Abstract
The HIV Prevention Trials Network (HPTN) is supported by the NIH to conduct randomized clinical trials to assess the efficacy of HIV prevention strategies and technologies to reduce HIV transmission between adults. A special focus of attention is on the use of antiretroviral drugs to prevent HIV transmission, both by reducing infectiousness among HIV-infected persons taking combination antiretroviral therapy (cART) and also by reducing susceptibility among HIV-uninfected persons taking antiretrovirals for pre-exposure prophylaxis. Studies may be developmental in nature to assess novel ideas for interventions or for assessing trial feasibility. However, pivotal efficacy trials to test HIV-specific prevention strategies and technologies are the main HPTN priority. Examples include a major protocol investigating the impact of expanded testing and linkage to care on HIV surveillance indicators in the USA (HPTN 065). Another protocol is addressing similar issues while also investigating how combinations of prevention approaches are best deployed to make a community-level impact in southern Africa (HPTN 071). HPTN 068 is evaluating a novel conditional cash transfer structural intervention to increase school completion rates in young girls and thereby reduce their HIV risk. Studies outside the US address the epidemic in most at-risk populations and include an assessment of opiate agonist therapy to reduce risk of HIV seroconversion among injection drug users (HTPN 058), methods to increase HIV testing rates (HTPN 043), as well as methods for reducing high-risk behaviors, and increasing adherence to cART in HIV-infected individuals (HPTN 062 and HPTN 063, respectively). The recent HPTN 052 study demonstrated that a 96% reduction in HIV transmission could be achieved between serodiscordant sexual partners by providing the infected partners with cART at a CD4+ cell count (350–550/µl) above the level that would usually qualify them for therapy in low- and middle-income countries. The immediate relevance to public health policy showcased in these trials is a paradigm for the HPTN: design and conduct of clinical trials using available licensed tools that can be rapidly translated for implementation (‘Prevention NOW!’).
Keywords: developing country, HIV/AIDS, prevention, randomized clinical trial, research collaboration, research infrastructure
The HIV Prevention Trials Network (HPTN) is a partnership between scientists and communities around the world to develop, evaluate and implement biomedical, behavioral and structural interventions that can be used immediately to reduce the transmission of HIV. The HPTN is primarily funded by the National Institute of Allergy and Infectious Diseases, with co-funding from the National Institute of Mental Health and the National Institute for Drug Abuse. The network performs a combination of observational and randomized controlled clinical trials, designed and conducted according to the highest scientific and ethical standards, to identify the best combinations of interventions for the populations at highest risk of HIV infection worldwide [101]. As of mid-2011, 12 clinical research sites (CRS) are participating in nine active trials; this includes 79 HIV testing, care and/or CRS in the USA and 31 CRS in 11 other countries in sub-Saharan Africa, Asia and South America. Through this work, major scientific discoveries have helped shape the field of HIV-prevention research both internationally and in the USA.
The roots of the HPTN are found in the establishment of the HIV Network for Prevention Trials (HIVNET) in 1993, sponsored by the Division of AIDS of the National Institute of Allergy and Infectious Diseases. HIVNET international site infrastructure was developed with a master contract from 1993 to 1999 held by Family Health International (renamed FHI 360), and domestic sites were nurtured similarly through Abt Associates. The international and domestic HIVNETs were designed as multicenter, multidisciplinary collaborative research networks for HIV-prevention efficacy trials, including vaccines, microbicides and mother-to-child transmission. As research needs expanded, HIVNET evolved into two separate networks in 1999: HPTN and the HIV Vaccine Trials Network. In 2006, there was another network evolution such that trials that originated in the HPTN became key components of the new Microbicides Trials Network (MTN), and new International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT). Collectively, the prevention-oriented networks (HPTN, HVTN, MTN and IMPAACT) evaluate the safety and effectiveness of promising interventions to prevent the transmission of HIV between sexual and/or needle-sharing partners, or from mother-to-baby during pregnancy, at birth and during breastfeeding. The key priority for all the prevention-focused networks is to assess the impact of prevention interventions on HIV incidence.
HPTN mission & rationale
The mission of the HPTN is to discover and develop interventions that can be used to prevent sexual and/or parenteral transmission of HIV around the world. This includes both development of new prevention technologies and the implementation of known efficacious interventions in real-world settings to determine study cost, coverage and population effectiveness.
The number of people living with HIV worldwide continues to grow, with an estimated 33 million people living with HIV in 2009 [102]. Three decades after HIV was discovered, the epidemic appears to have stabilized and has begun to show signs of decline in many parts of the world. In 33 countries, HIV incidence fell by more than 25% between 2001 and 2009 [102]. A total of 22 of these countries are in sub-Saharan Africa, where the epidemic has been fueled by sexual transmission [102]. The etiology of this decline is unknown; the impact of programs for HIV prevention and treatment may have contributed, but high death rates and subsequent declines in the infectious populations may also have been contributing factors to the drop in HIV prevalence. However, several regions and countries do not fit this declining trend. In Eastern Europe and Central Asia, HIV incidence increased by more than 25% between 2001 and 2009, highlighting the importance of the emerging HIV epidemic in these regions, which has been fueled primarily through injection drug users (IDUs) [1,102]. Furthermore, even after declines in incidence, many countries in sub-Saharan Africa still have exceedingly high HIV incidence rates, often greater than 1% per annum among sexually active adolescents and young adults [2]. While expanded availability of combination antiretroviral therapy (cART) has reduced morbidity and mortality, there is a long way to go before the clinical, social and public health impact are substantially diminished with a reduction in the numbers of new infections. Reduction in HIV incidence has been achieved in some parts of the world; it is promising that prevention research and implementation of new interventions will have an impact on the global pandemic, and may change its course altogether [3–13].
A striking characteristic of heterosexual HIV transmission risk is the disproportionate burden of HIV infection in women compared with men in the global epicenter of sub-Saharan Africa [14,102]. In countries where heterosexual transmission is dominant, there are often more than twice as many infections in young women compared with young men. In these settings, women acquire HIV infection on average of 5 to 10 years earlier than men, attributable to their comparatively older sexual partners [15,102]. Prevention interventions that are targeted to young women (and, when possible, their partners) will not only benefit adolescents and young adult women at high risk of HIV infection, but may also reduce transmission to their sexual partners and infants [15,16].
In the USA and in other countries, HIV incidence is increasing in some populations, such as men who have sex with men (MSM) [102]. The HPTN has focused its domestic prevention research agenda on the design and implementation of studies that evaluate the feasibility of multicomponent strategies for HIV prevention, particularly in MSM and vulnerable women [17–21].
HPTN: an international HIV research network
HPTN began as a cooperative agreement with NIH in 1999, and was renewed in 2006 as a 7-year award. The later award was given to an HPTN leadership group that focused on nonvaccine, non-microbicide, nonperinatal prevention interventions, those issues were addressed by the HVTN, the MTN and the IMPAACT networks, respectively. Rather, the HPTN has focused on cART for prevention, pre-exposure prophylaxis (PrEP), behavioral interventions, combination approaches and interventions among IDUs. The HIVNET and HPTN have led HIV prevention research efforts since 1993, with an anticipated renewal opportunity for 2013.
Notable HPTN achievements include the enhancement of clinical trial capabilities in nations with high burdens of HIV infection, including some of the world’s lowest-income nations (Table 1).
Table 1.
National Institute of Allergy and Infectious Diseases-supported clinical trials sites that are affiliated with the HIV Prevention Trials Network in 2011.
| Africa | Asia and South America | North America |
|---|---|---|
| Gaborone prevention/treatment trials CRS, Gaborone, Botswana | Instituto de Pesquisa Clinica Evandro Chagas CRS, Rio de Janeiro, RJ, Brazil | Bronx-Lebanon Hospital Center CRS, Bronx, NY, USA |
| KEMRI/CDC CRS, Kisumu, Kenya | Hospital Nossa Senhora da Conceicao CRS, Porto Alegre, RS, Brazil | The Fenway Institute CRS, Boston, MA, USA |
| University of North Carolina Lilongwe CRS, Malawi | Hospital Geral de Nova Iguaçu CRS, Nova Iguacu, RJ, Brazil | George Washington University CRS, Washington, DC, USA |
| College of Medicine JHU CRS, Blantyre, Malawi | Guangxi Centers for Disease Control and Prevention and for HIV/AIDS Prevention and Control CRS, Nanning, China | Harlem Prevention Center CRS, NY, USA |
| Wits HIV CRS, Johannesburg, Gauteng, South Africa | Heng County Center for Disease Control and Prevention CRS, Hengzhou, Guangxi, China | Hope Clinic of the Emory Vaccine Center CRS, Decatur, GA, USA |
| Emavundleni Desmond Tutu HIV Centre CRS, Cape Town, Western Cape, South Africa | Xinjiang CRS, Urumqi, China | Johns Hopkins Adult AIDS CRS, Baltimore, MD, USA |
| Soweto HPTN CRS, Johannesburg, Gauteng South Africa | NARI Pune CRS, Pune, Maharashtra, India | New Jersey Medical School CRS, Newark, NJ, USA |
| MRC/Wits Rural Public Health and Health Transitions Unit CRS, Bushbuckridge, Mpumalanga, South Africa | NARI Clinic at Gadikhana Dr. Kotnis Municipal Dispensary CRS, Pune, Maharashtra, India | NY Blood Center/Union Square CRS, NY, USA |
| Nyanga CRS, Cape Town, Western Cape South Africa | NARI Clinic at NIV CRS, Pune, Maharashtra, India | Ponce de Leon Center CRS, Atlanta, GA, USA |
| Makerere University-JHU Research Collaboration CRS, Kampala, Uganda | YRG CARE Medical Center, VHS Chennai CRS, Taramani, India | San Francisco Vaccine and Prevention CRS, San Francisco, CA, USA UCLA Vine Street CRS, Los Angeles, CA, USA |
| George Clinic CRS, Lusaka, Zambia | San Miguel CRS, Lima, Peru | UNC AIDS CRS, Chapel Hill, NC, USA |
| Kamwala Clinic CRS, Lusaka, Zambia | Asociacion Civil Selva Amazonica, CRS, Iquitos, Maynas, Peru | Wake County Health and Human Services CRS, Raleigh, NC, USA |
| Matero Reference Clinic CRS, Lusaka, Zambia | Chiang Mai University AIDS Prevention CRS, Thailand | |
| UZ-Parirenyatwa CRS, Harare, Zimbabwe | Silom Community Clinic CRS, Bangkok, Ratchathewi, Thailand |
CRS: Clinical research site; HPTN: HIV Prevention Trials Network; JHU: Johns Hopkins University.
Clinical and community research infrastructure development has included laboratory and data management, human resources, community advisory groups and field outreach. Strengthening the pool of local scientists in non-US countries has been accomplished through a strong partnership with the NIH Fogarty International Center’s AIDS International Training and Research Program. Training of clinical trial research staff, especially nurses from resource-constrained settings, has raised the level of quality science to meet International Committee for Harmonization Guidelines and Good Clinical Practice standards. In partnership with universities, ministries of health and health departments worldwide, the HPTN has supported its affiliated investigators who now have extensive clinical trials experience, local nursing and pharmacy management, proficient laboratories, community outreach and experience in data management systems. Much of this infrastructure has been built in lower-income nations (e.g., Malawi, Tanzania, Uganda and Zambia), in rural areas (China, Peru, Thailand and Zimbabwe), and in countries with hard-to-reach, impoverished subpopulations (Brazil, India, Russia, South Africa and the USA).
HPTN research agenda
The HPTN seeks to identify and test practical, safe and potentially effective approaches to prevent acquisition and/or spread of HIV either sexually or through IDU. The research agenda integrates biomedical and technological advances, such as use of antiretroviral drugs for HIV prevention and treatment for drug addiction, as well as behavioral and structural interventions that are focused on individuals or communities. These interventions may be designed to prevent infection or to reduce infectiousness of persons with acute, early or established HIV infection. Intervention trials are designed in both culturally appropriate and contextually relevant ways, ensuring the applicability of the results to public health practice.
HIV prevention is possible through:
-
▪
Avoiding or reducing exposure to HIV;
-
▪
Blocking HIV from entry once exposure has occurred;
-
▪
Reducing the viral load in an infectious person [102].
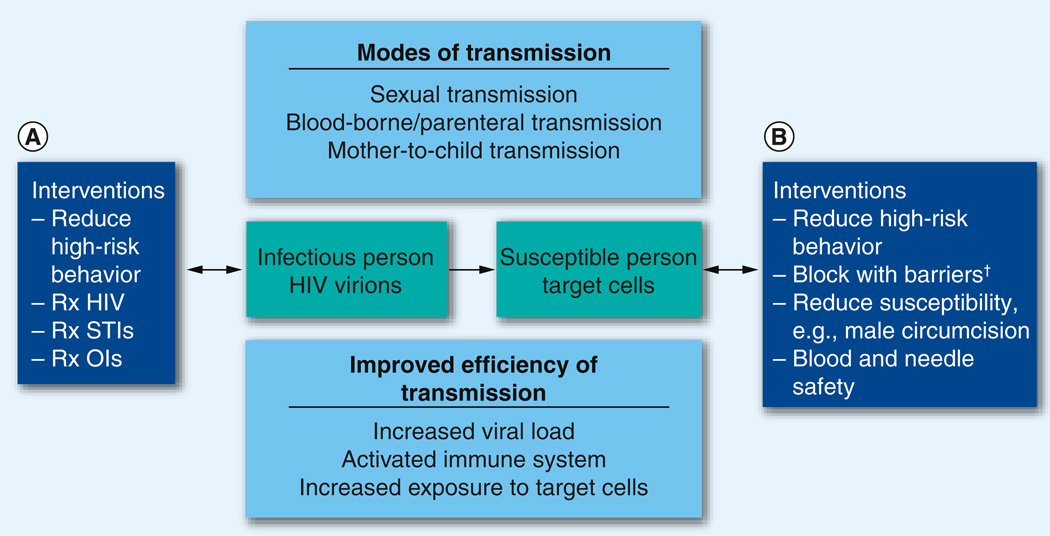
The HPTN scientific agenda focuses on all three of these approaches (Figure 1).
Figure 1. Conceptual model for HIV prevention.
An HIV-infected person can (A) become less infectious or (B) the HIV can be blocked from entering a susceptible individual. We test strategies marked ‘Interventions.’
†Barriers: physical, for example, condoms; chemical, for example, antiretroviral prophylaxis; or immunological, for example, HIV vaccines.
OI: Opportunistic infection; Rx: Treatment of; STI: Sexually transmitted infection.
HPTN studies are designed to evaluate interventions in all populations at risk of HIV infection though sexual or parenteral routes, including heterosexual discordant couples, adolescent girls, MSM, vulnerable women and IDU. A flagship study, HPTN 052, enrolled 1763 sero-discordant couples (i.e., one partner was HIV-infected and the other was HIV-uninfected) in nine countries (Botswana, Brazil, India, Kenya, Malawi, South Africa, Thailand, USA and Zimbabwe) [22]. Most (54%) participants were from Africa, which contributed disproportionately to the infection events. HIV-1-infected participants with CD4+ cell counts from 350–550 cells/µl were randomly assigned to receive cART either immediately (early therapy) or when CD4+ cell count declined below 250 cells/µl or at the onset of HIV-1-related symptoms (delayed therapy). The primary prevention end point was linked HIV-1 transmission in HIV-1-negative partners. This end point was designed to test the hypothesis that cART prevents HIV transmission to sexual partners. The primary clinical end point was the earliest occurrence of pulmonary tuberculosis, severe bacterial infection, a WHO stage 4 event or death, testing the hypothesis that earlier therapy would provide substantial benefit to the infected partner’s health. Among the 39 HIV-1 transmissions observed, 28 were linked to the infected partner by molecular analysis of HIV strains (incidence rate: 0.9/100 person-years; 95% CI: 0.6, 1.3). Of the 28 linked transmissions, only one occurred in the early therapy group (hazard ratio: 0.04; 95% CI: 0.01, 0.27; p < 0.001). In addition, the HIV-infected persons receiving early therapy had fewer treatment end points (hazard ratio: 0.59; 95% CI: 0.40, 0.88; p = 0.01), especially non-pulmonary tuberculosis. This 96% prevention efficacy represents the most efficacious intervention yet discovered in the HIV prevention field. The finding that early initiation of cART is an effective tool for HIV prevention provides a strong indication that global mobilization to increase testing and linkage to early cART could be an efficacious HIV-prevention strategy.
The treatment of substance abuse has been linked to the reduction of HIV risk behaviors for more than a decade [23,102], but no studies have been able to directly evaluate whether this type of intervention can prevent HIV infection. HPTN 058 addresses this question with a large, randomized controlled trial to determine whether an opiate agonist drug, co-formulated with an antagonist that renders it less valuable as a ‘street drug’ (e.g., buprenorphine/naloxone), can be used to treat heroin addiction, reduce injection drug use, and prevent HIV infection and death among opiate injectors in China and Thailand. While buprenorphine is an opiate agonist and can reduce the craving for heroin, the naloxone antagonist is not absorbed in sublingual dosing, but can block agonist effects if illicitly diverted and injected. Hence, this combination is acceptable in some parts of the world where public security and policy authorities do not want opiate agonists to be used when they might be diverted to the illegal market. Results from this ongoing study will advance the scientific understanding of HIV prevention among IDUs with a product that may be useful in venues without ready access to methadone substitution therapy.
Individuals with acute or early HIV infection may be responsible for a large proportion of all HIV transmission [24]. A study in sub-Saharan Africa suggests transmission by individuals with recent infections could be up to 10- to 100-times more efficient than transmission by those with more established infections [25]. HPTN 062 is evaluating the acceptability and feasibility of using an enhanced one-on-one counseling intervention to reduce risk behaviors among individuals with acute HIV infection in Malawi. A second behaviorally focused study (HPTN 063) is testing the feasibility of a counseling intervention in chronically infected individuals in Brazil, Thailand and Zambia.
Approximately 75% of young people infected with HIV (15–24 years of age) in sub-Saharan Africa are girls, and most of them become infected during adolescence [15]. Therefore, evaluations of structural interventions in this group remain a priority. The goal of HPTN 068 is to evaluate the impact of a multilevel HIV prevention intervention to address structural and social factors contributing to young South African women’s risk of HIV infection. Specifically, the Phase III efficacy trial HPTN 068 will assess whether the use of financial incentives to keep adolescent girls in school in South Africa can decrease their risks of acquiring HIV infection.
In the USA, HPTN 064 (also known as the ISIS study) is estimating HIV incidence among women living in ten distinct geographic areas of the USA that have high rates of HIV prevalence and poverty. This study uses innovative approaches of ethnographic mapping and community engagement to identify the areas of highest risk of HIV acquisition. A study of use and acceptability of tenofovir 1% microbicide gel for topical PrEP in women in the USA is the subject of HPTN 072, which builds on results from a South African study, CAPRISA 004 [26]. HPTN 061 (also known as the BROTHERS study) is testing the feasibility and acceptability of a multicomponent community-wide intervention for black MSM. The test, Link to Care Plus study (HPTN 065) will assess the public health impact of expanded HIV testing, linkage to care strategies, and approaches to improve treatment adherence on key indicators of the USA HIV epidemic; this study is being conducted in Washington, DC, and in the Bronx borough of New York City.
Results from recent studies have identified two new tools that can be used as PrEP: a vaginal tenofovir gel that is administered proximate to coitus in heterosexual young women (CAPRISA 004) and a daily oral tenofovir/emtricitabine regimen used in MSM (iPrEx), heterosexual men and women, and serodiscordant couples [26,27]. For the majority of ‘at-risk’ populations, HIV risk-taking is unlikely to occur on a daily basis. Therefore, it is hypothesized that less frequent drug dosing (i.e., intermittent PrEP) will result in higher acceptability, decreased drug costs and lower risk of toxicity. However, any benefit from intermittent PrEP compared with daily PrEP could be mitigated by an increased risk of HIV infection if intermittent PrEP were to provide insufficient drug concentrations in relevant tissues. HPTN is conducting three trials to evaluate new dosing regimens and new drugs for PrEP. The data generated by HPTN 066 will be used to develop a comprehensive multicompartment pharmacokinetic model for intermittent pre-exposure antiretroviral chemoprophylaxis. The purpose of the study is to establish the dose-proportionality of tenofovir/emtricitabine (serum and intracellular forms) with daily to weekly dosing. The purpose of HPTN 067 (the ADAPT study) is to identify dosing regimens that foster healthy sexual practices and pill-taking behavior in people at high risk of HIV infection. A challenging issue related to the use of PrEP is to avoid development of antiretroviral drug resistance in individuals who have unrecognized seroconversion or recent infection with ART drug resistance. Ideally, drugs used for PrEP would not be used as a part of a first-line treatment regimen. Maraviroc is not used in early therapy and meets these (and other) criteria as a potential PrEP drug agent. The purpose of HPTN 069 is to assess the safety and tolerability of maraviroccontaining regimens for PrEP, and to characterize the relative pharmcokinetic properties, and identify potential resistance issues. One study nearing completion is HPTN 043 (also called Project ACCEPT), which is testing whether a huge increase in HIV testing will result in a drop in community HIV incidence; 48 communities have been randomized in Thailand, Tanzania, South Africa and Zimbabwe [28–33]. Building on our knowledge that testing can be radically expanded successfully in rural and urban Africa, HPTN will now study a matrix of interventions based on cART for prevention and medical male circumcision to reduce HIV transmission in a community-randomized trial in Zambia and South Africa (HPTN 071) [34].
Organizational structure of the HPTN
Partner institutions in the HPTN leadership group
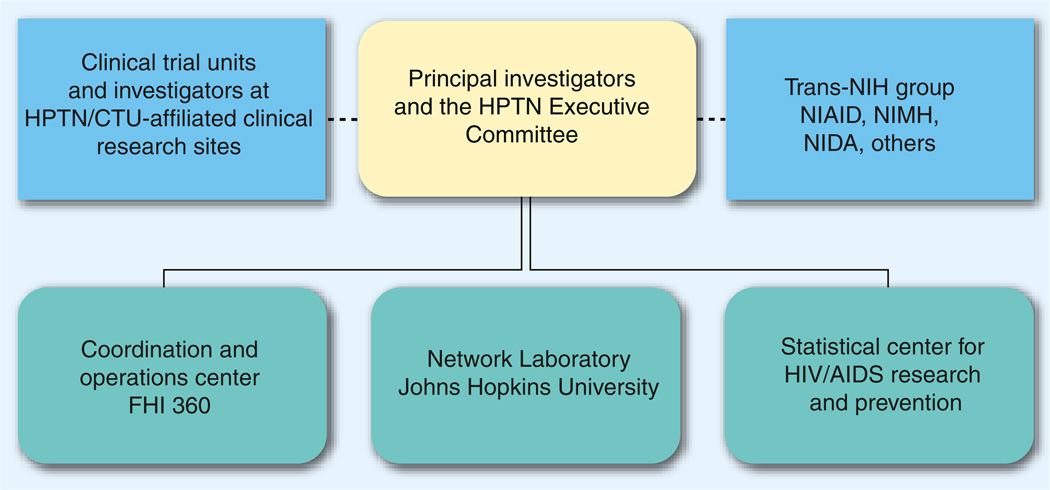
The HPTN Leadership Group consists of a partnership between FHI 360, which serves as a Coordinating and Operations center (CORE); Johns Hopkins University (JHU), which serves as the Network Laboratory (NL); and the Fred Hutchinson Cancer Research Center, which serves as the network’s Statistical Data Management Center (SDMC) (Figure 2). Other institutional partners include Vanderbilt University, TN, USA (primary affiliation for Vermund, Principal Investigator [PI] of HPTN and chair of the Executive Committee [EC]) and the Centre for the AIDS Programme of Research in South Africa (CAPRISA) in Durban, South Africa (primary affiliation for Abdool Karim, co-PI of HPTN). In 2012, leadership of the HPTN CORE and EC will rotate to Wafaa El-Sadr (Columbia University) and Myron Cohen (University of North Carolina).
Figure 2. Structure of HIV Prevention Trials Network leadership with operational components.
CTU: Clinical Trials Units; HPTN: HIV Prevention Trials Network.
Scientific committees & working groups
The HPTN leadership group governs the network with the help of scientific and operational committees, and scientific working groups (WG). The EC is chaired by the HPTN PI and includes members of the Trans-NIH Group. The EC sets the research priorities of the HPTN and directs its scientific activities. Through its members serving as liaisons to each scientific committee (SC) and WG, the EC assures that the specific areas of prevention science addressed by the individual SC/WG are effectively coordinated. SC/WGs include these areas of focus:
-
▪
MSM;
-
▪
Women-at-risk;
-
▪
Adolescents;
-
▪
Substance users;
-
▪
Community;
-
▪
Ethics;
-
▪
Biomedical tools;
-
▪
Combination approaches.
The EC includes investigators from the Clinical Trials Units (CTUs), CORE, SDMC, NL, NIH representatives, and chairs of scientific WG. A subset of the EC, the Prevention Management Group, is involved in the day-to-day operational oversight of all HPTN studies and helps troubleshoot problems that may arise.
The HPTN SCs and cross-cutting WGs serve as the network units for collaboration, communication, science development and trials management. Operations are organized to ensure good governance, streamlined science generation and review, and expeditious initiation and efficient conduct of protocols. Scientific priorities are kept current and timely, and studies are initiated and implemented in compliance with Division of AIDS policies. This includes state-of-the-art ethical and regulatory oversight and compliance.
Membership of all committees and groups reflect the diversity of the network, including representatives from central network operational components, CTUs and community representatives, as well as scientists and researchers. Members are chosen by soliciting nominations from the network members and from scientists in HIV-related fields external to the network. Criteria for selection include expertise in scientific areas that are of interest to the network, geographical diversity, and access to key populations.
Oversight committees
There are several key standing committees engaged in the review and oversight of HPTN activities. Two committees highlighted are the science review committee (SRC) and the study monitoring committee (SMC). The SRC is responsible for providing a thorough scientific, ethical and operational assessment of study concept plans and protocols. The SRC ensures that study protocols are statistically, operationally and ethically sound, as well as accurate, consistent, complete and, to the extent possible, standardized relative to other HPTN protocols. The SMC functions as an arm of the EC to provide a peer review of the conduct of all HPTN studies. Active HPTN studies are typically reviewed by the SMC within the first 4–6 months of study implementation and, thereafter, approximately every 6 months, including prior to data and safety monitoring board reviews. HTPN also uses a manuscript review committee to maximize the quality of work before submission to external peer review.
HPTN operational components
The HPTN components responsible for the operational aspects of the Network and funded through cooperative agreements with NIH are the CORE, SDMC, NL and the CTUs. The CORE is responsible for facilitating and managing the scientific agenda and research operations of the HPTN. The CORE staff are involved in the studies from concept to protocol development and in study conduct and publication of results. The CORE is also responsible for logistical and administrative support for the HPTN EC and other committees. FHI 360, an international research organization, with headquarters in Durham, North Carolina, is the CORE for the network.
The CORE’s scientific and operational responsibilities include:
-
▪
Leadership and governance;
-
▪
Scientific priority setting;
-
▪
Research management and support;
-
▪
Protocol development, review and pre-implementation activities;
-
▪
Assistance to CTUs during conduct of study;
-
▪
Community and research ethics programs;
-
▪
Communication and information dissemination [101];
-
▪
Financial management and support.
The SDMC is responsible for facilitating all aspects of study design, data collection, reporting and statistical analysis for HPTN trials. The SDMC manages the HPTN study databases and guides protocol teams on both the statistical components of study design and the collection and analysis of study data. The Statistical Center for HIV/AIDS Research and Prevention at the Fred Hutchinson Cancer Research Center in Seattle, Washington, USA, is the SDMC for the HPTN.
The SDMC’s specific responsibilities include:
-
▪
Member of network leadership;
-
▪
Statistical support and scientific leadership;
-
▪
Data management;
-
▪
Study monitoring reports;
-
▪
Central data repository (clinical and laboratory);
-
▪
Protocol operations;
-
▪
International IT support;
-
▪
Clinical data safety monitoring;
-
▪
Data assistance for specimen repository management.
The NL is responsible for overseeing collection, testing and reporting of results from biologic samples, assisting in the development and quality assessment of local laboratory capacity at CTUs, and identifying and implementing state-of-the-art assays and technologies to advance the scientific agenda of the Network. The HPTN NL is at the Johns Hopkins University School of Medicine.
The NL’s responsibilities include:
-
▪
Member of network leadership;
-
▪
Laboratory support to sites;
-
▪
Laboratory-related scientific leadership on protocols;
-
▪
Monitor local laboratory proficiency, with remediation assistance when needed.
Proposing new protocols
New scientific ideas are encouraged at all times in the HPTN. Concept capsules may be generated by site investigators or any external investigators collaborating with the HPTN. Ideas that address the mission and focus of the HPTN research agenda are summarized in a 3–5 page capsule that briefly describes the background/rationale, target study population and possible HIV prevention intervention(s), and should also include an estimated budget. Capsules are reviewed by three reviewers designated by the EC Chair, one of whom represents the NIH. Upon capsule approval, the incipient protocol team is formed and a detailed concept (10–15 pages) is developed. Upon WG and EC approval, and after funding planning with the NIH, protocol development is authorized.
Protocol teams & site selection committees
Protocol teams assume primary responsibility for scientific leadership in the development, implementation and day-to-day oversight of HPTN studies and dissemination of their results. The Protocol Chair provides scientific leadership during the development, implementation and reporting of the study and assumes responsibility for the projected protocol timeline and budget. Although individual protocol team members have different roles in fulfilling specific protocol team responsibilities, all members are expected to provide appropriate scientific, operational or site-specific input.
Once the concept for a new study has been approved by the EC and a first draft of the protocol is available, a Site Selection Committee is formed, composed of the following voting members: Protocol Chair and representatives from CORE, NL, SDMC and NIH. The Site Selection Committee creates a questionnaire that is submitted to all potential CTUs in the HPTN, unless the protocol requirements are applicable to only certain sites. The questionnaire solicits information pertinent to the CRS/CTU (hereafter referred to as ‘site’) ability to execute the protocol. Interested sites are evaluated and scored on predefined scoring criteria. The Site Selection Committee discusses any additional factors that seem relevant to a site’s consideration for the study and advise network leadership, which selects the sites.
Publication policy
Timely communication with the scientific community is an essential function of the HPTN and is generally accomplished by presentations at scientific meetings and the publication of manuscripts in peer-reviewed journals. The HPTN publication policy is designed to be flexible and to facilitate rapid and accurate dissemination of HPTN study results. HPTN protocol team members are responsible for publications and presentations that are reviewed by the manuscript review committee before submission for publication. All publications are on the HPTN website [101]; a few recent papers are referenced here [30,34–42].
In conclusion, epidemiological data highlight the necessity for a comprehensive HIV-prevention science agenda to study both sexually and parenterally mediated prevention strategies internationally and in the USA. The HPTN focuses on interventions in adults of potentially high and rapid global impact. HPTN focuses on existing technologies that have the potential to make an immediate impact on the HIV epidemic; ‘Prevention Now!’ is our motto. Such studies require the infrastructure of an international clinical research network such as HPTN that is well poised to address the next research questions to address the HIV pandemic.
Future perspective
The recent discoveries of the use of ART for Prevention in HPTN 052, a partially effective topical microbicide (1% tenofovir) and an oral chemoprophylactic agent (oral tenofovir/emtricitabine and oral tenofovir) as well as male circumcision have brought new hope to prevention efforts. However, combinations of interventions to prevent HIV infection will be more effective than a single approach, analogous to the use of highly active antiretroviral therapy to treat HIV infection. Evaluation of such combination approaches customized to prevent HIV infection in different populations at-risk will be the focus of HIV prevention research in the future. Research studies designed to evaluate such combinations of interventions will require a large number of participants in multiple research sites in areas of high HIV prevalence. The HPTN is well-poised to conduct this research and is already focusing on such approaches and developing research studies that will provide important data on how best to combine interventions for HIV prevention in different populations and settings to optimize the reduction in HIV incidence. Such studies will also provide data on cost–effectiveness.
Executive summary.
HIV Prevention Trials Network’s creation: vision & challenges
-
▪
The HIV Prevention Trials Network (HPTN) was created by the NIH to conduct Phase III efficacy clinical trials of novel technologies and approaches to prevent adult HIV transmission. In partnership of central resources and clinical trials units around the world, trials are in progress on a wide variety of strategies for preventing the spread of HIV.
HPTN’s contribution to advancing international HIV prevention research
-
▪
HPTN and its predecessor, HIV Network for Prevention Trials, has conducted dozens of trials over nearly two decades. Among them are: HIV Network for Prevention Trials 012, which discovered that single-dose nevirapine given to an HIV-infected pregnant woman and to her HIV-exposed infant could drop transmission to the newborn in half; HPTN 052, which demonstrated that combination antiretroviral therapy could reduce transmission from an infected sexual partner to an uninfected partner; and HPTN developmental studies, which demonstrated the promise of topical tenofovir 1% gel as a microbicide.
-
▪
HPTN has also done a number of developmental studies that have helped prepare clinical research sites, develop promising interventions, or accessed highest risk populations.
HPTN contributing new standards for HIV prevention
-
▪
It is now standard practice to include nevirapine in regimens to prevent mother-to-child transmission of HIV.
-
▪
Liberalization of the use of combination antiretroviral therapy is making possible the decline in transmission by reducing the number of infectious persons in communities.
Moving towards the future
-
▪
Combinations of prevention strategies are now being used to assess for synergism in combating HIV spread.
-
▪
Structural interventions are being combined with individual behavioral and biomedical approaches to make a greater community-level HIV prevention impact.
Acknowledgments
Supported by NIH grants UM1 AI068619 and U01 AI068619 to the HIV Prevention Trials Network (HPTN) Coordinating and Operations Center at Family Health International 360; UM1 AI068613 and U01 AI068613, to the HPTN Network Laboratory at Johns Hopkins University; and UM1 AI068617 and U01 AI068617, to the HPTN Statistical and Data Management Center at Fred Hutchinson Cancer Research Center.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Rodrigo C, Rajapakse S. Current status of HIV/AIDS in South Asia. J. Glob. Infect. Dis. 2009;1(2):93–101. doi: 10.4103/0974-777X.56249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padian NS, Mccoy SI, Karim SS, et al. HIV prevention transformed. the new prevention research agenda. Lancet. 2011;378(9787):269–278. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkers GK, Fauci AS. Controlling and ultimately ending the HIV/AIDS pandemic: a feasible goal. JAMA. 2010;304(3):350–351. doi: 10.1001/jama.2010.957. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Fidler S. HIV prevention 2010: where are we now and where are we going? Curr. Opin HIV AIDS. 2010;5(4):265–268. doi: 10.1097/COH.0b013e32833acafa. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin. Infect. Dis. 2010;50 Suppl. 3:S85–S95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Hellmann N, Levy JA, Decock K, Lange J. The spread, treatment and prevention of HIV-1: evolution of a global pandemic. J. Clin. Invest. 2008;118(4):1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieffenbach CW, Fauci AS. Thirty years of HIV and AIDS: future challenges and opportunities. Ann. Intern. Med. 2011;154(11):766–771. doi: 10.7326/0003-4819-154-11-201106070-00345. [DOI] [PubMed] [Google Scholar]
- 9.Hayes R, Kapiga S, Padian N, McCormack S, Wasserheit J. HIV prevention research: taking stock and the way forward. AIDS. 2010;24 Suppl. 4:S81–S92. doi: 10.1097/01.aids.0000390710.04255.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges and opportunities. Curr. HIV/AIDS Rep. 2011;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer KH. Antiretrovirals for HIV prevention: translating promise into praxis. Lancet. 2011;378(9787):206–208. doi: 10.1016/S0140-6736(11)61056-8. [DOI] [PubMed] [Google Scholar]
- 12.Mayer KH, Venkatesh KK. Chemoprophylaxis for HIV prevention: new opportunities and new questions. J. Acquir. Immune Defic. Syndr. 2010;55 Suppl. 2:S122–S127. doi: 10.1097/QAI.0b013e3181fbcb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schackman BR. Implementation science for the prevention and treatment of HIV/AIDS. J. Acquir. Immune Defic. Syndr. 2010;55 Suppl. 1:S27–S31. doi: 10.1097/QAI.0b013e3181f9c1da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez T, Finlayson T, Drake A, et al. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors – United States, National HIV Behavioral Surveillance System: men who have sex with men, November 2003–April 2005. MMWR Surveill. Summ. 2006;55(6):1–16. [PubMed] [Google Scholar]
- 15.Pettifor A, Rees H, Kleinschmidt I, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviours from a nationally representative household survey. AIDS. 2005;19:1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 16.Samson M, Lee U, Ndlebe A, et al. Final Report Executive Summary, Commissioned by the Economics and Finance Directorate, Department of Social Development; 2004. The Social and Economic Impact of South Africa’s Social Security System. [Google Scholar]
- 17.Buchbinder S. HIV epidemiology, testing strategies, and prevention interventions. Top HIV Med. 2010;18(2):38–44. [PubMed] [Google Scholar]
- 18.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America – forgotten but not gone. N. Engl. J. Med. 2010;362(11):967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodder SL, Justman J, Haley DF, et al. Challenges of a hidden epidemic: HIV prevention among women in the United States. J. Acquir. Immune Defic. Syndr. 2010;55 Suppl. 2:S69–S73. doi: 10.1097/QAI.0b013e3181fbbdf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer KH, Venkatesh KK. Antiretroviral therapy as HIV prevention: status and prospects. Am. J. Public Health. 2010;100(10):1867–1876. doi: 10.2105/AJPH.2009.184796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermund SH, Hodder SL, Justman JE, et al. Addressing research priorities for prevention of HIV infection in the United States. Clin. Infect. Dis. 2010;50 Suppl. 3:S149–S155. doi: 10.1086/651485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MS, Chen YQ, Mccauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boothe RE, Watters JK. How effective are risk-reduction interventions targeting injection drug users? AIDS. 1994;8(11):1515–1524. doi: 10.1097/00002030-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MS, Shaw GM, Mcmichael AJ, Haynes BF. Acute HIV-1 Infection. N. Engl. J. Med. 2011;364(20):1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 26.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirowodza A, Van Rooyen H, Joseph P, Sikotoyi S, Richter L, Coates T. Using participatory methods and geographic information systems (GIS) to prepare for an HIV community-based trial in Vulindlela, South Africa (Project Accept, HPTN 043) J. Community Psychol. 2009;37(1):41–57. doi: 10.1002/jcop.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khumalo-Sakutukwa G, Morin SF, Fritz K, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J. Acquir. Immune Defic. Syndr. 2008;49(4):422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect. Dis. 2011;11(7):525–532. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong LH, Rooyen HV, Modiba P, et al. Test and tell: correlates and consequences of testing and disclosure of HIV status in South Africa (HPTN 043 Project Accept) J. Acquir. Immune Defic. Syndr. 2009;50(2):215–222. doi: 10.1097/QAI.0b013e3181900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young SD, Hlavka Z, Modiba P, et al. HIV-related stigma, social norms and HIV testing in Soweto and Vulindlela, South Africa: National Institutes of Mental Health Project Accept (HPTN 043) J. Acquir. Immune Defic. Syndr. 2010;55(5):620–624. doi: 10.1097/QAI.0b013e3181fc6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genberg BL, Kulich M, Kawichai S, et al. HIV risk behaviors in sub-Saharan Africa and Northern Thailand: baseline behavioral data from Project Accept. J. Acquir. Immune Defic. Syndr. 2008;49(3):309–319. doi: 10.1097/QAI.0b013e3181893ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25(7):957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tedrow VA, Zelaya CE, Kennedy CE, et al. No ‘magic bullet’: exploring community mobilization strategies used in a multi-site community-based randomized controlled trial: project accept (HPTN 043) AIDS Behav. 2011 doi: 10.1007/s10461-011-0009-9. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cates W. HPTN 052 and the future of HIV treatment and prevention. Lancet. 2011;378(9787):224–225. doi: 10.1016/S0140-6736(11)61116-1. [DOI] [PubMed] [Google Scholar]
- 37.Mirochnick M, Nielsen-Saines K, Pilotto JH, et al. Nelfinavir and lamivudine pharmacokinetics during the first two weeks of life. Pediatr. Infect. Dis. J. 2011;30(9):769–772. doi: 10.1097/INF.0b013e3182242950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber CA, Whittington S, Cen L, Maslankowski L. Good intentions: risk factors for unintended pregnancies in the US cohort of a microbicide trial. Contraception. 2011;83(1):74–81. doi: 10.1016/j.contraception.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob ST, Baeten JM, Hughes JP, et al. A post-trial assessment of factors influencing study drug adherence in a randomized biomedical HIV-1 prevention trial. AIDS Behav. 2011;15(5):897–904. doi: 10.1007/s10461-010-9853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez J, Sal YRVG, Hughes JP, et al. Male circumcision and risk of HIV acquisition among MSM. AIDS. 2011;25(4):519–523. doi: 10.1097/QAD.0b013e328340fd81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bedoya CA, Mimiaga MJ, Beauchamp G, Donnell D, Mayer KH, Safren SA. Predictors of HIV transmission risk behavior and seroconversion among Latino men who have sex with men in project EXPLORE. AIDS Behav. 2011 doi: 10.1007/s10461-011-9911-4. Online First™ (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugarman J, Corneli A, Donnell D, et al. Are there adverse consequences of quizzing during informed consent for HIV research? J. Med. Ethics. 2011;37(11):693–697. doi: 10.1136/jme.2011.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Rennie S, Sugarman J. HIV Prevention Trials Network ethics guidance document: ethics guidance for research. 2009:1–67. doi: 10.1136/jme.2010.035444. www.hptn.org/ResearchEthics/HPTN_Ethics_Guidance.htm. [DOI] [PMC free article] [PubMed]
- 102.Unaids. Report on the global AIDS epidemic. 2010:359. www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf.