Abstract
Frontotemporal dementia (FTD) comprises a group of behavioral, language, and movement disorders. On the basis of the nature of the characteristic protein inclusions, frontotemporal lobar degeneration (FTLD) can be subdivided into the common FTLD-tau and FTLD-TDP as well as the less common FTLD-FUS and FTLD-UPS. Approximately 10% of cases of FTD are inherited in an autosomal-dominant manner. Mutations in seven genes cause FTD, with those in tau (MAPT), chromosome 9 open reading frame 72 (C9ORF72), and progranulin (GRN) being the most common. Mutations in MAPT give rise to FTLD-tau and mutations in C9ORF72 and GRN to FTLD-TDP. The other four genes are transactive response–DNA binding protein-43 (TARDBP), fused in sarcoma (FUS), valosin-containing protein (VCP), and charged multivesicular body protein 2B (CHMP2B). Mutations in TARDBP and VCP give rise to FTLD-TDP, mutations in FUS to FTLD-FUS, and mutations in CHMP2B to FTLD-UPS. The discovery that mutations in MAPT cause neurodegeneration and dementia has important implications for understanding Alzheimer disease.
Mutations in the tau (MAPT) gene account for ∼5% of frontotemporal dementia cases. They give rise to characteristic protein inclusions, providing insight into tau pathology in Alzheimer disease.
THE CONCEPT OF FRONTOTEMPORAL DEMENTIA: HISTORICAL OVERVIEW
Frontotemporal dementia (FTD) results from degeneration of the cortex of the frontal and temporal lobes, often in conjunction with the degeneration of subcortical brain regions. This gives rise to a spectrum of behavioral, language, and movement disorders. A link exists between FTD and forms of motor neuron disease (MND). Work on FTD stretches back to the end of the 19th century.
Arnold Pick was Professor of Neuropsychiatry at the German University in Prague from 1886 to 1921. In 1892, he described a 71-year-old man with behavioral disturbances, aphasia, and dementia (Pick 1892). At autopsy, marked atrophy of the left temporal lobe rather than the diffuse atrophy characteristic of senile dementia was present. Although Pick was doubtful of the primacy of these observations, his paper is considered to be the first description of lobar cortical atrophy. At the time, there was much interest in language abnormalities, following the description of motor and sensory aphasias (Broca 1861; Wernicke 1874; see also Freud 1891). A few years later, Déjerine and Sérieux (1897) described a case of sensory aphasia with bilateral temporal atrophy. Pick went on to report four additional cases with temporal lobe atrophy and language disturbances (Pick 1901, 1904). In 1906, he described a patient with disinhibition and mixed apraxia who had severe bilateral frontal and left-sided parietal atrophy, with a more moderate atrophy of the left temporal lobe (Pick 1906). Pick was mainly interested in comparing the clinical picture with the macroscopic appearance of the brain. He made no systematic attempt at identifying histopathological abnormalities. Alzheimer discovered the association of argyrophilic intracytoplasmic inclusions and ballooned neurons with lobar cortical atrophy, in the absence of the plaques and tangles he had described four years earlier (Alzheimer 1907, 1911). This revealed the existence of a second type of intraneuronal inclusion and established that different inclusions can characterize distinct clinical entities.
Richter proposed that lobar cortical atrophies are hereditary diseases (Richter 1918) and Gans, a pupil of Pick, linked his mentor’s name to cases of lobar cortical atrophy (Gans 1923). Additional examples of frontal and/or temporal cortical atrophy with or without argyrophilic inclusions were subsequently reported and the clinical condition was called “Pick’s disease” (Onari and Spatz 1926; Stertz 1926). Unlike Pick, who believed to have described atypical forms of senile dementia, Onari and Spatz considered Pick’s disease to be a distinct entity. One of their patients (Therese Mühlich) had already been described by Alzheimer. Carl Schneider proposed a three-stage model for the clinical course of Pick’s disease (Schneider 1927, 1929). In most individuals, the first stage is characterized by disinhibition and impaired judgement, although Schneider recognized that amnestic aphasia is the presenting symptom of temporal lobe atrophy. The second stage is dominated by progressive dementia and focal symptoms, such as apathy in frontal lobe atrophy and sensory aphasia in temporal lobe atrophy. Stereotyped perseverations of speech, movement, and facial expression also appear. The third stage is characterized by dementia and severe language problems, resulting in a vegetative state with flexion contractures. Schneider concluded that the argyrophilic inclusions and ballooned cells described by Alzheimer were diagnostic of Pick’s disease. Similar cases were described in the 1930s, when it became clear that lobar cortical atrophy has a high degree of heritability, irrespective of the presence of argyrophilic inclusions (Grünthal 1930; Verhaart 1930; Von Braunmühl and Leonhard 1934). The link between frontal lobe dementia and MND was also recognized (Meyer 1929; Von Braunmühl 1932). The early work was summarized and discussed by Van Mansvelt (1954) and Lüers and Spatz (1957).
Interest in the focal dementias waned after World War II, before it was rekindled in the 1970s and 1980s. Cases of Pick’s disease with argyrophilic inclusions and ballooned neurons (type A) were now distinguished from those with ballooned neurons lacking argyrophilic inclusions (type B) and those lacking both ballooned neurons and argyrophilic inclusions (type C) (Constantinidis 1974). Work by Brun, Gustafson, and Neary showed that some individuals with frontal lobe atrophy lacked a distinctive histopathology (Brun 1987; Gustafson 1987; Neary et al. 1988). Clinically, these patients suffered from a severe personality disorder, which is now known as behavioral-variant FTD (bvFTD). Mesulam described primary progressive aphasia (PPA), with an isolated language deficit as the most prominent presenting feature, in the absence of strokes or tumors (Mesulam 1982, 1987, 2001). PPA has been divided into three syndromes (Gorno-Tempini et al. 2011): (1) Semantic dementia (SD), also known as semantic variant PPA, a fluent aphasia with loss of word meaning (Snowden et al. 1989); (2) progressive nonfluent aphasia (PNFA), also known as nonfluent/agrammatic variant PPA, a disorder characterized by effortful, nonfluent speech (Grossman et al. 1996); and (3) logopenic progressive aphasia (LPA), also known as logopenic variant PPA, a nonfluent aphasia with deficits in word retrieval and sentence repetition (Gorno-Tempini et al. 2004b).
Symptoms correlate better with specific patterns of brain atrophy than with the underlying neuropathology. Prediction of the neuropathology based on clinical picture remains challenging. FTD is the third most common cause of early-onset dementia (disease onset <65 years), after Alzheimer disease and vascular dementia (Rossor et al. 2010). Approximately 40% of patients with FTD have a family history, but only 10% of cases are inherited in a dominant manner. Links exist with the corticobasal syndrome (CBS), progressive supranuclear palsy (PSP), parkinsonism, and MND.
CLINICAL PRESENTATIONS OF FRONTOTEMPORAL DEMENTIA
Behavioral-Variant Frontotemporal Dementia (bvFTD)
bvFTD comprises more than half of the cases of FTD and is the most heritable form. Presenting features are insidious and include progressive changes in the patient’s personality, interpersonal conduct, and emotional modulation (Gustafson 1987; Neary et al. 1988; Piguet et al. 2011a). A variable degree of language impairment is also present. Apathy manifesting as passivity, inertia, reduced motivation, and social withdrawal, associated with a lack of insight, is common. Disinhibition often coexists alongside apathy and manifests itself by impulsivity. Emotional blunting characterized by a lack of empathy is common. Abnormal eating behavior can be extensive, resulting in marked weight gain. Stereotypic and ritualistic behavior is common, as expressed by motor stereotyping, including humming, lip smacking, hand ruffling, and foot tapping. Neglect of self-care and impairment of other activities of daily living are common. Most patients are unable to manage their financial affairs. Memory is relatively spared in the early stages of bvFTD. By neuroimaging, four subtypes have been identified based on relative grey matter loss: frontal-dominant, frontotemporal, temporofrontoparietal, and temporal-dominant (Whitwell et al. 2009a). Combined frontotemporal and basal ganglion atrophy can also be present, as can atrophy of a number of other subcortical regions. bvFTD and Alzheimer disease lead to divergent network activity patterns, with atrophy in an anterior salience network in bvFTD and a posterior default mode network in Alzheimer disease (Zhou et al. 2010).
Semantic Dementia (SD)
SD is a progressive fluent aphasia, which is characterized by the loss of word meaning (Snowden et al. 1989; Hodges and Patterson 2007). Patients have difficulty in finding words, with anomia being a defining feature. They also complain of memory loss, but this does not in general reflect true amnesia. Although language deficits predominate, behavioral alterations also occur. SD is the least heritable FTD syndrome. A deficit in naming and word comprehension predominates, with the patient’s vocabulary being depleted of all but the most common words. However, speech is fluent and the syntax correct. This is often coupled with deficits in person recognition, especially when the right temporal lobe is affected. Although patients are insightful and can be distressed by their condition, lack of empathy and mental inflexibility are common. Restriction of food preferences is present without the overeating characteristic of bvFTD. Compulsive behavior is prominent and centers on visual objects (left temporal lobe predominance) or on letters, words, and symbols (right temporal lobe predominance). By neuroimaging of grey matter, bilateral, often asymmetric, anterior temporal lobe atrophy is most prominent. The hippocampus can also be affected (Mummery et al. 1999). White matter changes are found in the ventral language pathways and the temporal components of the dorsal language pathways (Galantucci et al. 2011).
Progressive Nonfluent Aphasia (PNFA)
PNFA is a disorder of expressive language. Patients lose the ability to speak fluently, with relative preservation of word comprehension and nonlinguistic cognition (Grossman et al. 1996). Several speech changes characterize PNFA. At an early stage, patients speak less than normal and in shorter sentences. They show speech apraxia, with effortful speech and phonological errors. Word finding difficulty is commonly observed, often resulting in muteness. Behavioral features similar to those of bvFTD may occur, but they are usually mild, with apathy being most common. As the disease progresses, extrapyramidal features become widespread and can lead to a change in diagnosis (Gorno-Tempini et al. 2004a). Heritability of PNFA is intermediate between that of bvFTD and SD. Neuroimaging shows a widening of the Sylvian fissure, with atrophy of left posterior frontal and insular regions (Neary et al. 2003; Nestor et al. 2003). In white matter, the most prominent changes are found in the dorsal language pathways (Galantucci et al. 2011).
Logopenic Progressive Aphasia (LPA)
LPA is a progressive nonfluent aphasia, which is characterized by a slow speech rate and word retrieval difficulties (Gorno-Tempini et al. 2004b, 2008). Repetition of phrases is also markedly impaired, in part as a result of limited auditory-verbal short-term memory. Single-word comprehension and semantic association are largely preserved. It differs from the fast output typical of patients with SD and the agrammatism and articulation deficits characteristic of PNFA. However, a language variant of Alzheimer disease overlaps with LPA (Galton et al. 2000; Alladi et al. 2007). It has been suggested that LPA and posterior cortical atrophy are clinical presentations of sporadic, early-onset Alzheimer disease (Migliaccio et al. 2009). This nonmemory phenotype characterizes about one quarter of patients (Van der Flier et al. 2011). Inheritance of the APOE ε4 allele appears not to be a risk factor for LPA and posterior cortical atrophy, distinguishing them from the more common amnestic forms of Alzheimer disease (Strittmatter and Roses 1995). Neuroimaging of LPA shows atrophy or hypoperfusion of the left posterior superior and middle temporal regions, and of the inferior parietal region (Gorno-Tempini et al. 2004b). Brain atrophy is located more posteriorly than in SD and PNFA. White matter changes are most marked in the temporoparietal component of the dorsal language pathway (Galantucci et al. 2011).
Frontotemporal Dementia and Corticobasal Syndrome (CBS)
CBS and PSP are atypical parkinsonian disorders. CBS is characterized by extrapyramidal symptoms consisting of progressive asymmetric rigidity and dystonia, and by signs of cortical dysfunction in the form of PNFA, apraxia, cortical sensory loss, alien limb syndrome, myoclonus, and hemineglect. For many years, the emphasis was on the extrapyramidal component, even though similarities with Pick’s disease were noticed early on (Rebeiz et al. 1968). More recent work has shown that patients with CBS can have aphasia or a behavioral disorder characteristic of bvFTD (Lippa et al. 1991; Kertesz et al. 1994). Pathologically, CBS is heterogenous, but its most common form is corticobasal degeneration (CBD). Some cases of CBS are dominantly inherited. Neuroimaging shows variable frontoparietal and basal ganglion atrophy (Whitwell et al. 2010).
Frontotemporal Dementia and Progressive Supranuclear Palsy (PSP)
The clinical presentation of PSP includes vertical supranuclear ophtalmoplegia with difficulty looking up, bradykinesia, axial dystonia and rigidity, pseudobulbar palsy and postural instability with backward falls (Steele et al. 1964; Litvan et al. 1996). More than half of the patients develop cognitive impairment. Apathy and emotional blunting, accompanied by mental slowness and reduced verbal fluency, are common. A small percentage of cases of PSP is inherited. By neuroimaging, atrophy in premotor and supplemental motor areas is observed, with sparing of the inferior frontal lobe (Whitwell et al. 2010). Some patients show PNFA with early apraxia of speech (Josephs et al. 2006). Three subtypes of PSP have been described: Richardson’s syndrome, PSP-parkinsonism, and PSP-pure akinesia with gait freezing (Williams and Lees 2009). Cognitive impairment and cortical atrophy are most prominent in Richardson’s syndrome, which corresponds to classical PSP.
Frontotemporal Dementia and Parkinsonism Linked to Chromosome 17 (FTDP-17)
In the 1980s and 1990s, dominantly inherited forms of FTD were identified (Ghetti et al. 2011). Extrapyramidal signs resembling CBS and PSP also featured prominently. Amyotrophy was present in some cases. These forms of inherited FTD were given different names according to their predominant clinical and pathological features, including familial Pick’s disease, disinhibition-dementia-parkinsonism-amyotrophy complex, familial progressive subcortical gliosis, familial presenile dementia with tangles, autosomal-dominant parkinsonism, dementia with pallido-ponto-nigral degeneration, and multiple system tauopathy with presenile dementia. Despite this heterogeneity, disease was linked to the long arm of chromosome 17 (Wilhelmsen et al. 1994). The syndrome received its name at a consensus conference during which 13 families were presented (Foster et al. 1997). FTDP-17 is divided into a dementia-dominant and a parkinsonism-dominant type. Neuroimaging shows variable frontotemporoparietal and basal ganglion atrophy (Whitwell et al. 2009b).
Frontotemporal Dementia with Motor Neuron Disease (MND)
MND can be associated with cognitive dysfunction (Morita et al. 1987). Mild frontal lobe involvement is found in 30% of cases and in ∼3% of cases FTD is present (Shaw 2010). A psychotic phase consisting of vivid delusions is an early sign. Behavioral and cognitive changes tend to predate MND. Bulbar signs are common and electromyography is as in MND. Inherited cases of FTD-MND have been linked to chromosome 9p21 (Vance et al. 2006). Neuroimaging shows posterior frontal lobe atrophy (Whitwell et al. 2006). Based on the presence of isolated upper MND in some cases, further subclassification of FTD-MND has been proposed (Josephs and Dickson 2007).
HISTOPATHOLOGY OF FRONTOTEMPORAL LOBAR DEGENERATION (FTLD)
FTLD-Tau
In 1986, the argyrophilic inclusions of Pick’s disease were shown to be immunoreactive for hyperphosphorylated tau (Pollock et al. 1986), a normally soluble microtubule-associated protein that stabilizes microtubules and promotes microtubule assembly. It followed the finding that the intracellular inclusions of Alzheimer disease stain for hyperphosphorylated tau (Brion et al. 1985; Grundke-Iqbal et al. 1986). In adult human brain, six tau isoforms are expressed from a single MAPT gene through alternative mRNA splicing (Fig. 1A) (Goedert et al. 1989a,b). Three isoforms have three repeats each and three isoforms have four repeats each. By 1992, the paired helical filament of Alzheimer disease had been shown to be made of the six tau isoforms, each full-length and hyperphosphorylated (Goedert et al. 1988, 1992; Wischik et al. 1988; Lee et al. 1991). Filamentous tau inclusions were subsequently shown to be characteristic of many cases of FTDP-17 (Spillantini et al. 1996, 1998a).
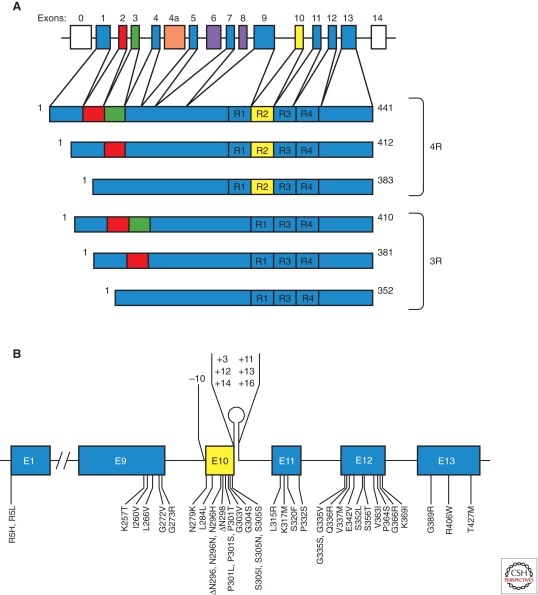
Figure 1.
MAPT and the six tau isoforms expressed in adult human brain and mutations in MAPT in frontotemporal dementia and parkinsonism linked to chromosome 17. (A) MAPT consists of 16 exons (E). Alternative mRNA splicing of E2 (red), E3 (green), and E10 (yellow) gives rise to six tau isoforms (352-441 amino acids). The constitutively spliced exons (E1, E4, E5, E7, E9, E11, E12, E13) are indicated in blue. E0, which is part of the promoter, and E14 are noncoding (white). E6 and E8 (violet) are not transcribed in human brain. E4a (orange) is only expressed in the peripheral nervous system. The repeats of tau (R1–R4) are shown, with three isoforms having four repeats each (4R) and three isoforms having three repeats each (3R). Each repeat is 31 or 32 amino acids in length. (B) Shown are 39 coding region mutations in E1, E9, E10, E11, E12, and E13 as well as seven intronic mutations flanking E10.
Around 40% of patients with FTD show tau inclusions (Fig. 2). They include most cases of PNFA, ∼45% of cases of bvFTD and some cases of SD (Piguet et al. 2011a). Most cases of LPA are characterized by focal Alzheimer disease pathology (Aβ plaques and tau tangles), as are some cases of SD and PNFA (Mesulam et al. 2008; Rabinovici et al. 2008). Focal Alzheimer disease pathology accounts for ∼25% of autopsy cases of PPA. A frontal variant of Alzheimer disease has also been described (Johnson et al. 1999). Tau inclusions are characteristic of Pick’s disease, PSP, and CBD, which belongs to the CBS spectrum (Goedert and Spillantini 2006). They are not typical of FTD-MND, even though MND can occur in conjunction with FTD and tauopathy (Fu et al. 2010).
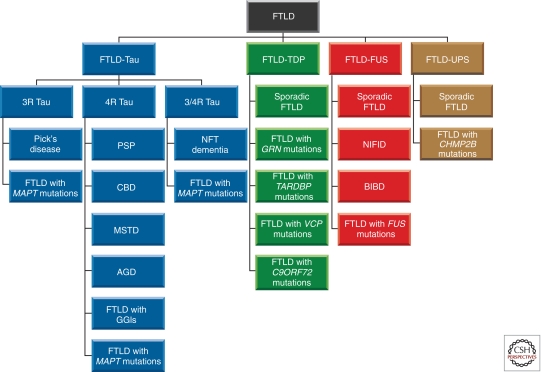
Figure 2.
Frontotemporal lobar degeneration (FTLD) molecular classification. Four subtypes (FTLD-Tau, FTLD-TDP, FTLD-FUS, and FTLD-UPS) can be distinguished, based on what is known about the major components that make up the pathological deposits (Tau protein, TDP-43, FUS and unknown protein). FTLD-Tau and FTLD-TDP are more common than FTLD-FUS and FTLD-UPS. Tau deposits are made of either three-repeat (3R), four-repeat (4R) or all six (3/4R) brain isoforms of tau. Together, FTLD-TDP, FTLD-FUS, and FTLD-UPS make up FTLD-U, which is characterized by the presence of tau-negative, ubiquitin-positive inclusions. In some cases of FTLD-U, the ubiquitinated protein is unknown; they are classified as FTLD-UPS, to indicate that the inclusions can currently only be identified by markers of the ubiquitin-proteasome system. Abbreviations: PSP, progressive supranuclear palsy; CBD, corticobasal degeneration; MSTD, multiple system tauopathy with presenile dementia; AGD, argyrophilic grain disease; GGI, globular glial inclusion; NIFID, neuronal intermediate filament inclusion disease; BIBD, basophilic inclusion body disease; UPS, ubiquitin-proteasome system.
The anatomical distribution of pathology rather than its molecular identity determines the nature of the clinical syndromes. In most cases of Alzheimer disease, the locus coeruleus, entorhinal cortex, and hippocampus are the initial targets of neurofibrillary pathology, with the neocortex becoming affected later (Braak and Del Tredici 2011). In Pick’s disease, tau inclusions predominate in the cerebral cortex, resulting in FTD (Piguet et al. 2011b). In PSP, patients with Richardson’s syndrome have a higher tau burden and a different distribution of inclusions than patients with PSP-parkinsonism (Williams et al. 2007). The subthalamic nucleus, substantia nigra, and globus pallidus are the most affected brain regions. In CBD, apraxia, rigidity, dystonia, and frontal lobe signs reflect the presence of neuronal and glial tau deposits in brainstem, basal ganglia, and cerebral cortex (Feany and Dickson 1995).
The repeats of tau form the core of the filaments whose isoform composition varies between diseases. The assembly of four-repeat tau into filaments is characteristic of PSP, CBD, and many cases of FTDP-17 (Fig. 3). It is also typical of argyrophilic grain disease and white matter tauopathy with globular glial inclusions, which belong to the FTD spectrum. A combination of neuronal and glial tau pathology is in evidence, with the glial pathology predominating in white matter tauopathy with globular glial inclusions (Kovacs et al. 2008). In contrast, in Pick’s disease and some cases of FTDP-17, three-repeat tau predominates in the neuronal inclusions (Fig. 3), whereas in Alzheimer disease, other diseases with extracellular deposits, Guam Parkinsonism-dementia complex, tangle-only dementia, and some cases of FTDP-17, both three- and four-repeat tau isoforms make up the neurofibrillary lesions. Distinct sets of tau isoforms in different neurodegenerative diseases and the presence of morphologically distinct filaments have led to the suggestion that self-propagating conformers of tau may exist (Goedert et al. 2010), akin to the prion strains accounting for the conformational variability of PrPSc (Colby and Prusiner 2011). In support, experimental evidence for the intercellular transfer of tau aggregates has been adduced (Clavaguera et al. 2009; Frost et al. 2009).
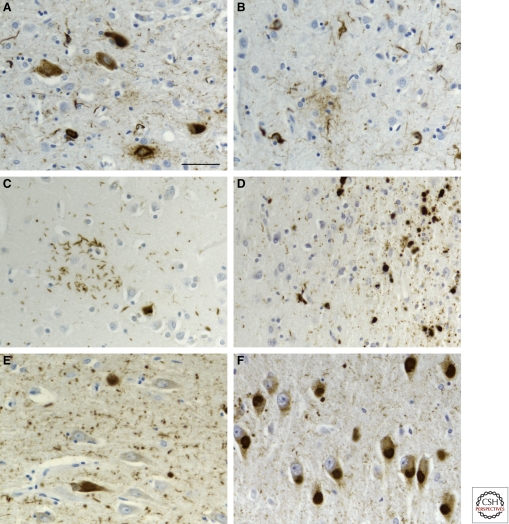
Figure 3.
FTLD-Tau. Inclusions in progressive supranuclear palsy (A,B), corticobasal degeneration (C), white matter tauopathy with globular glial inclusions (D), argyrophilic grain disease (E), and Pick’s disease (F). Progressive supranuclear palsy, corticobasal degeneration, white matter tauopathy with globular glial inclusions and argyrophilic grain disease are four-repeat tauopathies with abundant neuronal and glial tau filaments. Pick’s disease is a three-repeat tauopathy with abundant neuronal tau filaments. Scale bar, 50 µm.
FTLD-TDP
By 2006, most cases of FTLD were known to exhibit either tau-positive or tau-negative inclusions (FTLD-U) (Fig. 2). The latter were first described in patients with MND (Okamoto et al. 1991). Four histological subtypes (A–D) of FTLD-U can be distinguished (Fig. 4) (Mackenzie et al. 2006, 2011; Sampathu et al. 2006). Type A is associated with bvFTD and PFNA, type B with bvFTD and FTD-MND, type C with SD, and type D with familial inclusion body myopathy with Paget’s disease of the bone and frontotemporal dementia (IBMPFD).
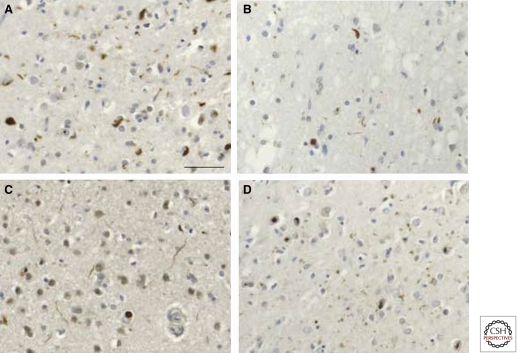
Figure 4.
FTLD-TDP: Histological subtypes (A–D). Type A is characterized by abundant TDP-43-immunoreactive compact neuronal cytoplasmic inclusions and short dystrophic neurites, often with neuronal intranuclear inclusions (A); type B by abundant compact and granular cytoplasmic inclusions (B); type C by abundant long dystrophic neurites (C); and type D by abundant lentiform neuronal intranuclear inclusions and many short dystrophic neurites (D). Scale bar, 50 μm.
In 2006, transactive response-DNA binding protein-43 (TDP-43) was identified as the major component of the inclusions in most cases of FTLD-U (Figs. 2 and 4), MND, and FTLD-MND (Arai et al. 2006; Neumann et al. 2006). Around 50% of patients with FTD have TDP-43 inclusions. They include most cases of SD, ∼45% of cases of bvFTD, as well as some cases of PNFA, LPA, FTDP-17, CBS, and PSP (Piguet et al. 2011a). Most cases of FTD-MND belong to the TDP-43 proteinopathy group. In Alzheimer disease, TDP-43 deposits are found in a minority of cases in conjunction with the characteristic plaques and tangles (Amador-Ortiz et al. 2007).
TDP-43 is a ubiquitously expressed 414 amino acid RNA-binding protein of the heterogenous nuclear ribonucleoprotein (hnRNP) family with two RNA recognition motifs, nuclear localization and export signals, and a carboxy-terminal glycine-rich region. The glycine-rich region is predicted to show a prion domain, based on an algorithm that identifies yeast prion domains (Cushman et al. 2010). TDP-43 binds to noncoding RNAs, introns, and the 3′-untranslated regions of mRNAs, indicating a role in the integration of gene regulation. It functions as a transcriptional repressor and splicing modulator. UV-crosslinking and immunoprecipitation analysis has shown that TDP-43 has thousands of potential targets, with a preference for long clusters of UG-rich intronic sequences (Polymenidou et al. 2011; Tollerey et al. 2011). It binds to ∼30% of the mouse transcriptome. TDP-43 negatively regulates its own mRNA and protein through binding to a long UG-rich region in its 3′-untranslated region (Ayala et al. 2011). It is predominantly nuclear, even though it normally shuttles between nucleus and cytoplasm. In FTD, TDP-43 it is found mainly in the cytoplasm in a hyperphosphorylated, ubiquitinated, and truncated form (Hasegawa et al. 2008).
FTLD-FUS
Most cases of FTLD-U show TDP-43 inclusions. In 2009, inclusions made of fused in sarcoma (FUS) were shown to account for the bulk of TDP-43-negative FTLD-U (Fig. 2) (Neumann et al. 2009a), following the discovery that mutations in FUS cause familial forms of ALS (Kwiatkowski et al. 2009; Vance et al. 2009). Less than 10% of cases of FTLD have FUS inclusions. They include atypical cases of FTLD-U, basophilic inclusion body disease, and neuronal intermediate filament inclusion body disease (Munoz et al. 2009; Neumann et al. 2009a,b). Neuroimaging of FTLD-FUS shows atrophy of frontoinsular and cingulate cortex, and of the head of the caudate nucleus (Josephs et al. 2010; Seelaar et al. 2010). FTD-FUS should be suspected when disease onset is before 40 years of age, in the absence of a family history of FTD, and the presence of caudate atrophy. This is reminiscent of a case from the early literature (Bonfiglio 1938). The existence of cases of PPA with FUS inclusions remains to be demonstrated.
FUS is a widely expressed 526 amino acid protein with an amino-terminal region rich in QGSY residues, a glycine-rich region, an RNA recognition motif, two RGG domains, and a zinc finger motif. Like TDP-43, it is a DNA/RNA-binding protein that is involved in transcriptional and translational regulation, as well as in mRNA splicing and transport. The predicted prion domain of FUS resides in the amino-terminal region (Cushman et al. 2010). In normal brain, FUS is concentrated in the nucleus, with smaller amounts in the cytoplasm. In FTD, the ability of FUS to shuttle to the nucleus is impaired, resulting in its cytoplasmic accumulation. FUS inclusions contain the full-length protein (Neumann et al. 2009a). Staining for TDP-43 and FUS appears to be mutually exclusive, suggestive of distinct subtypes of FTLD-U.
FTLD-UPS
FTLD-TDP and FTLD-FUS account for the majority of cases of FTLD-U. Additional forms remain to be discovered, because inclusions that are negative for TDP-43 and FUS, but positive for components of the ubiquitin-proteasome system (UPS), have been described (Fig. 2) (Holm et al. 2009).
GENETICS OF FRONTOTEMPORAL DEMENTIA
Dominantly inherited FTD is caused by mutations in seven genes. Mutations in MAPT (Hutton et al. 1998; Poorkaj et al. 1998; Spillantini et al. 1998b), chromosome 9 open reading frame 72 (C9ORF72) (DeJesus-Hernandez et al. 2011; Renton et al. 2011), and progranulin (GRN) (Baker et al. 2006; Cruts et al. 2006) are the most common. The other four genes are TARDBP (Benajiba et al. 2009; Kovacs et al. 2009), FUS (Ticozzi et al. 2009), valosin-containing protein (VCP) (Watts et al. 2004), and charged multivesicular body protein 2B (CHMP2B) (Skibinski et al. 2005).
Mutations in MAPT
Mutations in MAPT account for ∼5% of cases of FTD and are believed to cause disease through a gain of toxic function mechanism. Most mutations are located in exons 9–12 (which encode the repeats) and the adjacent introns (Fig. 1B). It remains to be determined whether the amino acid changes in codon 5 of exon 1 are pathogenic. Mutations fall into two largely nonoverlapping groups: those with a primary effect at the protein level and those influencing the alternative splicing of tau pre-mRNA. Mutations acting at the protein level change or delete single amino acids in tau. This reduces the ability of tau to interact with microtubules, suggesting that this interaction is crucial for preventing the self-assembly of tau (Hasegawa et al. 1998). Some mutations also promote the assembly of tau into filaments (Goedert et al. 1999; Nacharaju et al. 1999). Mutations having their primary effect at the RNA level are intronic or exonic and increase the alternative mRNA splicing of exon 10. This changes the ratio of 3- to 4-repeat isoforms, resulting in the relative overproduction of 4-repeat tau and the formation of filamentous inclusions made of 4-repeat tau.
Cases with MAPT mutations show abundant filamentous inclusions made of hyperphosphorylated tau in either nerve cells or in both nerve cells and glial cells. Clinical and neuropathological phenotypes similar or identical to those of Pick’s disease, PSP, CBD, and argyrophilic grain disease have been described. A given mutation can lead to different clinical syndromes in an individual family. Thus, mutation P301S in exon 10 of MAPT caused bvFTD in a father and CBD in his son (Bugiani et al. 1999), supporting the view that FTD and CBS are part of the same disease spectrum (Kertesz et al. 2000).
Haplotypes H1 and H2 characterize MAPT in populations of European descent. They result from a 900 kb inversion/noninversion (H1/H2) polymorphism (Stefansson et al. 2005). Inheritance of the H1 haplotype is a risk factor for PSP and CBD (Williams and Lees 2009). This was confirmed in a genome-wide association study of PSP, which also implicated proteins involved in vesicle traffic, the unfolded protein response and the innate immune system (Höglinger et al. 2011). Heterozygous microdeletions in the chromosomal region, which defines the H1 and H2 haplotypes, give rise to a clinical phenotype consisting of mental retardation, hypotonia, and a characteristic face (Koolen et al. 2006; Sharp et al. 2006; Shaw-Smith et al. 2006). Besides MAPT, the deleted region comprises five additional genes [corticotrophin-releasing hormone receptor 1 (CRHR1), intramembrane protease 5 (IMP5), NP 689679.1, NP 787078.1, and KIAA1267]. Deletions occur on the H2 haplotype through low-copy repeat-mediated nonallelic homologous recombination. An association has also been described between the H1 haplotype and idiopathic Parkinson’s disease (Pastor et al. 2000), a disease without tau inclusions. The elevated risk of PSP and CBD conferred by the H1 haplotype appears to promote MAPT transcription and incorporation of exon 10, resulting in increased levels of four-repeat tau (Caffrey et al. 2006).
Mutations in C9ORF72
The cause of chromosome 9p21-linked FTD-MND has been identified as a hexanucleotide (GGGGCC) expansion in the noncoding region of C9ORF72, a gene that encodes a protein of unknown function (DeJesus-Hernandez et al. 2011; Renton et al. 2011). The hexanucleotide expansion leads to the loss of an alternatively spliced transcript and the formation of nuclear RNA foci. The latter may be toxic. The repeat expansion in C9ORF72 is also a common cause of isolated FTD and MND.
Mutations in GRN
Mutations in GRN account for ∼5% of cases of FTD and cause disease by a loss of function mechanism. Progranulin is a 593 amino acid glycoprotein consisting of 7.5 tandem repeats of a 12-cysteine granulin motif. Although its function is only incompletely understood, progranulin may be a physiological antagonist of tumor necrosis α signaling (Tang et al. 2011). It has been reported to act on nerve cells by binding to sortilin following release from activated microglial cells (Hu et al. 2010). Mutations in GRN include gene deletions, as well as nonsense, frameshift, and splice-site mutations that cause premature termination, creating null alleles with the mutant RNAs being degraded by nonsense-mediated decay (Van Swieten and Heutink 2008). Known mutations result in haploinsufficiency, implying that progranulin is critical for the survival of neurons in adult brain. Reduced levels of plasma progranulin have been used to identify mutation carriers (Ghidoni et al. 2008). Mutations in GRN cause diseases belonging to the whole spectrum of FTD, with a predominance of bvFTD and PNFA (Yu et al. 2010). Parietal deficits and CBS have been observed (Spina et al. 2007). This is reflected in frontotemporoparietal cortical atrophy. Cases with GRN mutations show type A TDP-43 inclusions (Mackenzie et al. 2006), showing that a reduction in progranulin levels causes the accumulation of TDP-43. Unlike TARDBP mutations, mutations in GRN do not appear to cause MND. In a genome-wide study of FTLD-TDP, significant association was detected with three single nucleotide polymorphisms in the transmembrane protein 106B locus (TMEM106B) (Van Deerlin et al. 2010). It was most significant in patients with GRN mutations.
Mutations in TARDBP
Mutations in TARDBP are mostly associated with inherited forms of MND, consistent with the presence of TDP-43 inclusions in upper and lower motor neurons in patients with the disease (Gitcho et al. 2008; Sreedharan et al. 2008; Yokoseki et al. 2008). TARDBP mutations have also been described in two patients with bvFTD and SD who went on to develop MND (Benajiba et al. 2009). Histopathological changes were not documented. One patient with a K263E change in TARDBP developed FTD, supranuclear palsy, and chorea, in the absence of MND. Abundant neuronal and glial TDP-43 deposits were in evidence, especially in brainstem and subcortical nuclei (Kovacs et al. 2009). The mechanisms by which mutations in TARDBP cause neurodegeneration are unclear. Pathological assembly is associated with a marked reduction in nuclear TDP-43 staining (Neumann et al. 2006) and the cytoplasmic accumulation of TDP-43 is believed to be an early event (Giordana et al. 2010). A combination of loss of function and gain of toxic function mechanisms may be at play. Wild-type TDP-43 is prone to aggregation and disease-causing mutations increase its aggregation and toxicity (Johnson et al. 2009). Many disease-causing mutations are located in the carboxy-terminal domain of TDP-43.
Mutations in FUS
Mutations in FUS cause inherited forms of MND (Kwiatkowski et al. 2009; Vance et al. 2009). Patients have FUS inclusions in spinal cord and cerebral cortex. Cases of FTD and/or FTD-MND may also be caused by mutations in FUS (Ticozzi et al. 2009), but larger clinicopathological series must be awaited. Like mutant TDP-43, mutant FUS accumulates in the cytoplasm, where it is found in stress granules (Dormann et al. 2010; Nishimoto et al. 2010). Wild-type FUS is prone to aggregation, but disease-causing mutations do not increase its aggregation or toxicity (Sun et al. 2011). The mutations appear to cause cytoplasmic mislocalization instead. Although several disease-causing mutations are present in its amino-terminal region, most mutations are located in the carboxy-terminus of FUS.
Mutations in VCP
Mutations in VCP cause IBMPFD through what appears to be a gain of function (Watts et al. 2004), possibly as the result of a dominant negative effect. IBMPFD affects skeletal muscle, bone, and nervous system, with dementia developing in ∼30% of patients. It is characterized by the presence of type D TDP-43 inclusions (Neumann et al. 2007). Some missense mutations in VCP cause inherited MND (Johnson et al. 2010) and motor neuron abnormalities are present in many patients with IBMPFD. Furthermore, a missense mutation in vacuolar protein sorting 54 (Vps54), the homolog of VCP, causes motor neuron degeneration in the wobbler mouse, a model of MND (Schmitt-John et al. 2005). VCP belongs to the type II AAA+ (ATPases associated with a variety of activities) family and takes part in multiple cellular processes, including protein quality control, nuclear functions, and the regulation of membrane dynamics. It extracts ubiquitinated proteins from complexes, so that they can be degraded by the proteasome. VCP promotes autophagic protein degradation, with disease-causing mutations giving rise to defective autophagosome maturation (Ju and Weihl 2010). Transgenic mice expressing mutant VCP show many characteristics of IBMPFD, including involvement of skeletal muscle, bone and brain, and show increased activation of NF-κB signaling (Custer et al. 2010). In the brain of these mice, TDP-43 is redistributed from the nucleus to the cytoplasm, in the absence of nuclear inclusions. In a Drosophila model of IBMPFD, a genetic screen has identified TBPH, the fly ortholog of TDP-43, as one of three RNA-binding proteins that dominantly suppress degeneration (Ritson et al. 2010). In this model, VCP mutations lead to the redistribution of TDP-43 to the cytoplasm.
Mutations in CHMP2B
Mutations in CHMP2B appear to cause disease through a gain of toxic function mechanism (Skibinski et al. 2005). The early signs are those of bvFTD, with extrapyramidal symptoms developing later, resulting in a clinical picture of CBS (Gydesen et al. 2002). In a Danish family with a truncating CHMP2B mutation, the intracytoplasmic inclusions are ubiquitin-positive, but negative for TDP-43 and FUS (Holm et al. 2009). CHMP2B is a component of the endosomal-sorting complex required for transport-III (ESCRT-III), which is involved in the degradation of proteins in the endocytic and autophagic pathways. A disruption of these processes results in the accumulation of autophagosomes, possibly leading to FTD (Lee et al. 2007).
IMPLICATIONS FOR UNDERSTANDING ALZHEIMER DISEASE
For a long time, tau inclusions were believed by many to be epiphenomena of little relevance. Reasons underlying this negative stance were the absence of genetic evidence linking dysfunction of tau to neurodegeneration and the presence of tau pathology in diseases other than Alzheimer disease. Things changed with the identification of mutations in MAPT in cases of FTDP-17 with filamentous tau pathology, establishing that dysfunction of tau is sufficient to cause neurodegeneration and dementia (Hutton et al. 1998; Poorkaj et al. 1998; Spillantini et al. 1998b). Thus, a pathway leading from soluble to insoluble, filamentous tau is central to the neurodegenerative process in the human tauopathies. It is therefore important to understand the mechanisms underlying tau aggregation and its downstream consequences for cell function. With the benefit of hindsight, it is clear that Alzheimer’s description of silver-positive inclusions in cases with either presenile dementia or lobar cortical atrophy marked the beginning of the tauopathy field.
The crucial importance of FTDP-17T is that it proves that mutations in MAPT can lead to neurofibrillary assembly, neurodegeneration and dementia, in the absence of Aβ amyloid deposits. The morphologies of tau filaments observed in the various forms of FTDP-17T vary (Crowther and Goedert 2000). Some mutations, such as V337M and R406W, produce filaments that appear identical to the paired helical and straight filaments of Alzheimer disease (Spillantini et al. 1996; Reed et al. 1997; Hutton et al. 1998; Poorkaj et al. 1998). All six tau isoforms are affected by the mutations and are incorporated into the filaments, which give rise to a pattern of tau bands on SDS-PAGE identical to that seen in Alzheimer disease. Mutation G389R also affects all six tau isoforms and the majority of filaments resemble the straight filaments of Alzheimer disease (Murrell et al. 1999), despite the presence of Pick-like bodies by light microscopy. In contrast, in the case of mutations that increase the splicing of exon 10, the filaments appear as irregularly twisted ribbons, which are made of tau isoforms with four repeats (Spillantini et al. 1997). In the case of mutation P301L in exon 10, which affects only four-repeat tau isoforms, the majority of filaments consists of narrow, irregularly twisted ribbons, with a smaller number of straight filaments (Spillantini et al. 1998c).
Unlike Alzheimer disease and several other neurodegenerative diseases with tau inclusions, most cases of FTD lack extracellular deposits. However, focal Alzheimer disease pathology is diagnostic of a significant proportion of cases of PPA. Crossing mice transgenic for human mutant amyloid precursor protein with mice transgenic for human mutant tau results in increased tau deposition in some brain regions (Lewis et al. 2001). Similarly, in mice transgenic for the Danish mutant form of human BRI2 and mutant tau, the extracellular deposition of Dan-amyloid promotes tau phosphorylation and aggregation (Coomaraswamy et al. 2010). Phosphorylation of tau by GSK3β and AMP-activated protein kinase is a potential mechanism. This is consistent with the coexistence of extracellular amyloid deposits and intraneuronal tau inclusions in Alzheimer disease, familial British and Danish dementias, and in some diseases caused by mutations in the prion protein gene (Ghetti et al. 1994; Vidal et al. 2004; Goedert and Spillantini 2006). It suggests that extracellular amyloid deposits with a certain conformation trigger the intraneuronal assembly of tau into filaments.
Tau is required for Aβ toxicity in experimental models (Roberson et al. 2007). The absence of Aβ toxicity in mice lacking MAPT may result from a reduction in excitotoxicity, because of the decreased dendritic localization of the tyrosine kinase Fyn, resulting in hypophosphorylation of the NMDA receptor and a reduced interaction with postsynaptic density protein-95 (Ittner at al. 2010). Haploinsufficiency of p73, a member of the p53 protein family, has been found to be associated with the formation of tau aggregates in nerve cells and to potentiate Aβ toxicity, possibly through the activation of stress-activated protein kinases (Wetzel et al. 2008).
The intraneuronal pathology of Alzheimer disease may originate in a single cell and become self-sustaining, irrespective of upstream factors. Thus, injection of sonicated brain extract from mice with abundant tau inclusions into the cerebral cortex and hippocampus of transgenic mice lacking inclusions induces the assembly of human wild-type tau into filaments and leads to the spreading of pathology from the injection sites to neighboring brain regions (Clavaguera et al. 2009). Injection of brain extract immunodepleted of tau or divided into soluble and insoluble fractions shows that insoluble tau induces aggregation, in the absence of obvious signs of neurodegeneration. Parallel work has shown the transfer of aggregated tau between transfected cells (Frost et al. 2009). It thus appears that the tau species responsible for transmission and toxicity are not identical. An uncoupling of prion infective titre and neurotoxicity has been described (Sandberg et al. 2011).
Although tau inclusions form in many neurodegenerative diseases, their relevance for neurotoxicity remains a subject for debate. Studies using transgenic mice overexpressing human mutant tau in a conditional manner have reported a dissociation between tangle formation and nerve cell death (Santacruz et al. 2005). It appears that soluble hyperphosphorylated tau can contribute to nerve cell dysfunction prior to assembly into filaments. This is reminiscent of Drosophila and Caenorhabditis elegans lines expressing human wild-type or mutant tau, in which nerve cell loss and a reduced lifespan are observed, in the apparent absence of tau filaments (Wittmann et al. 2001; Kraemer et al. 2003). In genetic modifier screens in Drosophila, an increase in kinase activity enhanced tau toxicity, whereas an increase in phosphatase activity was beneficial (Feany et al. 2010). Activation of oxidative defences was also beneficial. In C. elegans, loss of Sut-2 (suppressor of tau pathology-2), eliminated the toxic effects of human mutant tau, possibly via an increase in autophagic clearance (Guthrie et al. 2009).
The main goal behind work on tauopathies is to transform the treatment of common neurodegenerative diseases through an understanding of the underlying molecular pathways. There is an unmet need for mechanism-based therapies of Alzheimer disease. Tau binds to microtubules and boosting this interaction may be beneficial. This may be achieved through a reduction of the hyperphosphorylation of tau (Le Corre et al. 2006). In Alzheimer disease, tau assembles into paired helical and straight filaments. The assembly pathway is being defined and inhibitors of aggregation are being developed (Pickhardt et al. 2005; Taniguchi et al. 2005). Immunotherapy has been shown to clear tau aggregates from transgenic mouse brain and to reduce functional impairment (Asuni et al. 2007). Because aggregation is a concentration-dependent process, a reduction in production and/or increased clearance of tau are also potential targets (Morris et al. 2011).
Footnotes
Editors: Dennis J. Selkoe, Eckhard Mandelkow, and David M. Holtzman
Additional Perspectives on The Biology of Alzheimer Disease available at www.perspectivesinmedicine.org
REFERENCES
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR 2007. Focal cortical presentations of Alzheimer’s disease. Brain 130: 2636–2645 [DOI] [PubMed] [Google Scholar]
- Alzheimer A 1907. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat 64: 146–148 [Google Scholar]
- Alzheimer A 1911. Über eigenartige Krankheitsfälle des späteren Alters. Z ges Neurol Psychiat 4: 356–385 [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW 2007. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61: 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. 2006. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611 [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quatermain D, Sigurdsson EM 2007. Immunotherapy targeting pathological tau conformers in a transgenic mouse model reduces brain pathology associated with functional improvements. J Neurosci 27: 9115–9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YM, De Conti L, Avendano-Vázquez SE, Dhir A, Romano M, D’Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, et al. 2011. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J 30: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. 2006. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442: 916–919 [DOI] [PubMed] [Google Scholar]
- Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, Legallic S, Salachas F, Hannequin D, Decousus M, et al. 2009. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol 65: 470–474 [DOI] [PubMed] [Google Scholar]
- Bonfiglio F 1938. Die umschriebene Atrophie der Basalganglien. Z Neurol 160: 306–333 [Google Scholar]
- Braak H, Del Tredici K 2011. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121: 171–181 [DOI] [PubMed] [Google Scholar]
- Brion JP, Passareiro H, Nunez J, Flament-Durand J 1985. Mise en évidence immunologique de la protéine tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Arch Biol 95: 229–235 [Google Scholar]
- Broca P 1861. Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau. Bull Soc Anthropol 2: 235–238 [Google Scholar]
- Brun A 1987. Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Arch Gerontol Geriatr 6: 193–208 [DOI] [PubMed] [Google Scholar]
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, et al. 1999. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in Tau. J Neuropathol Exp Neurol 58: 667–677 [DOI] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R 2006. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet 15: 3529–3537 [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11: 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Prusiner SB 2011. Prions. Cold Spring Harb Perspect Biol 3: a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis J, Richard J, Tissot R 1974. Pick’s disease. Histological and clinical correlations. Eur Neurol 11: 208–217 [DOI] [PubMed] [Google Scholar]
- Coomaraswamy J, Kilger E, Wölfing H, Schäfer C, Kaeser SA, Wegenast-Braun BM, Hefendehl JK, Wolburg H, Mazzella M, Ghiso J, et al. 2010. Modeling familial Danish dementia in mice supports the concept of the amyloid hypothesis of Alzheimer’s disease. Proc Natl Acad Sci 107: 7969–7974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA, Goedert M 2000. Abnormal tau-containing filaments in neurodegenerative diseases. J Struct Biol 130: 271–279 [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, et al. 2006. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442: 920–924 [DOI] [PubMed] [Google Scholar]
- Cushman M, Johnson BS, King OD, Gitler AD, Shorter J 2010. Prion-like disorders: Blurring the divide between transmissibility and infectivity. J Cell Sci 123: 1191–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer SK, Neumann M, Lu H, Wright AC, Taylor JP 2010. Transgenic mice expressing mutant forms of VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Hum Mol Genet 19: 1741–1755 [DOI] [PubMed] [Google Scholar]
- Déjerine J, Sérieux P 1897. Un cas de surdité verbale pure terminée par aphasie sensorielle, suivi d’autopsie. CR Acad Sci Paris 49: 1074–1077 [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. 2011. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IRA, Capell A, Schmid B, et al. 2010. ALS-associated fused in sarcoma (FUS) mutations disrupt transportin-mediated nuclear import. EMBO J 29: 21841–21857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB 2010. New approaches to the pathology and genetics of neurodegeneration. Am J Pathol 176: 2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Dickson DW 1995. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146: 1388–1396 [PMC free article] [PubMed] [Google Scholar]
- Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D’Amato CJ, Gilman S 1997. Frontotemporal dementia and parkinsonism linked to chromosome 17: A consensus conference. Ann Neurol 41: 706–715 [DOI] [PubMed] [Google Scholar]
- Freud S 1891. Zur Auffassung der Aphasien. Franz Deuticke, Leipzig und Wien [Google Scholar]
- Frost B, Jacks RL, Diamond MI 2009. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YJ, Nishihara Y, Kuroda S, Toyoshima Y, Ishihara T, Shinozaki M, Miyashita A, Piao YS, Tan CF, Tani T, et al. 2010. Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: A distinct neuropathological and biochemical disease entity. Acta Neuropathol 120: 21–32 [DOI] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, et al. 2011. White matter damage in primary progressive aphasias: A diffusion tensor tractography study. Brain 134: 3011–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR 2000. Atypical and typical presentations of Alzheimer’s disease: A clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123: 484–498 [DOI] [PubMed] [Google Scholar]
- Gans A 1923. Betrachtungen über Art und Ausbreitung des krankhaften Prozesses in einem Fall von Pickscher Atrophie des Stirnhirns. Z Neurol 80: 10–28 [Google Scholar]
- Ghetti B, Tagliavini F, Giaccone G, Bugiani O, Frangione B, Farlow MR, Dlouhy SR 1994. Familial Gerstmann-Sträussler-Scheinker disease with neurofibrillary tangles. Mol Neurobiol 8: 41–48 [DOI] [PubMed] [Google Scholar]
- Ghetti B, Wszolek ZW, Boeve BF, Spina S, Goedert M 2011. Frontotemporal dementia and parkinsonism linked to chromosome 17. In Neurodegeneration: The molecular pathology of dementia and movement disorders, 2nd ed. (ed. Dickson D, Weller RO), pp. 110–134 Blackwell, Oxford, UK [Google Scholar]
- Ghidoni R, Benussi L, Glionna M, Franzoni M, Binetti G 2008. Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology 71: 1235–1239 [DOI] [PubMed] [Google Scholar]
- Giordana MT, Piccinini M, Grifoni S, De Marco G, Vercellino M, Magistrello M, Pellerino A, Buccinna B, Lupino E, Rinaudo MT 2010. TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol 20: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL, Bigio EH, Caselli R, et al. 2008. TDP-43 A315 mutation in familial motor neuron disease. Ann Neurol 63: 535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG 2006. A century of Alzheimer’s disease. Science 314: 777–781 [DOI] [PubMed] [Google Scholar]
- Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A 1988. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci 85: 4051–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA 1989a. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: Differential expression of tau protein mRNAs in human brain. EMBO J 8: 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA 1989b. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3: 519–526 [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA 1992. Tau proteins of Alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron 8: 159–168 [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA 1999. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett 450: 306–311 [DOI] [PubMed] [Google Scholar]
- Goedert M, Clavaguera F, Tolnay M 2010. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci 33: 317–325 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL 2004a. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase 10: 426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL 2004b. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55: 335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL 2008. The logopenic/phonological variant of primary progressive aphasia. Neurology 71: 1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, et al. 2011. Classification of primary progressive aphasia and its variants. Neurology 76: 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding XS, Alavi A, Reivich M 1996. Progressive nonfluent aphasia: Language, cognitive, and PET mesasures contrasted with probable Alzheimer disease. J Cogn Neurosci 8: 135–154 [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI 1986. Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci 83: 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünthal E 1930. Über ein Brüderpaar mit Pickscher Krankheit. Z Neurol 129: 350–375 [Google Scholar]
- Gustafson L 1987. Frontal lobe degeneration of non-Alzheimer type. II. Clinical picture and differential diagnosis. Arch Gerontol Geriatr 6: 209–223 [DOI] [PubMed] [Google Scholar]
- Guthrie CR, Schellenberg GD, Kraemer BC 2009. SUT-2 potentiates tau-induced neurotoxity in Caenorhabditis elegans. Hum Mol Genet 18: 1825–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gydesen S, Brown JM, Brun A, Chakrabarti L, Gade A, Johannsen P, Rossor M, Thusgaard T, Grove A, Yancopoulou D, et al. 2002. Chromosome 3 linked frontotemporal dementia (FTD-3). Neurology 59: 1585–1594 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Smith MJ, Goedert M 1998. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 437: 207–210 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, et al. 2008. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64: 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K 2007. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurol 6: 1004–1014 [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Melhem NM, Dickson D, Sleiman PMA, Wang LS, Klei L, Rademakers R, de Silva R, Litvan I, Riley DC, et al. 2011. Common variants affect risk for the tauopathy progressive supranuclear palsy. Nat Genet 43: 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm IE, Isaacs AM, Mackenzie IRA 2009. Absence of FUS-immunoreactive pathology in frontotemporal dementia linked to chromosome 3 (FTD-3) caused by mutation in the CHMP2B gene. Acta Neuropathol 118: 719–720 [DOI] [PubMed] [Google Scholar]
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM 2010. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68: 654–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu M, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. 1998. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702–705 [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, Van Eersel J, Wölfing H, Chieng BC, Christie J, Napier IA, et al. 2010. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142: 387–397 [DOI] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW 1999. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol 56: 1233–1239 [DOI] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery MM, Shorter J, Gitler AD 2009. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and toxicity. J Biol Chem 284: 20329–20339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, et al. 2010. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68: 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW 2007. Frontotemporal lobar degeneration with upper motor neuron disease/primary lateral sclerosis. Neurology 69: 1800–1801 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, et al. 2006. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129: 1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Parisi JE, Petersen RC, Boeve BF, Jack CR, Dickson DW 2010. Caudate atrophy on MRI is a characteristic feature of FTLD-FUS. Eur J Neurol 17: 969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju JS, Weihl CC 2010. Inclusion body myopathy, Paget’s disease of the bone and frontotemporal dementia: A disorder of autophagy. Hum Mol Genet 19: R38–R45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Hudson L, Mackenzie IRA, Munoz DG 1994. The pathology and nosology of primary progressive aphasia. Neurology 44: 2065–2072 [DOI] [PubMed] [Google Scholar]
- Kertesz A, Martinez-Lage P, Davidson W, Munoz DG 2000. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 55: 1368–1375 [DOI] [PubMed] [Google Scholar]
- Koolen DA, Vissers LELM, Pfundt R, de Leeuw N, Knight SJL, Regan R, Kooy RF, Reyniers E, Romano C, Fichera M, et al. 2006. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet 38: 999–1001 [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Majtenyi K, Spina S, Murrell JR, Gelpi E, Höftberger R, Fraser G, Crowther RA, Goedert M, Budka H, et al. 2008. White matter tauopathy with globular glial inclusions: A distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67: 963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, Budka H, Ghetti B, Spina S 2009. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 24: 1843–1847 [DOI] [PubMed] [Google Scholar]
- Kraemer BC, Zhang B, Leverenz JB, Thomas JH, Trojanowski JQ, Schellenberg GD 2003. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci 100: 9980–9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. 2009. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323: 1205–1211 [DOI] [PubMed] [Google Scholar]
- Le Corre S, Klafki HW, Plesnila N, Hübinger G, Obermeier A, Sahagún H, Monse B, Seneci P, Lewis J, Eriksen J, et al. 2006. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci 103: 9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VMY, Balin BJ, Otvos L, Trojanowski JQ 1991. A68—a major subunit of paired helical filaments and derivatized forms of normal tau. Science 251: 675–678 [DOI] [PubMed] [Google Scholar]
- Lee JA, Beigneux A, Tariq Ahmad S, Young SG, Gao FB 2007. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol 17: 1561–1567 [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, et al. 2001. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293: 1487–1491 [DOI] [PubMed] [Google Scholar]
- Lippa CF, Cohen R, Smith TW, Drachman DA 1991. Primary progressive aphasia with focal neuronal achromasia. Neurology 41: 882–886 [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, et al. 1996. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 47: 1–9 [DOI] [PubMed] [Google Scholar]
- Lüers T, Spatz H 1957. Picksche Krankheit. In Handbuch der speziellen Anatomie und Histologie (ed. Henke F, Lubarsch O), Vol. 13, pp. 614–715 Springer, Berlin [Google Scholar]
- Mackenzie IRA, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA 2006. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration. Classification and relation to clinical phenotype. Acta Neuropathol 112: 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DMA, Lee VMY 2011. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122: 111–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM 1982. Slowly progressive aphasia without generalized dementia. Ann Neurol 11: 592–598 [DOI] [PubMed] [Google Scholar]
- Mesulam MM 1987. Primary progressive aphasia—differentiation from Alzheimer’s disease. Ann Neurol 22: 533–534 [DOI] [PubMed] [Google Scholar]
- Mesulam MM 2001. Primary progressive aphasia. Ann Neurol 49: 425–432 [PubMed] [Google Scholar]
- Mesulam MM, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, Weintraub S, Bigio EH 2008. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 63: 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A 1929. Über eine der amyotrophischen Lateralsklerose nahestehende Erkrankung mit psychischen Störungen. Z Neurol 121: 107–128 [Google Scholar]
- Migliaccio R, Agosta F, Rascovsky K, Karydas A, Bonasera S, Rabinovici GD, Miller BL, Gorno-Tempini ML 2009. Clinical syndromes associated with posterior atrophy. Neurology 73: 1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Kaiya H, Ikeda T, Namba M 1987. Presenile dementia combined with amyotrophy: A review of 34 Japanese cases. Arch Gerontol Geriatr 6: 263–277 [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L 2011. The many faces of tau. Neuron 70: 410–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJS, Vandenberghe R, Price CJ, Hodges JR 1999. Disrupted temporal lobe connections in semantic dementia. Brain 122: 61–73 [DOI] [PubMed] [Google Scholar]
- Munoz DG, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, Kuroda S, Mackenzie IR 2009. FUS pathology in basophilic inclusion body disease. Acta Neuropathol 118: 617–627 [DOI] [PubMed] [Google Scholar]
- Murrell JR, Spillantini MG, Zolo P, Guazzelli M, Smith MJ, Hasegawa M, Redi F, Crowther RA, Pietrini P, Ghetti B, et al. 1999. Tau gene mutation G389R causes a tauopathy with abundant Pick body-like inclusions and axonal deposits. J Neuropathol Exp Neurol 58: 1207–1226 [DOI] [PubMed] [Google Scholar]
- Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH 1999. Accelerated filament formation from tau protein with specific FTDP-17 mutations. FEBS Lett 447: 195–199 [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Northen B, Goulding P 1988. Dementia of frontal type. J Neurol Neurosurg Psychiatry 51: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, et al. 2003. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain 126: 2406–2418 [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR 2003. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain 126: 2406–2418 [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. 2006. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133 [DOI] [PubMed] [Google Scholar]
- Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS 2007. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol 66: 152–157 [DOI] [PubMed] [Google Scholar]
- Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA 2009a. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132: 2922–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann A, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IRA 2009b. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 118: 605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto Y, Ito D, Yagi T, Nihei Y, Tsunoda Y, Suzuki N 2010. Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43. J Biol Chem 285: 608–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Hirai S, Yamazaki T, Sun X, Nakazato Y 1991. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129: 233–236 [DOI] [PubMed] [Google Scholar]
- Onari K, Spatz H 1926. Anatomische Beiträge zur Lehre von der Pickschen umschriebenen Grosshirnrinden-Atrophie (“Picksche Krankheit”). Z Neurol 101: 470–511 [Google Scholar]
- Pastor P, Ezquerra M, Munoz E, Marti MJ, Blesa R, Tolosa E, Oliva R 2000. Significant association between the tau gene A0/A0 genotype and Parkinson’s disease. Ann Neurol 47: 242–245 [PubMed] [Google Scholar]
- Pick A 1892. Ueber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager med Wschr 17: 165–167 [Google Scholar]
- Pick A 1901. Senile Hirnatrophie als Grundlage von Herderscheinungen. Wiener klin Wschr 14: 403–404 [Google Scholar]
- Pick A 1904. Zur Symptomatologie der linksseitigen Schläfenlappenatrophie. Mschr Psychiat Neurol 16: 378–388 [Google Scholar]
- Pick A 1906. Über einen weiteren Symptomencomplex im Rahmen der Dementia senilis, bedingt durch umschriebene stärkere Hirnatrophie (gemischte Apraxie). Mschr Psychiat Neurol 19: 97–108 [Google Scholar]
- Pickhardt M, Gazova Z, von Bergen M, Khlistunova I, Wang Y, Hascher A, Mandelkow EM, Biernat J, Mandelkow E 2005. Anthraquinones inhibit tau aggregation and dissolve Alzheimer paired helical filaments in vitro and in cells. J Biol Chem 280: 3628–3635 [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR 2011a. Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurol 10: 162–172 [DOI] [PubMed] [Google Scholar]
- Piguet O, Halliday GW, Reid WGJ, Casey B, Carman R, Huang Y, Xuereb JH, Hodges JR, Kril JJ 2011b. Clinical phenotypes in autopsy-confirmed Pick disease. Neurology 76: 253–259 [DOI] [PubMed] [Google Scholar]
- Pollock NJ, Mirra SS, Binder LI, Hansen LA, Wood JG 1986. Filamentous aggregates in Pick’s disease, progressive supranuclear palsy, and Alzheimer’s disease share antigenic determinants with microtubule-associated protein, tau. Lancet 328: 1211. [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, et al. 2011. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci 14: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD 1998. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43: 815–825 [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, et al. 2008. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 64: 388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz JJ, Kolodny EM, Richardson EP 1968. Corticodentonigral degeneration with neuronal achromasia. Arch Neurol 18: 20–33 [DOI] [PubMed] [Google Scholar]
- Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, et al. 1997. Autosomal dominant dementia with widespread neurofibrillary tangles. Ann Neurol 42: 564–572 [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, Van Swieten JC, Myllykangas L, et al. 2011. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H 1918. Eine besondere Art von Stirnhirnschwund mit Verblödung. Z Neurol 38: 127–159 [Google Scholar]
- Ritson GP, Custer SK, Freibaum BD, Guinto JB, Geffel D, Moore J, Tang W, Winton MJ, Neumann M, Trojanowski JQ, et al. 2010. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J Neurosci 30: 7729–7739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L 2007. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316: 750–754 [DOI] [PubMed] [Google Scholar]
- Rossor MN, Fox NC, Mummery CM, Schott JM, Warren JD 2010. The diagnosis of young-onset dementia. Lancet Neurol 9, 793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VMY 2006. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169: 1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J 2011. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature 470: 540–542 [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, KKKotilinek L, Ingelsson M, Guimares A, DeTure M, Ramsden M, McGowan E, et al. 2005. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309: 476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-John T, Drepper C, Mussmann A, Hahn P, Kuhlmann M, Thiel C, Hafner M, Lengeling A, Heimann P, Jones JM, et al. 2005. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet 37: 1213–1215 [DOI] [PubMed] [Google Scholar]
- Schneider C 1927. Über Picksche Krankheit. Mschr Psychiat Neurol 65: 230–275 [Google Scholar]
- Schneider C 1929. Weitere Beiträge zur Lehre von der Pickschen Krankheit. Z Neurol 120: 340–384 [Google Scholar]
- Seelaar H, Klijnsma KY, de Koning I, Van der Lugt A, Chiu WZ, Azmani A, Rozemuller AJM, Van Swieten JC 2010. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol 257: 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, Hurst JA, Stewart H, Price SM, Blair E, Hennekam RC, et al. 2006. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet 38: 1038–1042 [DOI] [PubMed] [Google Scholar]
- Shaw CE 2010. Capturing VCP: Another molecular piece in the ALS jigsaw puzzle. Neuron 68: 812–814 [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, et al. 2006. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet 38: 1032–1037 [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Charkrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, et al. 2005. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37: 806–808 [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D 1989. Semantic dementia: A form of circumscribed atrophy. Behav Neurol 2: 167–182 [Google Scholar]
- Spillantini MG, Crowther RA, Goedert M 1996. Comparison of the neurofibrillary pathology in Alzheimer’s disease and familial presenile dementia with tangles. Acta Neuropathol 92: 42–48 [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B 1997. Familial multiple system tauopathy with presenile dementia: A disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci 94: 4113–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Bird TD, Ghetti B 1998a. Frontotemporal dementia and parkinsonism linked to chromosome 17: A new group of tauopathies. Brain Pathol 8: 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B 1998b. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci 95: 7737–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Kamphorst W, Heutink P, Van Swieten JC 1998c. Tau pathology in two Dutch families with mutations in the microtubule-binding region of tau. Am J Pathol 153: 1359–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina S, Murrell JR, Huey ED, Wassermann EM, Pietrini P, Grafman J, Ghetti B 2007. Corticobasal syndrome associated with the A90D progranulin mutation. J Neuropathol Exp Neurol 66: 892–900 [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, et al. 2008. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319: 1668–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Helgason A, Thorleifsson, Steintorsdottir V, Masson G, Barnard J, Baker A, Jonasdottir A, Ingason A, Gudnadottir VG, et al. 2005. A common inversion under selection in Europeans. Nat Genet 37: 129–137 [DOI] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J 1964. Progressive supranuclear palsy. Arch Neurol 10: 333–359 [DOI] [PubMed] [Google Scholar]
- Stertz G 1926. Über die Picksche Atrophie. Z Neurol 101: 729–749 [Google Scholar]
- Strittmatter WJ, Roses AD 1995. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci 92: 4725–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Diaz Z, Fang X, Hart MP, Chesi A, Shorter J, Gitler AD 2011. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol 9: e1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, Syed NM, Lai Y, Lin EA, Kong L, et al. 2011. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332: 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S, Suzuki N, Masuda M, Hisanaga SI, Iwatsubo T, Goedert M, Hasegawa M 2005. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols and porphyrins. J Biol Chem 280: 7614–7623 [DOI] [PubMed] [Google Scholar]
- Ticozzi N, Silani V, LeClerc AL, Keagle P, Gellera C, Ratti A, Taroni F, Kwiatkowski TJ, McKenna-Yasek DN, Sapp PC, et al. 2009. Analysis of FUS gene mutation in familial amyotrophic lateral sclerosis within an Italian cohort. Neurology 73: 1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Riese M, Cereda M, Kayikci M, König J, Hortobágyi T, Nishimura AL, Zupunski V, et al. 2011. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci 14: 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, et al. 2006. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.p2–21.3. Brain 129: 868–876 [DOI] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nihimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. 2009. Mutations in FUS, an RNA processing protein, cause familial amyotrophic sclerosis type 6. Science 323: 1208–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Sleiman PMA, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, et al. 2010. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42: 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier WM, Pijnenburg YAL, Fox NC, Scheltens P 2011. Early-onset versus late-onset Alzheimer’s disease: The case of the missing APOE ε4 allele. Lancet Neurol 10: 280–288 [DOI] [PubMed] [Google Scholar]
- Van Mansvelt J 1954. Pick’s disease. MJvd Loeff, Enschede [Google Scholar]
- Van Swieten JC, Heutink P 2008. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol 7: 965–974 [DOI] [PubMed] [Google Scholar]
- Verhaart WJC 1930. Over de ziekte van Pick. Nederl Tijdschr Geneesk 74: 5586–5598 [Google Scholar]
- Vidal R, Delisle MB, Ghetti B 2004. Neurodegeneration caused by proteins with an aberrant carboxyl-terminus. J Neuropathol Exp Neurol 63: 787–800 [DOI] [PubMed] [Google Scholar]
- Von Braunmühl A 1932. Picksche Krankheit und amyotrophische Lateralsklerose. Allg Z Psychiat 96: 364–366 [Google Scholar]
- Von Braunmühl A, Leonhard K 1934. Über ein Schwesternpaar mit Pickscher Krankheit. Z Neurol 150: 209–241 [Google Scholar]
- Watts GDJ, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE 2004. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36: 377–381 [DOI] [PubMed] [Google Scholar]
- Wernicke C 1874. Der aphasische Symptomencomplex: Eine psychologische Studie auf anatomischer Basis. Max Cohn & Weigert, Breslau [Google Scholar]
- Wetzel MK, Naska S, Laliberte CL, Rymar VV, Fujitani M, Biernaskie JA, Cole CJ, Lerch JP, Spring S, Wang SH, et al. 2008. p73 regulates neurodegeneration and phospho-tau accumulation in aging and Alzheimer’s disease. Neuron 59: 708–721 [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Senjem ML, Josephs KA 2006. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology 66: 102–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, Senjem ML, Shiung MM, Boeve BF, Knopman DS, et al. 2009a. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: A cluster analysis study. Brain 132: 2932–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Senjem ML, Baker M, Rademakers R, Ivnik RJ, Knopman DS, Wszolek ZK, Petersen RC, et al. 2009b. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 72: 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, et al. 2010a. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 74: 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, Senjem ML, Knopman DS, Petersen RC, Dickson DW, et al. 2010b. Imaging correlates of pathology in corticobasal syndrome. Neurology 75: 1879–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen KC, Lynch T, Pavlou E, Higgins M, Nygaard TG 1994. Localization of disinhibition-dementia-parkinsonism-amyotrophy complex to 17q21–22. Am J Hum Genet 55: 1159–1164 [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Lees AJ 2009. Progressive supranuclear palsy: Clinicopathological concepts and diagnostic challenges. Lancet Neurol 8: 270–279 [DOI] [PubMed] [Google Scholar]
- Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T 2007. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130: 1566–1576 [DOI] [PubMed] [Google Scholar]
- Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A 1988. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci 85: 4506–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek ZF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB 2001. Tauopathy in Drosophila: Neurodegeneration without neurofibrillary tangles. Science 293: 711–714 [DOI] [PubMed] [Google Scholar]
- Yokoseki A, Shiga A, Tan CF, Tagawa A, Kaneko H, Koyama A, Eguchi H, Tsujino A, Ikeuchi T, Kakita A, et al. 2008. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol 63: 538–542 [DOI] [PubMed] [Google Scholar]
- Yu CE, Bird TD, Bekris LM, Montine TJ, Leverenz JB, Steinbart E, Galloway NM, Feldman H, Woltjer R, Miller CA, et al. 2010. The spectrum of mutations in progranulin. Arch Neurol 67: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133: 1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]