Congenital LQTS comprises a distinct group of cardiac channelopathies characterized by delayed repolarization of the myocardium, QT prolongation, and increased risk for syncope, seizures, and sudden cardiac death in the setting of a structurally normal heart and otherwise healthy individual. From a beat-to-beat perspective, this repolarization abnormality almost always is without consequence. However, rarely, when caught off guard by triggers such as exertion, swimming, emotion, auditory stimuli such as an alarm clock, or during the postpartum period, the heart can spiral electrically out of control into a potentially life threatening and sometimes lethal dysrhythmia of torsade de pointes.
The prevalence of LQTS may be as high as 1 in 2500 persons, with approximately 75% of clinically robust LQTS due to either “loss-of-function”- or “gain-of-function”-producing mutations in 3 genes: KCNQ1-encoded IKs potassium channel(1) (LQT1, loss-of-function), KCNH2-encoded IKr potassium channel(2) (LQT2, loss-of-function), and SCN5A-encoded INa sodium channel(3) (LQT3, gain-of-function) that are critically responsible for the orchestration of the cardiac action potential. Since the first discoveries of mutations in these three critical ion-channel alpha sub-units, there are now a total of 12 LQTS-susceptibility genes. However, LQT4-12 accounts for less than 5% of LQTS cases. Nonetheless, the 4 newest additions to the LQTS-susceptibility gene family have taught us to stay close to the ion channel alpha subunits that underlie the three most common subtypes of LQTS. Three of the four genes: CAV3 (LQT9), SCN4B (LQT10), and SNTA1 (LQT12) encode proteins that function in part as sodium channel interacting proteins (ChIPs). When co-expressed with an otherwise intact sodium channel alpha subunit, mutant caveolin-3, mutant sodium channel beta 4 subunit, or mutant alpha syntrophin convert the NaV1.5 sodium channel complex into a late sodium current generating one(4)(5)(6). Similarly, mutations in AKAP9 disrupts the IKs ChIP, yotiao, causing an LQT1-like loss-of-function(7). Although it appears that no new LQTS gene is going to capture a sizable piece of the remnant of genotype negative LQTS, these discoveries remind us that the “Towbinesque” Final Common Pathway strategy is working and that staying close to the 3 most common LQTS-susceptibility genes and their encoded signalling pathway is worthwhile(8).
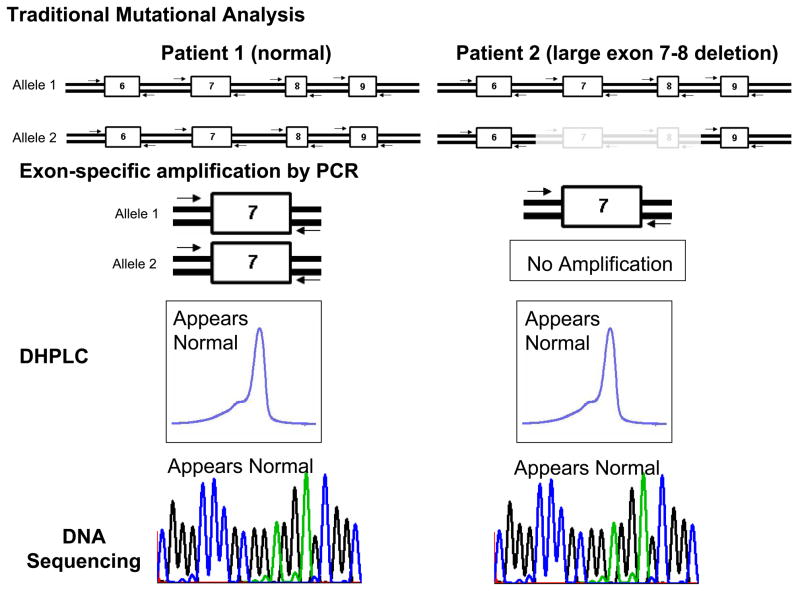
Now, Eddy and colleagues remind us that the street lamp still shineth over the two most common LQTS-susceptibility genes themselves with novel LQTS-causing mutations that escaped detection by traditional strategies being caught instead by multiplex ligation-dependent probe amplification (MLPA)(9). Until recently, traditional mutational analysis relied upon the techniques of exon-specific polymerase chain reaction (PCR), an intermediate mutation analysis platform such as single stranded conformation polymorphism (SSCP) or denaturing high performance liquid chromatography (DHPLC), and subsequent direct DNA sequencing to elucidate the genetic underpinnings of this disorder. These techniques work with great precision in detecting i) single nucleotide substitutions that produce missense, nonsense, and splice site mutations and ii) small insertion/deletions. However, large whole gene, multiple exon, or single exon deletions or duplications elude detection by this approach (Figure 1a).
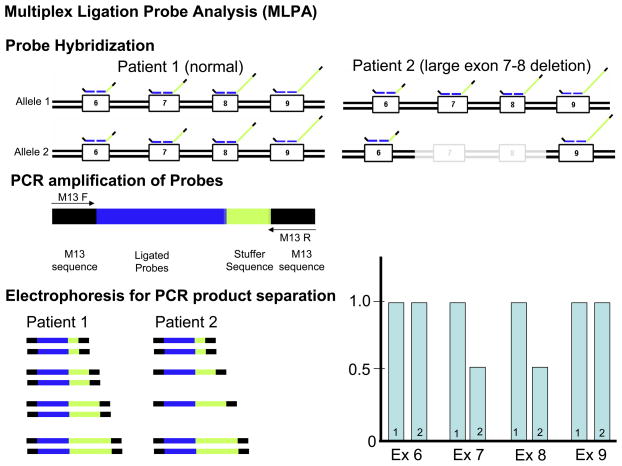
Figure 1. Traditional Mutational Analysis by DHPLC versus MLPA Detection for Large Insertion or Deletion Mutations.
Depicted is A) a traditional mutational analysis by DHPLC where exon specific PCR amplification of both the maternal (1) and paternal (2) alleles is achieved using specific primers designed to anneal to the exon flanking intronic sequences. In the case of normal patient (patient 1), PCR products representing both alleles will be produced. However, in patient 2 who hosts a large deletion, PCR primers will only anneal to the normal strand. In either patient, the resulting DHPLC and subsequent sequence will only be representative of the normal strand and the allele with the deletion will go unnoticed. B) Illustrates how multiplex ligation probe analysis (MLPA) can be used to detect such deletions or insertions by quantifying the copy number of target sequences (exons) as determined by specifically designed probes. Here, specific probes of varying length (as a result of the variable length “stuffer” sequence-shown in yellow) are first hybridized to each patient’s gene sequence. The probes are stripped from the genomic DNA and subsequently serve as template for PCR amplification using primers that specifically anneals to all of the probes allowing them to be simultaneously amplified in a single multiplex PCR reaction. Because of the varying length of the “stuffer” sequence, the amplified probes can be separated according to size by electrophoresis and viewed on a chromatogram. The amount of probe hybridizing to the patient’s genomic DNA determines the copy number for the specific targeted sequence (exons). In this example where exon 7 and 8 are deleted, less exon 7 and 8 specific probes have been hybridized and subsequently fewer copy numbers of template are detected. Conversely, a large insertion would produce more copy numbers of the target sequence(s).
In contrast to the traditional approach, MLPA relies on specifically engineered probes that are designed to bind to gene sequences (typically exonic sequences) and allows for the detection of copy number changes of the target sequence. Large deletion mutations for example, will result in a loss of copy number of the target (exon) while insertions are represented as an increase in copy number (Figure 1b). In 2006, Koopmann and colleagues provided the sentinel report for any cardiovascular disease of MLPA-exposed mutations demonstrating that large gene rearrangements in the LQT2-susceptibility gene KCNH2 may serve as a pathogenic basis for cases of seemingly genotype negative LQTS(10). The authors analyzed 21 patients that remained genotype negative following a coding (exonic) and splice site region mutational analysis of commonly involved LQTS genes, and found that 1 of the 21 (~5%) patients had a tandem duplication of 3,682 bp (involving 2.5 kb of intron 5, all of exon 6, and 121 bp of exon 7) in KCNH2 (LQT2). This large gene rearrangement mutation in KCNH2 co-segregated properly in this Dutch family with multiple effected individuals, including a 26-year-old female who experienced sudden unexpected nocturnal death. Consistent with LQT2 genotype, many of the cardiac events were auditory triggered. Most recently, MLPA was used to detect a 5.27 Mb deletion of chromosome 7 (7q36.1–q36.2) encompassing a whole gene deletion of KCNH2 in a 9-year-old with significant QT prolongation (QTc of 490 msec), renal hypoplasia, and mental retardation(11). In addition, using MLPA, Bhuiyan et al, identified a 1.1 kb deletion causing an in-frame deletion of 35 amino acids (deletion of exon 3) of the RYR2-encoded cardiac ryanodine receptor in a family with catecholaminergic polymorphic ventricular tachycardia (CPVT), another potentially lethal cardiac channelopathic disorder(12).
In this edition of Heart Rhythm, Eddy and colleagues used MLPA to identify two large multiple-exon deletions in KCNQ1 (deletion of exon 13–14) and KCNH2 (deletion of exons 6–14), and a large intragenic duplication in KCNH2 (duplication of exons 9–14) in 3 of 26 (11.5%) patients with a clinically robust diagnosis of LQTS. All patients were negative for traditional missense, nonsense, or small insertion/deletion coding region mutations of the three most common LQTS genes (KCNQ1, KCNH2, and SCN5A)(9). So far, the 3 large gene rearrangements of KCNH2 have been identified families with a remarkable history of autopsy negative sudden unexpected death in the young, totaling 6 cases between the three families. Perhaps, large gene rearrangements in KCNH2 confer a more “malignant” substrate, and this may have implications for postmortem genetic testing by MLPA in cases of autopsy negative sudden unexplained death (SUD) where traditional LQTS- and CPVT-associated mutations have been detected in approximately one-third of SUD cases(13)(14). Although the 6 sudden deaths involved adolescents and young adults, it is conceivable that such large gene rearrangements in these cardiac channel genes could provide a mechanism of channelopathic sudden infant death syndrome as well(15)(16). While most of the sudden deaths were inferred to be positive for each family’s respective mutation, Eddy and colleagues were able to perform a confirmatory “molecular autopsy” for the KCNH2 exon 6–14 deletion on one of the decedents using DNA isolated from her newborn screening Guthrie card. Expectantly, no large gene rearrangements have been reported in SCN5A from analysis of these LQTS cohorts as such a mutation would result almost certainly in “loss-of-function”. Perhaps, MLPA of SCN5A will catch additional Brugada syndrome mutations.
In terms of LQTS genetic testing, it will be interesting to see if MLPA continues to provide the answer for 5–10% of cases with an otherwise negative genetic test. If so, then additional surveillance of KCNQ1 and KCNH2 by MLPA might be expected to increase the maximal anticipated yield of the current clinically available genetic test by 1 – 3%. Although small in magnitude, it should be noted that this incremental increase is similar to that provided by analysis of the entire open reading frames for the nine genes responsible for LQT4–12.
Still, approximately 20% of LQTS remains genetically elusive. While targeting candidate genes that encode NaV1.5 ChIPs have yielded fruit recently, this present study reminds us that a renewed exploration of the two most common LQTS genes may be similarly fruitful. Besides traditional mutations involving the translated exons/canonical splice sites and now larger gene rearrangements, what other pathogenic mechanisms (promoter perturbations, intronic insults, and synonymous snares) within KCNQ1 and KCNH2 await discovery? For example, synonymous or “silent” (non-amino acid altering) single nucleotide substitutions residing within the exons could conceivably disrupt splice enhancer element sequences precipitating errant RNA splicing and subsequent exon skipping or intron inclusion. Such a mechanism has been reported for diseases like Marfan syndrome, familial adenomatous polyposis, and McArdle disease already(17)(18)(19). Although not directly illuminated by the street lamp, the shadows in its vicinity sure seem like a good place to cast more light.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Splawski I, Shen J, Timothy KW, et al. Genomic structure of three long QT syndrome genes: KVLQT1, HERG, and KCNE1. Genomics. 1998;51:86–97. doi: 10.1006/geno.1998.5361. [DOI] [PubMed] [Google Scholar]
- 2.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Shen J, Splawski I, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–11. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 4.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros-Domingo A, Kaku T, Tester DJ, et al. SCN4B-encoded sodium channel beta 4 subunit in congenital long-QT syndrome. Circulation. 2007 Jul 10;116(2):134–42. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda K, Valdivia CR, Medeiros-Domingo A, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proceedings of the National Academy of Sciences. 2008 doi: 10.1073/pnas.0801294105. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Marquardt ML, Tester DJ, et al. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20990–5. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles NE, Bowles KR, Towbin JA. The "final common pathway" hypothesis and inherited cardiovascular disease. The role of cytoskeletal proteins in dilated cardiomyopathy. Herz. 2000;25:168–75. doi: 10.1007/s000590050003. [DOI] [PubMed] [Google Scholar]
- 9.Eddy C-A, MacCormick JM, Chung S-K, et al. Identification of large gene deletions and duplications in KCNQ1 and KCNH2 in patients with long QT syndrome. Heart Rhythm. 2008 doi: 10.1016/j.hrthm.2008.05.033. In Press. Accepted Manuscript. [DOI] [PubMed] [Google Scholar]
- 10.Koopmann TT, Alders M, Jongbloed RJ, et al. Long QT syndrome caused by a large duplication in the KCNH2 (HERG) gene undetectable by current polymerase chain reaction-based exon-scanning methodologies. Heart Rhythm. 2006;3:52–55. doi: 10.1016/j.hrthm.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Caselli R, Mencarelli MA, Papa FT, et al. Delineation of the phenotype associated with 7q36.1q36.2 deletion: long QT syndrome, renal hypoplasia and mental retardation. American Journal of Medical Genetics Part A. 2008;146:1195–9. doi: 10.1002/ajmg.a.32197. [DOI] [PubMed] [Google Scholar]
- 12.Bhuiyan ZA, van den Berg MP, van Tintelen JP, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–76. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 13.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young.[see comment] Journal of the American College of Cardiology. 2007;49:240–6. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Tester DJ, Spoon DB, Valdivia HH, et al. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner's cases. Mayo Clin Proc. 2004;79:1380–84. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman MJ, Siu BL, Sturner WQ, et al. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. Jama. 2001;286:2264–9. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 16.Arnestad M, Crotti L, Rognum TO, et al. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–67. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Qian C, Francke U. Silent mutation induces exon skipping of fibrillin-1 gene in Marfan syndrome. Nature Genetics. 1997;16:328–9. doi: 10.1038/ng0897-328. [DOI] [PubMed] [Google Scholar]
- 18.Montera M, Piaggio F, Marchese C, et al. A silent mutation in exon 14 of the APC gene is associated with exon skipping in a FAP family. Journal of Medical Genetics. 2001;38:863–7. doi: 10.1136/jmg.38.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Cadenas I, Andreu AL, Gamez J, et al. Splicing mosaic of the myophosphorylase gene due to a silent mutation in McArdle disease.[see comment] Neurology. 2003;61:1432–4. doi: 10.1212/wnl.61.10.1432. [DOI] [PubMed] [Google Scholar]