Abstract
The steadily increasing frequency of insulin-dependent diabetes in several countries is best explained today by the decline of infections. Epidemiologic and animal data support this conclusion, which, however, requires confirmation by intervention trials in man. The mechanisms of the protective effect of infections on diabetes onset are diverse including competition for homeostatic factors and stimulation of regulatory T cells and of Toll-like receptors. These considerations might have interesting therapeutic applications for the prevention of the disease.
The decline of infectious disease over the past several decades may explain the increased frequency of insulin-dependent diabetes in developed countries.
Converging evidence suggests that the frequency of insulin-dependent diabetes (IDDM) is steadily increasing both in industrialized and developing countries (Bach 2002; Okada et al. 2010). This long-standing trend started in the 1970s in industrialized countries, and its current persistence is worrisome. It has led to a high incidence of IDDM in certain countries such as Finland, where the disease has indeed become a public health problem. Not only has IDDM incidence increased, but it is also affecting younger and younger children (under 5 and even under 2 yr of age, which had not been previously observed) (Patterson et al. 2009).
Several hypotheses have been put forward to explain this increase in frequency. Exposure to diabetes-causing viruses is a theoretical possibility despite the fact that no virus has been formally associated with the etiology of the disease and that it is difficult to see how such a virus could cause a global epidemic instantaneously. Changes in exposure to some environmental or chemical factors are also a potential cause, but this possibility is not a serious contender at the global level (Hettiarachchi et al. 2008). Variations in vitamin D intake have been suggested on the basis that vitamin D has a preventive effect on the disease in some experimental models (Adorini 2004). Collecting good epidemiological data on the subject has proven difficult.
In recent years, attention has been mostly focused on the possibility that changes in lifestyle are a major factor in the rise of IDDM frequency, as well as other immune diseases such as autoimmune diseases, allergic diseases (Bach 2002), and malignant proliferation of certain lymphoid cells (Greaves and Buffler 2009). Numerous lifestyle elements have changed in the last 20 years, and it is difficult to identify which may be the determinant factor. According to the hygiene hypothesis discussed in this article, the decline in infectious diseases as a result of better hygiene, health, and medical conditions plays a major role.
GEOGRAPHICAL DATA
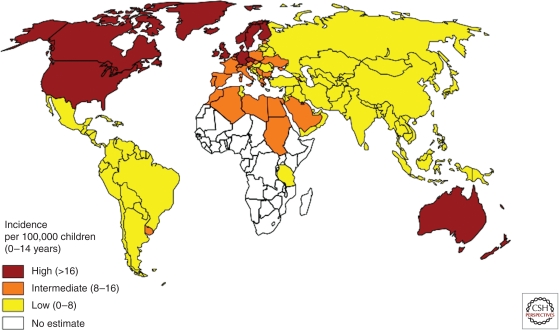
IDDM frequency varies considerably from one country to another (Fig. 1). In Europe, there is a marked North–South gradient that continues into Africa. IDDM occurs with high frequency in the Scandinavian countries, less so in Southern Europe, and is barely present in Africa. Similar data have been collected in North America, from Canada to Mexico.
Figure 1.
Frequency of Type 1 diabetes worldwide. Incidence of type 1 diabetes in children 0–14 yr. The data used in map creation are from www.eatlas.idf.org.
The fact that such geographical variations have been found in the case of other autoimmune diseases such as multiple sclerosis and inflammatory bowel disease is highly significant. It has also been found for allergic diseases, although the situation is more complex, probably because allergen exposure varies from one country to another. For instance, the frequency of allergic diseases is high in Brazil but is only moderate for autoimmune diseases.
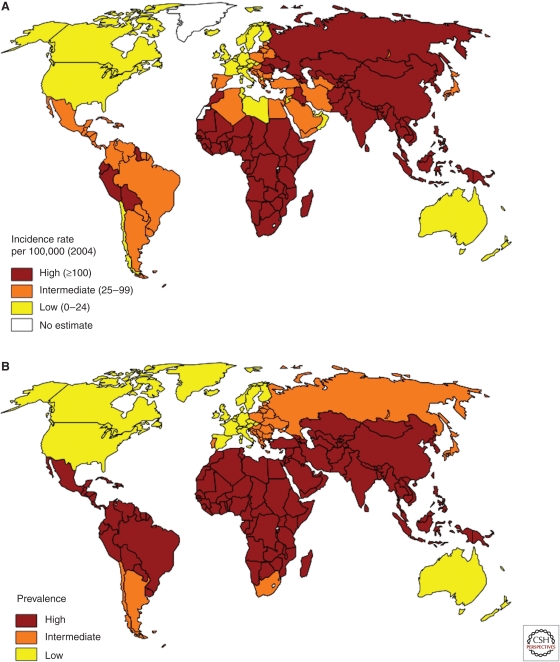
The geographical map of immune disease frequency is the mirror image of that for infectious disease distribution. Tuberculosis and childhood diarrheal diseases are more frequent in the southern countries compared with those of the north (Fig. 2). In the case of tuberculosis, a negative correlation between the frequency of the disease and IDDM has been directly shown (Airaghi and Tedeschi 2006).
Figure 2.
Frequency of tuberculosis and of childhood diarrheal diseases worldwide. (A) Prevalence of tuberculosis (the data used in map creation are from www.cdc.gov). (B) Incidence of childhood diarrheal diseases (the data used in map creation are from www.cdc.gov).
The variations mentioned above among countries and world regions had also been found in smaller geographical areas. The frequency of IDDM and allergic diseases is different in Finnish and Russian Karelia, two neighboring countries with the same ethnic background separated by only a few dozen kilometers. Finland has a threefold higher diabetes incidence and a fivefold higher allergy incidence (Kondrashova et al. 2005, 2007; Laatikainen et al. 2011). This is explained probably not by a North–South gradient but by a difference in social and economic development.
The variation in disease frequency among various countries suggests first of all the involvement of a genetic factor. In the case of diabetes, HLA susceptibility genes (specifically HLA-DR3) are less frequent in Japan than in Europe, which correlates with a lower IDDM frequency in Japan (Todd et al. 1990). Similarly, it is interesting to note that the abnormally high frequency of IDDM (and multiple sclerosis) in Sardinia compared with the rest of Italy is in great part due to a genetic factor. This is evidenced by the high frequency of disease among Sardinians living on the continent, in Lazio, where the frequency of the disease is lower (Muntoni et al. 1997). However, migration studies have shown that genetic differences only partially explain the differences in IDDM incidence among countries. In families that emigrated from countries with low IDDM incidence to a high IDDM country, the frequency of the disease increases sharply in first-generation migrants. This was observed for several migrant populations, in particular, Pakistanis in England (Bodansky et al. 1992; Soderstrom et al. 2011). Similar observations were made for multiple sclerosis (Ascherio and Munger 2008) and inflammatory bowel diseases (Cosnes et al. 2011). Within the United States, moving from Northern to Southern states, and vice versa, has an impact on the frequency of disease onset (Ascherio and Munger 2008). Although these observations are remarkable, they are only correlations. It is difficult a priori to assert that the increase in IDDM frequency affecting Pakistanis migrating to England is due to the loss of a protective factor present in their home environment or to the presence of an unfavorable factor in their English environment. There are many other lifestyle differences between Pakistan and England, in particular, food, housing conditions, and exposure to different chemical products.
EPIDEMIOLOGICAL EVIDENCE SUGGESTING THAT INFECTIONS HAVE A ROLE IN CONTROLLING THE ONSET OF IDDM AND OF AUTOIMMUNE AND ALLERGIC DISEASES IN GENERAL
The most remarkable argument in support of the hygiene hypothesis has been the reverse trend observed between infectious disease incidence and the incidence of autoimmune and allergic diseases described above (Bach 2002; Masoli et al. 2004). Other evidence can be found with a more detailed epidemiological analysis.
There exists a negative correlation between hygiene conditions and IDDM incidence. A study in Northern Ireland has shown that the lowest IDDM frequency was found in areas with the poorest hygiene conditions (among which are household crowding and inadequate sanitation) (Patterson et al. 1996).
It has also been shown that IDDM incidence rate is higher in children who have had the lowest level of exposure to common infectious diseases. This is the case of firstborns because they are exposed much later to infections brought into the home by their siblings. This has been observed for allergic diseases, which have been found to be more frequent in children who attended day care or school at a later age (Strachan 1989).
These data again show only correlations and are not proof of a causal relation. The nature of the infections most frequently found has not been clearly stated. Studies have reported a major role for common childhood infections, but the role of more severe infectious diseases cannot be ruled out. The question has been raised as to whether vaccinations have had an effect on IDDM frequency. No serious data that supports this notion has been obtained. The data published by the group of Classen suggesting an increased frequency of IDDM after BCG administration are not convincing (Classen and Classen 2003). As is seen below, in fact, vaccines may have a protective role.
INSIGHTS FROM EXPERIMENTAL MODELS
Experimental models for autoimmune diseases, among which is IDDM, offer the great advantage of allowing deliberate interventions rarely possible in humans. Experiments on non-obese diabetic (NOD) mice and Bio-Breeding (BB) rats are of great importance with regard to the hygiene hypothesis.
NOD mice spontaneously develop diabetes at the age of 2–3 mo, more frequently in females than males. The incidence of the disease and the rapidity of onset mainly depend on the way the animals are reared, in particular, their sanitary conditions. We and others have observed that when sanitary conditions are poor, the frequency of the disease decreases. It is possible to restore a high disease incidence both in females and males by decontamination by Caesarean delivery and raising newborns in isolators (Bach 2002). The effect is observed within the first generation. Inversely, it is possible to completely prevent diabetes onset by deliberately infecting mice previously raised in a clean environment with various infective agents such as bacteria (mycobacteria [Sadelain et al. 1990], viruses (murine hepatitis virus [Wilberz et al. 1991], lactate dehydrogenase virus [Takei et al. 1992], etc.), and parasites (pinworms [J-F Bach and L Chatenoud, unpubl.], schistosomes [Zaccone et al. 2009], etc.). Interestingly, the protective effect is clearer for long-term infections. Acute infection by the Nippostrongylus brasiliensis parasite, which induces a strong but transient TH2 reaction with a high percentage of eosinophils, does not confer protection (J-F Bach and L Chatenoud, unpubl.). Breeding BB rats in a germ-free facility also increases diabetes frequency, whereas the disease is prevented by various infections.
Similar data have been reported with other experimental models for autoimmune and allergic diseases. A Plasmodium infection completely prevents the development of systemic lupus erythematosus in F1 mice (NZB × NZW) (Greenwood et al. 1970). In spite of the explanations put forward (e.g., difference between spontaneous and induced models, complications associated with the use of transgenic mice with totally or partially deficient immunoregulation), the consistency of the data obtained with the induced models is not as good as in the case of spontaneous diabetes.
PRELIMINARY EVIDENCE FROM HUMAN THERAPEUTIC TRIALS
The best way to show a causal relationship between decreased infection and increased diabetes frequency, and more generally autoimmune and allergic disease frequencies, is to prove that a deliberate and well-characterized suppression of infections triggers a rise in autoimmunity or allergy. To date, no results are available for IDDM. Convincing evidence was collected in the case of atopic diseases. Helminthiases treatment in randomized therapeutic trials performed in countries of high incidence of these parasitic diseases has shown that the disappearance of the parasites is linked to an increase in atopic diseases (Lynch et al. 1993). A similar observation has been made in Southern Africa with respect to vaccination against Streptococcus pneumoniae, a pathogenic bacterium widely present in these countries (Klugman et al. 2003).
Inversely, the question may be asked as to whether infections can suppress the onset of certain autoimmune or allergic diseases in humans. Some patchy data exist to support this. The infection of newborns and their mothers by non-pathogenic lactobacilli (probiotics) prevents the onset of atopic dermatitis (Kalliomaki et al. 2001, 2003) and even induces the regression of an already established disease (Rosenfeldt et al. 2003). Although these therapeutic trials are still challenged (Taylor et al. 2007; Kopp et al. 2008), their converging results are intriguing. In this context, there are also the observations made in Argentina on multiple sclerosis patients that spontaneous infection by parasites slows the progression of the disease (Correale and Farez 2011). These results encouraged some investigators to deliberately infect some of their patients with a non-pathogenic parasite (Summers et al. 2005; Reddy and Fried 2009).
Concerning IDDM, trials involving treatment by BCG vaccination in the United States (Allen et al. 1999) and Israel (Shehadeh et al. 1994) and Q-fever vaccine in Australia (Silva et al. 2003) should be mentioned. No significant effect was observed, but the duration of the immune stimulation may have been too short to observe any effect on a chronic disease such as IDDM, as in the case of the N. brasiliensis treatment mentioned above. Additionally, these treatments were probably applied too late in the natural history of the disease (i.e., at the time of established hyperglycemia), later than what was performed successfully in NOD mice. We discuss this approach below, emphasizing that proof of concept that these approaches can lead to the development of therapeutic strategies should be collected using extracts of infectious agents or, better, specific chemical products, rather than live infectious agents even though they are considered nonpathogenic.
MECHANISMS UNDERLYING THE HYGIENE HYPOTHESIS
Studying the mechanisms by which the increase in autoimmune and allergic diseases is affected by a decrease in infections means essentially studying how infections provide a protective mechanism against such diseases.
Identification of Infectious Agents and Their Protective Constituents
Identification of relevant infectious agents is difficult and, to date, without a definitive answer. Most likely, a high number of pathogenic and non-pathogenic infectious agents can provide protection against autoimmune and allergic diseases. As mentioned above, common as well as severe infectious diseases can have this protective effect. Epidemiological data to identify with any certainty the respective roles of severe and chronic infections are not available. All that is known is that the earlier in a subject’s life the infections occur, the more protective they are (Krämer et al. 1999; McLeod et al. 2011). Studies of migrants have shown that the loss of the protective effect of infectious agents observed in families displaced from a country of low incidence to a country of high incidence of autoimmune and allergic diseases was no longer observed in subjects who were older than 8–10 yr at the time of migration. Similarly in mice, the protective effect of various infectious agents against diabetes is only observed when the animals are infected at a relatively young age. Diabetes is a chronic disease that develops over several months in mice and several years in humans, and, obviously, the preventive role of infections is expressed only during the early stages of immune events, that is to say, quite a long time before the clinical appearance of the disease.
Another question is that of the role of gut bacteria. A particularly important role is currently given to the microbiota in the control of autoimmune diseases (Round and Mazmanian 2009; Chervonsky 2010). It would be interesting to know whether commensal germs have a protective role and how changes in the composition of the gut flora influence the onset of autoimmune and allergic diseases. This question is a matter of intensive research today. In NOD mice, recent results indicate that diabetes frequency can be decreased using lactobacilli initially derived from the gut (Calcinaro et al. 2005; Aumeunier et al. 2010). Similarly, Dan Litman’s group has shown that a single commensal bacteria, segmented filamentous bacteria (SFB), is sufficient to drive the appearance of CD4+ T helper cells that produce interleukin (IL)-17 and IL-22 (TH17 cells) in the lamina propria, thereby influencing the microbiota equilibrium (Ivanov et al. 2009). Interestingly, recent data suggest that in some colonies, protection from autoimmune diabetes in NOD females segregates with SFB (Kriegel et al. 2011). More robust evidence was obtained in inflammatory bowel disease: The composition of the intestinal flora of patients suffering from Crohn’s disease is abnormal, lacking Faecalibacterium prausnitzii bacteria (Sokol et al. 2008). The latter were shown to prevent the occurrence of experimentally induced inflammatory colitis in mice (Sokol et al. 2008). In any case, a microbiota effect alone cannot explain the protective action of infectious agents. Reference should be made here to the numerous data mentioned above showing that NOD mice are protected against diabetes by many pathogens that are not associated with the gut.
Role of Anti-Infectious Immune Responses on Lymphocyte Homeostasis and Immunoregulation
Pathogen-induced immune responses are usually intensive and involve a wide diversity of pathways. The antigens carried by the infectious agents are strong, inducing both humoral and cellular TH1, TH2, and TH17 responses. It is easy to understand how such responses can compete with autoimmune and allergic responses elicited, respectively, by self-antigens and allergens, which are for the greater part weak antigens.
The phenomenon of antigen competition was observed long ago (Okada et al. 2010), but its underlying mechanisms have not yet been clearly identified. The involvement of antigen-presenting cells was mentioned several years ago, in particular, in the binding of antigenic peptides to major histocompatibility complex molecules.
In recent years, attention has been focused on the effect of homeostatic factors. It is known that lymphocyte proliferation and differentiation are stimulated by various homeostatic factors including recognition of complexes formed by self-peptides and major histocompatibility complex molecules and several cytokines, primarily IL-7 and IL-2. A hypothesis was suggested that immune responses elicited by the strong antigens carried by infectious agents compete for consumption of homeostatic factors with the immune responses elicited by weak antigens, such as self-antigens and allergens (Bach 2002). This hypothesis is strong, although as yet there is little experimental evidence to support it. The work of Sarvetnik’s group on IDDM and NOD mice is noteworthy in this context. It was suggested in this work that NOD mice are lymphopenic and harbor among residual T cells rapidly dividing lymphocytes giving rise to diabetogenic effector cells under the influence of homeostatic factors such as IL-21 and that complete Freund’s adjuvant induces lymphocytosis competing for homeostatic factors (King et al. 2004).
Bystander suppression should also be mentioned. It is a phenomenon by which regulatory cells induced by a given antigen extend their suppression potential to immune responses against antigens distinct from the ones that induced them (Miller et al. 1991). It is not yet clear which, among the various regulatory T-cells, are the ones involved in bystander suppression. The phenomenon is well documented for IL-10- and TGF-β-producing cells (Bach 2003). The question is still unresolved for other types of adaptive regulatory T cells and more so for the CD25+ FOXP3+ natural regulatory T cells.
We have performed several studies on NOD mice using as a substitute infection a polyvalent bacterial extract previously shown to prevent the onset of diabetes (Alyanakian et al. 2006). Results showed that its protective effect was abolished by the administration of anti-TGF-β antibodies but not by the administration of antibodies against the interleukin-10 receptor. We have also shown that the protective effect of the bacterial extract was no longer observed in NOD CD28−/− mice deficient in CD25+ FOXP3+ regulatory T cells (J-F Bach and L Chatenoud, unpubl.). Taken together, these results suggest a role for TGFβ-dependent regulatory T cells and natural regulatory cells in the protective effect of infections against diabetes. Experiments performed with CD1d−/− mice deficient in NKT cells also indicate a certain involvement of NKT cells in the protection phenomenon (Alyanakian et al. 2006).
Stimulatory Role of Toll-Like Receptors
Converging evidence indicates a central role for Toll-like receptors (TLR) in the induction of immune responses. Their involvement is dispensable as shown by the fact that immune competence was maintained in double-mutant mice deficient in Myd88 and TRIFF pathways (Gavin et al. 2006). The question asked is whether the absence of TLR function, following knockout of TLR genes or their adaptor molecules, had a preventive effect on autoimmune and allergic diseases. In the case of diabetes, knockout of the Myd88 gene when mice were raised in axenic conditions did not modify the incidence of the disease (Wen et al. 2008). Conversely, in Specific Pathogen Free animals, diabetes did not develop in Myd88−/− mice, a fact probably explained by the development of infections linked to the immune deficiency created by the Myd88 gene invalidation (Wen et al. 2008; Aumeunier et al. 2010).
In contrast, and more unexpectedly, the administration of various TLR agonists to NOD mice before they were 6 wk old completely prevented diabetes onset (Quintana et al. 2000; Aumeunier et al. 2010). This prevention, observed for TLR2, TLR3, TLR4, TLR7, and TLR9 agonists, was also reported in the case of ovalbumin-induced allergic asthma (Aumeunier et al. 2010). Recent results indicate that the suppression is due to yet unclear mechanisms involving the induction of immunoregulatory cytokines such as TGF-β and IL-10, NKT cell induction in the case of TLR3 agonists, and CD25+ FOXP3+ natural regulatory T cells in the case of TLR4 agonists (Aumeunier et al. 2010).
Other Mechanisms
The mechanisms mentioned above are probably not exclusive. Other phenomena linked to innate immunity may be involved, as suggested by the immunosuppressive activity observed in some parasitic diseases. Certain components of infectious agents that nonspecifically (independent of immunogenicity) stimulate various cells of the lymphoid system could be involved. An illustration of this type of mechanism is given by the stimulation elicited by the TIM-1 protein, a receptor for the hepatitis A virus expressed on the surface of TH2 cells (McIntire et al. 2003). Recently, it has been shown that patients expressing the long form of the TIM-1 receptor (compared with those expressing the short form) had a lower frequency of atopic diseases and a higher susceptibility toward severe forms of hepatitis A (Chatenoud and Bach 2011; Kim et al. 2011). The hepatitis A virus receptors of these patients have a stronger affinity for the virus, which explains both the greater severity of the hepatitis and the better protection against secondary atopic diseases once hepatitis develops.
To date, no such data exist for type 1 diabetes, but this possibility should be kept in mind given the great similarity of the protective effect provided by infections against type 1 diabetes and allergic diseases.
CONCLUSION AND THERAPEUTIC PERSPECTIVES
Although a definitive proof has not yet been obtained, it is highly likely that the decline of infections is one of the major explanations for the increased frequency of IDDM in developed countries. This does not exclude the involvement of other factors, although no robust data have yet been collected. The most frequently involved infections still remain to be identified as well as the epidemiological characteristics of the protective effect during the natural course of the disease.
Such considerations are relevant not only at the epidemiological level but also at the fundamental level with regard to the pathophysiology of the disease. They can also open new therapeutic perspectives. The administration of live infectious agents is a possibility, especially in the case of probiotics, but its effectiveness is still unclear. The administration of pathogenic infectious agents or agents suspected of developing into pathogens is harder to imagine. The best solution would be to administer bacterial extracts, or better, well-characterized chemical compounds proven effective in experimental models. Bacterial extracts such as the one we have used in NOD mice (Alyanakian et al. 2006) have the advantage that their safety has been shown on hundreds of thousands of children and adult subjects (Schaad et al. 2002; Steurer-Stey et al. 2007). Their disadvantage is that they are not sufficiently standardized. Well-characterized chemical compounds, and foremost TLR agonists, would be preferable because they can be developed like any other drug, but they would probably need to be administered at relatively high doses, which might pose safety issues. Insofar as this treatment would be applied as a preventive measure to healthy subjects, mostly children, any risk, even small, is unacceptable.
Footnotes
Editors: Jeffrey A. Bluestone, Mark A. Atkinson, and Peter R. Arvan
Additional Perspectives on Type 1 Diabetes available at www.perspectivesinmedicine.org
REFERENCES
- Adorini L 2004. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. Kidney Int 65: 1538. [DOI] [PubMed] [Google Scholar]
- Airaghi L, Tedeschi A 2006. Negative association between occurrence of type 1 diabetes and tuberculosis incidence at population level. Acta Diabetol 43: 43–45 [DOI] [PubMed] [Google Scholar]
- Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP 1999. Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care 22: 1703–1707 [DOI] [PubMed] [Google Scholar]
- Alyanakian MA, Grela F, Aumeunier A, Chiavaroli C, Gouarin C, Bardel E, Normier G, Chatenoud L, Thieblemont N, Bach JF 2006. Transforming growth factor-β and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55: 179–185 [PubMed] [Google Scholar]
- Ascherio A, Munger K 2008. Epidemiology of multiple sclerosis: From risk factors to prevention. Semin Neurol 28: 17–28 [DOI] [PubMed] [Google Scholar]
- Aumeunier A, Grela F, Ramadan A, Pham Van L, Bardel E, Gomez Alcala A, Jeannin P, Akira S, Bach JF, Thieblemont N 2010. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS One 5: e11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JF 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347: 911–20 [DOI] [PubMed] [Google Scholar]
- Bach JF 2003. Regulatory T cells under scrutiny. 2003. Nat Rev Immunol 3: 189–198 [DOI] [PubMed] [Google Scholar]
- Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R 1992. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. Br Med J 304: 1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, et al. 2005. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 48: 1565–1575 [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Bach JF 2011. Genetic control of hepatitis A severity and susceptibility to allergy. J Clin Invest 121: 848–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervonsky AV 2010. Influence of microbial environment on autoimmunity. Nat Immunol 11: 28–35 [DOI] [PubMed] [Google Scholar]
- Classen JB, Classen DC 2003. Clustering of cases of type 1 diabetes mellitus occurring 2–4 years after vaccination is consistent with clustering after infections and progression to type 1 diabetes mellitus in autoantibody positive individuals. J Pediatr Endocrinol Metab 16: 495–508 [DOI] [PubMed] [Google Scholar]
- Correale J, Farez MF 2011. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol 233: 6–11 [DOI] [PubMed] [Google Scholar]
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A 2011. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140: 1785–1794 [DOI] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D 2006. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 314: 1936–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M, Buffler PA 2009. Infections in early life and risk of childhood ALL. Br J Cancer 100: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM, Herrick EM, Voller A 1970. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature 226: 266–267 [DOI] [PubMed] [Google Scholar]
- Hettiarachchi KD, Zimmet PZ, Myers MA 2008. Dietary toxins, endoplasmic reticulum (ER) stress and diabetes. Curr Diabetes Rev 4: 146–156 [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E 2001. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 357: 1076–1079 [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E 2003. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361: 1869–1871 [DOI] [PubMed] [Google Scholar]
- Kim HY, Eyheramonho MB, Pichavant M, Gonzalez Cambaceres C, Matangkasombut P, Cervio G, Kuperman S, Moreiro R, Konduru K, Manangeeswaran M, et al. 2011. A polymorphism in TIM1 is associated with susceptibility to severe hepatitis A virus infection in humans. J Clin Invest 121: 1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117: 265–277 [DOI] [PubMed] [Google Scholar]
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 349: 1341–1348 [DOI] [PubMed] [Google Scholar]
- Kondrashova A, Reunanen A, Romanov A, Karvonen A, Viskari H, Vesikari T, Ilonen J, Knip M, Hyoty H 2005. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med 37: 67–72 [DOI] [PubMed] [Google Scholar]
- Kondrashova A, Viskari H, Kulmala P, Romanov A, Ilonen J, Hyoty H, Knip M 2007. Signs of β-cell autoimmunity in nondiabetic schoolchildren: A comparison between Russian Karelia with a low incidence of type 1 diabetes and Finland with a high incidence rate. Diabetes Care 30: 95–100 [DOI] [PubMed] [Google Scholar]
- Kopp MV, Hennemuth I, Heinzmann A, Urbanek R 2008. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: No clinical effects of Lactobacillus GG supplementation. Pediatrics 121: e850–e856 [DOI] [PubMed] [Google Scholar]
- Krämer U, Heinrich J, Wjst M, Wichmann HE 1999. Age of entry to day nursery and allergy in later childhood. The Lancet 353: 450–454 [DOI] [PubMed] [Google Scholar]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D 2011. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci 108: 11548–11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen T, von Hertzen L, Koskinen JP, Makela MJ, Jousilahti P, Kosunen TU, Vlasoff T, Ahlstrom M, Vartiainen E, Haahtela T 2011. Allergy gap between Finnish and Russian Karelia on increase. Allergy 66: 886–892 [DOI] [PubMed] [Google Scholar]
- Lynch NR, Hagel I, Perez M, Di Prisco MC, Lopez R, Alvarez N 1993. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol 92: 404–411 [DOI] [PubMed] [Google Scholar]
- Masoli M, Fabian D, Holt S, Beasley R 2004. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 59: 469–478 [DOI] [PubMed] [Google Scholar]
- McIntire JJ, Umetsu SE, Macaubas C, Hoyte EG, Cinnioglu C, Cavalli-Sforza LL, Barsh GS, Hallmayer JF, Underhill PA, Risch NJ, et al. 2003. Immunology: Hepatitis A virus link to atopic disease. Nature 425: 576. [DOI] [PubMed] [Google Scholar]
- McLeod JG, Hammond SR, Kurtzke JF 2011. Migration and multiple sclerosis in immigrants to Australia from United Kingdom and Ireland: A reassessment. I. Risk of MS by age at immigration. J Neurol 258:1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Lider O, Weiner HL 1991. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med 174: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni S, Fonte MT, Stoduto S, Marietti G, Bizzarri C, Crino A, Ciampalini P, Multari G, Suppa MA, Matteoli MC, et al. 1997. Incidence of insulin-dependent diabetes mellitus among Sardinian-heritage children born in Lazio region, Italy. Lancet 349: 160–162 [DOI] [PubMed] [Google Scholar]
- Okada H, Kuhn C, Feillet H, Bach JF 2010. The “hygiene hypothesis” for autoimmune and allergic diseases: An update. Clin Exp Immunol 160: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CC, Carson DJ, Hadden DR 1996. Epidemiology of childhood IDDM in Northern Ireland 1989–1994: Low incidence in areas with highest population density and most household crowding. Northern Ireland Diabetes Study Group. Diabetologia 39: 1063–1069 [DOI] [PubMed] [Google Scholar]
- Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G 2009. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet 373: 2027–2033 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Rotem A, Carmi P, Cohen IR 2000. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: Modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol 165: 6148–6155 [DOI] [PubMed] [Google Scholar]
- Reddy A, Fried B 2009. An update on the use of helminths to treat Crohn’s and other autoimmune diseases. Parasitol Res 104: 217–221 [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, Paerregaard A 2003. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 111: 389–395 [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain MW, Qin HY, Lauzon J, Singh B 1990. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39: 583–589 [DOI] [PubMed] [Google Scholar]
- Schaad UB, Mütterlein R, Goffin H 2002. Immunostimulation with OM-85 in children with recurrent infections of the upper respiratory tract: A double-blind, placebo-controlled multicenter study. Chest 122: 2042–2049 [DOI] [PubMed] [Google Scholar]
- Shehadeh N, Calcinaro F, Bradley BJ, Bruchim I, Vardi P, Lafferty KJ 1994. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet 343: 706–707 [DOI] [PubMed] [Google Scholar]
- Silva DG, Charlton B, Cowden W, Petrovsky N 2003. Prevention of autoimmune diabetes through immunostimulation with Q fever complement-fixing antigen. Ann NY Acad Sci 1005: 423–430 [DOI] [PubMed] [Google Scholar]
- Soderstrom U, Aman J, Hjern A 2011. Being born in Sweden increases the risk for type 1 diabetes–a study of migration of children to Sweden as a natural experiment. Acta Paediatr 10.1111/j.1651-2227.2011.02410.x [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, et al. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci 105: 16731–16736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steurer-Stey C, Lagler L, Straub DA, Steurer J, Bachmann LM 2007. Oral purified bacterial extracts in acute respiratory tract infections in childhood: A systematic quantitative review. Eur J Pediatr 166: 365–376 [DOI] [PubMed] [Google Scholar]
- Strachan DP 1989. Hay fever, hygiene, and household size. BMJ 299: 1259–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV 2005. Trichuris suis therapy in Crohn’s disease. Gut 54: 87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei I, Asaba Y, Kasatani T, Maruyama T, Watanabe K, Yanagawa T, Saruta T, Ishii T 1992. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun 5: 665–673 [DOI] [PubMed] [Google Scholar]
- Taylor AL, Dunstan JA, Prescott SL 2007. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: A randomized controlled trial. J Allergy Clin Immunol 119: 184–191 [DOI] [PubMed] [Google Scholar]
- Todd JA, Fukui Y, Kitagawa T, Sasazuki T 1990. The A3 allele of the HLA-DQA1 locus is associated with susceptibility to type 1 diabetes in Japanese. Proc Natl Acad Sci 87: 1094–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. 2008. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilberz S, Partke HJ, Dagnaes-hansen F, Herberg L 1991. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34: 2–5 [DOI] [PubMed] [Google Scholar]
- Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A 2009. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol 39: 1098–1107 [DOI] [PubMed] [Google Scholar]