Background: Efflux of macrophages limits inflammation.
Results: Macrophage integrin αMβ2 is cleaved during exiting from inflammatory sites, released αMβ2 retains ligand binding capabilities, and inhibition of its metalloproteinase-mediated cleavage impairs macrophage efflux.
Conclusion: Metalloproteinase-mediated proteolysis of integrin β2 promotes macrophage efflux from inflammatory sites.
Significance: Regulated proteolysis of integrin β2 during inflammation demonstrates the potential of this mechanism to contribute to resolution of inflammation.
Keywords: Fibrin, Inflammation, Integrin, Leukocyte, Proteolytic Enzymes, Integrin Activation
Abstract
Macrophage exiting from inflammatory sites is critical to limit the local innate immune response. With tissue insult, resident tissue macrophages rapidly efflux to lymph nodes where they modulate the adaptive immune response, and inflammatory macrophages attracted to the site of injury then exit during the resolution phase. However, the mechanisms that regulate macrophage efflux are poorly understood. This study has investigated soluble forms of integrin β2 whose levels are elevated in experimental peritonitis at times when macrophages are exiting the peritoneum, suggesting that its proteolytic shedding may be involved in macrophage efflux. Both constitutive and inducible metalloproteinase-dependent shedding of integrin β2 from mouse macrophages are demonstrated. Soluble integrin β2 is primarily released as a heterodimeric complex with αM that retains its ability to bind its ligands intracellular adhesion molecule-1, fibrin, and collagen and thus may serve as a soluble antagonist. In a model of accelerated exiting, administration of a metalloproteinase inhibitor prevents macrophage efflux by 50% and impedes loss of macrophage integrin β2 from the cell surface. Exiting of peritoneal macrophages in mice lacking integrin β2 is accelerated, and antibody disruption of integrin β2-substrate interactions can reverse 50% of the metalloprotease inhibitor blockade of macrophage exiting. Thus, our study demonstrates the ability of metalloproteinase-mediated shedding of integrin β2 to promote macrophage efflux from inflammatory sites, and the release of soluble integrin heterodimers may also limit local inflammation.
Introduction
Integrin β2 has been shown to play a critical role in leukocyte recruitment to sites of inflammation. In particular, the requirement for integrin β2 to induce rapid arrest of leukocytes on the vessel wall at sites of inflammation has highlighted the importance of β2 activation of integrin avidity in this process (1, 2). Avidity, or the overall strength of cellular adhesiveness, is controlled by the intrinsic affinity of the individual receptor-ligand bonds and the number of these bonds (valency), and avidity can be altered by integrin density, geometric conformation, and lateral mobility in the cell membrane (3). Leukocyte recruitment also requires the integrin-ligand bond to be dynamic to allow de-adhesion. Generation of constitutively active integrin αLβ2 (lymphocyte function-associated antigen 1 (LFA-1))2 dramatically impaired deactivation and de-adhesion from its ligand intercellular adhesion molecule (ICAM)-1 (4). Thus, both activation and deactivation of leukocyte integrin heterodimers are needed to promote firm adhesion and de-adhesion during a normal inflammatory response.
An alternative mechanism that could functionally act in a manner similar to integrin deactivation, and could also induce an immediate change in integrin density, is proteolytic shedding of leukocyte integrins. Although integrin proteolysis has not been shown to regulate leukocyte trafficking, cleavage of another adhesion molecule, l-selectin, selectively limits early neutrophil influx (5). Shedding of other adhesion molecules can also elicit rapid changes in cellular responses, instantly lower adhesion molecule density, and release soluble forms of their extracellular domains that can act as antagonists (6, 7). In an effort to detect novel proteins proteolytically cleaved from the surface of inflammatory macrophages, we identified the extracellular domain of integrin β2 in two proteomic screens using different inflammatory stimuli (8).3 Identification of increased levels of soluble integrin β2 under inflammatory conditions suggested a possible function in leukocyte activation and/or trafficking.
Previous studies have also demonstrated soluble forms of integrin β2 associated with the loss of integrin β2, or one of its α subunits, from the leukocyte cell surface during inflammation (9–12). Time-dependent loss of integrin αMβ2 (macrophage-1 antigen or Mac-1) from infiltrating neutrophils in the reperfused canine myocardium was shown to be associated with the appearance in lymph of soluble heterodimers, which had lower molecular weights than cellular heterodimers (9). In human blister fluid, truncated ectodomains of αLβ2 (LFA-1) were detected, and neutrophils and monocytes showed a loss of cell surface αLβ2 (10). More recently, shedding of integrin β2 complexed with different α subunits was shown to be a part of synovial inflammation in rheumatoid arthritis and spondyloarthritis but not in osteoarthritis (12). These authors further demonstrated the ability of soluble integrin β2 to inhibit monocytic cell adhesion to ICAM-1. Thus, soluble integrin β2 with the ability to buffer leukocyte adhesion has been identified in physiological fluids from multiple inflammatory states in vivo, but the functional significance of in vivo shedding from the cell surface has not been investigated. Analysis of molecular mechanisms involved in the resolution of acute inflammation has also identified the loss of macrophage surface αM (CD11b) in a distinct macrophage subpopulation that may represent specialized “pro-resolving” macrophages (13, 14). Together these studies raise the possibility that leukocyte shedding of integrin β2 heterodimers could play a role in leukocyte recruitment to inflammatory sites and/or the resolution of inflammation.
This study biochemically documents proteolytic shedding of the integrin β2 ectodomain from the surface of mouse macrophages, and it identifies zinc-dependent metalloproteinases as major regulators of this cleavage. Soluble integrin β2 retains its ability to bind its substrates, and thus may serve as a soluble antagonist. Analysis of peritoneal lavage fluid following administration of the sterile irritant thioglycollate shows that in vivo release of soluble integrin β2 is most marked at times when macrophages are exiting the peritoneal cavity. Furthermore, interference with proteolytic shedding is sufficient to significantly reduce macrophage exiting in a model of accelerated macrophage exiting from the peritoneal cavity. Diminished exiting can be partially rescued by disruption of integrin β2-substrate interactions, and peritoneal macrophage exiting in mice lacking integrin β2 is accelerated. Together, these data establish a functional role for integrin β2 heterodimer shedding in macrophage efflux from sites of inflammation.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6J mice were from The Jackson Laboratory. Integrin β2 null mice were previously described (15) and were backcrossed 10 times onto the C57BL/6J background as were the Mmp9 null mice obtained from Robert Senior (Washington University). Hematopoietic chimeras lacking Adam17 were recently described (5), and floxed Adam10 mice (16) were provided by Howard Crawford and were crossed with LysM-Cre transgenic mice (The Jackson Laboratory). Animals were housed in a pathogen-free facility, and procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Peritonitis Model
Thioglycollate-induced peritonitis was initiated by intraperitoneal injection of 1 ml of 4% sterile thioglycollate (BD Biosciences). Peritoneal cells were collected by injection of 5 ml of PBS, 5 mm EDTA, and the lavage fluid was saved following cell removal by centrifugation.
Cell Culture of Mouse Macrophages
Thioglycollate-elicited peritoneal macrophages were plated on bacterial plastic dishes in RPMI 1640 medium with 10% FCS. The metalloproteinase inhibitor GM6001 (Elastin Products; 50 μm in DMSO) or a mixture of metalloproteinase-sparing protease inhibitors (Sigma P8340; containing 4-(2-aminoethyl)benzenesulfonyl fluoride, aprotinin, leupeptin, bestatin, pepstatin A, and E-64) were added in some experiments. Macrophage lysates were prepared in Nonidet P-40 lysis buffer.
Human Monocyte Cultures
Human peripheral blood mononuclear cells were isolated from citrated blood by Ficoll-Paque Plus (Amersham Biosciences) separation and enriched for monocytes by negative selection with monocyte isolation kit II (Miltenyi Biotech). Monocytes were resuspended in Opti-MEM at 4 × 106/ml in 15-ml polypropylene tubes and incubated with activating CD18 MEM-48 antibody (10 μg/ml) or control IgG or in the presence of ultrapure LPS for 4 h at 37 °C. Monocytes were evaluated by FACS and conditioned media by ELISA.
Soluble Integrin β2 Binding to Its Ligands
Polysorb microtiter plates were coated with different amounts of purified human fibrinogen (American Diagnostica), bovine collagen type I (Cohesion), and fibronectin (Invitrogen). For fibrinogen, bovine thrombin (Innovative Research; 6 μl of solution at 1.2 mg/ml, activity 1900 NIH units/mg) was added at 4 °C and left at RT for 30 min to allow fibrin formation. Collagen gels were allowed to form (1 h at 37 °C) after neutralization. For rICAM-1, plates were coated with goat anti-human IgG (Fc-specific) at 0.5 μg/well in 50 mm sodium bicarbonate buffer (pH 9.2) for 16 h at 4 °C, and nonspecific binding was blocked with 0.5% (w/v) BSA. Thereafter, 50 μl of different concentrations of recombinant ICAM-1/Fc (R&D Systems) in PBS containing 0.1% (w/v) BSA was added to the plate and incubated overnight at 4 °C. Lavage or conditioned media were added to the extracellular matrix- or ICAM-1-coated wells and incubated for 4 h at 37 °C and assayed for depletion of soluble integrin β2 levels by ELISA.
Macrophage Binding to Integrin β2 Ligands
Plates were coated with extracellular matrix proteins or ICAM-1 as described above, and 4-day elicited peritoneal macrophages were added to the plate at 10 × 106/ml, 100 μl/well, and incubated for 4 h at 37 °C. Media were assayed for soluble integrin β2 by ELISA.
Model of Accelerated Macrophage Exiting
As published previously, LPS (1 μg of LPS in 200 μl of PBS) (17) or TNF-α (0.5 μg in 200 μl of PBS) was given by intraperitoneal injection 3 days after thioglycollate injection, and peritoneal cells were harvested 4 h later as described for the peritonitis model. For mice that received GM6001, it was dissolved in 4% carboxymethylcellulose in PBS to give a final concentration of 1.5 mg/ml, and peritoneal injection of the GM6001 (200 μl/mouse, or 0.3 mg/mouse) was 20 min before LPS injection. For blocking integrin β2 antibody experiments, 50 μg of 2E6 Fab fragments or control hamster IgG was administered 20 min before LPS or TNF-α together with GM6001 or with carboxymethylcellulose control. Fab fragments of 2E6 were generated following purification of the IgG from hybridoma supernatants using protein G-Sepharose (Pierce) and Fab preparation kit (Pierce). Control hamster IgG was purchased from eBioscience.
Flow Cytometry Analyses
Antibodies for FACS analysis were from either BD Biosciences or from eBioscience and included the following: phycoerythrin (PE)-B220 (RA3-6B2); PE-CD3 (145–2C11); PE- or FITC-Ly6G; PE- or FITC-F4/80; PE-CD18, and PECy5-CD11b (M1/70). Typically, 200,000 cells were stained after nonspecific binding was blocked by anti-CD16/32. Stained cells were analyzed on a FACScan (Becton, Dickinson and Co.), and 10,000–20,000 events were collected for each analysis. Flow data were analyzed using FlowJo software (Tree Star Inc.).
ELISAs for Soluble Integrin β2 and Integrin αMβ2 Complexes
ELISAs for integrin β2 or αMβ2 complexes utilized monoclonal antibodies to mouse integrin β2 (MAB2618, R&D Systems) and integrin αM (clone M1/70, Pharmingen) as capture antibodies, and biotin anti-mouse integrin β2 (clone C71/16, Pharmingen) as the detection antibody with streptavidin-HRP (Jackson ImmunoResearch). Antibodies used for depletion of specific integrins from Pharmingen included integrin β2 (clone M18/12), integrin αM (clone M1/70), integrin αL (clone 2D7), and integrin αX (clone HL3).
Protein Analysis and Immunoprecipitation of Soluble Integrin β2 Complexes
Cell lysates and conditioned media were separated by 4–12% gradient gel SDS-PAGE and analyzed by Western blot. For immunoprecipitation, peritoneal lavage was incubated in 96-well plates coated with antibodies to integrin β2, integrin αM, integrin αL, and integrin αX overnight at 4 °C and eluted with SDS sample buffer. Immunoblots were probed with rat monoclonal anti-integrin β2 (MAB2618) or goat polyclonal anti-integrin β2 (AF2619) from R&D Systems.
Statistics
Statistical analysis was performed using a two-tailed Student's t test using InStat (GraphPad software). All values are presented as the means ± S.E. with p < 0.05 considered significant unless otherwise noted.
RESULTS
Proteolytic Release of Integrin β2 by Mouse Resident and Elicited Peritoneal Macrophages
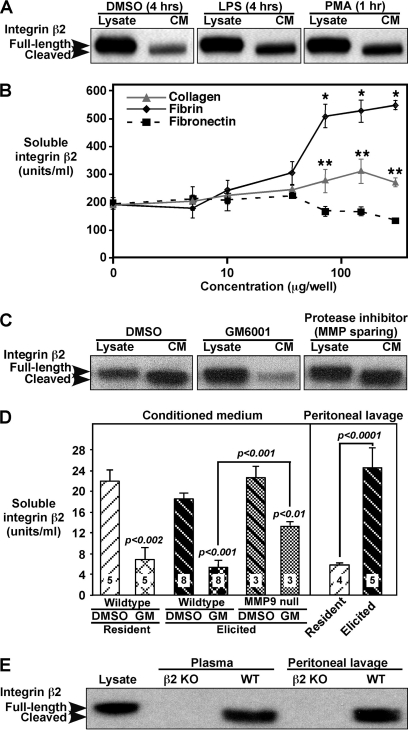
We have proposed that proteolytic shedding of cell surface proteins contributes to regulation of leukocyte recruitment to inflammatory stimuli (6). To characterize properties of cleaved integrin β2 identified in macrophage proteomics screens, elicited mouse peritoneal macrophages (stimulated to emigrate into the peritoneal cavity by injection of the sterile irritant thioglycollate) were harvested 4 days after thioglycollate and activated in vitro with different stimuli. Without stimulation, soluble integrin β2 is detected in conditioned medium, and both LPS and PMA stimulation lead to a 2-fold increase in integrin β2 in the medium (Fig. 1A). Analysis of the ratio of shed integrin β2 in conditioned medium to lysate integrin β2 shows ∼1–4% is shed in 4–6-h conditioned medium and 5–20% after 24 h. These percentages are even more significant considering that up to 90% of total cellular integrin β2 is intracellular, as shown by comparing flow cytometry of fixed versus fixed and permeabilized macrophages and consistent with previous reports of significant intracellular stores of αMβ2 (18). Adhesion of peritoneal macrophages to increasing amounts of integrin β2 physiological ligands fibrin and collagen, but not another matrix protein fibronectin, also leads to a dose-dependent increase in soluble integrin β2 (Fig. 1B). As compared with macrophage lysates, soluble integrin β2 shows a small molecular mass reduction to ∼90 kDa under all conditions (Fig. 1, A, C, and E), and this small shift is consistent with cleavage within the ectodomain.
FIGURE 1.
Integrin β2 on mouse peritoneal macrophages is constitutively and inducibly shed from the cell surface. A–D, mouse peritoneal macrophages were harvested 4 days post-thioglycollate (elicited macrophages). Cell extracts (lysate, 30 μg) and conditioned medium (CM, 20 μl) were separated by 4–12% gradient SDS-PAGE and analyzed by Western blotting with anti-mouse integrin β2 or by ELISA. A, control diluent (4 h), LPS (100 ng/ml, 4 h), and phorbol 12-myristate 13-acetate (PMA) (100 ng/ml, 1 h) treatment of elicited macrophages plated on bacterial plastic dishes for 16 h before treatment. B, elicited macrophages were plated on tissue culture plates or increasing concentrations of fibrin-, fibronectin-, or collagen-coated tissue culture plates for 4 h, and conditioned medium was evaluated by ELISA. Means ± S.E. (n = 4/condition). *, p < 0.0001; **, p < 0.015 relative to medium from macrophages on plastic. C, elicited macrophages were incubated with diluent control (DMSO), the metalloproteinase inhibitor GM6001 (50 μm), or a mixture of metalloproteinase-sparing protease inhibitors, and lysates (15 μg) and conditioned medium (CM, 30 μl) were evaluated by Western blot. D, left panel, mouse resident peritoneal macrophages and elicited macrophages were plated as described for A, and 4 h media were collected and analyzed by ELISA for soluble integrin β2. The p values for GM6001 treatments versus paired DMSO conditions are shown above the GM6001 (GM) bars, and comparisons between different groups are indicated above connecting bars. Right panel, integrin β2 levels were determined by ELISA for peritoneal lavage fluid collected with resident and 4-day elicited macrophages. Means ± S.E. (n indicated in bars). E, Western analysis of peritoneal lavage fluid and plasma from wild type (WT) C57BL/6 mice and integrin β2 knock-out mice (KO) shows a lower molecular mass band for integrin β2 relative to lysate only in WT mice. Western data in this figure are representative of more than four replicate experiments.
Because LPS stimulation and adhesion to integrin ligands can both lead to integrin activation, we asked whether integrin β2 activation alone could induce its proteolytic release. We utilized well characterized antibodies to human integrins that can induce integrin activation and also detect the activated epitope. As shown in supplemental Fig. 1, human monocytes release soluble integrin β2 during a 4-h incubation in suspension, and levels released are increased 3-fold by incubation with LPS. However, addition of integrin β2-activating antibody MEM-48 did not increase soluble integrin β2 (supplemental Fig. 1A) despite a 4-fold increase in the activated epitope (supplemental Fig. 1B). Thus, integrin β2 activation is not sufficient to enhance its shedding.
To evaluate protease families involved in integrin β2 cleavage, the broad spectrum metalloproteinase inhibitor GM6001 and a mixture of metalloproteinase-sparing protease inhibitors were added to adherent cultures of peritoneal macrophages (Fig. 1C). GM6001 almost completely inhibits shedding of integrin β2 with soluble levels reduced by 80–90% (Fig. 1D), suggesting a matrix metalloproteinase (MMP) or ADAM (A Disintegrin And Metalloproteinase) protease. In contrast, the matrix metalloproteinase-sparing protease inhibitors have no effect on soluble integrin β2 (Fig. 1C), ruling out serine, cysteine, aspartic acid, and aminopeptidases as major contributors.
Soluble integrin β2 is detected in peritoneal fluid collected in vivo with resident (no stimulation) and elicited macrophages (4 days following thioglycollate injection) with higher levels of soluble integrin β2 following thioglycollate injection (Fig. 1D, right panel). In vitro culture of the peritoneal macrophages also leads to release of soluble integrin β2, which is significantly reduced in the presence of GM6001 (Fig. 1D, left panel). The decrease in molecular weight of soluble integrin β2 as compared with cell lysates is seen in both plasma and peritoneal lavage fluid (Fig. 1E), suggesting proteolytic cleavage of integrin β2 in vivo. No signals are detected by Western blot in fluids from β2 knock-out mice (Fig. 1E), establishing the specificity of the antibody-based data. Together, these experiments establish that integrin β2 is cleaved in vitro and in vivo in a metalloproteinase-dependent manner, and cleavage leads to the release of a soluble form of integrin β2.
Because we previously identified soluble integrin β2 in a proteomics screen of medium from macrophages overexpressing active MMP-9 (8), we asked whether MMP-9 is required for soluble integrin β2 release. As shown in Fig. 1D (left panel), MMP-9 null peritoneal macrophages in vitro release soluble integrin β2 at levels comparable with wild type peritoneal macrophages. However, GM6001 blocks integrin β2 release from MMP-9 null macrophages less efficiently than for wild type (Fig. 1D). Table 1 shows that levels of soluble integrin β2 are also comparable in peritoneal fluid from MMP-9 null and wild type mice. Both the pro- and active forms of MMP-9 have previously been demonstrated in peritoneal fluid following thioglycollate injection (19). Peritoneal fluid from mice deficient in myeloid ADAM10 and ADAM17, two enzymes that have been shown to shed many cell surface proteins (6, 20), was also tested (Table 1), and their deletion also did not significantly alter shedding of integrin β2. The decreased efficiency of GM6001-mediated blockade of soluble integrin β2 shedding from MMP-9 null macrophages suggests that increased levels of nonmetalloproteinase enzymes may compensate for the loss of MMP-9. Thus, although MMP-9 is capable of cleaving integrin β2 (8), neither this MMP nor ADAM10 and ADAM17 are required, suggesting that additional enzymes participate in shedding of integrin β2.
TABLE 1.
Levels of soluble integrin β2 in the peritoneal fluid are not altered by the absence of MMP-9 or by ADAM10 or ADAM17 myeloid deficiency
Integrin β2 levels in the peritoneal fluid (units/ml) collected 3 days following thioglycollate injection or 3 days following thioglycollate and an additional 4-h post-injection of LPS were measured by ELISA. The means ± S.E. are shown for the indicated number of mice. ND indicates not determined.
| Enzyme genotype | Soluble integrin β2 in 3-day post-thioglycollate peritoneal fluid | n | Soluble integrin β2 in 3-day post-thioglycollate also with 4-h LPS peritoneal fluid |
|---|---|---|---|
| Wild type MMP-9 | 29.73 ± 2.74 | 4 | 79.07 ± 3.67 |
| MMP-9 null | 29.97 ± 7.82 | 4 | 72.85 ± 10.6 |
| Wild type ADAM10 | 28.33 ± 3.28 | 5 | ND |
| Myeloid-deficient ADAM10 | 28.05 ± 3.74 | 5 | ND |
| Wild type ADAM17 hematopoietic chimeras | 37.86 ± 3.71 | 5 | 69.32 ± 4.87 |
| ADAM17-deficient hematopoietic chimeras | 36.36 ± 2.12 | 5 | 66.32 ± 4.18 |
Soluble Mouse Integrin β2 Is Primarily Released as a Complex with Integrin aM and the Soluble Complex Retains Ligand-binding Capacity
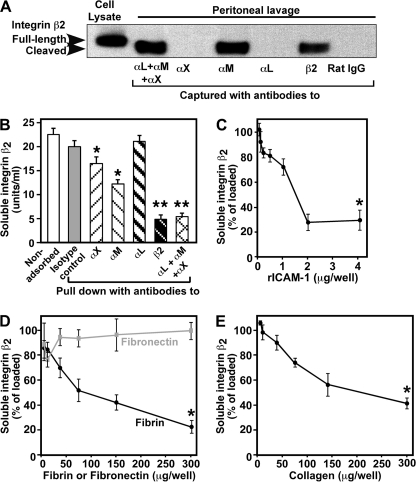
Leukocyte β2 integrins form heterodimers with one of four α subunits as follows: αL (LFA-1, CD18/CD11a), αM (Mac-1/CR3, CD18/CD11b), αX (CD18/CD11c), and αD (CD18/CD11d). Because many integrin β2 functions depend on the presence of the α subunit (21), we asked whether peritoneal lavage fluid contained integrin β2 complexed with one or more of its potential α subunits. Immunoprecipitation shows that integrin β2 is primarily complexed with integrin αM (Fig. 2A). Because we were unable to detect the different α subunits by Western analysis using multiple commercially available antibodies to mouse integrins, and because the β2 ELISA is a more sensitive method for detection of soluble form than immunoblotting, we performed immunoprecipitation followed by ELISA to quantify the contribution of different α subunits. Immunodepletion of peritoneal lavage fluid (Fig. 2B) with antibodies either to integrin β2 or combined antibodies to αL, αM, and αX remove >70% (70.0 ± 11.1 and 72.5 ± 7.8%, respectively, in three independent experiments) of the total soluble integrin β2, whereas antibodies to αM and αX are able to deplete by 40.0 ± 5.3 and 26.5 ± 0.2%, respectively.
FIGURE 2.
Soluble integrin β2 from peritoneal lavage is primarily complexed with integrin αM, and adsorbed ligands deplete integrin β2 in a concentration-dependent manner. A, mouse peritoneal lavage fluid from wild type mice was collected 4 days after thioglycollate and was immuno-captured with antibodies to individual and combined integrin α subunits, integrin β2 subunit, and control rat IgG. The captured complexes were separated by 4–12% gradient SDS-PAGE and analyzed by Western blotting with polyclonal anti-mouse integrin β2 antibody. B, soluble integrin β2 from peritoneal lavage collected from three different mice was subjected to multiple depletions with antibodies against integrin β2 and individual and combined antibodies to α subunits. The remaining unbound soluble integrin β2 was collected and measured using the mouse β2 ELISA. *, p < 0.002; **, p = 0.0002 relative to isotype control. C–E, quantification by ELISA is shown of soluble integrin β2 remaining following 4 h of incubation of peritoneal lavage fluid from four to five different mice with plates coated with increasing amounts of the following. C, recombinant mouse rICAM-1/Fc at concentrations of 0.01–2 μg/well (*, p < 0.001); D, purified human fibrinogen at 0.05–300 μg/well treated with thrombin to generate fibrin (*, p = 0.0013), and purified fibronectin at the same concentrations (not significant); E, purified bovine type I collagen at 0.05–300 μg/well (*, p < 0.001). Means ± S.E.
To assess whether the shed integrin β2 complex still retains biological function, its ability to bind to its in vivo ligands, ICAM-1, fibrin, and collagen, was evaluated. Recombinant mouse ICAM-1-coated plates (Fig. 2C), fibrin (Fig. 2D), and collagen (Fig. 2E) dose-dependently deplete integrin β2. However, fibronectin, which is not an integrin β2 ligand, failed to remove any significant amount of soluble integrin β2 (Fig. 2D). Thus, soluble integrin β2 is released as a complex primarily with integrin αM, and this complex readily binds its ligands.
Metalloproteinase Inhibition Blocks LPS-induced Macrophage Exit from the Peritoneum and Increases β2 Surface Levels Specifically on Retained Macrophages
Sterile peritonitis models have been a valuable approach to identify specific mediators and cell types involved in regulating the acute inflammatory response because the models phenocopy macrophage exiting, inflammatory neutrophil and monocyte infiltration, and subsequent self-resolving phases of the response (14). A single peritoneal injection of the sterile irritant thioglycollate leads to the exit of almost all of the resident peritoneal macrophages within 4 h and their migration to the draining lymph nodes. Following the exit of resident peritoneal macrophages, inflammatory monocytes infiltrate to levels that peak at 3–4 days, followed by a slow exiting of recruited monocyte/macrophages over 4–14 days to the omentum and draining lymph nodes (22, 23). Evaluation of peritoneal lavage fluid collected at different times using this model demonstrates that β2 integrin levels were highest at times of peritoneal macrophage exiting (Table 2). This led us to hypothesize that macrophages may shed integrin β2 to leave the peritoneal cavity.
TABLE 2.
Levels of soluble integrin β2 in the peritoneal fluid are elevated at times of macrophage exiting
Integrin β2 levels in the peritoneal fluid (units/ml) collected at the indicated time points were measured by ELISA. The means ± S.E. are shown for 9–14 individual mice.
| Time point | Soluble integrin β2 | n | Condition |
|---|---|---|---|
| 0 | 9.96 ± 3.12 | 14 | No stimulation |
| 4 h | 28.62 ± 9.00 | 14 | Resident macrophage exiting |
| 1 day | 12.26 ± 1.97 | 9 | Leukocyte infiltration |
| 4 day | 29.62 ± 6.40 | 10 | Initiation of slow exiting |
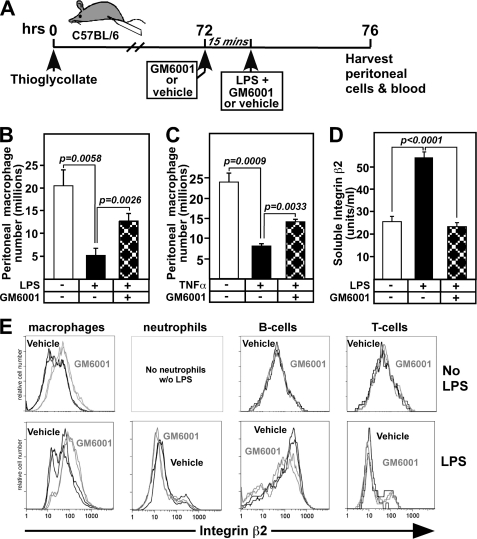
To test this hypothesis, we used a model of accelerated macrophage exiting from the peritoneal cavity stimulated by LPS activation of thioglycollate-elicited macrophages (17, 24) shown in Fig. 3A. In this model, LPS promotes macrophage adhesion and migration across the mesothelium into the lymphatics in a fibrin-dependent manner (24). To block β2 integrin cleavage, the metalloprotease inhibitor GM6001 was injected 15 min prior to LPS administration, and cells remaining in the peritoneal cavity 4 h after LPS were collected and analyzed (Fig. 3). Macrophages that remain in the peritoneal cavity are used to assess exiting because it is difficult to quantitatively evaluate macrophage content in lymph nodes and omentum around the peritoneal cavity. LPS promotes exiting of 15 million macrophages, although 5 million remain in the peritoneum. However, in the presence of GM6001, macrophage exiting is reduced to 7 million, a reduction of 50% (Fig. 3B). Because TNF-α is downstream of LPS stimulation, we also tested whether TNF-α was sufficient to induce macrophage exiting and integrin β2 shedding. As shown in Fig. 3C, TNF-α stimulates macrophage exiting to a similar extent as LPS and was inhibited by GM6001, suggesting that they may both act through a common molecular mechanism.
FIGURE 3.
In vivo administration of an MMP/ADAM inhibitor GM6001 prevents LPS-induced macrophage exiting from the peritoneum and release of soluble integrin β2, while increasing β2 surface expression on macrophages. A, mice received thioglycollate and after 3 days (3–5 mice per group) were injected with the metalloprotease inhibitor GM6001 or vehicle 15 min prior to administration of vehicle or 1 μg of LPS, all by intraperitoneal injections. Four hours later, cells from the peritoneal cavity were harvested, counted, and stained for flow cytometric analysis of cell distributions. B, macrophage (F4/80+, Ly6G−) numbers in the peritoneal cavity were determined. C, peritoneal macrophages were collected as shown in A except that vehicle or 0.5 μg of TNF-α was administered 15 min following injection of GM6001. Macrophage (F4/80+, Ly6G−) numbers in the peritoneal cavity were determined. D, peritoneal lavage fluids were analyzed 4 h following injection of PBS, LPS or LPS with GM6001 by αMβ2 ELISA. E, representative FACS histograms of two mice from each group show the mean fluorescence intensity with (gray) or without (black) GM6001 for integrin β2 on macrophages, neutrophils, B-cells, and T-cells in the presence (lower panel) or absence (upper panel) of LPS. Means ± S.E. are shown, and data are representative of three different experiments.
GM6001 also prevents the release of soluble integrin β2 into peritoneal fluid (Fig. 3D). Furthermore, surface expression of β2 integrin is specifically elevated 5-fold on macrophages of mice treated with GM6001, but no difference in β2 expression is observed on neutrophils, B-cells, or T-cells (Fig. 3E). These data are consistent with our hypothesis that β2 integrin, in the absence of GM6001, is shed from macrophages as they exit the peritoneal cavity. However, it is unclear to what extent the cleavage of β2 integrin and/or other metalloproteinase substrates are responsible for macrophage exiting.
Integrin β2 Deficiency Accelerates Macrophage Exiting and GM6001 Inhibits Exiting That Is Partially Rescued by Disruption of Integrin β2 Substrate Interactions
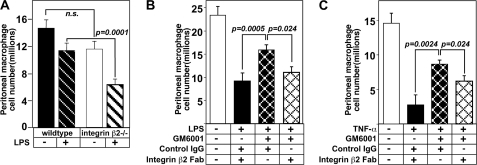
If cleavage of β2 integrin is functionally involved in promoting macrophage exiting, we would predict that absence of β2 integrin would accelerate their exit. We tested this possibility in mice either +/+ or −/− for β2 integrin (Fig. 4A). As predicted, mice lacking β2 integrin showed twice as many macrophages exiting the peritoneal cavity at 2 h after LPS injection.
FIGURE 4.
In vivo administration of disrupting integrin β2 antibody 2E6 promotes macrophage exit from the peritoneum prevented by the metalloproteinase inhibitor GM6001. A, mice either integrin β2 +/+ or −/− received an LPS injection as described in Fig. 3A, and then cells were harvested after 2 h to evaluate exiting (n = 4–5/group; n.s. = not significant). B and C, experimental protocol to measure macrophage exiting was the same as in Fig. 3A except that the 15-min incubation with GM6001 or vehicle control also included treatment with 150 μg of 2E6 Fab fragments or hamster isotype IgG control prior to LPS injection. Macrophage cell number was quantitated by FACS analysis (F4/80+, Ly6G−). The numbers of macrophages remaining in the peritoneal cavity with different treatments is shown: B, following LPS administration (n = 3–4/group); C, after TNF-α stimulation (n = 3/group). The data are expressed as the number of macrophages that remain in the peritoneal cavity after 2 or 4 h. Means ± S.E. are shown, and the data are representative of two replicate experiments.
Another strategy to evaluate the role of proteolytic shedding of integrin β2 in peritoneal macrophage exiting is to ask whether an antibody capable of disrupting integrin β2 interactions with its ligands, antibody 2E6 (25), can reverse a significant portion of macrophage exiting inhibited by GM6001. To avoid dimer formation and associated signaling from whole IgG, Fab fragments of 2E6 or a hamster isotype control IgG were injected with GM6001 15 min prior to LPS injection. GM6001 with control IgG shows the expected retention of macrophages in the peritoneal cavity (Fig. 4B). However, administration of 2E6 Fab fragments promotes macrophage exiting from the peritoneum despite the presence of GM6001 and is able to rescue ∼50% of the macrophage exiting blocked by GM6001 following LPS (Fig. 4B) and TNF-α stimulation (Fig. 4C). Together these data demonstrate that proteolytic shedding of integrin β2 significantly contributes to the regulation of macrophage exiting during the inflammatory responses.
DISCUSSION
Our study highlights the contribution of metalloproteinase-mediated cleavage of integrin β2 to macrophage exiting from an inflammatory site, a previously unrecognized mechanism for controlling macrophage trafficking and limiting the local response to injury. Defects in the process of inflammatory cell exiting can promote progression to chronic inflammation and persistent tissue injury. However, because disrupting integrin β2-substrate interactions only restored 50% of the LPS-induced macrophage exiting blocked by GM6001 (Fig. 4), cleavage of other macrophage proteins is likely involved in regulation of macrophage exiting. It is also unclear what the specific ligand is that integrin αMβ2 binds to within the peritoneal cavity, and which signaling pathways are involved in initiation of shedding and macrophage exiting.
Extravascular fibrin deposition is an early and persistent hallmark of the inflammatory response in the peritonitis model and promotes macrophage cell-cell and cell-fibrin interactions, thus serving as a provisional matrix for recruited macrophages (24, 26, 27). Deficiency in fibrin(ogen) does not prevent macrophage infiltration in response to thioglycollate, but it does impair macrophage activation (28) and adhesion to the peritoneal cavity mesothelial lining (24). However, our detection of soluble forms of two fibrin(ogen)-binding integrin dimers, αMβ2 and αXβ2, in peritoneal fluid suggests that macrophage binding to fibrin may promote their shedding. Mac-1 deficient macrophages (mice lacking integrin αM) did not exit the peritoneal cavity in the accelerated exiting model (17), although we observed more rapid exiting in the same model with mice lacking integrin β2. Outside-in signaling (29) and binding to low density lipoprotein receptor-related protein (LRP)-1, which is required for migration (30), are both impaired in the absence of integrin αM. Thus, although Mac-1 null mice still express αXβ2 that could mediate fibrin binding, their macrophages would be impaired in “outside-in” signaling from fibrin. It would also be interesting in future experiments to evaluate whether αXβ2 is shed in Mac-1-deficient mice and the extent of shedding in wild type and mutant mice of the most recently identified β2 integrin for which reagents have been lacking, αDβ2, which can also bind fibrinogen (31). In contrast, macrophages lacking integrin β2 would not express any of the fibrin-binding integrin heterodimers, and thus they would not need to shed the β2 integrins to leave and could utilize integrin α4β1 for migration (32, 33). It is interesting to note that the integrin dimer that does not bind fibrin, αLβ2 (LFA-1), is expressed on recruited macrophages but is not shed into peritoneal lavage fluid. Thus, we propose that LPS and TNF-α may promote integrin activation and fibrin adhesion leading to shedding of integrins αMβ2 and αXβ2 and enhancement of macrophage efflux (Fig. 5).
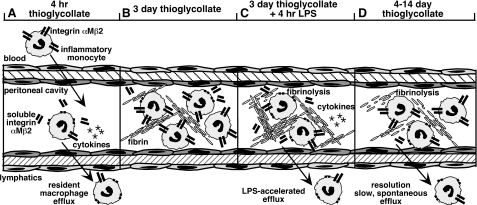
FIGURE 5.
A diagram depicting peritoneal macrophage efflux following different treatments. A, in response to injection of the sterile irritant thioglycollate, resident macrophages proteolytically shed integrin αMβ2 (CD11b/CD18) and exit the peritoneal cavity to the draining lymph nodes. By 4 h after thioglycollate injection, almost all of the resident macrophages have exited the peritoneum, and maximal levels of cytokines are detected. Neutrophils (data not shown) and monocytes begin to emigrate into the peritoneal cavity from the blood, and the inflammatory monocytes express low levels of CD11b. B, 3 days after thioglycollate, the recruited macrophages are the major cell population, and CD11b levels on the cell surface are elevated relative to that observed after 1 day. Fibrin deposits are detected within the peritoneal cavity. C, injection of LPS (or TNF-α) into the peritoneal cavity 3 days after thioglycollate stimulates accelerated efflux, a process that is enhanced by proteolytic shedding of integrin αMβ2 and fibrinolysis. Cytokines are acutely released in response to LPS similar to the initial response to thioglycollate shown in A. The injection of LPS and/or cytokines released may promote macrophage adhesion to deposited fibrin, which may induce macrophage shedding of integrin αMβ2 and rapid efflux. D, macrophage inflammatory response to thioglycollate slowly resolves spontaneously over 4–14 days. Both fibrinolysis and shedding of integrin αMβ2 likely contribute to this slow efflux.
Macrophage exiting from the peritoneal cavity is impaired by deletion of components of another protease pathway, the plasminogen activation cascade, and specifically by deletion of tissue plasminogen activator (tPA) (34). tPA is expressed on the macrophage cell surface and specifically interacts with integrin αMβ2 (34). Cao et al. (34) further suggested that the endocytic receptor LRP-1 associates with integrin αMβ2 through a mechanism in which tPA-plasminogen activator inhibitor-1 complexes bridge LRP-1 with αMβ2 and promote the internalization of αMβ2, thus facilitating cell detachment. However, an alternative explanation for their data is that rather than being internalized, αMβ2 is cleaved from the cell surface, a possibility that is more consistent with the low inhibition of macrophage exiting observed following administration of the LRP-1 inhibitor RAP (34). Fibrinolysis is required for optimal exiting from the peritoneal cavity (27), and it seems likely that fibrinolysis mediated by tPA may contribute to the nonmetalloproteinase component of macrophage efflux (Fig. 5).
Although our in vitro analysis of shedding with matrix metalloproteinase-sparing inhibitors suggests that >90% of integrin β2 shedding is mediated by MMPs or ADAMs, other proteases may contribute under different conditions. The serine proteases elastase, proteinase-3, and cathepsin G were recently reported to cleave human neutrophil integrins αMβ2 (11). In addition to release of the ∼90-kDa fragment of integrin β2 representing the majority of the extracellular domain, Zen et al. (11) also detected a fragment of ∼50 kDa. However, in our analysis of peritoneal fluid, plasma, and conditioned media, only the ∼90-kDa form of soluble integrin β2 has been detected, suggesting that integrin β2 cleavage by serine proteases may be neutrophil-specific and/or human-specific.
Based on our identification of soluble integrin β2 in a proteomics screen of medium from macrophages overexpressing active MMP-9, this enzyme was a strong candidate. Similarly, a significant number of leukocyte cell surface adhesion proteins are shed by ADAM10 and ADAM17 (6, 20). However, analysis of mice lacking MMP-9, or with myeloid deficiency of ADAM10 or ADAM17, suggests that these three enzymes are not major contributors to integrin β2 cleavage from the macrophage cell surface under conditions comparable with the model of accelerated macrophage exiting from the peritoneal cavity. Although multiple enzymes appear to cleave the same substrates in vitro, it is becoming increasingly clear that in vivo cleavage is both cell- and context-specific. For example, we recently demonstrated context specificity in ADAM17 cleavage of l-selectin that is required for limiting neutrophil rolling on endothelial cells, but neutrophil l-selectin levels are reduced >90% during neutrophil extravasation independent of ADAM17 (5). So, despite the almost complete lack of l-selectin shedding in Adam17 null cultured cells in vitro (35), conditions exist in vivo that utilize distinct enzymes not revealed in studies of cultured cells. Thus, additional candidate metalloproteinases need to be evaluated in vivo.
Rapid efflux of resident macrophages from an inflammatory site is an important component of the adaptive immune response (Fig. 5). In studies of accelerated macrophage efflux, pro-inflammatory cytokine release is induced with subsequent neutrophil influx (data not shown) in a manner similar to the initial response to thioglycollate (36). In contrast, the slow and spontaneous exiting of macrophages with the resolution of inflammation induced by thioglycollate or zymosan proceeds over 4–14 days after the stimulus and is associated with the release of anti-inflammatory cytokines and pro-resolving lipid mediators (14). The pro-resolving lipid mediators lipoxin A4 and resolvin E1 stimulate monocyte shedding of l-selectin in the blood helping to reduce monocyte influx (37). We show that peritoneal levels of soluble integrin β2 are elevated both at the time of initial exiting of resident macrophages and during the slow resolution phase. As shown by studies of synovial fluids (12), the release of soluble integrin β2 may buffer leukocyte adhesion thus further limiting leukocyte influx and/or activation. In addition, soluble complexes may retain signaling capabilities.
Defects in the initial efflux of resident macrophages can have significant implications for controlling the inflammatory stimuli, whereas impaired exiting of inflammatory macrophages during the resolution phase can promote progression to chronic inflammation and persistent tissue injury. Thus, there is a need to identify pathways that regulate macrophage efflux as therapeutic targets, and our study now adds integrin β2 shedding to the list of mediators of macrophage efflux from inflammatory sites.
Acknowledgments
We thank Howard Crawford for providing the floxed Adam10 mice and Robert Senior for Mmp9 null mice.
This work was supported, in whole or in part, by National Institutes of Health Grants 5P01 HL018645 (to E. W. R., J. M. H., and J. W. H.), R01 HL067267 and HL081795 (to E. W. R.), and 5P01 HL030086 (to J. W. H.). This work was also supported by Grant P30 DK017047 from the Mass Spectrometry Core, Diabetes Education and Research Center, University of Washington.

This article contains supplemental Fig. 1.
W. Yan, I. G. Gomez, C. L. Wilson, R. Aebersold, and E. W. Raines, unpublished data.
- LFA-1
- lymphocyte function-associated antigen-1
- ADAM
- A Disintegrin And Metalloproteinase
- ICAM-1
- intracellular adhesion molecule-1
- LRP
- low-density lipoprotein receptor-related protein
- Mac-1
- macrophage-1 antigen
- MMP
- matrix metalloproteinase
- PE
- phycoerythrin
- tPA
- tissue plasminogen activator.
REFERENCES
- 1. Alon R., Feigelson S. (2002) From rolling to arrest on blood vessels. Leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin. Immunol. 14, 93–104 [DOI] [PubMed] [Google Scholar]
- 2. Alon R., Dustin M. L. (2007) Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26, 17–27 [DOI] [PubMed] [Google Scholar]
- 3. Carman C. V., Springer T. A. (2003) Integrin avidity regulation. Are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 15, 547–556 [DOI] [PubMed] [Google Scholar]
- 4. Semmrich M., Smith A., Feterowski C., Beer S., Engelhardt B., Busch D. H., Bartsch B., Laschinger M., Hogg N., Pfeffer K., Holzmann B. (2005) Importance of integrin LFA-1 deactivation for the generation of immune responses. J. Exp. Med. 201, 1987–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang J., Zarbock A., Gomez I., Wilson C. L., Lefort C. T., Stadtmann A., Bell B., Huang L. C., Ley K., Raines E. W. (2011) Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood 118, 786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garton K. J., Gough P. J., Raines E. W. (2006) Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J. Leukocyte Biol. 79, 1105–1116 [DOI] [PubMed] [Google Scholar]
- 7. Murphy G., Murthy A., Khokha R. (2008) Clipping, shedding, and RIPping keep immunity on cue. Trends Immunol. 29, 75–82 [DOI] [PubMed] [Google Scholar]
- 8. Vaisar T., Kassim S. Y., Gomez I. G., Green P. S., Hargarten S., Gough P. J., Parks W. C., Wilson C. L., Raines E. W., Heinecke J. W. (2009) MMP-9 sheds the β2 integrin subunit (CD18) from macrophages. Mol. Cell. Proteomics 8, 1044–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Youker K. A., Beirne J., Lee J., Michael L. H., Smith C. W., Entman M. L. (2000) Time-dependent loss of Mac-1 from infiltrating neutrophils in the reperfused myocardium. J. Immunol. 164, 2752–2758 [DOI] [PubMed] [Google Scholar]
- 10. Evans B. J., McDowall A., Taylor P. C., Hogg N., Haskard D. O., Landis R. C. (2006) Shedding of lymphocyte function-associated antigen-1 (LFA-1) in a human inflammatory response. Blood 107, 3593–3599 [DOI] [PubMed] [Google Scholar]
- 11. Zen K., Guo Y. L., Li L. M., Bian Z., Zhang C. Y., Liu Y. (2011) Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood 117, 4885–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gjelstrup L. C., Boesen T., Kragstrup T. W., Jørgensen A., Klein N. J., Thiel S., Deleuran B. W., Vorup-Jensen T. (2010) Shedding of large functionally active CD11/CD18 integrin complexes from leukocyte membranes during synovial inflammation distinguishes three types of arthritis through differential epitope exposure. J. Immunol. 185, 4154–4168 [DOI] [PubMed] [Google Scholar]
- 13. Schif-Zuck S., Gross N., Assi S., Rostoker R., Serhan C. N., Ariel A. (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages. Modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., Serhan C. N. (2005) Molecular circuits of resolution. Formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 [DOI] [PubMed] [Google Scholar]
- 15. Scharffetter-Kochanek K., Lu H., Norman K., van Nood N., Munoz F., Grabbe S., McArthur M., Lorenzo I., Kaplan S., Ley K., Smith C. W., Montgomery C. A., Rich S., Beaudet A. L. (1998) Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 188, 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibb D. R., El Shikh M., Kang D. J., Rowe W. J., El Sayed R., Cichy J., Yagita H., Tew J. G., Dempsey P. J., Crawford H. C., Conrad D. H. (2010) ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J. Exp. Med. 207, 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao C., Lawrence D. A., Strickland D. K., Zhang L. (2005) A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106, 3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller L. J., Bainton D. F., Borregaard N., Springer T. A. (1987) Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J. Clin. Invest. 80, 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong Y., Hart E., Shchurin A., Hoover-Plow J. (2008) Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J. Clin. Invest. 118, 3012–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reiss K., Saftig P. (2009) The “a disintegrin and metalloprotease” (ADAM) family of sheddases. Physiological and cellular functions. Semin. Cell Dev. Biol. 20, 126–137 [DOI] [PubMed] [Google Scholar]
- 21. Solovjov D. A., Pluskota E., Plow E. F. (2005) Distinct roles for the α and β subunits in the functions of integrin αMβ2. J. Biol. Chem. 280, 1336–1345 [DOI] [PubMed] [Google Scholar]
- 22. Melnicoff M. J., Horan P. K., Morahan P. S. (1989) Kinetics of changes in peritoneal cell populations following acute inflammation. Cell. Immunol. 118, 178–191 [DOI] [PubMed] [Google Scholar]
- 23. Bellingan G. J., Caldwell H., Howie S. E., Dransfield I., Haslett C. (1996) In vivo fate of the inflammatory macrophage during the resolution of inflammation. Inflammatory macrophages do not die locally but emigrate to the draining lymph nodes. J. Immunol. 157, 2577–2585 [PubMed] [Google Scholar]
- 24. Szaba F. M., Smiley S. T. (2002) Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 99, 1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwata A., Harlan J. M., Vedder N. B., Winn R. K. (2002) The caspase inhibitor z-VAD is more effective than CD18 adhesion blockade in reducing muscle ischemia-reperfusion injury. Implication for clinical trials. Blood 100, 2077–2080 [DOI] [PubMed] [Google Scholar]
- 26. Colvin R. B., Dvorak H. F. (1975) Fibrinogen/fibrin on the surface of macrophages. Detection, distribution, binding requirements, and possible role in macrophage adherence phenomena. J. Exp. Med. 142, 1377–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook A. D., Vlahos R., Massa C. M., Braine E. L., Lenzo J. C., Turner A. L., Way K. J., Hamilton J. A. (2006) The effect of tissue type-plasminogen activator deletion and associated fibrin(ogen) deposition on macrophage localization in peritoneal inflammation. Thromb. Haemost. 95, 659–667 [PubMed] [Google Scholar]
- 28. Flick M. J., Du X., Witte D. P., Jirousková M., Soloviev D. A., Busuttil S. J., Plow E. F., Degen J. L. (2004) Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest. 113, 1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lefort C. T., Hyun Y. M., Schultz J. B., Law F. Y., Waugh R. E., Knauf P. A., Kim M. (2009) Outside-in signal transmission by conformational changes in integrin Mac-1. J. Immunol. 183, 6460–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ranganathan S., Cao C., Catania J., Migliorini M., Zhang L., Strickland D. K. (2011) Molecular basis for the interaction of low density lipoprotein receptor-related protein 1 (LRP1) with integrin αMβ2: identification of binding sites within αMβ2 for LRP1. J. Biol. Chem. 286, 30535–30541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yakubenko V. P., Yadav S. P., Ugarova T. P. (2006) Integrin αDβ2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood 107, 1643–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Issekutz T. B. (1995) In vivo blood monocyte migration to acute inflammatory reactions, IL-1α, TNF-α, IFN-γ, and C5a utilizes LFA-1, Mac-1, and VLA-4. The relative importance of each integrin. J. Immunol. 154, 6533–6540 [PubMed] [Google Scholar]
- 33. Ulyanova T., Priestley G. V., Banerjee E. R., Papayannopoulou T. (2007) Unique and redundant roles of α4 and β2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Exp. Hematol. 35, 1256–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao C., Lawrence D. A., Li Y., Von Arnim C. A., Herz J., Su E. J., Makarova A., Hyman B. T., Strickland D. K., Zhang L. (2006) Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 25, 1860–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 [DOI] [PubMed] [Google Scholar]
- 36. Matsukawa A., Kudo S., Maeda T., Numata K., Watanabe H., Takeda K., Akira S., Ito T. (2005) Stat3 in resident macrophages as a repressor protein of inflammatory response. J. Immunol. 175, 3354–3359 [DOI] [PubMed] [Google Scholar]
- 37. Filep J. G., Zouki C., Petasis N. A., Hachicha M., Serhan C. N. (1999) Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood. Modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood 94, 4132–4142 [PubMed] [Google Scholar]