Abstract
Mitochondria are highly dynamic organelles with complex structural features which play several important cellular functions, such as the production of energy by oxidative phosphorylation, the regulation of calcium homeostasis, or the control of programmed cell death (PCD). Given its essential role in neuronal viability, alterations in mitochondrial biology can lead to neuron dysfunction and cell death. Defects in mitochondrial respiration have long been implicated in the etiology and pathogenesis of Parkinson's disease (PD). However, the role of mitochondria in PD extends well beyond defective respiration and also involves perturbations in mitochondrial dynamics, leading to alterations in mitochondrial morphology, intracellular trafficking, or quality control. Whether a primary or secondary event, mitochondrial dysfunction holds promise as a potential therapeutic target to halt the progression of dopaminergic neurodegeneration in PD.
Dopaminergic neurons of the substantia nigra pars compacta (SNpc), which are significantly reduced in Parkinson's disease, are particularly susceptible to mitochondrial alterations.
Mitochondria are intracellular membrane-enclosed organelles found by the hundreds in most eukaryotic cells, in which they perform a number of crucial functions such as pyruvate oxidation, the Krebs cycle, the metabolism of amino acids, fatty acids, steroids, and, most importantly, the generation of energy as adenosine triphosphate (ATP). The latter function is exerted by means of the mitochondrial electron-transport chain and the oxidative-phosphorylation system (Fig. 1). From an evolutionary point of view, mitochondria are believed to originate from a symbiotic relationship established more than a billion years ago between primordial eukaryotic cells lacking the ability to use oxygen metabolically and primitive aerobic bacteria capable of oxidative phosphorylation. Through this symbiotic relationship, which became permanent, bacteria evolved into mitochondria and the host cell acquired the ability to metabolically use oxygen, a much more efficient way to produce energy than anaerobic glycolysis (DiMauro and Schon 2003).
Figure 1.
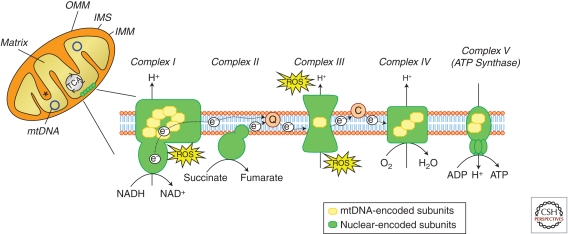
Schematic representation of mitochondrial compartmentalization. Mitochondria are divided in four compartments: the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM), and the matrix. The respiratory chain is localized at the IMM whereas the mitochondrial DNA (mtDNA) is located in the matrix. The citric acid cycle (or Krebs cycle or TCA cycle) takes place within the mitochondrial matrix. Asterisk indicates mitochondrial cristae. The respiratory chain, also known as the electron transport chain (ETC) or oxidative phosphorylation system (OXPHOS), is composed of approximately 100 proteins, 13 of which are encoded by the mtDNA. The remaining components are encoded by the nuclear DNA and imported into the mitochondria. It consists of five protein complexes; complex I (NADH dehydrogenase) and complex II (succinate dehydrogenase) receive electrons (e−) from intermediary metabolism, which are then transferred to coenzyme Q and subsequently delivered to complex III (cytochrome c reductase). The electron shuttling protein cytochrome c then transfers the electrons to complex IV (cytochrome c oxidase), which constitutes the final step in the ETC in which molecular oxygen is reduced to water. The electron transport is coupled to proton (H+) pumping across the IMM by complexes I, III, and IV. The resulting proton gradient drives ATP synthesis through complex V (ATP synthase). Reactive oxygen species (ROS), in the form of superoxide (O2), can be generated by the exit of electrons at the level of complex I and III. C, cytochrome c; Q, coenzyme Q. (Images based on Larsson 2010.)
Structurally, mitochondria contain four compartments: the outer mitochondrial membrane (OMM), the inner mitochondrial membrane (IMM), the intermembrane space (IMS), and the matrix (i.e., the region inside the inner membrane) (Fig. 1). The IMM, in which the electron transport chain (ETC) is located, is highly folded and protrudes into the matrix by invaginations called mitochondria cristae, which greatly increase the surface area of the IMM and thus the efficiency of the ETC. Mitochondria are the only organelles of the cell besides the nucleus that contain their own DNA (i.e., mitochondrial DNA, mtDNA), and their own machinery for synthesizing RNA and proteins. Each mitochondrion contains several copies of the small circular mitochondrial genome in its matrix, which encodes for 13 mitochondrial proteins that are all components of the oxidative phosphorylation system. The majority of proteins required to build and maintain functional mitochondria are therefore encoded by nuclear DNA, synthesized in the cytosol, and imported into mitochondria, where they are targeted to one of the four mitochondrial compartments.
In addition to their function in supplying cellular energy, mitochondria play a vital role in calcium homeostasis and contain several molecules involved in PCD. Furthermore, mitochondria are dynamic organelles which actively divide, fuse with one another, and undergo regulated turnover, all of which is important for the maintenance of mitochondrial function and quality control. In neurons, mitochondria are actively transported throughout axons and dendrites to facilitate their recruitment to critical subcellular compartments distant from the cell body. Alterations in any of these mitochondrial features can potentially cause disease and have been linked to the pathogenesis of Parkinson's disease (PD).
THE MITOCHONDRIAL OXIDATIVE PHOSPHORYLATION SYSTEM
Mitochondria are usually considered the “powerhouses of the cell” because of the production of ATP, via the combined efforts of the tricarboxylic acid cycle and the respiratory chain/oxidative phosphorylation system. The respiratory chain, located in the IMM, consists of five multimeric protein complexes: reduced nicotinamide adenine dinucleotide (NADH) dehydrogenase-ubiquinone oxidoreductase (complex I, approximately 46 subunits), succinate dehydrogenase-ubiquinone oxidoreductase (complex II, four subunits), ubiquinone-cytochrome c oxidoreductase (complex III, 11 subunits), cytochrome c oxidase (complex IV, 13 subunits), and ATP synthase (complex V, approximately 16 subunits) (Fig. 1). The respiratory chain also requires two small electron carriers, ubiquinone/coenzyme Q and cytochrome c. ATP synthesis involves two coordinated processes: electrons derived from energy substrates (such as NADH and FADH2) are transported through the different mitochondrial complexes to molecular oxygen, thereby producing water; at the same time, protons are pumped across the mitochondrial inner membrane (i.e., from the matrix to the IMS) by complexes I, III, and IV, generating an electrochemical gradient (termed mitochondrial membrane potential, Δψ). ATP is produced by the influx of these protons back into the mitochondrial matrix through complex V (ATP synthase). ATP is the main form of energy used by the cell and, once produced in the mitochondrion, it is exported to the cytosol by the adenine nucleotide translocator (ANT) in exchange for cytosolic ADP.
Defective mitochondrial respiration, in particular at the level of complex I, has long been associated with the pathogenesis of PD. Evidence of this involvement first emerged following the observation that accidental exposure of drug abusers to 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine (MPTP), an inhibitor of mitochondrial complex I, resulted in an acute and irreversible parkinsonian syndrome almost indistinguishable from PD (Langston et al. 1983). It was subsequently shown that MPTP selectively kills dopaminergic neurons of the substantia nigra pars compacta (SNpc), the type of cells that preferentially degenerate in PD, when injected into nonhuman primates and mice (Dauer and Przedborski 2003). Similarly, chronic infusion of the potent complex I inhibitor rotenone to rats has been reported to produce nigrostriatal dopaminergic neurodegeneration (Betarbet et al. 2000). A link between complex I dysfunction and PD was further established when several groups reported reduced complex I activity in the brain, platelets, and skeletal muscle of patients with sporadic PD (Parker et al. 1989; Schapira et al. 1990). In addition, cell lines engineered to contain mitochondria derived from platelets of PD patients (cybrids) were also shown to exhibit reduced complex I activity (Swerdlow et al. 1996). Supporting an instrumental role for complex I dysfunction in PD-related dopaminergic neurodegeneration, the feeding of the mitochondrial ETC directly at complex II by means of the ketone body D-β-hydroxybutyrate was shown to bypass complex I blockade, enhance oxidative phosphorylation, and attenuate dopaminergic neurodegeneration in MPTP-intoxicated mice (Tieu et al. 2003). Also, virally mediated expression of yeast’s single-unit NADH-quinone oxidoreductase, which is insensitive to complex I inhibitors, into the substantia nigra of rats has been shown to protect against rotenone-induced dopaminergic nigrostriatal impairment (Marella et al. 2008). Finally, methylene blue, an alternative electron carrier able to deliver electrons directly from NADH to cytochrome c, thus bypassing complex I blockade, attenuates mitochondrial dysfunction, behavioral alterations, and dopaminergic neurodegeneration in rotenone-intoxicated rats (Wen et al. 2011). Reinforcing a potential role for complex I defects in PD, most of the pesticides that have been epidemiologically linked to an increased risk of PD cause complex I dysfunction (Sherer et al. 2002; Schuh et al. 2005, 2009; Richardson et al. 2009).
Consequences of Complex I Blockade
One of the expected consequences of impaired mitochondrial respiration is a reduction in ATP production and subsequent bioenergetic failure. Supporting this view, MPP+ (MPTP’s active metabolite) causes a rapid and profound depletion of cellular ATP levels in isolated hepatocytes (Di Monte et al. 1986), in brain synaptosomal preparations (Scotcher et al. 1990) and in whole mouse brain tissues (Chan et al. 1991). In mice, however, MPTP causes only a mild (∼20%) and transient reduction in striatal and midbrain ATP levels (Chan et al. 1991). It appears that complex I activity should be reduced by more than 50% to cause significant ATP depletion in nonsynaptic brain mitochondria (Davey and Clark 1996). Because complex I activity is only reduced by 25–30% in PD patients (Parker et al. 1989; Schapira et al. 1990), this argues against a major role for ATP depletion in PD-related dopaminergic neurodegeneration.
Another consequence of impaired mitochondrial respiration is an increased production of reactive oxygen species (ROS). In a normal situation, small amounts of molecular oxygen in the mitochondria, rather than being converted to water, are reduced to ROS such as superoxide radicals (Zhou et al. 2008). However, thanks to the arsenal of antioxidants inside the mitochondria, including the mitochondrial isoform of the ROS-scavenging enzyme superoxide dismutase (SOD2), the basal levels of ROS byproducts of mitochondrial respiration are minimal (Zhou et al. 2008). Following complex I blockade, however, the amount of ROS generated by the ETC increases dramatically, likely because of a higher rate of molecular oxygen reduction into superoxide radical in response to the hampered terminal step of electron transfer from the highest potential iron–sulfur cluster of complex I to ubiquinone (Ramsay et al. 1987). In agreement with this, MPP+ and rotenone increase ROS production in isolated brain mitochondria in proportion to the degree of complex I inhibition (Perier et al 2005). Increased ROS can oxidatively damage virtually all biological macromolecules, including proteins, lipids, and DNA. For instance, complex I inhibition results in the inactivation of the mitochondrial enzyme aconitase, which is essential to maintain normal metabolic function, by oxidation of the iron–sulfur clusters contained in this enzyme (Liang and Patel 2004). Also, oxidative damage to catalytic subunits of complex I, which correlates with a misassembly and dysfunction of this complex, has been observed in frontal cortex postmortem samples from PD patients (Keeney et al. 2006). Furthermore, MPTP intoxication to mice leads to the peroxidation of the IMM phospholipid cardiolipin, thereby disrupting the normal binding of cytochrome c to the mitochondrial inner membrane and thus facilitating the pro-apoptotic release of cytochrome c to the cytosol (Perier et al. 2005). Mitochondria-derived ROS have also been shown to damage lysosomal membranes in MPTP-intoxicated mice, leading to an impairment of lysosomal function and defective autophagic degradation in these animals (Dehay et al. 2010). In addition to proteins and lipids, MPTP-intoxicated mice also exhibit oxidative damage to nuclear and mitochondrial DNA (Hoang et al. 2009). Relevant to PD, oxidative damage to proteins, lipids, and DNA have been observed in postmortem brain samples from PD patients (Dauer and Przedborski 2003). In addition, PD-linked protein DJ-1, mutations of which cause an autosomal recessive form of PD (Bonifati et al. 2003), has been identified as a mitochondrial peroxiredoxin-like peroxidase, able to scavenge mitochondrial ROS; its deficiency in mutant mice results in increased mitochondrial ROS production (Andres-Mateos et al. 2007). Supporting a pathogenic role for mitochondria-derived ROS in the context of PD, transgenic mice overexpressing human catalase (an antioxidant enzyme normally localized in the peroxisome) specifically targeted to the mitochondria exhibit an attenuation of MPTP-induced mitochondrial ROS and reduced dopaminergic cell death (Perier et al. 2010). It is important to note, however, that increased ROS levels in the context of PD may also emanate from sources other than mitochondria, including neighboring glial cells (Zhou et al. 2008).
MITOCHONDRIAL DNA
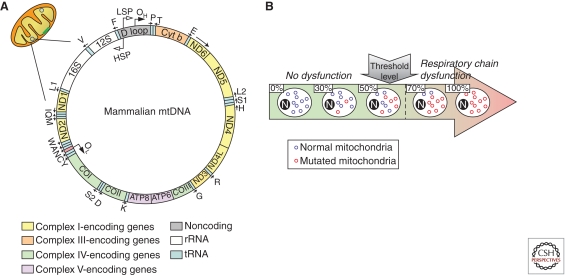
The human mitochondrial genome (mtDNA) consists of a 16.6 kb multicopy, double-stranded, circular molecule containing 37 genes of which 13 encode subunits of the respiratory chain and 24 are required for mtDNA translation within the organelle (two ribosomal RNAs and 22 transfer RNAs) (Fig. 2). Mitochondrial genetics differs from mendelian genetics in several aspects (DiMauro and Schon 2003). For instance, mtDNA is transmitted through the maternal line, which implies that a mother carrying an mtDNA mutation will pass it on to all her children, but only her daughters will transmit it to their progeny. Also, each mitochondrion contains several copies of mtDNA, resulting in thousands of mtDNA molecules per cell. In normal subjects, all mtDNAs are identical (homoplasmy). In contrast, pathogenic mtDNA mutations are usually present in some, but not all, of these genomes. In the latter situation, cells and tissues, and even individual mitochondria, can harbor both normal and mutant mtDNA (heteroplasmy). In the case of heteroplasmy, a minimal number of mutant mtDNAs is required to cause mitochondrial dysfunction and clinical signs, a phenomenon known as the threshold effect (Fig. 2). The threshold for disease is lower in tissues that are highly dependent on oxidative metabolism, such as brain, heart, or skeletal muscle, rendering these tissues especially vulnerable to the effects of pathogenic mtDNA mutations.
Figure 2.
Mitochondrial DNA (mtDNA). (A) Mammalian mtDNA is a double-stranded circular molecule containing 37 genes: two ribosomal RNAs (12S and 16S rRNA), 22 transfer RNAs (tRNAs: F, V, L1, I, M, W, D, K, G, R, H, S1, L2, T, P, E, S2, Y, C, N, A, Q), and 13 encoding subunits of the respiratory chain, including seven subunits of complex I (ND1, 2, 3, 4, 4L, 5, and 6), one subunit of complex III (cytochrome b), three subunits of cytochrome c oxidase (COX I, II, and III) and two subunits of ATP synthase (ATP6 and ATP8). (B) In a normal situation, all mtDNAs within a cell are identical (homoplasmy). In a pathological situation linked to pathogenic mtDNA mutations, cells can harbor both normal and mutant mtDNA (heteroplasmy). In the latter case, a minimal number of mutated mtDNAs is required to cause mitochondrial dysfunction and clinical signs (threshold effect). (Images based on Larsson 2010.)
Transfer of mtDNA from platelets of PD patients into cells depleted of their own mtDNA recapitulates complex I deficiency and other pathogenic features of PD (Swerdlow et al. 1996; Gu et al. 1998; Trimmer et al. 2004). This observation indicates that PD-derived mtDNA encodes pathogenic information, raising the possibility that mitochondrial alterations in PD may be inherited from the mitochondrial genome or related to somatic mtDNA alterations acquired during aging. Whereas maternally inherited parkinsonism associated with mtDNA mutations is rare (Thyagarajan et al. 2000), several studies suggest that acquired mtDNA abnormalities may contribute to PD pathogenesis. In humans, mutations in mtDNA polymerase γ (POLG), the enzyme responsible for the synthesis and proofreading of mtDNA, are associated with levodopa-responsive parkinsonism, usually as part of a more complex syndrome (Luoma et al. 2004; Davidzon et al. 2006). In these families, affected individuals exhibit reduced striatal dopamine uptake on positron emission tomography (PET) analysis and severe loss of pigmented neurons in the substantia nigra at postmortem examination, although no Lewy bodies are observed (Luoma et al. 2004). In all these patients, POLG mutations result in the accumulation of multiple mtDNA deletions in muscle (Luoma et al. 2004; Davidzon et al. 2006). Remarkably, mtDNA deletions have been observed in individual dopaminergic neurons microdissected from the substantia nigra of postmortem human brains from aged individuals and idiopathic PD patients (Bender et al. 2006; Kraytsberg et al. 2006). Different SNpc dopaminergic neurons from the same individual contained unique mtDNA deletions, indicating that these deletions were acquired throughout life (Bender et al. 2006). Furthermore, high levels of mtDNA deletions in these neurons were associated with decreased histochemical activity of mitochondrial complex IV, suggesting that the accumulation of mtDNA deletions over a certain threshold in SNpc dopaminergic neurons may cause mitochondrial functional defects (Bender et al. 2006; Kraytsberg et al. 2006). Mitochondrial ROS may be responsible for the high level of somatic mtDNA alterations occurring in SNpc dopaminergic neurons. Because of its proximity to the site of ROS production within the mitochondria, mtDNA can be oxidatively damaged during aging or following increased mitochondrial ROS production linked to complex I defects. Consistent with this view, mutant mice overexpressing the antioxidant enzyme catalase specifically in the mitochondria exhibit reduced accumulation of mtDNA mutations during aging (Vermulst et al. 2007). In addition, increased ROS production following MPTP intoxication to mice results in oxidative damage to striatal mtDNA (Hoang et al. 2009). Although it remains to be determined whether mtDNA alterations represent a primary or secondary event in PD, mice with a conditional disruption in dopaminergic neurons of mitochondrial transcription factor A (TFAM), which regulates mtDNA transcription, exhibit reduced mtDNA expression, respiratory chain defects, and slowly progressive levodopa-responsive motor deficits associated with progressive nigrostriatal denervation (Ekstrand et al. 2007), suggesting that impaired mtDNA expression may primarily contribute to the pathogenesis of PD.
MITOCHONDRIAL DYNAMICS
Mitochondria are not autonomous, rigidly structured organelles, but highly dynamic structures, which continually fuse and divide, move along the cell, and undergo regulated turnover, all of which ensure an adequate mitochondrial function at the appropriate time and subcellular location in order to adapt to changes in cellular requirements. Stressing the importance of these processes, defects in mitochondrial dynamics lead to neurological diseases and may contribute to PD.
Mitochondrial Fusion and Fission
The hundreds of mitochondria within a cell undergo continual cycles of fusion (the combination of two mitochondria into a single organelle) and fission (the separation of long, tubular mitochondria into two or more smaller parts), resulting in a wide range of mitochondrial morphologies (Fig. 3A; Detmer and Chan 2007; Knott et al. 2008). The adequate balance between fusion and fission is crucial for the maintenance of mitochondrial function. For instance, mitochondrial fusion is required for the proper respiratory activity of the mitochondria and has been associated with cell survival (Chen et al. 2007). In addition, the functionality of damaged mitochondria can be restored by exchanging mitochondrial genomes and gene products by fusion with neighboring, intact mitochondria, thereby attenuating the potential deleterious effects of misfolded proteins or mutated mtDNAs (Detmer and Chan 2007; Schon and Przedborski 2011). The proper localization of mitochondria to nerve terminals also depends on the correct balance between mitochondrial fusion and fission, as fragmentation of the mitochondrial network by fission appears to facilitate the recruitment of mitochondria to nerve terminals (Brown et al. 2006).
Figure 3.
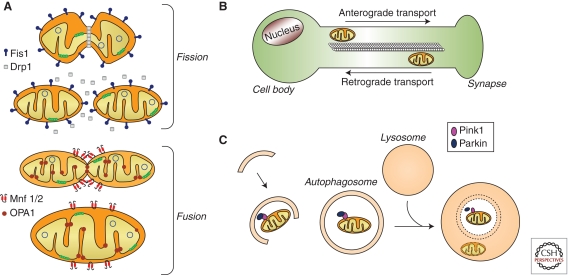
Mitochondrial dynamics. (A) Mitochondrial fusion and fission control mitochondrial number and size. Fission is mediated by dynamin-related protein 1 (Drp1) and mitochondrial fission-1 protein (Fis1). Mitofusins (Mnf) 1 and 2 are involved in the fusion of the OMM, whereas protein optic atrophy type 1 (OPA1) regulates the fusion of the IMM. (B) In neurons, mitochondria are recruited to subcellular compartments distant from the cell body, such axons and dendrites, by active transport along microtubules and actin filaments. Distinct molecular motors transport the mitochondria in anterograde or retrograde directions. (C) Selective autophagic degradation of mitochondria (i.e., mitophagy) involves the recruitment of damaged mitochondria into a pre-autophagosome structure via a PINK1/Parkin-dependent process. Targeted mitochondria are then sequestered into double-membrane-bounded autophagosomes and subsequently delivered to lysosomes for degradation.
At the molecular level, mitochondrial fusion and fission are regulated by a series of GTPases: mitofusins 1 and 2 (Mfn1, Mfn2) for outer membrane fusion, optic atrophy 1 (OPA1) for inner membrane fusion, and dynamin-related protein 1 (Drp1) for mitochondrial fission (Fig. 3A; Chen and Chan 2009). Alterations in this molecular machinery lead to defects in mitochondrial function and can cause cell death and disease. For instance, mutations in Mfn2 and OPA1 result in defective mitochondrial fusion and cause inherited neurodegenerative disorders such as Charcot–Marie–Tooth disease type 2A or dominant optic atrophy, respectively (Delettre et al. 2000; Zuchner et al. 2004). Also, defective mitochondrial fission linked to mutations in Drp1 results in neurons with elongated mitochondria largely absent from synapses and unable to maintain normal neurotransmission during intense stimulation (Verstreken et al. 2005). On the other hand, excessive Drp1-mediated mitochondrial fission is associated with apoptosis, probably resulting from the enhanced release of mitochondrial pro-apoptotic molecules, such as cytochrome c, to the cytosol (Frank et al. 2001).
In the context of PD, cell death induced by parkinsonian neurotoxins 6-OHDA, rotenone, and MPP+ in cultured neurons is associated with mitochondrial fragmentation (Barsoum et al. 2006; Meuer et al. 2007; Gomez-Lazaro et al. 2008). Supporting a pathogenic role for the latter, genetic inhibition of pro-fission Drp1 or overexpression of pro-fusion Mfn1 prevent cell death induced by these neurotoxins (Barsoum et al. 2006; Meuer et al. 2007; Gomez-Lazaro et al. 2008). Furthermore, pathogenic mutations in Parkin (an E3 ubiquitin ligase) and PINK1 (PTEN-induced putative kinase-1, a mitochondrially targeted kinase), which cause autosomal recessive forms of PD (Vila and Przedborski 2004), are associated with an increase in dysfunctional, fragmented mitochondria that can be rescued by pharmacological or genetic inactivation of Drp1 (Lutz et al. 2009; Cui et al. 2010). Also, DJ-1 deficiency in cultured cells results in ROS-dependent mitochondrial fragmentation, which can be prevented by PINK1 and Parkin overexpression (Irrcher et al. 2010). In addition, α-synuclein binding to mitochondrial membranes causes a Drp1-independent mitochondrial fragmentation that can be prevented by PINK1, Parkin, or DJ-1 overexpression (Kamp et al. 2010; Nakamura et al. 2011). However, alterations in mitochondrial fusion/fission balance have not yet been directly shown in PD.
Mitochondrial Motility and Regional Distribution
In neurons, mitochondria are actively recruited to subcellular sites distant from the cell body, such as the axonal and dendritic processes, reflecting the high levels of ATP required for synaptic transmission and the need to regulate Ca2+ homeostasis during intense synaptic activity (Keating 2008). Neuronal populations selectively vulnerable to PD, such as SNpc dopaminergic neurons, are characterized by having long, thin axons with little or no myelination (Braak et al. 2004). This feature may render these neurons more susceptible to potential alterations in mitochondrial motility, because of the high metabolic demands for the transmission of impulses along poorly myelinated axons and the long distances that mitochondria have to travel between cell bodies and axon terminals in these neurons (Vives-Bauza et al. 2010a). Indeed, compared to nondopaminergic neurons, SNpc dopaminergic neurons exhibit: (i) three times slower mitochondrial axonal transport (Kim-Han et al. 2011), (ii) a reduced number of mitochondria in cell body and dendrites (Liang et al. 2007), and (iii) smaller mitochondrial size (Kim-Han et al. 2011).
Mitochondria move along cytoskeletal tracks, such as microtubules and actin filaments, using distinct molecular motors (Fig. 3B). Short-range mitochondrial transport along actin filaments requires myosin motors whereas long-range transport on microtubules requires dynein/dynactin for retrograde transport and kinesins for anterograde transport (Hollenbeck 1996; Jung et al. 2004; Schon and Przedborski 2011). Relevant to PD, MPP+ was shown to impair kinesin-mediated anterograde fast axonal transport in isolated squid axoplasm (Morfini et al. 2007), and overall axonal motility of mitochondria, but not of other moving particles, in murine mesencephalic cultures (Kim-Han et al. 2011). In addition, proteins linked to familial forms of PD, such as PINK1, Parkin, α-synuclein, or LRRK2, have been reported to interact with, and potentially impair, microtubule-mediated trafficking (Schon and Przedborski 2011). However, direct evidence of mitochondrial trafficking alterations in PD patients is still lacking, probably because of the technical difficulties in analyzing mitochondrial transport in human-derived material.
To reach nerve terminals, tubular mitochondria is disgregated by fission into smaller, more motile fragments (Brown et al. 2006). Accordingly, synaptic mitochondria appear mostly punctate, rather than tubular (Brown et al. 2006). However, punctate synaptic mitochondria have limited ability to buffer Ca2+, compared to elongated nonsynaptic mitochondria, and appear more susceptible to Ca2+ overload (Brown et al. 2006). Synaptic mitochondria are also more sensitive to complex I inhibition than their nonsynaptic counterparts. In nonsynaptic mitochondria, complex I can be inhibited by 70% before major changes in mitochondrial respiration and ATP production are detected (Davey and Clark 1996). In contrast, in synaptic mitochondria, this threshold is lowered to 25%, which is within the range of complex I impairment found in PD patients (Davey et al. 1998). Synaptic mitochondria also exhibit lower levels of cardiolipin than their nonsynaptic counterparts (Kiebish et al. 2008), which may lower the threshold for the pro-apoptotic release of cytochrome c. These observations may explain, at least in part, the apparent increased susceptibility of striatal dopaminergic terminals, compared to dopaminergic nigral cell bodies, to the degenerative process in PD (Cheng et al. 2010).
Mitochondrial Turnover
Selective autophagic degradation of mitochondria, termed mitophagy, is necessary for the steady-state turnover of mitochondria, the adjustment of mitochondrion numbers to changing metabolic demands, or the removal of damaged mitochondria (Kim et al. 2007; Youle and Narendra 2011). Using the core autophagic machinery, mitophagy involves the sequestration of targeted mitochondria into double-membrane-bounded structures known as autophagosomes (Fig. 3C). Subsequently, autophagosomes fuse with lysosomes (i.e., cytoplasmic membrane-enclosed organelles that contain a wide variety of hydrolytic enzymes) in which sequestered mitochondria are degraded. Functional mitochondrial alterations, such as loss of Δψ or permeabilization of the OMM, trigger mitophagy, probably in an attempt to limit potential deleterious effects associated with damaged mitochondria such as excessive ROS production or enhanced release of mitochondrial pro-apoptotic factors (Tait and Green 2010).
In PD, overall autophagic degradation, including mitophagy, seems to be impaired. Indeed, in experimental PD models and postmortem PD brain samples, abnormal mitochondria readily accumulate in the cytosol of affected neurons (Dehay et al. 2010; Vila et al. 2011), indicating that it cannot be efficiently degraded through mitophagy. Accumulation of dysfunctional mitochondria may contribute to dopaminergic cell death by an increased production of ROS and an enhanced release of mitochondrial apoptogenic factors (Vila et al. 2001; Vila and Przedborski 2003; Perier et al. 2005, 2007). Defective autophagy in PD originates, at least in part (Martinez-Vicente et al. 2008), from a pathogenic reduction in the amount of functional lysosomes (Chu et al. 2009; Dehay et al. 2010). Lysosomal breakdown in PD appears secondary to the abnormal permeabilization of lysosomal membranes by mitochondrially driven oxidative attack (Dehay et al. 2010), leading to a vicious cycle in which increased ROS production from dysfunctional mitochondria contributes to defective autophagy by oxidatively damaging lysosomal membranes, thereby resulting in a further accumulation of altered mitochondria which cannot be degraded through mitophagy. Supporting a pathogenic role for decreased autophagy/mitophagy in PD, pharmacological restoration of lysosomal-mediated degradation by rapamycin is able to reduce the cytosolic accumulation of undegraded autophagosomes (which contain abnormal mitochondria) and attenuate dopaminergic neurodegeneration in MPTP-intoxicated mice (Dehay et al. 2010; Bové et al. 2011). Besides a general impairment of autophagic degradation, specific defects in mitophagy may also occur in PD. For instance, PD-linked mutations in PINK1 and Parkin have been shown to disrupt the coordinated normal regulatory role of these molecules at promoting autophagic degradation of dysfunctional mitochondria, thereby leading to defective mitophagy (Geisler et al. 2010; Narendra et al. 2010; Vives-Bauza et al. 2010b).
MITOCHONDRIA AND CALCIUM HOMEOSTASIS
Intracellular calcium (Ca2+) regulates an array of cellular processes and is important for signal transduction. In neurons, Ca2+ acts as the main second messenger to transmit depolarization status and synaptic activity to the biochemical machinery of neurons (Gleichmann and Mattson 2011). The concentration of cytosolic free Ca2+ in resting neurons (∼100 nm) is 10,000-fold lower than the concentration of Ca2+ in the extracellular space (∼1.2 mm) (Gleichmann and Mattson 2011). This concentration gradient leads to a significant increase in cytosolic Ca2+ after depolarization, rendering Ca2+ regulation a critical process in neurons. To maintain Ca2+ homeostasis, Ca2+ entering neurons is rapidly sequestered in intracellular organelles, such as the mitochondria and the endoplasmic reticulum (ER), or pumped back across the plasma membrane concentration gradient, all of which require high levels of energy in the form of ATP. The ability to accumulate, retain, and release Ca2+ is a fundamental property of mitochondria. Accumulation of Ca2+ within the mitochondrial matrix depends on both Ca2+ uptake into the mitochondria through an electrogenic uniporter, as well as extrusion of Ca2+ from the mitochondria through Na+/Ca2+ and H+/Ca2+ antiporters (Szabadkai et al. 2006; De Stefani et al. 2011). The most significant intracellular storage site of Ca2+, however, is the ER, and there is a significant interplay between mitochondria and ER in relation to Ca2+. These two organelles are linked, both biochemically and physically (Csordas et al. 2006; de Brito and Scorrano 2008), which facilitates efficient Ca2+ transmission from the ER to the mitochondria. The accumulation of Ca2+ in the mitochondria leads to the activation of oxidative phosphorylation and subsequent increase in ATP production (Gleichmann and Mattson 2011), thus helping to meet the metabolic demands associated with neuronal electrical activity.
During normal synaptic activity, intracellular Ca2+ concentrations increase only transiently (seconds to a few minutes) and have no adverse effects on neurons. However, unlike most neurons in the brain, adult SNpc dopaminergic neurons are autonomously active, generating action potentials in a clock-like manner in the absence of synaptic input (Chan et al. 2007). The pacemaking activity of these neurons is driven by voltage-dependent L-type Ca2+ channels, leading to sustained elevations in cytosolic Ca2+ concentrations in these cells (Chan et al. 2007). The large Ca2+-buffering burden created by pacemaking activity in SNpc dopaminergic neurons ultimately compromises mitochondrial function, resulting in mitochondrial oxidative stress and oscillations in mitochondrial potential, the latter being associated with compromised ATP production (Guzman et al. 2010). Supporting a pathogenic role for increased Ca2+ load linked to pacemaking activity, the L-type Ca2+ channel antagonist isradipine is able to attenuate rotenone-induced dendritic loss in adult ventral midbrain slices and to reduce SNpc dopaminergic neurodegeneration in MPTP-intoxicated mice (Chan et al. 2007). These observations suggest that sustained mitochondrial Ca2+ overload in adult SNpc dopaminergic neurons may render these cells selectively vulnerable to PD. In agreement with this, neighboring dopaminergic neurons in the ventral tegmental area (VTA), which do not rely on L-type Ca2+ channels for pacemaking, are relatively preserved in PD (Chan et al. 2010). In addition, expression of Ca2+-buffering protein calbindin in selected SNpc dopaminergic populations is negatively correlated with PD-linked cell loss (German et al. 1992; Damier et al. 1999).
Other studies further support a role for alterations in mitochondrial Ca2+ homeostasis in PD. For instance, cybrid cells containing mtDNA from PD patients exhibit lower mitochondrial Ca2+ sequestration than control cells following carbachol-stimulated Ca2+ entry (Sheehan et al. 1997). Similarly, parkinsonian neurotoxins MPP+ and rotenone cause diminished mitochondrial Ca2+ uptake and increased cytosolic free Ca2+ in cultured cells (Frei and Richter 1986; Sousa et al. 2003; Wang and Xu 2005). Also, exogenously applied oligomeric, but not monomeric, α-synuclein to cultured dopaminergic neurons was shown to increase intracellular Ca2+ levels through a pore-mediated influx of extracellular Ca2+, leading to increased mitochondrial Ca2+-buffering burden and apoptotic cell death (Danzer et al. 2007). Furthermore, PD-related protein PINK1 appears to regulate the physiological release of Ca2+ from the mitochondria via the mitochondrial Na+/Ca2+ exchanger (Gandhi et al. 2009). Indeed, ablation of PINK1 in dopaminergic neurons leads to impaired Ca2+ efflux from mitochondria, accumulation of mitochondrial Ca2+, increased production of mitochondrial ROS, decreased mitochondrial respiration, reduced Δψ, and a lowered threshold for Ca2+-dependent opening of the mitochondrial permeability transition pore complex, overall resulting in increased apoptosis (Gandhi et al. 2009). Supporting a role for increased Ca2+ load in PD-related cell death in vivo, pharmacological or genetic inhibition of Ca2+-sensitive proteases (i.e., calpains) has been shown to attenuate dopaminergic neurodegeneration in MPTP-intoxicated mice (Crocker et al. 2003).
MITOCHONDRIA AND PROGRAMMED CELL DEATH
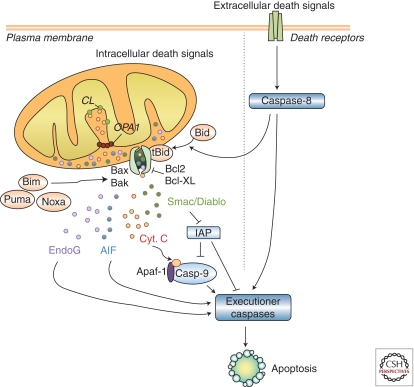
PCD, a physiologic process in which molecular programs intrinsic to the cell are activated to cause its own destruction, is a fundamental property of all pluricellular organisms and is crucial for their development, organ morphogenesis, tissue homeostasis, and defense against infected or damaged cells. However, excessive PCD or abnormal reactivation of PCD in adulthood can lead to neurodegeneration (Vila and Przedborski 2003). Mitochondria play a central role in the regulation of PCD, as they contain several molecules which, when abnormally released into the cytosol following mitochondrial outer membrane permeabilization (MOMP), activate caspase-dependent or caspase-independent PCD pathways (Fig. 4). MOMP represents the point-of-no-return in mitochondrial-dependent PCD and is highly regulated by a series of proteins of the Bcl-2 family that either prevent (e.g., Bcl-2 and Bcl-xL) or promote (e.g., Bax and Bak) MOMP and subsequent cell death (Vila and Przedborski 2003). Although the exact mechanism by which pro-apoptotic proteins, such as Bax, induce MOMP is still a matter of debate, it requires the translocation and insertion of these proteins into mitochondrial membranes, whence they can elicit the release of mitochondrial apoptogenic factors, such as cytochrome c, by at least two distinct described mechanisms: one involving the opening of the so-called mitochondrial permeability transition pore complex, and another dependent on the formation of channels directly by these proteins into mitochondrial membranes (Galluzzi et al. 2009).
Figure 4.
Mitochondrial-dependent apoptosis. Apoptosis can result from the activation of two distinct molecular cascades, known as the extrinsic (or death receptor) and the intrinsic (or mitochondrial) pathways. Both pathways, which can converge at the level of mitochondria, involve the activation of initiator caspases (caspase-8 and -9, respectively) that catalyze the proteolytic maturation of downstream executioner caspases, such as caspase-3, which are the final effectors of cell death. Mitochondrial outer membrane permeabilization (MOMP) represents the point-of-no-return in the mitochondrial apoptotic pathway. Following MOMP, mitochondrial apoptogenic factors such as cytochrome c, Smac/Diablo, endonuclease G, or apoptosis-inducing factor (AIF) are released to the cytosol. Once into the cytosol, these factors can initiate cell death in a caspase-dependent or a caspase-independent manner. Released cytochrome c interacts with two other cytosolic protein factors, Apaf-1 and procaspase-9, to activate caspase-3. Smac/Diablo can interact with several inhibitors of apoptosis (IAPs), thereby relieving the inhibitory effect of IAPs on initiator (e.g., caspase-9) and effector (e.g., caspase-3) caspases. AIF and endonuclease G can translocate to the nucleus and induce caspase-independent DNA fragmentation. MOMP is highly regulated by anti-apoptotic (e.g., Bcl-2 and Bcl-xL) and pro-apoptotic (e.g., Bax and Bak) protein members of the Bcl-2 family. Structurally, all these proteins share up to four Bcl-2-homology domains (BH1–BH4). In addition to multidomain Bcl-2 family members, there are molecules that share sequence homology only with the BH3 domain (such as Bid, Bim, Puma, and Noxa) which can induce cell death either by activating multidomain pro-apoptotic proteins or by inactivating anti-apoptotic proteins. Bid is activated following its cleavage by caspase-8, thus linking the extrinsic and intrinsic pathways at the level of the mitochondria. Whereas several components of the mitochondrial apoptotic pathway have been implicated in the pathogenesis of PD, the participation of the extrinsic pathway in PD has not been consistently shown (Perier et al. 2011). AIF, apoptosis-inducing factor; Casp-9, caspase-9; CL, cardiolipin; OPA1, optic atrophy type 1; Cyt. c, cytochrome c; EndoG, endonuclease G; IAP, inhibitor of apoptosis; tBid, truncated Bid.
In experimental PD models, dopaminergic neurodegeneration appears to occur, at least in part, through activation of mitochondria-dependent PCD pathways (Vila and Przedborski 2003; Perier et al. 2011). In MPTP-intoxicated mice, there is a time-dependent, region-specific mitochondrial release of cytochrome c followed by activation of caspase-9, caspase-3, and apoptotic nigral cell death (Perier et al. 2005). All these MPTP-induced molecular events, including dopaminergic neurodegeneration, are regulated by pro-apoptotic protein Bax, as they coincide with Bax mitochondrial translocation and are prevented by genetic ablation of Bax (Vila et al. 2001; Perier et al. 2005, 2007). Further supporting the involvement of mitochondria-dependent PCD in PD, dopaminergic neurodegeneration caused by MPTP in mice can also be attenuated by targeting other molecules of this pathway, such as caspase-9 or Apaf-1 (Mochizuki et al. 2001; Viswanath et al. 2001) or by overexpressing Bcl-2 (Offen et al. 1998; Yang et al. 1998). Importantly, complex I inhibition by either MPP+, rotenone, or pathogenic complex I mutations does not directly trigger mitochondrial cytochrome c release but instead increases the “releasable” soluble pool of cytochrome c in the mitochondrial IMS that can subsequently be released to the cytosol by activated Bax (Perier et al. 2005). This effect is mediated by peroxidation of the IMM phospholipid cardiolipin, which disrupts the normal binding of cytochrome c to the mitochondrial inner membrane (Perier et al. 2005). In addition to its detachment from the mitochondrial inner membrane, cytochrome c release also requires, in other cellular settings, a remodeling of mitochondrial cristae mediated by OPA1 (Scorrano et al. 2002; Frezza et al. 2006).
A role for mitochondria-dependent PCD in PD is further reinforced by the finding that many of the mutated nuclear genes associated with familial forms of PD either directly or indirectly affect mitochondria-dependent PCD pathways (Vila et al. 2008). For instance, overexpression of α-synuclein in vivo kills dopaminergic neurons by apoptosis through activation of caspase-9 and caspase-3 (Yamada et al. 2004). Furthermore, aggregated, but not nonaggregated, α-synuclein induces cytochrome c release in isolated rat brain mitochondria (Parihar et al. 2008). In cell lines, PD-linked mutations in LRRK2 lead to mitochondria-dependent PCD through the release of cytochrome c (Iaccarino et al. 2007). Also, overexpression of wild-type PINK1, but not of PD-associated PINK1 mutants, is able to attenuate cytochrome c release, caspase activation, and apoptosis induced by parkinsonian neurotoxins or hydrogen peroxide in cultured cells (Petit et al. 2005; Wang et al. 2007). Similarly, Parkin was shown to prevent ceramide-induced cytochrome c release, caspase activation, and apoptotic cell death in vitro (Darios et al. 2003), an effect that was abolished by PD-causing Parkin mutations (Darios et al. 2003). Overall, these results suggest that activation of mitochondria-dependent PCD pathways contributes to dopaminergic neurodegeneration in PD.
MITOCHONDRIA AND AGING
Increasing age is the most consistent risk factor for PD and mitochondria have long been suspected to play an essential role in aging. Indeed, it has been postulated that mitochondrial ROS accumulation in multiple tissues over the years may result in mtDNA alterations, mitochondrial dysfunction, and cell death, leading to the decline in tissue function associated with aging (Wallace 2005). Consistent with this view, genetic ablation of the pro-apoptotic mitochondrial ROS-producing protein p66Shc extends the lifespan of mutant mice (Migliaccio et al. 1999; Pinton et al. 2007). In addition, mutant mice overexpressing the antioxidant enzyme catalase specifically in the mitochondria exhibit reduced accumulation of mtDNA mutations (Vermulst et al. 2007) and increased lifespan (Schriner et al. 2005). Conversely, mutant mice with proofreading-deficient forms of POLG accumulate high levels of mtDNA alterations in all tissues and exhibit decreased mitochondrial respiration, increased apoptosis, accelerated aging, and reduced lifespan (Trifunovic et al. 2004; Kujoth et al. 2005). Some of these molecular events may underlie the link between mitochondria, aging, and PD. For instance, as mentioned earlier in this paper, mutant mice overexpressing mitochondrial catalase not only exhibit extended lifespan but are also more resistant to MPTP-induced dopaminergic cell death (Perier et al. 2010). Also, high levels of mtDNA deletions, which are responsible for the premature aging phenotype and shortened lifespan observed in the POLG mutant mice (Vermulst et al. 2007, 2008), have also been detected in SNpc dopaminergic neurons from postmortem human brains from aged individuals and PD patients (Bender et al. 2006; Kraytsberg et al. 2006). Furthermore, mutations in POLG are associated with parkinsonism in humans (Luoma et al. 2004; Davidzon et al. 2006).
Other potential links between mitochondria, aging, and PD are provided by members of the sirtuin family of protein deacetylases, which promote longevity in several organisms. Three of the seven mammalian sirtuins (SIRT3, 4, and 5) are targeted to mitochondria, and SIRT1 promotes mitochondrial biogenesis by deacetylating and activating PGC-1α (peroxisome proliferator-activated receptor-γ coactivator 1α), a transcriptional coactivator of nuclear genes encoding mitochondrial proteins (Guarente 2008). Pharmacological activation of SIRT1 protects dopaminergic neurons from midbrain slice cultures against MPP+ intoxication (Okawara et al. 2007). Downstream from SIRT1, genetic ablation of PGC-1α in mutant mice increases the sensitivity of these animals to MPTP-induced dopaminergic cell death (St Pierre et al. 2006). Conversely, overexpression of PGC-1α results in increased expression of nuclear-encoded subunits of the mitochondrial respiratory chain and blocks neuronal loss induced by mutant α-synuclein, rotenone, or paraquat in cellular disease models (St Pierre et al. 2006; Zheng et al. 2010). Further linking PGC-1α with PD, a newly identified partner of PD-related protein Parkin, named PARIS (Parkin-interacting substrate), was shown to be a repressor of PGC-1α expression (Shin et al. 2011). Supporting a pathogenic role for PARIS-induced PGC-1α repression, overexpression of PARIS in the substantia nigra of mice leads to dopaminergic cell death that can be reversed by PGC-1α co-expression (Shin et al. 2011). Finally, indicating a link between mitochondria quality control, aging, and PD, treatment with rapamycin, a pharmacological compound able to activate autophagy/mitophagy, has been shown to extend lifespan in several species, including aged mice (Harrison et al. 2009; Anisimov et al. 2010), and to attenuate dopaminergic neurodegeneration in MPTP-intoxicated mice (Dehay et al. 2010; Bové et al. 2011).
CONCLUSIONS
Given the essential role of mitochondria in cell viability, alterations in mitochondria biology can lead to cell dysfunction and cell death. Neurons are particularly vulnerable to mitochondrial impairment because of their dependence for energy on the mitochondrial metabolism of pyruvate produced from glucose by the glycolytic pathway or the need to recruit mitochondria to axons and dendrites. Dopaminergic SNpc neurons, in particular, are especially susceptible to mitochondrial alterations because of the increased mitochondrial Ca2+-buffering burden created by autonomous pacemaking activity in these cells and their long, poorly myelinated axons. Defects in mitochondrial respiration have indeed been implicated for a long time in the pathogenesis of PD. However, the role of mitochondria in PD seems to extend well beyond a sole deficit in respiration (Fig. 5). Although it remains to be shown whether mitochondrial alterations in PD constitute a primary or a secondary event, or are just part of a larger multifactorial pathogenic process, the targeting of mitochondrial dysfunction holds promise for the development of novel therapeutic strategies aimed at halting or slowing down the progression of dopaminergic neurodegeneration in this currently incurable neurodegenerative disorder.
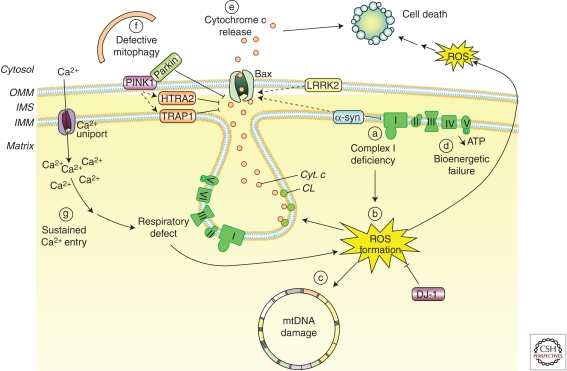
Figure 5.
Mitochondrial dysfunction in PD. Alterations in several aspects of mitochondria biology have been linked to the pathogenesis of PD, such as: (a) reduced complex I activity, (b) increased production of mitochondria-derived ROS, (c) ROS-mediated mtDNA damage, (d) bioenergetic failure, (e) Bax-mediated cytochrome c release and activation of mitochondria-dependent apoptotic pathways, (f) defective mitophagy, or (g) increased mitochondrial Ca2+-buffering burden. Many of the mutated nuclear genes linked to familial forms of PD, including PINK1, Parkin, α-synuclein, DJ-1, or LRRK2, have been shown to affect many of these mitochondrial features (see main text for details). CL, cardiolipin; Cyt. c, cytochrome c; HTRA2, high temperature requirement A2; IMM, inner mitochondrial membrane; IMS, intermembrane space; LRRK2, leucine-rich-repeat kinase 2; OMM, outer mitochondrial membrane PINK1, phosphatase, and tensin homolog-induced kinase 1; ROS, reactive oxygen species; TRAP1, tumor necrosis factor receptor-associated protein 1; α-syn, alpha-synuclein.
ACKNOWLEDGMENTS
This work was supported by European Commission Marie Curie Excellence Grant (M.V.), European Commission Marie Curie International Reintegration Grant (M.V.), Fundació la Caixa, Spain (M.V.), FIS-ISCIII, Spain (M.V. and C.P.), MICINN, Spain (M.V.), and Ramón y Cajal Program from MICINN (C.P.).
Footnotes
Editor: Serge Przedborski
Additional Perspectives on Parkinson's Disease available at www.perspectivesinmedicine.org
REFERENCES
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, et al. 2007. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci 104: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV 2010. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 176: 2092–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, et al. 2006. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. 2006. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38: 515–517 [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT 2000. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306 [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, Van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al. 2003. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259 [DOI] [PubMed] [Google Scholar]
- Bové J, Martinez-Vicente M, Vila M 2011. Fighting neurodegeneration with rapamycin: Mechanistic insights. Nat Rev Neurosci 12: 437–452 [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K 2004. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 318: 121–134 [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW 2006. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem 281: 11658–11668 [DOI] [PubMed] [Google Scholar]
- Chan P, DeLanney LE, Irwin I, Langston JW, Di Monte D 1991. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine in mouse brain. J Neurochem 57: 348–351 [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ 2007. “Rejuvenation” protects neurons in mouse models of Parkinson's disease. Nature 447: 1081–1086 [DOI] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ 2010. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson's disease. Mov Disord 25 (Suppl 1): S63–S70 [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC 2009. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18: R169–R176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC 2007. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130: 548–562 [DOI] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE 2010. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67: 715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH 2009. Alterations in lysosomal and proteasomal markers in Parkinson's disease: Relationship to alpha-synuclein inclusions. Neurobiol Dis 35: 385–398 [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Smith PD, Jackson-Lewis V, Lamba WR, Hayley SP, Grimm E, Callaghan SM, Slack RS, Melloni E, Przedborski S, et al. 2003. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J Neurosci 23: 4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G 2006. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K 2010. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 285: 11740–11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM 1999. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122: 1437–1448 [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M 2007. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci 27: 9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A 2003. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet 12: 517–526 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S 2003. Parkinson's disease: Mechanisms and models. Neuron 39: 889–909 [DOI] [PubMed] [Google Scholar]
- Davey GP, Clark JB 1996. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem 66: 1617–1624 [DOI] [PubMed] [Google Scholar]
- Davey GP, Peuchen S, Clark JB 1998. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem 273: 12753–12757 [DOI] [PubMed] [Google Scholar]
- Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, DiMauro S 2006. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol 59: 859–862 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R 2011. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, Bové J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M 2010. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci 30: 12535–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP 2000. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210 [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC 2007. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879 [DOI] [PubMed] [Google Scholar]
- Di Monte D, Jewell SA, Ekstrom G, Sandy MS, Smith MT 1986. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridinium (MPP+) cause rapid ATP depletion in isolated hepatocytes. Biochem Biophys Res Commun 137: 310–315 [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA 2003. Mechanisms of disease: Mitochondrial respiratory-chain diseases. N Engl J Med 348: 2656–2668 [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, et al. 2007. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci 104: 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ 2001. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525 [DOI] [PubMed] [Google Scholar]
- Frei B, Richter C 1986. N-methyl-4-phenylpyridine (MMP+) together with 6-hydroxydopamine or dopamine stimulates Ca2+ release from mitochondria. FEBS Lett 198: 99–102 [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, et al. 2006. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189 [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Blomgren K, Kroemer G 2009. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 10: 481–494 [DOI] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY 2009. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33: 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W 2010. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131 [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, Sonsalla PK, Brooks BA 1992. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: Sparing of calbindin-D28k-containing cells. Ann NY Acad Sci 648: 42–62 [DOI] [PubMed] [Google Scholar]
- Gleichmann M, Mattson MP 2011. Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal 14: 1261–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordan J, Schrader M 2008. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med 44: 1960–1969 [DOI] [PubMed] [Google Scholar]
- Gu M, Cooper JM, Taanman JW, Schapira AH 1998. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson's disease. Ann Neurol 44: 177–186 [DOI] [PubMed] [Google Scholar]
- Guarente L 2008. Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell 132: 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ 2010. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468: 696–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Choi DK, Nagai M, Wu DC, Nagata T, Prou D, Wilson GL, Vila M, Jackson-Lewis V, Dawson VL, et al. 2009. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson disease. Free Radic Biol Med 47: 1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ 1996. The pattern and mechanism of mitochondrial transport in axons. Front Biosci 1: d91–d102 [DOI] [PubMed] [Google Scholar]
- Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P 2007. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum Mol Genet 16: 1319–1326 [DOI] [PubMed] [Google Scholar]
- Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, et al. 2010. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet 19: 3734–3746 [DOI] [PubMed] [Google Scholar]
- Jung C, Chylinski TM, Pimenta A, Ortiz D, Shea TB 2004. Neurofilament transport is dependent on actin and myosin. J Neurosci 24: 9486–9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, et al. 2010. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 29: 3571–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating DJ 2008. Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem 104: 298–305 [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP Jr 2006. Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26: 5256–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Lunceford A, Clarke CF, Moon H, Chuang JH, Seyfried TN 2008. Lipidomic analysis and electron transport chain activities in C57BL/6J mouse brain mitochondria. J Neurochem 106: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Rodriguez-Enriquez S, Lemasters JJ 2007. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Han JS, Antenor-Dorsey JA, O’Malley KL 2011. The Parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci 31: 7212–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E 2008. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9: 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K 2006. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38: 518–520 [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484 [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Irwin I 1983. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219: 979–980 [DOI] [PubMed] [Google Scholar]
- Larsson NG 2010. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem 79: 683–706 [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M 2004. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson's disease. J Neurochem 90: 1076–1084 [DOI] [PubMed] [Google Scholar]
- Liang CL, Wang TT, Luby-Phelps K, German DC 2007. Mitochondria mass is low in mouse substantia nigra dopamine neurons: Implications for Parkinson's disease. Exp Neurol 203: 370–380 [DOI] [PubMed] [Google Scholar]
- Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, et al. 2004. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: Clinical and molecular genetic study. Lancet 364: 875–882 [DOI] [PubMed] [Google Scholar]
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, et al. 2009. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 284: 22938–22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, Yagi T 2008. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson's disease. PLoS ONE 3: e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, et al. 2008. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 118: 777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L, Braus GH, Dietz GP, Jakobs S, Bahr M, et al. 2007. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ 14: 651–661 [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG 1999. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313 [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Hayakawa H, Migita M, Shibata M, Tanaka R, Suzuki A, Shimo-Nakanishi Y, Urabe T, Yamada M, Tamayose K, et al. 2001. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc Natl Acad Sci 98: 10918–10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Opalach K, Serulle Y, Moreira JE, Sugimori M, Llinas RR, Brady ST 2007. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci 104: 2442–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, et al. 2011. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J Biol Chem 286: 20710–20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ 2010. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, Beart PM, Cheung NS, Pascoe CJ, Hochman A, Gorodin S, Melamed E, Bernard R, Bernard O 1998. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Proc Natl Acad Sci 95: 5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A 2007. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol 73: 550–560 [DOI] [PubMed] [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P 2008. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65: 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD Jr, Boyson SJ, Parks JK 1989. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol 26: 719–723 [DOI] [PubMed] [Google Scholar]
- Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M 2005. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci 102: 19126–19131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bové J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V, Rathke-Hartlieb S, Bouillet P, Strasser A, Schulz JB, et al. 2007. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson's disease. Proc Natl Acad Sci 104: 8161–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bové J, Dehay B, Jackson-Lewis V, Rabinovitch PS, Przedborski S, Vila M 2010. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann Neurol 68: 184–192 [DOI] [PubMed] [Google Scholar]
- Perier C, Bové J, Vila M 2011. Mitochondria and programmed cell death in Parkinson's disease: Apoptosis and beyond. Antioxid Redox Signal 10.1089/ars.2011.4074 [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, et al. 2005. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem 280: 34025–34032 [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, et al. 2007. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant pp66Shc. Science 315: 659–663 [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Kowal AT, Johnson MK, Salach JI, Singer TP 1987. The inhibition site of MPP+, the neurotoxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine is near the Q-binding site of NADH dehydrogenase. Arch Biochem Biophys 259: 645–649 [DOI] [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, Winnik B, O’Suilleabhain P, Diaz-Arrastia R, Reisch J, German DC 2009. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol 66: 870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD 1990. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem 54: 823–827 [DOI] [PubMed] [Google Scholar]
- Schon EA, Przedborski S 2011. Mitochondria: The next (neurode)generation. Neuron 70: 1033–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. 2005. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911 [DOI] [PubMed] [Google Scholar]
- Schuh RA, Kristian T, Gupta RK, Flaws JA, Fiskum G 2005. Methoxychlor inhibits brain mitochondrial respiration and increases hydrogen peroxide production and CREB phosphorylation. Toxicol Sci 88: 495–504 [DOI] [PubMed] [Google Scholar]
- Schuh RA, Richardson JR, Gupta RK, Flaws JA, Fiskum G 2009. Effects of the organochlorine pesticide methoxychlor on dopamine metabolites and transporters in the mouse brain. Neurotoxicology 30: 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ 2002. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Developmental Cell 2: 55–67 [DOI] [PubMed] [Google Scholar]
- Scotcher KP, Irwin I, DeLanney LE, Langston JW, Di Monte D 1990. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on ATP levels of mouse brain synaptosomes. J Neurochem 54: 1295–1301 [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB 1997. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson's disease. J Neurochem 68: 1221–1233 [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT 2002. Environment, mitochondria, and Parkinson's disease. Neuroscientist 8: 192–197 [DOI] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM 2011. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell 144: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa SC, Maciel EN, Vercesi AE, Castilho RF 2003. Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett 543: 179–183 [DOI] [PubMed] [Google Scholar]
- St Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, et al. 2006. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408 [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP Jr, Davis RE, Parker WD Jr 1996. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol 40: 663–671 [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R 2006. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta 1763: 442–449 [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR 2010. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632 [DOI] [PubMed] [Google Scholar]
- Thyagarajan D, Bressman S, Bruno C, Przedborski S, Shanske S, Lynch T, Fahn S, DiMauro S 2000. A novel mitochondrial 12SrRNA point mutation in parkinsonism, deafness, and neuropathy. Ann Neurol 48: 730–736 [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R 2003. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112: 892–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly Y, Gidlof S, Oldfors A, Wibom R, et al. 2004. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423 [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Borland MK, Keeney PM, Bennett JP Jr, Parker WD Jr 2004. Parkinson's disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem 88: 800–812 [DOI] [PubMed] [Google Scholar]
- Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA 2007. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet 39: 540–543 [DOI] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA 2008. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet 40: 392–394 [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ 2005. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47: 365–378 [DOI] [PubMed] [Google Scholar]
- Vila M, Przedborski S 2003. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 4: 365–375 [DOI] [PubMed] [Google Scholar]
- Vila M, Przedborski S 2004. Genetic clues to the pathogenesis of Parkinson's disease. Nat Med 10 (Suppl 1): S58–S62 [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S 2001. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci 98: 2837–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Ramonet D, Perier C 2008. Mitochondrial alterations in Parkinson's disease: New clues. J Neurochem 107: 317–328 [DOI] [PubMed] [Google Scholar]
- Vila M, Bové J, Dehay B, Rodriguez-Muela N, Boya P 2011. Lysosomal membrane permeabilization in Parkinson disease. Autophagy 7: 98–100 [DOI] [PubMed] [Google Scholar]
- Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK 2001. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci 21: 9519–9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Tocilescu M, Devries RL, Alessi DM, Jackson-Lewis V, Przedborski S 2010a. Control of mitochondrial integrity in Parkinson's disease. Prog Brain Res 183: 99–113 [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. 2010b. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci 107: 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet 39: 359–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Xu JX 2005. Possible involvement of Ca2+ signaling in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci Lett 376: 127–132 [DOI] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST 2007. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol Dis 28: 216–226 [DOI] [PubMed] [Google Scholar]
- Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, et al. 2011. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem 286: 16504–16515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H 2004. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: Resemblance to pathogenetic changes in Parkinson's disease. J Neurochem 91: 451–461 [DOI] [PubMed] [Google Scholar]
- Yang LC, Matthews RT, Schulz JB, Klockgether T, Liao AW, Martinou JC, Penney JB Jr, Hyman BT, Beal MF 1998. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci 18: 8145–8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP 2011. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, et al. 2010. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med 2: 52ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Przedborski S 2008. Oxidative stress in Parkinson's disease: A mechanism of pathogenic and therapeutic significance. Ann NY Acad Sci 1147: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, et al. 2004. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36: 449–451 [DOI] [PubMed] [Google Scholar]