Background: TGFβ1-induced pY654-β-catenin correlates with epithelial mesenchymal transition (EMT) and pulmonary fibrosis, but whether pY654-β-catenin is functionally important is unknown.
Results: β-Catenin point mutants reveal that pY654 is critical to EMT, and pY654-β-catenin accumulation is blocked by axin-dependent β-catenin turnover.
Conclusion: Raised axin levels in vivo attenuate EMT and fibrosis after bleomycin injury.
Significance: Targeting axin levels could attenuate fibrosis without blocking TGFβ1 homeostatic functions.
Keywords: Epithelial Mesenchymal Transition, Signaling, Src, Transforming Growth Factor β (TGFβ), Wnt Signaling
Abstract
Epithelial to mesenchymal transition (EMT) and pulmonary fibrogenesis require epithelial integrin α3β1-mediated cross-talk between TGFβ1 and Wnt signaling pathways. One hallmark of this cross-talk is pY654-β-catenin accumulation, but whether pY654-β-catenin is a biomarker of fibrogenesis or functionally important is unknown. To clarify further the role of β-catenin in fibrosis, we explored pY654-β-catenin generation and function. α3β1 was required for TGFβ1-mediated activation of Src family kinases, and Src inhibition blocked both pY654 and EMT in primary alveolar epithelial cells (AECs). TGFβ1 stimulated β-catenin/Lef1-dependent promoter activity comparably in immortalized AECs stably expressing WT β-catenin as well as Y654E or Y654F β-catenin point mutants. But EMT was abrogated in the Tyr to Phe mutant. pY654-β-catenin was sensitive to the axin β-catenin turnover pathway as inhibition of tankyrase 1 led to high AEC axin levels, loss of pY654-β-catenin, and inhibition of EMT ex vivo. Mice given a tankyrase inhibitor (50 mg/kg orally) daily for 7 days beginning 10 days after intratracheal bleomycin had improved survival over controls. Treated mice developed raised axin levels in the lung that abrogated pY654-β-catenin and attenuated lung Snail1, Twist1, α-smooth muscle actin, and type I collagen accumulation. Total β-catenin levels were unaltered. These findings identify Src kinase(s) as a mediator of TGFβ1-induced pY654-β-catenin, provide evidence that pY654-β-catenin levels are a critical determinant of EMT and fibrogenesis, and suggest regulation of axin levels as a novel therapeutic approach to fibrotic disorders.
Introduction
Progressive pulmonary fibrosis has proven to be an intractable process characterized by the repeated appearance of foci of wound-like lesions in the lung parenchyma that undergo scarification. As in other organs, fibrosis of the lung is thought to be driven in part by sustained TGFβ1 signaling (1). TGFβ1 signaling is regulated at multiple levels from production and activation of TGFβ1 to formation of a Smad transcriptional complex with various Smad co-regulators that promote or restrain transcriptional activity (2, 3). Hence, cells respond in a variety of different ways to active TGFβ1 depending on convergence with other signaling pathways. In epithelial cells the extracellular matrix is an important determinant of the cellular response to TGFβ1. The fibrillar matrix proteins such as type I collagen (Col1)2 and fibronectin (Fn) promote TGFβ1 activation and mesenchymal expansion whereas basement membrane proteins such as the laminins suppress activation (4, 5). Epithelial cells may respond to active TGFβ1 both by signaling responses that recruit and activate mesenchymal cells and by extensive reprogramming to a more mesenchymal phenotype, the overall process termed epithelial to mesenchymal transition (EMT) (6). The mechanisms by which the extracellular matrix regulates EMT and fibrosis are not fully understood but appear to involve signaling through integrin receptors (7).
We have previously provided evidence that EMT develops in vivo during experimental lung fibrosis and is an important contributor to fibrogenesis. We elucidated an important role for an integrin in this process (4, 8). The epithelial integrin, α3β1, binds laminin and also associates with E-cadherin and via these interactions acts to sense disruptions in cell-cell or cell-matrix contacts. In the presence of active TGFβ1 and disrupted cell contacts, α3β1 and E-cadherin associate with TGFβ1 receptors and induce β-catenin phosphorylation at a specific tyrosine (Tyr-654) and complexes of this catenin with pSmad2 (8). Formation of this integrin-dependent complex in AECs strongly correlates with fibrogenesis and myofibroblast expansion in vivo in mice. Nuclear pY654-β-catenin/pSmad2 complexes localize to interstitial myofibroblasts in biopsied lungs of idiopathic pulmonary fibrosis (IPF) patients, but are not found in normal or emphysematous lungs (8).
Although accumulation of pY654-β-catenin in lungs correlates with active fibrogenesis, it remains unclear whether pY654-β-catenin is simply a biomarker for the complicated signaling that follows TGFβ1 activation or is an important determinant of the fibrogenic response. That the latter is possible is suggested by previous reports that phosphorylation of Y654-β-catenin promotes both its dissociation from E-cadherin and its physical association with TATA-binding proteins known to enhance β-catenin/TCF transcriptional activity (9, 10). Thus, acting in concert with cytoplasmic stabilization of β-catenin, e.g. through Wnt signaling, pY654 could promote nuclear translocation and transcriptional activity of β-catenin on its target genes. Prior studies have provided evidence of active Wnt signaling during experimental and human fibrosis (11–13), and recent observations indicate that one function of Wnt signaling in the lung is likely an epithelial cytoprotective effect following injury (14). It is also unclear mechanistically why the epithelial integrin α3β1 is required for TGFβ1-induced Tyr-654 phosphorylation. To clarify these uncertainties, in this study we have explored the regulation and importance of pY654-β-catenin accumulation ex vivo in AECs and in vivo in mice following bleomycin-induced lung injury.
EXPERIMENTAL PROCEDURES
Reagents
Inhibitors SU6656 (Src), PP2 (Src), PP3 (control for PP2), SB431542 (TGFβ receptor 1 (TBRI)), SIS3 (Smad3), and phospho-Smad2 antibody were from Calbiochem. Recombinant EGF and M2-FLAG, α-SMA, and β-actin monoclonal antibodies were from Sigma-Aldrich. 9B11-Myc and Snail monoclonal antibodies, pY416-Src, and total β-catenin polyclonal antibodies were from Cell Signaling. Col1 and vimentin polyclonal antibodies were from Abcam. Monoclonal Twist and GAPDH and secondary HRP-conjugated antibodies were from Santa Cruz Biotechnology. Polyclonal pro-surfactant protein C antibody was from Millipore. pY654-β-catenin monoclonal IgG antibody was from the University of Iowa Hybridoma Bank. Keratinocyte growth factor was from Peprotech. TGFβ1 was from R&D Systems. Small airway basal medium and supplemented small airway growth medium were from Lonza.
Plasmid and Viral Constructs
FLAG-Smad3 plasmid was obtained from Addgene (plasmid 12638). Mouse β-catenin cDNAs encoding WT or the Y654E and Y654F mutations were a gift from Dr. Mireia Duñach (Universitat Autonoma de Barcelona), His tag was substituted by a Myc tag and then cloned into a pENTR vector (Gateway Technology, Invitrogen) and recombined into a modified version of pRV-GFP pDEST vector enabling retrovirus-mediated expression (provided by Dr. Mark Ansel, University of California, San Francisco (UCSF)). Retrovirus was produced in Phoenix-E packaging cells, concentrated by centrifugation, and added to cells in suspension in the presence of Polybrene (6.5 μg/ml). Lenti-TOPflash was a gift from Dr. Jean Y. J Wang (UCSF), and replication-deficient lentivirus was produced by the UCSF Lentiviral Core Facility. Adenovirus expressing cre recombinase (AdenoCre) or GFP as a control was obtained from University of Iowa Vector Core.
Cells and Cell Culture
AECTs were generated by isolating AECs from temperature-sensitive SV40 T antigen-immortalized mice (Immortomouse, Charles River Laboratory) crossed with mice homozygous for floxed β-catenin (β-Ctnfl/fl; Jackson Laboratory). AECTs were maintained on Matrigel (BD Biosciences) in small airway growth medium with 5% FBS. Cells were infected in suspension with Polybrene, plated onto Fn, and puromycin (10 mg/ml)-selected. After selection, cells were maintained on Matrigel. Preliminary experiments indicated that cre recombinase-mediation deletion of AECT β-catenin resulted in slow growth and eventual cell death. Hence, AECTs were stably infected with WT or mutant β-catenin prior to cre recombinase exposure. For experiments, cells encoding the various forms of β-catenin were seeded on Fn-coated plates and infected with AdenoCre (75 units/cell) prior to experiments. Primary mouse type II AEC isolation and culture were performed as described previously (8).
Immunoblotting, Immunoprecipitation, and Immunofluorescence
Tissue and cells were lysed in radioimmuneprecipitation assay buffer supplemented with proteinase and phosphatase inhibitors and immunoprecipitated as described previously (15). Cells were fixed and stained with various antibodies and IgG isotype controls as described previously (15). Where indicated, composite images comprising multiple ×20 images were tiled using 10% image overlap by Axiovision 4.7 software.
β-Catenin/TCF Signaling Assays
A549 cells were transfected with a 4× TOPflash reporter plasmid (Millipore) together with 1/10 CMV-β-galactosidase (Clontech) using Lipofectamine LTX or Lipofectamine 2000 (Invitrogen). AECT-TOPflash were obtained as described above. The Luciferase Assay System (Promega) was used and emitted light quantified in a Fluostar Optima (BMG Labtech). A549 luciferase activity was normalized with a Luminescent β-Galactosidase Detection kit II (Clontech).
Bleomycin Fibrosis Model
Six- to 8-week-old female C57BL/6 mice were endotracheally instilled with saline or 2.0 units/kg bleomycin (Sigma). Cohorts of mice were given FT4100 dissolved in dimethyl sulfoxide (10 mm) in a dose of 50 mg/kg mixed with 1% methylcellulose (Sigma) and administered daily by gavage beginning on day 10 after bleomycin. Controls received vehicle alone in the same formulation. Mice were killed 17 days after injury.
Statistics
Statistical analysis of group variance was performed by using the unpaired, two-way Student t test. Statistical analysis of group survival differences was performed using χ2 contingency testing. p < 0.05 was considered statistically significant.
RESULTS
pY654-β-catenin Accumulation Is Src Kinase-dependent
We first determined the time course of Y654-β-catenin phosphorylation after TGFβ1 stimulation in A549 lung adenocarcinoma cells. Smad2 phosphorylation was detectable within 15 min of TGFβ1 stimulation whereas pY654 was not apparent until 2 h, but was maintained at least up to 24 h (Fig. 1A). As described previously, pSmad2 co-precipitates with pY654-β-catenin. Most subsequent experiments examining pY654-β-catenin were conducted 2 h after TGFβ1 stimulation.
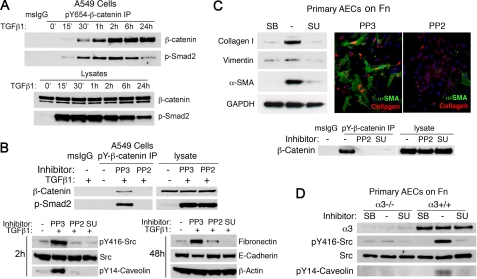
FIGURE 1.
Src activation mediates TGFβ1-induced pY654-β-catenin generation. A, pY654-β-catenin and p-Smad2 in immunoprecipitates and lysates were measured at various times after TGFβ1 stimulation. B, upper, pY654-β-catenin and its complex with p-Smad2 at 2 h were blocked by Src inhibitor PP2 (10 μm) but not inactive PP3. Lower, TGFβ1 activates Src family kinase(s) as revealed by p416-Src accumulation. PP2 or SU6656 (5 μm) blocked p416-Src and p14-caveolin1 at 2 h and attenuated Fn accumulation at 48 h. C, primary AECs were plated on Fn to allow latent TGFβ1 activation. Upper, AECs were cultured on Fn for 4 days. SB431542 and Src inhibitors inhibited TGFβ1-induced Col1, vimentin, and α-SMA as judged by immunoblotting and immunostaining. Lower, AECs were treated with TGFβ1 for 2 h ± PP2 or SU6656, and pY654β-catenin was detected by immunoprecipitation. D, WT or α3 integrin-null AECs plated on Fn were maintained in the presence of SB431542 (10 μm) for 2 days and then treated with TGFβ1 (4 ng/ml) plus dimethyl sulfoxide (control) or SU6656 (5 μm) for 4 h. Cell lysates were immunoblotted for Src kinase activation (pY416) and pY14-caveolin-1.
Y654-β-catenin is a known target of Src kinase, and Src family kinase activation is reported downstream of TGFβ1 in several models (7, 10, 16). Indeed Src kinase(s) activation proved crucial for TGFβ-induced phosphorylation of β-catenin. PP2, but not its inactive analog PP3, abrogated pY654 (Fig. 1B). SU6656, a structurally distinct Src inhibitor, had similar effects. A549 cells were pretreated 1 h with Src inhibitors and then with TGFβ1. At 2 h Src activation was easily detected, and both Src inhibitors blocked activation as judged by inhibition of pY416 Src and pY14-caveolin-1 (Fig. 1B, lower left), a known Src kinase substrate (17).
Src inhibition resulted also in a marked decrease in EMT markers in both A549 and primary AECs (Fig. 1, B, lower right, and C, upper), consistent with previous findings in other cell lines and recent studies of AECs (18–22). To test the effects of Src inhibition on primary lung AECs, cells from WT mice were seeded on a Fn matrix to activate endogenous latent TGFβ1 (4). Cells were cultured in the presence of SB431542, an inhibitor of TBRI, or in the presence or absence of Src inhibitors. Both PP2 and SU6656 blocked pY654-β-catenin accumulation (Fig. 1C, lower). We found that Y654-β-catenin phosphorylation also accumulates in H358 cells after TGFβ1 stimulation. Src inhibition had similar inhibitory effects on both β-catenin phosphorylation and EMT.
The functional effects of Src inhibition on primary AEC pY654-β-catenin accumulation and EMT are similar to those previously reported with epithelial specific deletion of α3β1 (8). Given the known association of Src kinases in integrin signaling, we tested the effects of α3 integrin deletion on Src pathway activation. WT or α3-null AECs were isolated, plated on Fn for 24 h, and then stimulated with TGFβ1 for 4 h. Src activation and pY654-β-catenin were easily detected in WT AECs but were not detected in α3-null cells (Fig. 1, D, and C, lower). Collectively, these observations indicate that the requirement for α3β1 in TGFβ1 signaling leading to EMT can be explained at least in part by the critical dependence on the integrin for TGFβ1-mediated Src family kinase(s) activation and subsequent Tyr-654 phosphorylation.
EMT Requires pY654 β-Catenin
To define further the role of β-catenin tyrosine phosphorylation as a mediator of EMT, we isolated AECs from mice containing a β-catenin exon flanked by loxP sites (floxed-β-catenin) and plated cells on Fn while being exposed to AdenoCre, or a control AdenoGFP. Cells with abrogated β-catenin expression showed an impaired expression of EMT markers such is α-SMA (Fig. 2A), consistent with prior studies demonstrating that knockdown of β-catenin suppressed EMT in kidney epithelial cells (23). Next, we capitalized on cre-dependent deletion of endogenous β-catenin to engineer cells expressing only WT or specific β-catenin point mutants. Because primary AECs do not grow well we first established immortalized derivatives of AECs capable of continued growth ex vivo. For this purpose flox-β-catenin mice were immortalized by introducing a widely expressed SV40 large T transgene (24). Then, primary AECs isolated from these mice, termed AECTs, were plated on Matrigel. These isolated cells were found to grow slowly but stably for many weeks and to form large epithelial clusters (Fig. 2B, upper left). Immunostained cytospins revealed that almost all of the cells highly expressed the integrin α6β4 whereas only 15–20% of the cells were positive for the type II cell marker pro-surfactant protein C (Fig. 2B, upper right) and few if any for the Clara cell marker CCSP. This phenotype matches a progenitor cell phenotype recently reported in adult murine lungs (25). In four independent experiments, cell lines emerging from culture of AECs isolated from mice expressing large T antigen highly expressed α6β4 and were largely deficient in type II or Clara cell lineage markers.
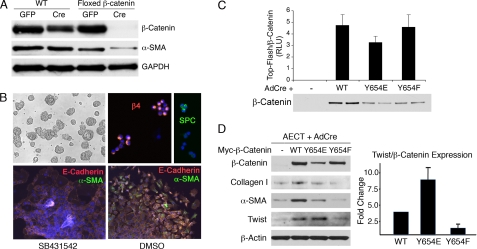
FIGURE 2.
pY654-β-catenin is required for EMT. A, AECs from WT or fl/fl β-catenin mice were treated with AdenoCre or AdenoGFP and seeded on Fn. After 4 days, cells were lysed and immunoblotted with the indicated antibodies. Absence of β-catenin correlated with decreased α-SMA expression. B, AECTs cultured on Matrigel form large cell aggregates (upper left). Cytospins of single cells were stained for β4 integrin or pro-surfactant protein C markers (upper right). Lower, AECTs seeded for 4 days on Fn in the presence (left) or absence (right) of SB431542 were stained for EMT markers E-cadherin (orange) and α-SMA (green). In the absence of inhibitor, AECTs undergo robust EMT. C, AECT-TOPflash expressing the indicated versions of exogenous β-catenin were treated with AdenoCre to remove endogenous β-catenin and seeded on Fn. After 4 days, starved cells were treated with LiCl (20 mm) and TGFβ1 (4 ng/ml) for 24 h. TOPflash luciferase activity is expressed as relative light units (RLU) and normalized to β-catenin expression. Data shown are representative of four independent experiments. Immunoblot of β-catenin (duplicate lanes) from one of the experiments is shown. D, left, AECTs expressing WT and mutant β-catenins were treated with AdenoCre and seeded on Fn. Cells were starved and treated with LiCl (20 mm) for 3 days. Twist1, α-SMA, and Col1 were induced in the presence of WT or Y654E-β-catenin, but not in the absence of β-catenin nor replacement by Y654F mutant. Right, Twist expression was normalized to total β-catenin expression, average of three independent experiments (p < 0.05).
When AECTs were removed from Matrigel and plated onto Fn in the presence of TGFβ1 inhibitors, the cells spread extensively but maintained adherens junctions. When TGFβ1 signaling was permitted, AECTs robustly underwent EMT as judged by redistribution of E-cadherin with loss of cell-cell contacts and the appearance of α-SMA (Fig. 2B). AECTs were subsequently used to examine the influence of the Y654-catenin residue on TGFβ1 signaling.
A Y654E mutation mimics its phosphorylated form, whereas a Y654F mutation mimics the nonphosphorylated form of β-catenin (26). It has been reported that the Y654F mutant fails to interact with TATA-binding proteins, whereas this interaction is enhanced in the Y654E mutant (9). To express and easily recognize these versions of Y654-β-catenin mutants, a Myc tag was introduced in the N terminus of both WT and the two β-catenin mutant forms, and then they were subcloned into a retroviral expression vector containing an internal ribosome entry site (IRES) enhanced GFP. The resultant viruses were used to insert the WT or mutants into flox-β-catenin AECTs. Preliminary experiments indicated that efficient transduction of the viruses required culturing the cells on Fn where the cells separate. It was found that once virus was integrated the cells could be replated on Matrigel where they recovered a strong epithelial clustered phenotype within 1 week and maintained stable GFP expression thereafter (Fig. 2B).
To determine whether different β-catenin forms mediated canonical β-catenin signaling comparably, the AECTs expressing each β-catenin form were stably infected with a lentivirus encoding TOPflash that contains multiple TCF response elements and is widely used as a reporter of β-catenin/TCF transcriptional activity (27, 28). AECT-TOPflash lines containing the WT or mutant β-catenins were plated on Fn in the presence of AdenoCre virus, resulting in an almost complete loss of endogenous β-catenin (Fig. 2C). The mutant forms could be distinguished from endogenous β-catenin by the slightly larger size of the protein containing the ∼9.5-kDa Myc tag. Cells were initially maintained in the presence of a TBRI inhibitor (SB431542) for 48 h to allow their spreading on Fn while avoiding activation of TGFβ1-induced pathways. Inhibitors were then removed and the cells exposed to active TGFβ1. After 24 h in the presence of LiCl and TGFβ1, the different forms of β-catenin showed no significant differences in TOPflash reporter activation when corrected for the levels of β-catenin expression (Fig. 2C). These observations confirm that in AECTs the Y654F mutant β-catenin is capable of nuclear translocation and activation of comparable canonical TCF transcriptional activity to that of WT and Y654E-β-catenins, consistent with recent reports (29). We attempted to verify nuclear translocation of Y654F-β-catenin by immunostaining with Myc antibodies, but convincing nuclear catenin staining was not apparent with any of the β-catenin forms.
In contrast, after 3 days on Fn without TGFβ1 inhibition, AECTs carrying the different mutants showed obvious differences in their EMT responses. Although all cells spread on Fn comparably, there was a clear induction of the EMT biomarkers α-SMA and Col1 as well as the canonical EMT transcription factor Twist only in cells expressing WT or Y654E mutant β-catenin. Cells expressing the Y654F mutant expressed EMT markers at levels similar to AECTs missing β-catenin (Fig. 2D). Among the cells expressing the different β-catenin forms, Y654E proved to be most efficient in EMT induction, as illustrated by the Twist/β-catenin protein expression ratio quantified and pooled from three independent experiments. Y654F-β-catenin was markedly deficient in Twist induction. Importantly, addition of LiCl, which stabilizes β-catenin and amplified TOPflash signaling of both WT and Y654 mutant forms of Myc-β-catenin (Fig. 2C), only enhanced TGFβ1-induced EMT of the WT and Y654E mutant but not the Y654F mutant (supplemental Fig. S1), indicating that Wnt pathway signaling alone is insufficient for EMT.
pY654-β-catenin Is Not Required for TGFβ1-induced TOPflash Activity
Because pY654-β-catenin accumulates in TGFβ1-stimulated epithelial cells (Fig. 1) and is capable of promoting TCF transcriptional activity (Fig. 2C), we asked whether Tyr-654 phosphorylation was required for TGFβ1-induced TCF signaling, as reflected by TOPflash activity. AECT-TOPflash cells were allowed to spread on Fn in the presence of a TBRI kinase inhibitor (SB431542) for 48 h and then treated for 24 h with TGFβ1 with or without continued kinase inhibition. Little TOPflash activity was detected in TGFβ1-stimulated cells, but when LiCl was added to inhibit GSK3β, TOPflash activity was easily detected and further induced by TGFβ1 (Fig. 3A, left). Both TBRI kinase inhibition and Smad3 signaling inhibitor, SIS3, abrogated most of the TGFβ1-induced TOPflash activity. In contrast, concentrations of the Src inhibitor PP2 that block pY654-β-catenin accumulation (Fig. 1) had no effect (Fig. 3A).
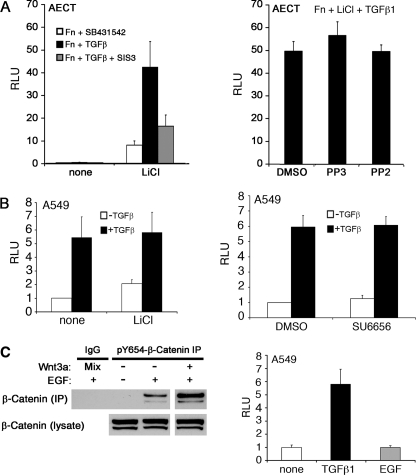
FIGURE 3.
pY654-β-catenin does not significantly contribute to TGFβ1-induced TCF/β-catenin reporter activity. A, AECTs carrying the TOPflash reporter were seeded on Fn. Cells were lysed, and TOPflash activity was measured after treating with the indicated factors for 24 h: 20 mm LiCl, 4 ng/ml TGFβ1, 1 μm SIS3, 10 μm SB431542, 10 μm PP2 and PP3. B, A549 cells expressing TOPflash were treated with TGFβ1 ± LiCl or SU6656, and RLU was determined after 24 h. Relative activity normalized to β-gal expression. C, left, A549 cells were treated with control PBS or EGF (50 ng/ml) with or without Wnt3a for 2 h, and the pY654-β-catenin immunoprecipitates and the lysates were blotted for β-catenin. Right, A549 cells expressing TOPflash were treated with TGFβ1 (4 ng/ml) or EGF (50 ng/ml), and RLU was determined after 24 h. Relative activity was normalized to β-gal expression.
In contrast to AECT cells, A549 cell TOPflash activity was clearly inducible by TGFβ1 in the absence of LiCl. Indeed, the addition of LiCl to these cells only modestly increased reporter activity over TGFβ1, suggesting that Wnt signaling is already active. The structurally distinct Src kinase inhibitor SU6656 again had no consistent effect on TGFβ1-induced TOPflash activity in A549 cells (Fig. 3B, right). In contrast, similar experiments with H358 cells indicated that SU6656 inhibited ∼50% of the increase in TOPflash activity after TGFβ1 stimulation. The reasons for the variable sensitivity to Src kinase inhibitors among different cell lines are unclear but likely involve pathways distinct from pY654-β-catenin accumulation per se as this species is inhibited in all cells tested to date.
To define further whether induction of pY654-β-catenin could be dissociated from TCF signaling, cells were stimulated with EGF, a cytokine previously reported to induce pY654-β-catenin (30). We observed that EGF induced accumulation of pY654-β-catenin in A549 cells but, opposite from TGFβ1 (Fig. 3A), did not measurably activate the TOPflash reporter (Fig. 3C). EGF induction of pY654-β-catenin was also Src kinase-dependent. Collectively, these findings indicate that whereas all forms of Y654-β-catenin, when stabilized by Wnt signaling, can activate TCF transcriptional activity, pY654-β-catenin accumulates at levels functionally important to EMT that are below that reportable by TOPflash activity. We next asked whether the classical pathway of axin-mediated β-catenin turnover could regulate the low levels of pY654-β-catenin generated by cytokine signaling.
Axin Regulates EMT in AECs ex Vivo
Raising cellular axin levels by prolonging the half-life of axin through tankyrase inhibition has been shown to promote β-catenin turnover and impair Wnt signaling (31). XAV939, a small molecule tankyrase inhibitor indentified in this study and termed FT4001 in this paper, promotes an increase of axin-GSK3β interactions, the reported rate-limiting step in β-catenin turnover (31). AECs were isolated and plated on Fn. Cells were cultured without or with 5 mm FT4001, and then axin and pY654-β-catenin levels were measured after 72–96 h. As expected, FT4001 increased cellular levels of axin 1, which is minimally detectable in untreated AECs (Fig. 4B, left). The tankyrase inhibitor had a clear inhibitory effect on the expected pY654β-catenin accumulation, inhibiting 75–80% of control levels in four independent experiments (Fig. 4A). In parallel experiments immunoblotting revealed a ∼70% reduction in the α-SMA expression induced by TGFβ1 in these cells (Fig. 4B). Immunostaining confirmed the inhibitory effect of FT4001 on both α-SMA and Col1 expression induced by culture of primary AECs on Fn for 96 h (Fig. 4C). Similar effects were found in AECTs. These findings confirm the predicted relationship between pY654-β-catenin accumulation and EMT in primary AECs and empowered testing of FT4001 in vivo in a model of experimental fibrosis.
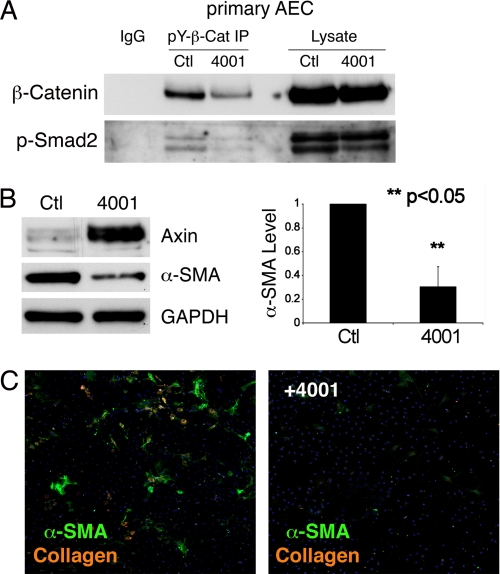
FIGURE 4.
Raised epithelial cell axin levels abrogate EMT. Primary AECs were plated on Fn and treated with either FT4001 (5 mm) or vehicle control. After 3 days: A, pY654-β-catenin and its complex with p-Smad2 detected by immunoprecipitation are decreased in the FT4001-treated cells, whereas total β-catenin and p-Smad2 levels are unaffected. B, immunoblots showed increased axin and decreased α-SMA proteins after FT4001 treatment (left). Quantification of α-SMA in 3 independent experiment confirms ∼75% loss of α-SMA with high axin levels (p < 0.05) (right). C, AECs were cultured on Fn without or with FT4001 (5 mm). After 3 days, immunostaining revealed suppression of Col1 (orange) and α-SMA (green) expression by FT4001.
Tankyrase Inhibition Improves Survival after Bleomycin-induced Lung Injury
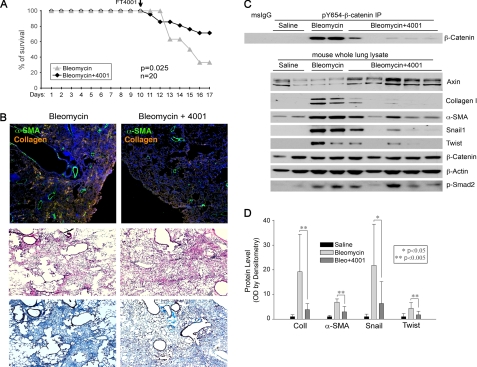
Preliminary experiments tested doses of FT4001 administered daily by gavage in normal mice and determined 50 mg/kg as the minimum effective dose in raising axin levels in extracts of lungs from mice treated for 7 days. Higher doses of FT4001 had little additive effect on axin levels, possibly due to the largely insoluble nature of FT4001 in aqueous solution. To explore a possible protective role of FT4001, adult female C57BL/6 mice were injected intratracheally with bleomycin in doses found to result in lethality in the majority of mice after 21 days (2.5 units/kg). To avoid possible effects of FT4001 on the early injury and inflammatory phase after bleomycin, FT4001 (50 mg/kg) was administered to 20 mice beginning after 10 days, during the fibrogenic phase (Fig. 5A). Control mice received vehicle alone by gavage. Treatment was repeated every day for 6 days. After this period, a significant increase in survival was observed in the FT4001-treated mice versus control (Fig. 5A).
FIGURE 5.
Tankyrase inhibition attenuates TGFβ1-stimulated EMT, bleomycin-induced injury, and fibrogenesis. A, mice injected intratracheally with bleomycin (2.5 units/kg). After 10 days, FT4001 50 mg/kg or vehicle alone was administered daily by gavage for 7 days. FT4001 significantly increased survival of bleomycin-treated mice by day 17 (p = 0.025). B, upper, Col1 (orange) and α-SMA (green) accumulation in bleomycin-injured lungs treated with FT4001 or vehicle. Images are composites of 20× fields tiled as described under “Experimental Procedures.” Middle and lower, representative H&E and Masson's trichrome staining of serial sections of lungs from vehicle- and FT4100-treated mice. Similar results were seen in lungs of three pairs of control and treated mice. C, pY654-β-catenin accumulation and EMT markers measured in protein extracts from snap-frozen lungs. pY654-β-catenin is strongly increased in bleomycin-treated mice and attenuated by FT4001. Axin and EMT markers (Col1, α-SMA, Snail1, Twist) are detected by immunoblotting. TGFβ1 signaling marker (p-Smad2) and total β-catenin are also shown. D, quantification (mean ± S.D. (error bars)) of EMT markers from three independent experiments (9 total mice given bleomycin and 15 given bleomycin + FT4001) analyzed by immunoblotting whole lung extracts. Data are expressed as relative -fold change over saline values (set at 1).
Tankyrase Inhibition Attenuates in Vivo pY654-β-catenin Accumulation and Fibrogenesis
In parallel experiments mice were given intratracheal bleomycin in a lower dose (2.0 units/kg) previously found to induce fibrosis in C57BL/6 mice but to allow >80% survival. Mice were again given daily FT4001 beginning day 10 after bleomycin, and the lungs were harvested for analysis on day 17. Immunoprecipitation confirmed accumulation of pY654-β-catenin in lungs of mice given bleomycin (Fig. 5C). Mice given FT4001 showed much less or no detectable accumulation of the pY654 form. In contrast, no decrease in total lung β-catenin was observed (Fig. 5C). Although there was overall a clear decrease in pY654 accumulation, there was significant variability among the FT4001-treated mice. The increase in lung axin levels was modest compared with that of cultured cells (Fig. 4B), and some mice showed little or no increase in total lung axin levels. Moreover, it was not always the case that total lung axin levels predicted whole lung pY654-β-catenin levels. We attempted to localize axin 1 and 2 proteins in situ but were unable to immunostain cellular axin convincingly in either untreated or treated mice with available antibodies. Despite these limitations there was an overall clear decrease in pY654-β-catenin levels in FT4001-treated mice in each of four independent experiments.
To assess the effects of FT4001 on fibrogenesis, Col1, α-SMA, Snail1, and Twist1 levels in extracts of injured lungs were assessed by immunoblotting (normalized to total protein), and quantification of the individual proteins from each of three experiments was pooled for statistical analysis. Mice treated with FT4001 showed consistently decreased Col1 and α-SMA protein levels compared with mice given bleomycin but no drug (Fig. 5, C and D). Likewise, the canonical EMT transcription factors Snail and Twist1 were easily detected in the injured lungs at day 17 but not in saline-treated mice. Bleomycin-injured mice treated with FT4001 had almost no Snail (p < 0.05) or Twist1 (p < 0.005) accumulation (Fig. 5, C and D). The relatively large SDs for Col1 and Snail reflect variability in the degree of fibrosis in cohorts of mice given a single dose of bleomycin.
Fibrogenesis was also assessed morphologically (Fig. 5B). Low power images of regions of injured lungs from bleomcyin-treated mice on day 17 are shown. As judged by H&E staining, lungs of both vehicle- and FT4001-treated mice contained comparable regions of extensive injury, but trichrome staining revealed attenuated collagen accumulation in lungs of FT4001-treated mice. Sections of lungs from mice treated with either vehicle or FT4001 were also immunostained for Col1 and α-SMA (top panel). Accumulation of α-SMA and Col1 was attenuated in lungs of mice given FT4001, consistent with the biochemical assays and improved survival.
DISCUSSION
β-Catenin has a dual role as transcription factor and as part of the structural protein network that regulates adherens junctions (32). Tyrosine phosphorylation of β-catenin on Tyr-654, located in the last of 12 armadillo repeat domains in β-catenin, is a link between these two functions because pY654 is an important determinant of the physical association of β-catenin with E-cadherin and hence the availability of β-catenin for signaling. Generation of pY654 has been shown to diminish β-catenin association with E-cadherin 10-fold (26). In addition, the β-catenin C-terminal tail interacts with the terminal armadillo repeat domains, preventing the interaction of this region with other proteins, such as protein kinase A (33). Phosphorylation of β-catenin at Tyr-654 decreases the armadillo-C-terminal tail association, uncovering these residues (10). These known functions of pY654, as well as other tyrosine phosphorylation sites on β-catenin, appear to re-enforce the capacity of β-catenin to translocate to the nucleus and mediate activation of classic TCF target genes (32). In the context of TGFβ1 signaling, this would be expected to promote pathways of cross-talk between Smad- and β-catenin-mediated transcription reported previously (23, 34–36).
However, prior studies leave open the possibility that pY654-β-catenin has a distinctive signaling function in EMT. This idea is supported by prior findings tightly linking the epithelial integrin α3β1 with both pY654-β-catenin accumulation and EMT ex vivo and in vivo (8, 23) coupled with findings reported here that a critical function of the integrin is mediating Src kinase activation after TGFβ1 signaling, Src kinase(s) activity being the principal pathway of Tyr-654 phosphorylation, and a requirement for EMT (Fig. 1) (21, 22). In addition, expression of the pY654F-β-catenin mutant incapable of phosphorylation as the only β-catenin species in AECs had no inhibitory effect on TCF signaling compared with WT β-catenin in response to TGFβ1, and yet Y654F-β-catenin failed to induce several markers of EMT including Twist1, α-SMA, and Col1 (Fig. 2). These observations dissociate classical TCF/β-catenin signaling from the functional effects of pY654-β-catenin on EMT. This dissociation is also revealed by our observation that TGFβ1 signaling in AECTs promotes pY654-β-catenin phosphorylation (detected biochemically) and EMT under conditions where TCF-mediated TOPflash activity is unmeasurable (Figs. 1C and 3A). Nonetheless, pY654-β-catenin co-immunoprecipitates with TCF3 and is clearly regulated by the axin pathway of β-catenin turnover (Fig. 4), indicating that Wnt signaling or other mechanisms of GSK3β inactivation by TGFβ1 could be expected to promote pY654-β-catenin accumulation. We favor the view that a principal function of enhanced Wnt signaling induced by TGFβ1 (Fig. 3), and possibly other cytokines implicated in EMT, is to promote the Src kinase-dependent accumulation of pY654-β-catenin that then directly interacts with the Smad pathway to effect EMT. Clearly, definition of the DNA binding specificity of pY654-β-catenin contrasted with nonphosphorylated β-catenin will be an important area for future investigation.
Although Src activity appears critical to pY654-β-catenin accumulation and EMT, our data do not exclude other targets of active Src kinase(s) as being important in EMT. For example, activation of FAK and Src from ligand-mediated integrin activation is also reported to support myofibroblast differentiation (7, 37). Internalization of E-cadherin is dependent on Src kinase-mediated phosphorylation of its cytoplasmic tail, and we have previously reported defective internalization of α3β1/E-cadherin cell surface complexes in response to TGFβ1 in α3-null cells (23, 38). Finally, although Src kinase activity is not required for β-catenin/TCF transcriptional activity, active Src may promote β-catenin signaling by altering cell contacts or in some systems inactivating GSK3β apparently independently of Wnt ligands (39). Hence, the role of Src kinase in β-catenin signaling is complex and seemingly cell type-dependent. Despite this complexity, tyrosine phosphorylation of β-catenin at Tyr-654 appears to be a consistent, required function of Src activation in TGFβ1-initiated EMT.
Inhibition of β-catenin signaling has been previously reported to inhibit bleomycin-induced pulmonary fibrosis in mice. Henderson et al. identified a small molecule, ICG-001, that inhibits the TCF/β-catenin signaling pathway by blocking the interaction of β-catenin with the transcriptional co-activator CBP. This approach resulted in an increase of survival in the bleomycin model and attenuated fibrogenesis, supporting the idea of β-catenin as a potential target for fibrotic disorders (40). Because CBP is a co-activator for many transcription factors, however, the ultimate specificity of ICG-001 in vivo is uncertain. Our data support these prior observations by demonstrating that a completely independent mechanism of attenuating β-catenin signaling improves survival and decreases fibrosis after bleomycin-induced lung injury. Importantly, although Wnt signaling could be expected to promote β-catenin signaling in the bleomycin model as discussed above, both of the in vivo approaches used to block β-catenin signaling and fibrogenesis act very downstream of Wnt ligands. Indeed, it is conceivable that Wnt ligands are not critical to the fibrogenic response in this model. Inhibition of GSK3β could arise more directly through TGFβ1 (or other cytokine)-dependent direct inhibition of GSK3β activity (41). Still, it is likely that induction of Wnt ligands contribute to the overall fibrotic process as suggested by prior studies in several models of tissue fibrosis as well as evidence of enhanced Wnt activity in IPF (13, 42–44).
Although raised axin levels block EMT ex vivo and block accumulation of Snail1 and Twist1 in vivo, the mechanism of inhibition of fibrogenesis is not necessarily through attenuation of EMT. The primary source of Col1 in fibrotic lungs is likely to be fibroblasts. The acquisition of Snail expression by adult fibroblasts was described previously as a mechanism of promoting migration and matrix production by fibroblasts (45). Although our attempts to block Col1 or α-SMA production by raising axin levels in cultured primary lung fibroblasts had little effect,3 this may not be true in vivo. AECs could be contributing to collagen production directly through EMT, but also could be promoting type I collagen expression in resident fibroblasts through cytokine/chemokine signaling activated by EMT. The role of enhanced cross-talk between epithelial cells and fibroblasts as a result of EMT is not well defined. Yet the fact that either genetic or small molecule inhibitors of EMT ex vivo suppress fibrogenesis in vivo further supports the importance of the EMT signaling pathway in fibrosis. Up-regulation of Snail and Twist is observed in IPF, and enhanced Snail expression is observed in IPF lung epithelial cells (46, 47). A recent report that selective Snail deletion in hepatic epithelial cells (hepatocytes) blocks experimental liver fibrosis underscores the importance of Snail signaling in epithelial cells during fibrogenesis (48). Given the importance of TGFβ1 signaling in many physiological processes, identification of a domain of TGFβ1 signaling specifically required for fibrosis invites a more targeted approach to this disorder, and our studies raise the novel possibility of manipulating lung axin levels as one such approach.
Supplementary Material
Acknowledgments
We thank Alexis Brumwell and Maria Sereda for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 44712 (to H. A. C.) and K08HL085290 (to K. K. K.).

This article contains supplemental Fig. S1.
Y. Xi, unpublished observation.
- Col1
- type I collagen
- AEC
- alveolar epithelial cell
- AdenoCre
- adenovirus expressing cre recombinase
- AECT
- T antigen-immortalized AEC
- EMT
- epithelial mesenchymal transition
- Fn
- fibronectin
- GSK3β
- glycogen synthase kinase 3β
- IPF
- idiopathic pulmonary fibrosis
- α-SMA
- α-smooth muscle actin
- TBRI
- TGFβ receptor 1
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- PP3
- 1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- TCF
- T cell factor.
REFERENCES
- 1. Selman M., King T. E., Pardo A. (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 134, 136–151 [DOI] [PubMed] [Google Scholar]
- 2. Shi Y., Massagué J. (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 3. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 4. Kim K. K., Kugler M. C., Wolters P. J., Robillard L., Galvez M. G., Brumwell A. N., Sheppard D., Chapman H. A. (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U.S.A. 103, 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinz B. (2009) Tissue stiffness, latent TGF-β1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr. Rheumatol. Rep. 11, 120–126 [DOI] [PubMed] [Google Scholar]
- 6. Chapman H. A. (2011) Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu. Rev. Physiol. 73, 413–435 [DOI] [PubMed] [Google Scholar]
- 7. Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M., Thomas P. E. (2003) Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 278, 12384–12389 [DOI] [PubMed] [Google Scholar]
- 8. Kim K. K., Wei Y., Szekeres C., Kugler M. C., Wolters P. J., Hill M. L., Frank J. A., Brumwell A. N., Wheeler S. E., Kreidberg J. A., Chapman H. A. (2009) Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J. Clin. Invest. 119, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simoneau M., Coulombe G., Vandal G., Vézina A., Rivard N. (2011) SHP-1 inhibits β-catenin function by inducing its degradation and interfering with its association with TATA-binding protein. Cell. Signal. 23, 269–279 [DOI] [PubMed] [Google Scholar]
- 10. Piedra J., Martinez D., Castano J., Miravet S., Dunach M., de Herreros A. G. (2001) Regulation of β-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 276, 20436–20443 [DOI] [PubMed] [Google Scholar]
- 11. Chilosi M., Poletti V., Zamò A., Lestani M., Montagna L., Piccoli P., Pedron S., Bertaso M., Scarpa A., Murer B., Cancellieri A., Maestro R., Semenzato G., Doglioni C. (2003) Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 162, 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Königshoff M., Eickelberg O. (2010) WNT signaling in lung disease: a failure or a regeneration signal? Am. J. Respir. Cell Mol. Biol. 42, 21–31 [DOI] [PubMed] [Google Scholar]
- 13. Königshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O. V., Jahn A., Rose F., Fink L., Seeger W., Schaefer L., Günther A., Eickelberg O. (2009) WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is up-regulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 119, 772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flozak A. S., Lam A. P., Russell S., Jain M., Peled O. N., Sheppard K. A., Beri R., Mutlu G. M., Budinger G. R., Gottardi C. J. (2010) β-Catenin/T cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J. Biol. Chem. 285, 3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang F., Tom C. C., Kugler M. C., Ching T. T., Kreidberg J. A., Wei Y., Chapman H. A. (2003) Distinct ligand binding sites in integrin α3β1 regulate matrix adhesion and cell-cell contact. J. Cell Biol. 163, 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pechkovsky D. V., Scaffidi A. K., Hackett T. L., Ballard J., Shaheen F., Thompson P. J., Thannickal V. J., Knight D. A. (2008) Transforming growth factor β1 induces αvβ3 integrin expression in human lung fibroblasts via a β3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J. Biol. Chem. 283, 12898–12908 [DOI] [PubMed] [Google Scholar]
- 17. Lee H., Volonte D., Galbiati F., Iyengar P., Lublin D. M., Bregman D. B., Wilson M. T., Campos-Gonzalez R., Bouzahzah B., Pestell R. G., Scherer P. E., Lisanti M. P. (2000) Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol. Endocrinol. 14, 1750–1775 [DOI] [PubMed] [Google Scholar]
- 18. Behrens J., Vakaet L., Friis R., Winterhager E., Van Roy F., Mareel M. M., Birchmeier W. (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120, 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyer B., Roche S., Denoyelle M., Thiery J. P. (1997) Src and Ras are involved in separate pathways in epithelial cell scattering. EMBO J. 16, 5904–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avizienyte E., Wyke A. W., Jones R. J., McLean G. W., Westhoff M. A., Brunton V. G., Frame M. C. (2002) Src-induced deregulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4, 632–638 [DOI] [PubMed] [Google Scholar]
- 21. Zhong Q., Zhou B., Ann D. K., Minoo P., Liu Y., Banfalvi A., Krishnaveni M. S., Dubourd M., Demaio L., Willis B. C., Kim K. J., duBois R. M., Crandall E. D., Beers M. F., Borok Z. (2011) Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 45, 498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanjore H., Cheng D. S., Degryse A. L., Zoz D. F., Abdolrasulnia R., Lawson W. E., Blackwell T. S. (2011) Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J. Biol. Chem. 286, 30972–30980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y., Kugler M. C., Wei Y., Kim K. K., Li X., Brumwell A. N., Chapman H. A. (2009) Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J. Cell Biol. 184, 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jat P. S., Noble M. D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., Kioussis D. (1991) Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. U.S.A. 88, 5096–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapman H. A., Li X., Alexander J. P., Brumwell A., Lorizio W., Tan K., Sonnenberg A., Wei Y., Vu T. H. (2011) Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121, 2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roura S., Miravet S., Piedra J., García de Herreros A., Duñach M. (1999) Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 274, 36734–36740 [DOI] [PubMed] [Google Scholar]
- 27. Minami Y., Stuart S. A., Ikawa T., Jiang Y., Banno A., Hunton I. C., Young D. J., Naoe T., Murre C., Jamieson C. H., Wang J. Y. (2008) BCR-ABL-transformed GMP as myeloid leukemic stem cells. Proc. Natl. Acad. Sci. U.S.A. 105, 17967–17972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 29. Howard S, Deroo T., Fujita Y, Itasaki N. (2011) A positive role of cadherin in Wnt/β-catenin signaling during epithelial-mesenchymal transition. PloS One 6, e23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shibamoto S., Hayakawa M., Takeuchi K., Hori T., Oku N., Miyazawa K., Kitamura N., Takeichi M., Ito F. (1994) Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes. Commun. 1, 295–305 [DOI] [PubMed] [Google Scholar]
- 31. Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C. J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M. W., Lengauer C., Finan P. M., Tallarico J. A., Bouwmeester T., Porter J. A., Bauer A., Cong F. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signaling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- 32. Brembeck F. H., Rosário M., Birchmeier W. (2006) Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr. Opin. Genet. Dev. 16, 51–59 [DOI] [PubMed] [Google Scholar]
- 33. van Veelen W., Le N. H., Helvensteijn W., Blonden L., Theeuwes M., Bakker E. R., Franken P. F., van Gurp L., Meijlink F., van der Valk M. A., Kuipers E. J., Fodde R., Smits R. (2011) β-Catenin tyrosine 654 phosphorylation increases Wnt signaling and intestinal tumorigenesis. Gut 60, 1204–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lei S., Dubeykovskiy A., Chakladar A., Wojtukiewicz L., Wang T. C. (2004) The murine gastrin promoter is synergistically activated by transforming growth factor-β/Smad and Wnt signaling pathways. J. Biol. Chem. 279, 42492–42502 [DOI] [PubMed] [Google Scholar]
- 35. Eger A., Stockinger A., Park J., Langkopf E., Mikula M., Gotzmann J., Mikulits W., Beug H., Foisner R. (2004) β-Catenin and TGFβ signaling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene 23, 2672–2680 [DOI] [PubMed] [Google Scholar]
- 36. Medici D., Hay E. D., Goodenough D. A. (2006) Cooperation between snail and LEF-1 transcription factors is essential for TGF-β1-induced epithelial-mesenchymal transition. Mol. Biol. Cell 17, 1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Avizienyte E., Frame M. C. (2005) Src and FAK signaling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 17, 542–547 [DOI] [PubMed] [Google Scholar]
- 38. Fujita Y., Krause G., Scheffner M., Zechner D., Leddy H. E., Behrens J., Sommer T., Birchmeier W. (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222–231 [DOI] [PubMed] [Google Scholar]
- 39. Burkhalter R. J., Symowicz J., Hudson L. G., Gottardi C. J., Stack M. S. (2011) Integrin regulation of β-catenin signaling in ovarian carcinoma. J. Biol. Chem. 286, 23467–23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henderson W. R., Jr., Chi E. Y., Ye X., Nguyen C., Tien Y. T., Zhou B., Borok Z., Knight D. A., Kahn M. (2010) Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. U.S.A. 107, 14309–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang X., Yu S. X., Lu Y., Bast R. C., Jr., Woodgett J. R., Mills G. B. (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. U.S.A. 97, 11960–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng J. H., She H., Han Y. P., Wang J., Xiong S., Asahina K., Tsukamoto H. (2008) Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol 294, G39–49 [DOI] [PubMed] [Google Scholar]
- 43. He W., Dai C., Li Y., Zeng G., Monga S. P., Liu Y. (2009) Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bergmann C., Akhmetshina A., Dees C., Palumbo K., Zerr P., Beyer C., Zwerina J., Distler O., Schett G., Distler J. H. (2011) Inhibition of glycogen synthase kinase 3β induces dermal fibrosis by activation of the canonical Wnt pathway. Ann. Rheum. Dis. 70, 2191–2198 [DOI] [PubMed] [Google Scholar]
- 45. Rowe R. G., Li X. Y., Hu Y., Saunders T. L., Virtanen I., Garcia de Herreros A., Becker K. F., Ingvarsen S., Engelholm L. H., Bommer G. T., Fearon E. R., Weiss S. J. (2009) Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 184, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pozharskaya V., Torres-González E., Rojas M., Gal A., Amin M., Dollard S., Roman J., Stecenko A. A., Mora A. L. (2009) Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PloS One 4, e7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jayachandran A., Königshoff M., Yu H., Rupniewska E., Hecker M., Klepetko W., Seeger W., Eickelberg O. (2009) SNAI transcription factors mediate epithelial-mesenchymal transition in lung fibrosis. Thorax 64, 1053–1061 [DOI] [PubMed] [Google Scholar]
- 48. Rowe R. G., Lin Y., Shimizu-Hirota R., Hanada S., Neilson E. G., Greenson J. K., Weiss S. J. (2011) Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol. Cell. Biol. 31, 2392–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.