of epithelial injuries, digesting the damaged tissue through production of MMPs and other ECM-degrading enzymes, as well as synthesizing and modulating elements of the wound provisional ECM (Tomasek et al. 2002; Hinz and Gabbiani 2003; Thannickal et al. 2004; Duffield 2010). Under conditions in which the wound healing program is properly executed, the provisional ECM is degraded and the myofibroblasts are removed by apoptosis during tissue regrowth; however, when repair mechanisms are disrupted, chronically activated myofibroblasts produce a fibrotic ECM that is not easily degraded by proteases (Thannickal et al. 2004). This aberrant ECM interferes with normal cell function, leading to tissue disruption, altered response to apoptotic signals, and increased cellular proliferation, an ideal context for tumor formation and progression (Massague 2008). The presence of fibrosis significantly increases the risk of subsequent appearance of tumors in the lung (Perez-Moreno and Fuchs 2006; Radisky and Radisky 2010), liver (Acloque et al. 2009; Sanjabi et al. 2009), and breast (Boyd et al. 2005; Fuxe et al. 2010).
A causative link between fibrosis and cancer has been best studied in the lung (Thannickal et al. 2004), kidney (Zeisberg and Kalluri 2004), and liver (Elsharkawy and Mann 2007). Breast fibrosis, by comparison, is a much less studied phenomenon, in part because characterization of fibrosis in nonmalignant breast tissue has not been as precisely defined as with fibrosis of the lung, kidney, or liver. One indication that breast fibrosis might also predispose to cancer, however, is the identification of increased risk of tumor incidence in women with mammographically dense breasts (Boyd et al. 2005); several investigations of dense breast tissue have shown that these areas contain increased collagen deposition, presence of activated fibroblasts, and other early markers of fibrosis (Wellings and Wolfe 1978; Buchanan et al. 1981; Bright et al. 1988; Urbanski et al. 1988; Bartow et al. 1990; Boyd et al. 2000). Another indication is that the appearance of myofibroblasts is a frequent component of the breast cancer reactive stroma (Lagace et al. 1985; Sappino et al. 1988). Given the role of reactive stroma in tumor promotion (Bissell and Radisky 2001), targeting processes involved in myofibroblast formation may be essential for preventing cancer recurrence.
7. EMT AND THE CANCER STEM CELL PHENOTYPE
Stem cells are characterized as having low proliferative rates, existing as minority populations within tissues in defined tissue compartments, or niches, and having responses to extracellular stimuli that are distinct from those of the more differentiated cells within the organ (Fuchs et al. 2004). Stem cells show self-renewal and can divide symmetrically, to produce additional stem cells, or asymmetrically, to generate progenitor cells that can subsequently differentiate into the many different cell types within the organ. More recently, cells with stem/progenitor characteristics have been found to play critical roles in tumor formation and progression (Kakarala and Wicha 2008). Cancer subpopulations of highly malignant tumor-initiating cells, designated cancer stem cells (CSCs), were first identified in human acute myeloid leukemia (Lapidot et al. 1994). Subsequent studies have identified CSCs in solid tumors, including breast, brain, colon, and pancreas (reviewed in Hollier et al. 2009).
The identification of cells within tumors that possess CSC characteristics has resulted in increased efforts to identify treatments that selectively target this subpopulation. Some studies have provided evidence that traditional treatments for breast cancer that target the bulk of the tumor such as surgery, conventional chemotherapy, and radiation, may be less effective toward the CSCs, because of their decreased proliferation rate and altered response to environmental stimuli (Creighton et al. 2009). Although identification of therapies that selectively target CSCs is a potentially important goal, a parallel and perhaps equally effective approach would be to target the mechanisms that generate and maintain the CSC population in tumors. A significant advance toward this goal was provided by a recent series of studies showing that induction of EMT in human or mouse mammary epithelial can result in the generation of cells with stem cell properties, such as self-renewal and resistance to toxicants (Mani et al. 2008; Morel et al. 2008; Santisteban et al. 2009). Characterization of these model systems provides an experimental platform to explore the processes involved in CSC development.
8. CANCER IMMUNOEDITING AND EMT
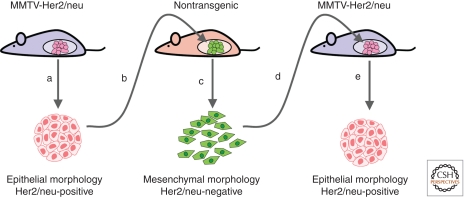
Immune responses can eliminate premalignant and malignant cells in a process called immunosurveillance, and this has been identified as a key factor in reducing the earliest stages of tumor development (Dunn et al. 2004). However, aberrant activation of the immune system can also promote tumor development, as chronic activation of inflammatory responses has been directly linked to development of cancer (Tan and Coussens 2007; Mantovani et al. 2008). The interaction of tumors with the immune system progresses through an initial stage in which the immune system can effectively target and kill tumor cells. However, as the tumor-immune interaction evolves, tumors can acquire the ability to evade immune targeting entirely, by becoming less immunogenic or more immune suppressive (Dunn et al. 2004; Reiman et al. 2007). Evaluation of immunoediting in a mouse model of breast cancer relapse identified activation of EMT as a mechanism for immune escape (Fig. 5) (Reiman et al. 2010). Transgenic mice that express the cell-surface rat neu oncogene under control of the mammary epithelial cell-specific mouse mammary tumor virus (MMTV) promoter develop tumors with a highly epithelial morphology; transplantation of these tumors into nontransgenic syngeneic mice stimulated a T-cell-dependent rejection, followed by relapse of phenotypically mesenchymal tumors enriched in neu-negative variant cells (Knutson et al. 2006; Kmieciak et al. 2007). Activation of the EMT program had led to down-regulation of the epithelial-specific MMTV promoter and consequent loss of expression of the neu oncogene, allowing tumor growth without immune reaction. Subsequent investigations revealed that CD8 T cells were required for outgrowth of the neu-negative mesenchymal variants suggesting local induction of EMT rather than selection (Santisteban et al. 2009), and that the induction of EMT was associated with TGF-β and TNF-α signaling pathways (Asiedu et al. 2011). Detailed characterization of tumor cells isolated from relapsed mice showed that these tumors had adopted CSC characteristics, including altered cell-surface marker profiles, enhanced mammosphere formation, and substantially increased tumorigenicity (Santisteban et al. 2009). Immunoedited mesenchymal tumor cells also had elevated expression of drug transporters, DNA repair enzymes, and enhanced resistance to chemotherapy and radiation. Histopathologic analysis of tumors formed by injection of neu-negative cells into MMTV-neu mice showed a predominant epithelial phenotype with the reacquisition of neu expression, suggesting that the immune-induced EMT was fully reversible through the reverse process, MET (Santisteban et al. 2009). These studies identify EMT as an intrinsic mechanism that can allow tumor cells to avoid immune surveillance.
Figure 5.
EMT facilitates evasion of immunosurveillance. (A) Tumor cells isolated from MMTV-Her2/neu mice have an epithelial morphology and express the cell-surface rat Her2/neu antigen. (B) When reimplanted into nontransgenic mice, these tumor cells are initially targeted by the immune system, but eventually regrow. (C) Isolated tumor cells from the xenografted mice show a mesenchymal morphology and do not display the Her2/neu antigen. (D) Reinjection of these cells into MMTV-Her2/neu mice leads to rapid tumor regrowth. (E) Isolation of secondary tumors reveals an epithelial morphology and reexpression of Her2/neu. (Adapted from Reiman et al. 2010.)
9. CONCLUSIONS
EMT is, therefore, both an essential step in development and normal morphogenic processes, as well as a mechanism that facilitates pathological processes such as fibrosis and chronic inflammation and many stages of tumor progression. Accordingly, differentiating between how the EMT program differs between normal development and disease has become an important area of investigation. Such studies have been complicated by a relatively recent controversy concerning the specific role of EMT in disease (Duffield 2010; Popov and Schuppan 2010; Zeisberg and Duffield 2010; Kriz et al. 2011). Part of this controversy results from differing definitions of EMT, and particularly from the diagnostic use of specific markers that may not be universally involved in all EMT processes. Such narrow definitions are likely to be too limited—even in well-established developmental EMT, there are few universal rules (Shook and Keller 2003). Moreover, it is likely that many studies of EMT in fibrosis or cancer progression have reflected the cellular consequences of an incomplete activation of the EMT program. Unlike developmental EMT, which represents a controlled and organized morphogenic program, the chaotic microenvironment of fibrotic tissues or developing tumors contains many different, dysregulated signaling processes, inducing an uncoordinated and incomplete EMT. As a consequence, these EMT-activated cells may acquire significant fibrotic or tumor-promoting abilities even as they retain many of their original characteristics, making it difficult to distinguish them from the original tumor mass from which they are derived.
However, aside from the semantic debates, there remain unanswered questions pertaining to the extent of EMT involvement in some aspects of disease progression, which could be addressed through the use of appropriate model systems. For example, although EMT of epithelial cells offers one potential source of myofibroblasts that accumulate in fibrosis, there remain competing theories, including recruitment of circulating fibrocytes or activation of resident fibroblasts (Scotton and Chambers 2007). Such questions have been difficult to address experimentally, because except in relatively uncommon types of cancer, active EMT is not readily detected using conventional imaging methodologies, such as immunohistochemistry of paraffin-fixed tissue samples (Iwatsuki et al. 2010). Future studies are needed to address these questions using lineage-tracing approaches and new methods of in vivo imaging.
Footnotes
Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant
Additional Perspectives on Mammalian Development available at www.cshperspectives.org
REFERENCES
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA 2009. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest 119: 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL 2011. TGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res 71: 4707–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA 2005. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 132: 3151–3161 [DOI] [PubMed] [Google Scholar]
- Bartow SA, Pathak DR, Mettler FA 1990. Radiographic microcalcification and parenchymal patterns as indicators of histologic “high-risk” benign breast disease. Cancer 66: 1721–1725 [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D 2001. Putting tumours in context. Nat Rev Cancer 1: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NF, Jensen HM, Cooke G, Han HL, Lockwood GA, Miller AB 2000. Mammographic densities and the prevalence and incidence of histological types of benign breast disease. Reference Pathologists of the Canadian National Breast Screening Study. Eur J Cancer Prev 9: 15–24 [DOI] [PubMed] [Google Scholar]
- Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD 2005. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6: 798–808 [DOI] [PubMed] [Google Scholar]
- Bright RA, Morrison AS, Brisson J, Burstein NA, Sadowsky NS, Kopans DB, Meyer JE 1988. Relationship between mammographic and histologic features of breast tissue in women with benign biopsies. Cancer 61: 266–271 [DOI] [PubMed] [Google Scholar]
- Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C 2011. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest 121: 1064–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JB, Weisberg BF, Sandoz JP, Gray LA Sr, Bland KI 1981. Selected prognostic variables for mammographic parenchymal patterns. Cancer 47: 2135–2137 [DOI] [PubMed] [Google Scholar]
- Cao J, Chiarelli C, Richman O, Zarrabi K, Kozarekar P, Zucker S 2008. Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J Biol Chem 283: 6232–6240 [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T 2001. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol 21: 8184–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson L, Brownfield D, Garbe JC, Kuhn I, Stampfer MR, Bissell MJ, LaBarge MA 2011. Self-organization is a dynamic and lineage-intrinsic property of mammary epithelial cells. Proc Natl Acad Sci 108: 3264–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Nelson CM, Khauv D, Bennett S, Radisky ES, Hirai Y, Bissell MJ, Radisky DC 2009. Homology with vesicle fusion mediator syntaxin-1a predicts determinants of epimorphin/syntaxin-2 function in mammary epithelial morphogenesis. J Biol Chem 284: 6877–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Lovett DH 2003. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 162: 1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH 2006. Matrix metalloproteinase 2 and basement membrane integrity: A unifying mechanism for progressive renal injury. FASEB J 20: 1898–1900 [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM 2002. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 295: 2387–2392 [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, Stack MS, Hudson LG 2008. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res 68: 4606–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. 2009. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci 106: 13820–13825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM 2001. Taking cell-matrix adhesions to the third dimension. Science 294: 1708–1712 [DOI] [PubMed] [Google Scholar]
- de Herreros AG, Peiro S, Nassour M, Savagner P 2010. Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J Mammary Gland Biol Neoplasia 15: 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Modugno F, DeMonte L, Balsamo M, Bronzi G, Nicotra MR, Alessio M, Jager E, Condeelis JS, Santoni A, Natali PG, et al. 2007. Molecular cloning of hMena (ENAH) and its splice variant hMena + 11a: Eidermal growth factor increases their expression and stimulates hMena + 11a phosphorylation in breast cancer cell lines. Cancer Res 67: 2657–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL 2006. Neural crest delamination and migration: Integrating regulations of cell interactions, locomotion, survival and fate. Adv Exp Med Biol 589: 45–77 [DOI] [PubMed] [Google Scholar]
- Duffield JS 2010. Epithelial to mesenchymal transition in injury of solid organs: Fact or artifact? Gastroenterology 139: 1081–1083 [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21: 137–148 [DOI] [PubMed] [Google Scholar]
- Elsharkawy AM, Mann DA 2007. Nuclear factor-κB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 46: 590–597 [DOI] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ 2004. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res 6: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiegen D, Haeusler LC, Blumenstein L, Herbrand U, Dvorsky R, Vetter IR, Ahmadian MR 2004. Alternative splicing of Rac1 generates Rac1b, a self-activating GTPase. J Biol Chem 279: 4743–4749 [DOI] [PubMed] [Google Scholar]
- Franco DL, Mainez J, Vega S, Sancho P, Murillo MM, de Frutos CA, Del Castillo G, Lopez-Blau C, Fabregat I, Nieto MA 2010. Snail1 suppresses TGF-β-induced apoptosis and is sufficient to trigger EMT in hepatocytes. J Cell Sci 123: 3467–3477 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G 2004. Socializing with the neighbors: Stem cells and their niche. Cell 116: 769–778 [DOI] [PubMed] [Google Scholar]
- Fuxe J, Vincent T, Garcia de Herreros A 2010. Transcriptional crosstalk between TGFβ and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 9: 2363–2374 [DOI] [PubMed] [Google Scholar]
- Gertler F, Condeelis J 2011. Metastasis: Tumor cells becoming MENAcing. Trends Cell Biol 21: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G 2003. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 14: 538–546 [DOI] [PubMed] [Google Scholar]
- Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ 1998. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol 140: 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Radisky D, Boudreau R, Simian M, Stevens ME, Oka Y, Takebe K, Niwa S, Bissell MJ 2001. Epimorphin mediates mammary luminal morphogenesis through control of C/EBPβ. J Cell Biol 153: 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollier BG, Evans K, Mani SA 2009. The epithelial-to-mesenchymal transition and cancer stem cells: A coalition against cancer therapies. J Mammary Gland Biol 14: 29–43 [DOI] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW 2007. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol 213: 374–383 [DOI] [PubMed] [Google Scholar]
- Illman SA, Lehti K, Keski-Oja J, Lohi J 2006. Epilysin (MMP-28) induces TGF-β mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci 119: 3856–3865 [DOI] [PubMed] [Google Scholar]
- Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M 2010. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 101: 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P, Brazao R, Boavida MG, Gespach C, Chastre E 1999. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene 18: 6835–6839 [DOI] [PubMed] [Google Scholar]
- Kakarala M, Wicha MS 2008. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 26: 2813–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmieciak M, Knutson KL, Dumur CI, Manjili MH 2007. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol 37: 675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, Gad EA, Smorlesi A, Disis ML 2006. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol 177: 1526–1533 [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H, Werb Z 2006. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn 235: 3404–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W, Kaissling B, Le Hir M 2011. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: Fact or fantasy? J Clin Invest 121: 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarge MA, Nelson CM, Villadsen R, Fridriksdottir A, Ruth JR, Stampfer MR, Petersen OW, Bissell MJ 2009. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr Biol 1: 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace R, Grimaud JA, Schurch W, Seemayer TA 1985. Myofibroblastic stromal reaction in carcinoma of the breast: Variations of collagenous matrix and structural glycoproteins. Virchows Arch A Pathol Anat Histopathol 408: 49–59 [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648 [DOI] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ 1997a. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139: 1861–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ 1997b. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem 272: 5007–5015 [DOI] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Nieto MA 2009. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1: 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. 2008. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F 2008. Cancer-related inflammation. Nature 454: 436–444 [DOI] [PubMed] [Google Scholar]
- Massague J 2008. TGFβ in cancer. Cell 134: 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos P, Collard JG, Jordan P 2003. Tumor-related alternatively spliced Rac1b is not regulated by Rho-GDP dissociation inhibitors and exhibits selective downstream signaling. J Biol Chem 278: 50442–50448 [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A 2008. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 3: e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Khauv D, Bissell MJ, Radisky DC 2008. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem 105: 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA 2010. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 27: 347–376 [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M 2001. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114: 111–118 [DOI] [PubMed] [Google Scholar]
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA 2008. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68: 3645–3654 [DOI] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL 2005. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science 307: 1603–1609 [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nature Rev 8: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E 2006. Catenins: Keeping cells from getting their signals crossed. Dev Cell 11: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino MS, Balsamo M, Di Modugno F, Mottolese M, Alessio M, Melucci E, Milella M, McConkey DJ, Philippar U, Gertler FB, et al. 2008. Human Mena + 11a isoform serves as a marker of epithelial phenotype and sensitivity to epidermal growth factor receptor inhibition in human pancreatic cancer cell lines. Clin Cancer Res 14: 4943–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov Y, Schuppan D 2010. Epithelial-to-mesenchymal transition in liver fibrosis: Dead or alive? Gastroenterology 139: 722–725 [DOI] [PubMed] [Google Scholar]
- Przybylo JA, Radisky DC 2007. Matrix metalloproteinase-induced epithelial-mesenchymal transition: Tumor progression at Snail’s pace. Int J Biochem Cell Biol 39: 1082–1088 [DOI] [PubMed] [Google Scholar]
- Radisky DC 2005. Epithelial-mesenchymal transition. J Cell Sci 118: 4325–4326 [DOI] [PubMed] [Google Scholar]
- Radisky ES, Radisky DC 2010. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia 15: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Hirai Y, Bissell MJ 2003. Delivering the message: Epimorphin and mammary epithelial morphogenesis. Trends Cell Biol 13: 426–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Stallings-Mann M, Hirai Y, Bissell MJ 2009. Single proteins might have dual but related functions in intracellular and extracellular microenvironments. Nat Rev 10: 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman JM, Kmieciak M, Manjili MH, Knutson KL 2007. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol 17: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman JM, Knutson KL, Radisky DC 2010. Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res 70: 3005–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RG, Li XY, Hu Y, Saunders TL, Virtanen I, Garcia de Herreros A, Becker KF, Ingvarsen S, Engelholm LH, Bommer GT, et al. 2009. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J Cell Biol 184: 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan RB, Heimark RL, Camenisch TD, Klewer SE 2005. Epithelial-mesenchymal transformation in the embryonic heart. In Rise and fall of epithelial phenotype (ed. Savagner P), pp. 40–55 Springer, New York [Google Scholar]
- Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA 2009. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 9: 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, et al. 2009. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 69: 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G 1988. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 41: 707–712 [DOI] [PubMed] [Google Scholar]
- Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E 2000. Rac1 in human breast cancer: Overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19: 3013–3020 [DOI] [PubMed] [Google Scholar]
- Scotton CJ, Chambers RC 2007. Molecular targets in pulmonary fibrosis: The myofibroblast in focus. Chest 132: 1311–1321 [DOI] [PubMed] [Google Scholar]
- Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, Miyazono K, Saitoh M 2011. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J 30: 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook D, Keller R 2003. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev 120: 1351–1383 [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Stoker AW, Bissell MJ 1989. Evaluation of the cocarcinogenic effect of wounding in Rous sarcoma virus tumorigenesis. Cancer Res 49: 6419–6424 [PubMed] [Google Scholar]
- Sieweke MH, Thompson NL, Sporn MB, Bissell MJ 1990. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-β. Science 248: 1656–1660 [DOI] [PubMed] [Google Scholar]
- Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ 2004. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene 23: 9369–9380 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z 2001. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z 1999. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98: 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Bissell MJ, Werb Z 2000. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 19: 1102–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z 2006. Hormonal and local control of mammary branching morphogenesis. Differentiation 74: 365–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z 1994. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol 125: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Bissell MJ, Werb Z 1995. Mammary gland tumor formation in transgenic mice overexpressing stromelysin-1. Semin Cancer Biol 6: 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TT, Coussens LM 2007. Humoral immunity, inflammation and cancer. Curr Opin Immunol 19: 209–216 [DOI] [PubMed] [Google Scholar]
- Tan TK, Zheng G, Hsu TT, Wang Y, Lee VW, Tian X, Wang Y, Cao Q, Wang Y, Harris DC 2010. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol 176: 1256–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavanez JP, Valcarcel J 2010. A splicing mastermind for EMT. EMBO J 29: 3217–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ 2004. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA 2009. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev 3: 349–363 [DOI] [PubMed] [Google Scholar]
- Urbanski S, Jensen HM, Cooke G, McFarlane D, Shannon P, Kruikov V, Boyd NF 1988. The association of histological and radiological indicators of breast cancer risk. Br J Cancer 58: 474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YA, Shen K, Wang Y, Brooks SC 2005. Retinoic acid signaling is required for proper morphogenesis of mammary gland. Dev Dyn 234: 892–899 [DOI] [PubMed] [Google Scholar]
- Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y, Carstens RP 2010. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J 29: 3286–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings SR, Wolfe JN 1978. Correlative studies of the histological and radiographic appearance of the breast parenchyma. Radiology 129: 299–306 [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Pino G 2007. Matrix metalloproteinases as mediators of primary and secondary cataracts. Expert Rev Ophthalmol 2: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z 2002. Stromal effects on mammary gland development and breast cancer. Science 296: 1046–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z 2003. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol 162: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian W, Schwertfeger KL, Vargo-Gogola T, Rosen JM 2005. Pleiotropic effects of FGFR1 on cell proliferation, survival, and migration in a 3D mammary epithelial cell model. J Cell Biol 171: 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA 2008. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell 14: 818–829 [DOI] [PubMed] [Google Scholar]
- Yang L, Pang Y, Moses HL 2010. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31: 220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Duffield JS 2010. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol 21: 1247–1253 [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Kalluri R 2004. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181 [DOI] [PubMed] [Google Scholar]
- Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, Succar L, Rangan GK, Hu M, Henderson BR, et al. 2009. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-β1 in renal tubular epithelial cells. Am J Pathol 175: 580–591 [DOI] [PMC free article] [PubMed] [Google Scholar]