Abstract
Peroxiredoxins (Prxs) contain an active site cysteine that is sensitive to oxidation by H2O2. Mammalian cells express six Prx isoforms that are localized to various cellular compartments. The oxidized active site cysteine of Prx can be reduced by a cellular thiol, thus enabling Prx to function as a locally constrained peroxidase. Regulation of Prx via phosphorylation in response to extracellular signals allows the local accumulation of H2O2 and thereby enables its messenger function. The fact that the oxidation state of the active site cysteine of Prx can be transferred to other proteins that are less intrinsically susceptible to H2O2 also allows Prx to function as an H2O2 sensor.
Keywords: Cell Signaling, Peroxiredoxin, Post-translational Modification, Reactive Oxygen Species (ROS), Redox Signaling, Hydrogen Peroxide, Intracellular Messenger, Local Peroxide
Introduction
An unusual antioxidant protein, now called peroxiredoxin (Prx),2 was initially identified on the basis of its capacity to protect proteins from oxidative damage induced by reactive oxygen species (ROS) produced in the presence of DTT. It was named “protector protein” or “thiol-specific antioxidant” before being renamed Prx (1–5). The ROS were generated as the result of the reduction of molecular oxygen by DTT to superoxide and H2O2, which were further reduced to hydroxyl radicals in the presence of trace amounts of contaminating metal (iron or copper) ions (6). Analysis of purified yeast Prx revealed that it did not contain conventional redox centers such as metals, heme, flavin, or selenocysteine. Prx therefore did not resemble any antioxidant known at the time. It was subsequently found that (i) Prx is present in all biological kingdoms from bacteria to mammals; (ii) two cysteine residues, corresponding to Cys47 and Cys170 of yeast Prx, are highly conserved among Prx family members; (iii) Prxs are homodimers arranged in a head-to-tail orientation; and (iv) Cys47–SH of Prx is specifically oxidized by H2O2 to cysteine sulfenic acid (Cys–SOH), which is resolved by reaction with Cys170–SH of the adjacent monomer, resulting in the formation of a disulfide linkage, Cys47–S-S–Cys170 (7). The antioxidant function of Prx was attributed to the fact that the disulfide could be reduced by DTT, resulting in completion of a catalytic cycle. Thioredoxin (Trx) was eventually shown to be the biological donor of reducing equivalents for the catalytic function of Prx (8), with peroxynitrite (ONOO−) in addition to H2O2 and lipid peroxides also being found to be reduced by Prx enzymes (9). The conserved Cys residue corresponding to Cys47 of yeast Prx was later referred to as the peroxidatic Cys (CP) to reflect its sensitivity to oxidation by peroxides, and the conserved Cys residue corresponding to Cys170 was designated the resolving Cys (CR) (10).
Given that H2O2 reacts with the thiolate (deprotonated) form of cysteine, oxidation of cysteine is enhanced by a microenvironment that lowers the normally high pKa (∼8.6) of cysteine thiols to a value below neutral pH. The pKa values of the CP residue of members of the Prx family are in the range of 5–6 (11–16). However, whereas most deprotonated thiols react with H2O2 with a rate constant of ∼20 m−1 s−1 (17), the CP residue of Prxs reacts with H2O2 with rate constants of 1 × 105 to 1 × 107 m−1 s−1 (14, 17). The low pKa alone thus appears to be insufficient to account for the high reactivity of Prx with peroxide. The unusually high activity was recently attributed to the fact that Prx enzymes activate not only the thiolate of CP but also the peroxide substrate via a hydrogen bonding network formed by four amino acids (Pro, Thr, Arg, and Glu, Gln, or His) conserved in the active site of all Prx enzymes (18).
Mechanism of Peroxidase Reaction
On the basis of the location or absence of the CR residue, Prxs are classified into 2-Cys, atypical 2-Cys, and 1-Cys Prx subfamilies (4, 5, 19). Mammalian cells express six isoforms of Prx: four 2-Cys Prx isoforms (Prxs I–IV), one atypical 2-Cys Prx isoform (Prx V), and one 1-Cys Prx isoform (Prx VI). These isoforms vary in subcellular localization, with Prx I, II, and VI being localized mainly in the cytosol; Prx III being restricted to mitochondria; Prx IV being found predominantly in the endoplasmic reticulum (ER); and Prx V being present in the cytosol, mitochondria, and peroxisomes. As discussed below, however, Prx localization is multifarious, being dependent on the cell type and environment.
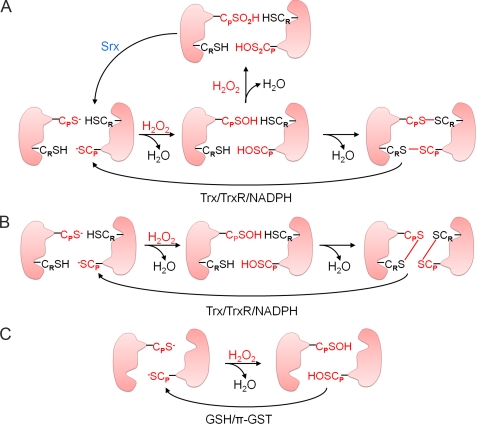
The peroxidase reaction mechanisms of mammalian Prx enzymes are shown in Fig. 1. As demonstrated first with yeast Prx, the conserved CP–SH of 2-Cys Prx is selectively oxidized by H2O2 to the CP–SOH intermediate. An intermolecular disulfide is then formed with CR–SH and is ultimately reduced by Trx (Fig. 1A). Eukaryotic members of the 2-Cys Prx subgroup are distinct from most prokaryotic members in that they contain two structural motifs (GGLG and YF) whose interaction restricts the ability of CP–SOH to approach CR–SH and thereby hinders disulfide formation (10, 18, 20). As a result, CP–SOH of eukaryotic 2-Cys Prxs can react with a second H2O2 molecule and become hyperoxidized to cysteine sulfinic acid (CP–SO2H) before it is able to react with CR–SH (21, 22). Such hyperoxidation results in the inactivation of peroxidase activity and in the formation of higher order molecular aggregates that exhibit protein chaperone function (23–25). The CP–SO2H moiety of 2-Cys Prxs can be reduced in an ATP-dependent reaction catalyzed by sulfiredoxin (Srx) (Fig. 1A) (26). Recent evidence has shown that 2-Cys Prxs from several prokaryotic organisms, including cyanobacteria, contain the GGLG and YF motifs and undergo hyperoxidation at the CP residue (27) and that cyanobacteria express a eukaryote-like Srx protein (28).
FIGURE 1.
Reaction mechanisms of Prx enzymes. The reaction mechanisms of 2-Cys Prx (A), atypical 2-Cys Prx (B), and 1-Cys Prx (C) enzymes are shown. TrxR, Trx reductase.
Prxs V and VI, the mammalian members of the atypical 2-Cys and 1-Cys Prx subgroups, respectively, also exist in an antiparallel dimeric form (29–33). During the catalytic cycles of Prxs V and VI (Fig. 1, B and C), the CP–SH residue is first oxidized by peroxides, as with 2-Cys Prxs. For Prx V, the resulting CP–SOH then reacts with CR–SH of the same subunit to form an intramolecular disulfide linkage, which is also reduced by Trx as in 2-Cys Prx (Fig. 1B). In the catalytic cycle of Prx VI, Cys–SOH does not form a disulfide because of the unavailability of another Cys–SH nearby. CP–SOH of oxidized Prx VI is reduced by GSH in the presence of the Pi isoform of GST but not by Trx or glutaredoxin (29, 30, 34–36). Prxs V and VI are less sensitive to hyperoxidation than are 2-Cys Prxs, and hyperoxidized forms of Prxs V and VI are not reduced by Srx (37).
Reduction of Locally Produced Peroxides by Concerted Action of 2-Cys Prx and Srx
The role of Prx enzymes as peroxidases has been demonstrated by increasing or decreasing their expression levels in various cell lines and evaluating the sensitivity of the cells to oxidative insults. Prx-deficient mice have also been generated by targeting the gene for each isoform. These mice develop to adulthood with no overt phenotype when maintained under normal laboratory conditions. Prx II knock-out (KO) mice subsequently develop hemolytic anemia (38), and ablation of Prx I resulted in spontaneous tumor formation in aging mice in one study (39). These phenotypes of Prx KO mice may not be related to the peroxidase activity of the targeted protein, however. As described below, certain Prx enzymes also function as local regulators of H2O2, which serves as an intracellular messenger. The tumor suppressor function of Prx I was attributed to such regulation of H2O2 (40, 41).
An in vivo antioxidant function of Prx I was recently demonstrated in the livers of ethanol-fed mice (42). The production of ROS and oxidative stress play a central role in the pathogenesis of alcoholic liver disease. Ethanol consumption induces the accumulation of CYP2E1 (cytochrome P450 2E1), a major contributor to ethanol-induced ROS production in the liver. CYP2E1 catalyzes the oxidation of a wide variety of low molecular weight compounds, including ethanol (43). Elevated CYP2E1 levels alone result in oxidative stress because electron transfer from the donor system (NADPH and NADPH-dependent cytochrome P450 reductase) to CYP2E1 is not perfectly coupled and is therefore leaky (44). Such leaked electrons react with O2 to produce superoxide, which is converted to H2O2 or reacts with nitric oxide to produce peroxynitrite. Ethanol feeding in mice was found to markedly increase the abundance of Srx protein and mRNA in the liver. However, it had no significant effect on the hepatic abundance of the six Prx isoforms. Analysis of Nrf2 KO mice indicated that the ethanol-induced up-regulation of Srx expression is mediated mainly via a pathway dependent on the transcription factor Nrf2 (42).
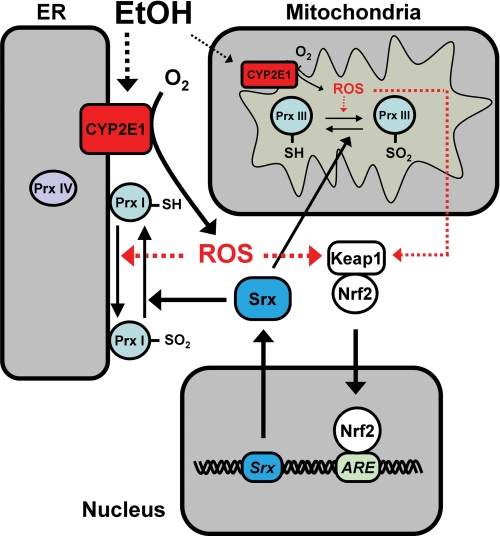
During elimination of peroxides, all 2-Cys Prxs undergo unavoidable hyperoxidation. Among Prxs I-IV, however, only Prx I was found to be hyperoxidized (to a moderate extent) in the livers of ethanol-fed mice, with this effect being markedly enhanced in Srx KO mice (Fig. 2) (42). These observations suggested that Prx I is the most active 2-Cys Prx in elimination of ROS in the livers of ethanol-fed mice and that, despite the elevated expression of Srx in such mice, the capacity of Srx is not sufficient to counteract the hyperoxidation of Prx I that occurs during ROS reduction. The preferential hyperoxidation of Prx I also occurs despite the fact that both Prxs I and II are cytosolic proteins and that Prx II is more prone to hyperoxidation than is Prx I in most cell types (41, 45). A protease protection assay showed that a large fraction of Prx I, but not of Prx II, is located at the cytoplasmic side of the ER membrane, where CYP2E1 is embedded (42). The selective burden of ROS removal borne by Prx I is thus likely attributable to its proximity to the ROS-generating CYP2E1 (Fig. 2). In addition to inducing the expression of Srx in the liver, ethanol feeding elicited the translocation of some Srx molecules to microsomes. However, the capacity of Srx located near the surface of the ER was not sufficient to fully counteract the hyperoxidation of Prx I, with consequent accumulation of a small amount of Prx I–SO2. Chronic ethanol feeding in Srx KO mice resulted in hyperoxidation of 30–50% of total Prx I, which likely represents virtually all ER-bound Prx I molecules (42).
FIGURE 2.
Antioxidant roles of Prx I, Prx III, and Srx in ethanol-fed mouse liver. EtOH feeding increases the abundance of CYP2E1 in the ER. A large proportion of Prx I is present at the cytosolic side of the ER membrane, where CYP2E1 is located. Prx I is therefore preferentially engaged in the reduction of ROS produced by CYP2E1 and becomes hyperoxidized. Reactivation of such hyperoxidized Prx I requires the action of Srx, the expression of which is greatly increased via an Nrf2- and antioxidant regulatory element (ARE)-dependent pathway in the livers of ethanol-fed mice. Ethanol feeding also increases the abundance of CYP2E1 and the production of ROS in mitochondria, likely resulting in hyperoxidation of Prx III that is reversed in part by translocation of Srx from the cytosol into mitochondria.
Ethanol feeding also increases the abundance of CYP2E1 and the production of ROS in mitochondria (46), likely resulting in the hyperoxidation of Prx III, a member of the 2-Cys Prx subfamily that is specifically localized to mitochondria (Fig. 2). However, Prx III–SO2 was not detected in the livers of ethanol-fed wild-type mice, probably because increased oxidative stress resulted in the translocation of Srx into mitochondria (47), and the capacity of mitochondrial Srx was then sufficient to counteract the hyperoxidation of Prx III. A small proportion of Prx III was hyperoxidized in the livers of ethanol-fed Srx-deficient mice, suggesting that Prx III is vulnerable to hyperoxidation by ethanol-induced ROS and that Prx III–SO2 accumulates only in the absence of Srx. Mammalian cells also contain a mitochondrion-specific Trx system (48), suggesting that Prx III together with mitochondrial Trx might provide a primary line of defense against H2O2 produced by the mitochondrial respiratory chain.
Ethanol-induced oxidative damage in the liver includes protein modification such as carbonylation, addition of 4-hydroxynonenal, and nitration of tyrosine to yield 3-nitrotyrosine (49). The pivotal roles of Srx and Prx I in protection of the liver against ethanol-induced oxidative stress were apparent in mice deficient in Srx or Prx I. Subjection of such mice to chronic ethanol feeding thus resulted in more severe oxidative damage to the liver, as revealed by protein carbonylation and by the formation of 4-hydroxynonenal and 3-nitrotyrosine adducts, compared with that observed in ethanol-fed wild-type mice (42).
Regulation by Prx I of H2O2 Produced Locally at Plasma Membrane
Many mammalian cell types produce H2O2 for the purpose of intracellular signaling in response to stimulation through various cell surface receptors (50, 51). For example, in cells stimulated with growth factors such as PDGF or EGF, the production of H2O2 is required for propagation of growth factor signaling (52, 53). Hydrogen peroxide is distinct from other messenger molecules in that it acts not by binding to effectors but by oxidizing their critical residues, most notably cysteine, as exemplified by the inhibition of protein-tyrosine phosphatases (PTPs) and the tumor suppressor PTEN (phosphatase and tensin homolog) as a result of oxidation of their catalytic Cys by H2O2 produced in response to growth factor stimulation (19, 54). Indeed, the activation of protein-tyrosine kinases (PTKs) is not sufficient to increase the steady-state level of protein tyrosine phosphorylation in cells; the concurrent inhibition of PTPs by H2O2 is also required. It had remained unclear, however, how a toxic molecule like H2O2 is able to oxidize effector proteins selectively without inflicting damage on other proteins and lipids. One possible explanation was localized production of H2O2. Evidence indicates that NADPH oxidase (Nox) is largely responsible for receptor-dependent H2O2 production (55, 56) and that Nox1 and Nox2 are activated within lipid rafts, which serve as a platform for the assembly of various signaling proteins, including receptor PTKs and Src family kinases (57).
Proteins such as PTPs and PTEN have a low pKa thiol that can be selectively oxidized by H2O2 with a rate constant of ∼20 m−1 s−1 (17). Given such a moderate rate of oxidation, for H2O2 to serve as a signaling molecule, its concentration must increase rapidly above a certain threshold (10–100 μm) and remain elevated long enough for it to oxidize effector molecules (58). However, in most cells, H2O2-eliminating enzymes are present at large concentrations in the cytosol to ensure that the toxic molecule remains at low intracellular levels (<0.1 μm) (58). The localized production of H2O2 might therefore not be sufficient to support its messenger function without protection from local H2O2-eliminating enzymes.
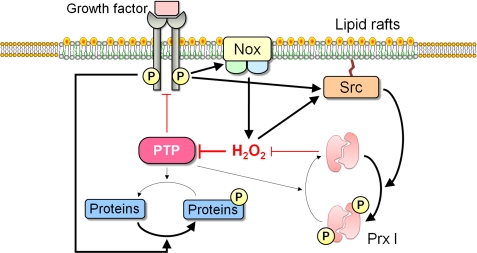
Although Prxs I and II are present mostly in the cytosolic fraction, a small proportion of these enzymes was shown to be constitutively associated with detergent-resistant lipid rafts (41). Prx I, but not Prx II, was also found to be phosphorylated (at Tyr194) by PTKs of the Src family in several cell types stimulated via growth factor receptors, including those for PDGF and EGF, or via immune receptors such as the T cell and B cell receptors. This phosphorylation induced inactivation of the peroxidase activity of Prx I and was confined to Prx I molecules associated with lipid rafts; it was not observed with Prx I present in the cytosol. The spatially confined inactivation of Prx I thus provides a means for the generation of favorable H2O2 gradients around lipid rafts, where signaling proteins are concentrated, while minimizing the general accumulation of H2O2 to toxic levels and disturbance of global redox potential. Prx I phosphorylation was enhanced by increasing Nox1 expression, supporting the notion that Prx I phosphorylation occurs in the cellular microdomains where H2O2 is produced. The phosphorylation of Prx I at Tyr194 was also detected at the wound edge during repair of a cutaneous injury in mice. This finding likely reflects the fact that growth factors such as EGF, PDGF, FGF, TGF-α, keratinocyte growth factor, and insulin-like growth factor-1 act as key inducers of the proliferation of keratinocytes and fibroblasts at the wound edge (59).
A model depicting the mechanism underlying H2O2 accumulation around lipid rafts and its role in intracellular signaling is shown in Fig. 3. Engagement of a growth factor receptor or an immune receptor thus induces the production of H2O2 through activation of Nox present in lipid rafts. The activated receptor also induces the phosphorylation of raft-associated Prx I at Tyr194 through activation of a Src family PTK, resulting in inactivation of Prx I, which would otherwise destroy the newly generated H2O2. Inactivation of Prx I allows the accumulation of H2O2 around lipid rafts, which in turn promotes further phosphorylation and inactivation of Prx I both by activating Src kinases and by inactivating PTPs. These two positive feedback loops allow the sustained H2O2 signaling necessary for the regulation of biological responses. The inhibition of PTPs by H2O2 is critical for growth factor signaling because activation of receptor PTKs alone is not sufficient to achieve the levels of protein tyrosine phosphorylation necessary for such signaling, with the concurrent inhibition of PTPs by H2O2 preventing the futile cycle of phosphorylation and dephosphorylation (52–54). Reversible inactivation of the inositol lipid phosphatase PTEN by H2O2 is also necessary for signaling mediated by phosphoinositide 3-kinase (60, 61).
FIGURE 3.
Model for mechanism underlying H2O2 accumulation around lipid rafts and its role in intracellular signaling. See text for details.
In contrast to Prx I, Prx II was found to be inactivated not via tyrosine phosphorylation but rather via hyperoxidation of its peroxidatic cysteine and only under conditions of sustained oxidative stress. Hyperoxidized Prx I or II could not be detected even when the extent of PDGF-induced H2O2 generation was increased by overexpressing the PDGF receptor or Nox1 (together with two additional proteins, Noxo1 and Noxa1, which are necessary for activation of Nox1) (41). Although both Prxs I and II are associated with lipid rafts, inactivation of Prx I, which is more efficient as a peroxidase than is Prx II, might be sufficient to allow local accumulation of H2O2 for signal propagation. The role of raft-associated Prx II remains unclear. Given that the accumulation of a small amount of H2O2 at lipid rafts can trigger rapid signal amplification through the action of the positive feedback loops shown in Fig. 3, the presence of both Prxs II and I might provide a safeguard to prevent inappropriate activation of signaling cascades by spurious production of H2O2 at rafts. Functional differences between Prxs I and II are also apparent in the phenotypes of the corresponding KO mice: mice lacking Prx I thus manifest an increased susceptibility to spontaneous cancer, whereas those deficient in Prx II do not (39, 40). Prx I has thus been suggested to play a tumor suppressor role, and this suggestion was further supported by the observation that Prx I deficiency in mice increases the susceptibility to Ras- or ErbB2-induced breast cancer (40).
The model shown in Fig. 3 also exemplifies a double-negative feedback loop, in which PTPs suppress receptor PTK activation, and H2O2 mediates coupling of receptor PTK activation with PTP inhibition. Computer simulation has recently been applied to analyze the signaling system involving a receptor PTK, a PTP, Prx I, and H2O2 (62). It was suggested previously that the plasma membrane is partitioned into 50–300-nm-wide domains by the combined action of actin-based membrane skeleton “fences” and anchored-transmembrane protein “pickets” (63), with proteins and lipids being highly mobile within these membrane domains. However, the hopping of signaling proteins across the fences is thought to occur with low probability and therefore becomes the rate-limiting factor in lateral spread of receptor signaling. The computer simulation indicated that the propagation of growth factor signaling initiated by a local source is possible as a result of the tight H2O2-dependent coupling between the receptor PTK and PTP. In addition, the simulation predicted that the phosphorylation-dependent regulation of Prx I activity contributes to lowering the threshold for later propagation of signaling but not substantially to its axial propagation in the cytoplasm (62).
Role of Prx IV as H2O2 Sensor for Protein Folding in ER
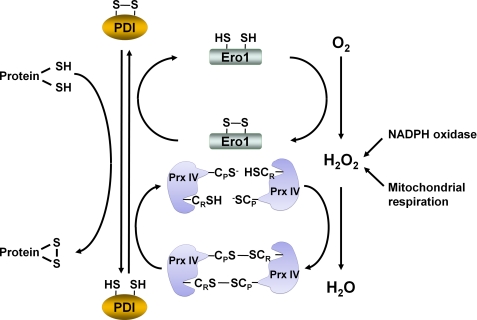
Prx IV is an ER-resident protein (64). The ER is responsible for maintaining redox conditions that facilitate the formation of disulfide bonds, which involves the transfer of electrons from the reduced cysteines of unfolded proteins to oxidoreductases of the protein-disulfide isomerase (PDI) family. Completion of the catalytic cycle requires re-formation of the intramolecular disulfide of PDI. Ero1 (ER membrane-associated oxidoreductin 1) was found to participate in PDI reoxidation and disulfide bond formation (65). During the PDI oxidation reaction, Ero1 mediates the flavin-dependent transfer of electrons directly to molecular oxygen, producing one molecule of H2O2 for every disulfide bond formed (Fig. 4). The H2O2 produced by Ero1 can be reduced by Prx IV. Given that the ER does not contain Trx, it has been unclear, however, how the inevitable formation of the disulfide CP–S-S–CR within Prx IV can be reversed to support peroxidase activity. A novel solution to this problem has been provided by the finding that PDI physically interacts with Prx IV and serves as the disulfide reductase of oxidized Prx IV (66, 67). Indeed, PDI itself was oxidized more efficiently by Prx IV than by Ero1 (67). In addition to providing a pathway for maintaining the catalytic activity of Prx IV, the coupling of the oxidation of Prx IV by H2O2 to the formation of a disulfide ensures that two disulfide bonds are formed for every oxygen molecule reduced (Fig. 4).
FIGURE 4.
Role of Prx IV as H2O2 sensor for protein folding in ER. Oxidation of thiols to disulfide linkages during protein folding in the ER is achieved with the use of H2O2 produced by oxidant-generating sources such as Ero1, NADPH oxidase, and mitochondria. Ero1 produces H2O2 by transferring electrons from reduced PDI to O2. In the proposed model, CP–SH and CR–SH of Prx IV are first oxidized by H2O2 to form a disulfide. The oxidized state of Prx IV is then transferred to PDI thiols, thereby converting them to a disulfide and finally resulting in the formation of a disulfide in the protein to be folded. Prx IV in the ER physically interacts with PDI. It is thus thought to function as a sensor of H2O2 in PDI-mediated protein folding.
Despite its key role in protein folding, Ero1 was found not to be essential in mice (68), suggesting the existence of alternative sources of H2O2 molecules for protein folding. Nox4, which is present in the ER, was proposed as a candidate for such a source. ER-associated mitochondria also can provide H2O2 to support ER function. It was proposed that H2O2 molecules provided by these sources are used to oxidize Prx IV and that the oxidized state of Prx IV is then transferred to PDI thiols, thereby converting them to a disulfide and finally resulting in the formation of a disulfide in the protein to be folded (Fig. 4) (66, 67). Prx IV thus functions as a sensor of H2O2 in PDI-mediated protein folding. This sensor function of Prx IV is possible because the active site of Prx is configured to favor oxidation by peroxides but not for reaction with other oxidants or disulfide-containing proteins. The peroxide sensor function of thiol-based peroxidases has been demonstrated previously for the oxidation of Yap1 (a transcription factor that controls antioxidant gene expression) by Orp1 (a Prx-related glutathione peroxidase) in Saccharomyces cerevisiae (69, 70). Similarly, in Schizosaccharomyces pombe, Tpx1 (a 2-Cys Prx) serves as a peroxide sensor for Pap1, the homolog of Yap1 (71, 72). In plants, the nuclear-encoded chloroplast 2-Cys Prx was shown to regulate the transcription activity of Rap2.4 (73).
Other Localized Functions of Prx
A somewhat unusual disulfide reductase activity of Prx I has been described recently in relation to regulation of the six-transmembrane protein GDE2 (74). GDE2, which controls the onset and progression of the differentiation of spinal motor neurons through its extracellular glycerophosphodiester phosphodiesterase activity, is normally inactive because of the presence of an intramolecular disulfide bond between Cys25 and Cys576. However, GDE2 associates with Prx I through its N-terminal 38 amino acids, resulting in the activation of GDE2 through Prx I-catalyzed reduction of the intramolecular disulfide bond. This finding thus uncovered a novel disulfide reductase activity of Prx I. Prx I-dependent activation of GDE2 should result in the formation of the intermolecular disulfide CP–S-S–CR within Prx I, suggesting that the progression of neuronal differentiation relies on the effective recycling of Prx I to the active reduced form by Trx.
Prx VI is the prototype and the only mammalian 1-Cys member of the Prx family. Major differences from other Prxs include the use of GSH instead of Trx as the physiological reductant, heterodimerization with Pi GST as part of the catalytic cycle, and the ability to hydrolyze phospholipids as a result of Ca2+-independent phospholipase A2 (PLA2) activity (35, 36, 75–77). Prx VI is thus a bifunctional protein and has separate active site residues for peroxidase (Cys47, the CP residue) and PLA2 (Ser32) activities. Prx VI is found in both cytosolic and lysosomal compartments in lung alveolar epithelium. It is capable of reducing a broad spectrum of peroxides and provides protection against oxidative stress through its peroxidase activity (78), and it participates in the turnover of lung surfactant phospholipids through its PLA2 activity (79). Given that the pH optimum for the PLA2 activity of the enzyme is in the acidic range (80) and that the peroxidase activity is expressed at cytosolic pH, the subcellular localization of Prx VI is likely to affect the balance between the two activities. The localization of Prx VI to lysosome-like organelles in lung epithelial cells was shown recently to require the activity of the mitogen-activated protein kinases ERK and p38 but not to involve Prx VI phosphorylation. Rather, ERK and p38 appear to regulate the subcellular localization of Prx VI through activation of 14-3-3ϵ as a chaperone protein that binds to Prx VI, resulting in entry of Prx VI into the vesicular pathway and its further targeting to lysosomal compartments (81).
Concluding Remarks
The structure of Prx provides a specific environment that renders CP highly sensitive to oxidation by H2O2 while at the same time protecting it from reaction with other cellular oxidants. Six different Prx isozymes are found in various locations of mammalian cells, allowing Prx to function as a peroxidase as well as a sensor of H2O2 in specialized cellular compartments. As exemplified by the tyrosine phosphorylation-dependent inactivation of Prx I, the peroxidase function of Prx can be regulated via various post-translational modifications. Such modifications also include threonine and serine phosphorylation (82, 83), lysine acetylation (84), cysteine glutathionylation (85, 86), cysteine nitrosylation (87), and limited proteolysis (88), although the biological relevance of these reactions remains unclear at the present time. Prxs also interact with numerous proteins, which might direct their cellular localization and sensitivity to oxidation (5).
This work was supported by National Honor Scientist Program Grant 2009-0052293 and Bio R&D Program Grant M10642040001-07N4204-00110 (to S. G. R.) from the National Research Foundation of Korea. This is the second article in the Thematic Minireview Series on Redox Sensing and Regulation.
- Prx
- peroxiredoxin
- ROS
- reactive oxygen species
- Trx
- thioredoxin
- CP
- peroxidatic Cys
- CR
- resolving Cys
- ER
- endoplasmic reticulum
- Srx
- sulfiredoxin
- KO
- knock-out
- PTP
- protein-tyrosine phosphatase
- PTK
- protein-tyrosine kinase
- Nox
- NADPH oxidase
- PDI
- protein-disulfide isomerase
- PLA2
- phospholipase A2.
REFERENCES
- 1. Kim I. H., Kim K., Rhee S. G. (1989) Induction of an antioxidant protein of Saccharomyces cerevisiae by O2, Fe3+, or 2-mercaptoethanol. Proc. Natl. Acad. Sci. U.S.A. 86, 6018–6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. (1988) The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 263, 4704–4711 [PubMed] [Google Scholar]
- 3. Chae H. Z., Kim I. H., Kim K., Rhee S. G. (1993) Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J. Biol. Chem. 268, 16815–16821 [PubMed] [Google Scholar]
- 4. Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. (1994) Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. U.S.A. 91, 7017–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhee S. G., Woo H. A. (2011) Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 15, 781–794 [DOI] [PubMed] [Google Scholar]
- 6. Kim K., Rhee S. G., Stadtman E. R. (1985) Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J. Biol. Chem. 260, 15394–15397 [PubMed] [Google Scholar]
- 7. Chae H. Z., Uhm T. B., Rhee S. G. (1994) Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc. Natl. Acad. Sci. U.S.A. 91, 7022–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chae H. Z., Chung S. J., Rhee S. G. (1994) Thioredoxin-dependent peroxide reductase from yeast. J. Biol. Chem. 269, 27670–27678 [PubMed] [Google Scholar]
- 9. Bryk R., Griffin P., Nathan C. (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 [DOI] [PubMed] [Google Scholar]
- 10. Wood Z. A., Poole L. B., Karplus P. A. (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300, 650–653 [DOI] [PubMed] [Google Scholar]
- 11. Hugo M., Turell L., Manta B., Botti H., Monteiro G., Netto L. E., Alvarez B., Radi R., Trujillo M. (2009) Thiol and sulfenic acid oxidation of AhpE, the one-cysteine peroxiredoxin from Mycobacterium tuberculosis: kinetics, acidity constants, and conformational dynamics. Biochemistry 48, 9416–9426 [DOI] [PubMed] [Google Scholar]
- 12. Nelson K. J., Parsonage D., Hall A., Karplus P. A., Poole L. B. (2008) Cysteine pKa values for the bacterial peroxiredoxin AhpC. Biochemistry 47, 12860–12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogusucu R., Rettori D., Munhoz D. C., Netto L. E., Augusto O. (2007) Reactions of yeast thioredoxin peroxidases I and II with hydrogen peroxide and peroxynitrite: rate constants by competitive kinetics. Free Radic. Biol. Med. 42, 326–334 [DOI] [PubMed] [Google Scholar]
- 14. Peskin A. V., Low F. M., Paton L. N., Maghzal G. J., Hampton M. B., Winterbourn C. C. (2007) The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 282, 11885–11892 [DOI] [PubMed] [Google Scholar]
- 15. Trujillo M., Ferrer-Sueta G., Thomson L., Flohé L., Radi R. (2007) Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell. Biochem. 44, 83–113 [DOI] [PubMed] [Google Scholar]
- 16. Knoops B., Goemaere J., Van der Eecken V., Declercq J. P. (2011) Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid. Redox Signal. 15, 817–829 [DOI] [PubMed] [Google Scholar]
- 17. Winterbourn C. C. (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 [DOI] [PubMed] [Google Scholar]
- 18. Hall A., Nelson K., Poole L. B., Karplus P. A. (2011) Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid. Redox Signal. 15, 795–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhee S. G., Chae H. Z., Kim K. (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 38, 1543–1552 [DOI] [PubMed] [Google Scholar]
- 20. Wood Z. A., Schröder E., Robin Harris J., Poole L. B. (2003) Structure, mechanism, and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32–40 [DOI] [PubMed] [Google Scholar]
- 21. Yang K. S., Kang S. W., Woo H. A., Hwang S. C., Chae H. Z., Kim K., Rhee S. G. (2002) Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine sulfinic acid. J. Biol. Chem. 277, 38029–38036 [DOI] [PubMed] [Google Scholar]
- 22. Rabilloud T., Heller M., Gasnier F., Luche S., Rey C., Aebersold R., Benahmed M., Louisot P., Lunardi J. (2002) Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem. 277, 19396–19401 [DOI] [PubMed] [Google Scholar]
- 23. Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 24. Moon J. C., Hah Y. S., Kim W. Y., Jung B. G., Jang H. H., Lee J. R., Kim S. Y., Lee Y. M., Jeon M. G., Kim C. W., Cho M. J., Lee S. Y. (2005) Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 280, 28775–28784 [DOI] [PubMed] [Google Scholar]
- 25. Lim J. C., Choi H. I., Park Y. S., Nam H. W., Woo H. A., Kwon K. S., Kim Y. S., Rhee S. G., Kim K., Chae H. Z. (2008) Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine-sulfonic acid for enhanced molecular chaperone activity. J. Biol. Chem. 283, 28873–28880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biteau B., Labarre J., Toledano M. B. (2003) ATP-dependent reduction of cysteine sulfinic acid by S. cerevisiae sulfiredoxin. Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 27. Pascual M. B., Mata-Cabana A., Florencio F. J., Lindahl M., Cejudo F. J. (2010) Overoxidation of 2-Cys peroxiredoxin in prokaryotes. Cyanobacterial 2-Cys peroxiredoxins sensitive to oxidative stress. J. Biol. Chem. 285, 34485–34492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boileau C., Eme L., Brochier-Armanet C., Janicki A., Zhang C. C., Latifi A. (2011) A eukaryotic-like sulfiredoxin involved in oxidative stress responses and in the reduction of the sulfinic form of 2-Cys peroxiredoxin in the cyanobacterium Anabaena PCC 7120. New Phytol. 191, 1108–1118 [DOI] [PubMed] [Google Scholar]
- 29. Choi H. J., Kang S. W., Yang C. H., Rhee S. G., Ryu S. E. (1998) Crystal structure of a novel human peroxidase enzyme at 2.0 Å resolution. Nat. Struct. Biol. 5, 400–406 [DOI] [PubMed] [Google Scholar]
- 30. Montemartini M., Kalisz H. M., Hecht H. J., Steinert P., Flohé L. (1999) Activation of active-site cysteine residues in the peroxiredoxin-type tryparedoxin peroxidase of Crithidia fasciculata. Eur. J. Biochem. 264, 516–524 [DOI] [PubMed] [Google Scholar]
- 31. Hirotsu S., Abe Y., Okada K., Nagahara N., Hori H., Nishino T., Hakoshima T. (1999) Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc. Natl. Acad. Sci. U.S.A. 96, 12333–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seo M. S., Kang S. W., Kim K., Baines I. C., Lee T. H., Rhee S. G. (2000) Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 275, 20346–20354 [DOI] [PubMed] [Google Scholar]
- 33. Smeets A., Marchand C., Linard D., Knoops B., Declercq J. P. (2008) The crystal structures of oxidized forms of human peroxiredoxin 5 with an intramolecular disulfide bond confirm the proposed enzymatic mechanism for atypical 2-Cys peroxiredoxins. Arch. Biochem. Biophys. 477, 98–104 [DOI] [PubMed] [Google Scholar]
- 34. Fisher A. B. (2011) Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 15, 831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang S. W., Baines I. C., Rhee S. G. (1998) Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem. 273, 6303–6311 [DOI] [PubMed] [Google Scholar]
- 36. Manevich Y., Feinstein S. I., Fisher A. B. (2004) Activation of the antioxidant enzyme 1-Cys peroxiredoxin requires glutathionylation mediated by heterodimerization with Pi GST. Proc. Natl. Acad. Sci. U.S.A. 101, 3780–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woo H. A., Jeong W., Chang T. S., Park K. J., Park S. J., Yang J. S., Rhee S. G. (2005) Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-Cys peroxiredoxins. J. Biol. Chem. 280, 3125–3128 [DOI] [PubMed] [Google Scholar]
- 38. Lee T. H., Kim S. U., Yu S. L., Kim S. H., Park D. S., Moon H. B., Dho S. H., Kwon K. S., Kwon H. J., Han Y. H., Jeong S., Kang S. W., Shin H. S., Lee K. K., Rhee S. G., Yu D. Y. (2003) Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038 [DOI] [PubMed] [Google Scholar]
- 39. Neumann C. A., Krause D. S., Carman C. V., Das S., Dubey D. P., Abraham J. L., Bronson R. T., Fujiwara Y., Orkin S. H., Van Etten R. A. (2003) Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defense and tumor suppression. Nature 424, 561–565 [DOI] [PubMed] [Google Scholar]
- 40. Cao J., Schulte J., Knight A., Leslie N. R., Zagozdzon A., Bronson R., Manevich Y., Beeson C., Neumann C. A. (2009) Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 28, 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woo H. A., Yim S. H., Shin D. H., Kang D., Yu D. Y., Rhee S. G. (2010) Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140, 517–528 [DOI] [PubMed] [Google Scholar]
- 42. Bae S. H., Sung S. H., Cho E. J., Lee S. K., Lee H. E., Woo H. A., Yu D. Y., Kil I. S., Rhee S. G. (2011) Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology 53, 945–953 [DOI] [PubMed] [Google Scholar]
- 43. Song B. J. (1996) Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology, and clinical relevance: 1996 update. Alcohol. Clin. Exp. Res. 20, 138A–146A [DOI] [PubMed] [Google Scholar]
- 44. Rashba-Step J., Turro N. J., Cederbaum A. I. (1993) Increased NADPH- and NADH-dependent production of superoxide and hydroxyl radical by microsomes after chronic ethanol treatment. Arch. Biochem. Biophys. 300, 401–408 [DOI] [PubMed] [Google Scholar]
- 45. Seo J. H., Lim J. C., Lee D. Y., Kim K. S., Piszczek G., Nam H. W., Kim Y. S., Ahn T., Yun C. H., Kim K., Chock P. B., Chae H. Z. (2009) Novel protective mechanism against irreversible hyperoxidation of peroxiredoxin. Nα-terminal acetylation of human peroxiredoxin II. J. Biol. Chem. 284, 13455–13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cederbaum A. I., Lu Y., Wu D. (2009) Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 83, 519–548 [DOI] [PubMed] [Google Scholar]
- 47. Noh Y. H., Baek J. Y., Jeong W., Rhee S. G., Chang T. S. (2009) Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. J. Biol. Chem. 284, 8470–8477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee S. R., Kim J. R., Kwon K. S., Yoon H. W., Levine R. L., Ginsburg A., Rhee S. G. (1999) Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 274, 4722–4734 [DOI] [PubMed] [Google Scholar]
- 49. Dey A., Cederbaum A. I. (2006) Alcohol and oxidative liver injury. Hepatology 43, S63–S74 [DOI] [PubMed] [Google Scholar]
- 50. Rhee S. G. (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 [DOI] [PubMed] [Google Scholar]
- 51. D'Autréaux B., Toledano M. B. (2007) ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 [DOI] [PubMed] [Google Scholar]
- 52. Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 [DOI] [PubMed] [Google Scholar]
- 53. Bae Y. S., Kang S. W., Seo M. S., Baines I. C., Tekle E., Chock P. B., Rhee S. G. (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 [PubMed] [Google Scholar]
- 54. Tonks N. K. (2005) Redox redux: revisiting PTPs and the control of cell signaling. Cell 121, 667–670 [DOI] [PubMed] [Google Scholar]
- 55. Oakley F. D., Abbott D., Li Q., Engelhardt J. (2009) Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 11, 1313–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lambeth J. D. (2004) NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 [DOI] [PubMed] [Google Scholar]
- 57. Ushio-Fukai M. (2009) Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 11, 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stone J. R., Yang S. (2006) Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270 [DOI] [PubMed] [Google Scholar]
- 59. Singer A. J., Clark R. A. (1999) Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 [DOI] [PubMed] [Google Scholar]
- 60. Kwon J., Lee S. R., Yang K. S., Ahn Y., Kim Y. J., Stadtman E. R., Rhee S. G. (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. U.S.A. 101, 16419–16424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leslie N. R., Bennett D., Lindsay Y. E., Stewart H., Gray A., Downes C. P. (2003) Redox regulation of PI 3-kinase signaling via inactivation of PTEN. EMBO J. 22, 5501–5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grecco H. E., Schmick M., Bastiaens P. I. (2011) Signaling from the living plasma membrane. Cell 144, 897–909 [DOI] [PubMed] [Google Scholar]
- 63. Kusumi A., Sako Y. (1996) Cell surface organization by the membrane skeleton. Curr. Opin. Cell Biol. 8, 566–574 [DOI] [PubMed] [Google Scholar]
- 64. Tavender T. J., Sheppard A. M., Bulleid N. J. (2008) Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme forming oligomeric complexes in human cells. Biochem. J. 411, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tu B. P., Weissman J. S. (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zito E., Melo E. P., Yang Y., Wahlander Å., Neubert T. A., Ron D. (2010) Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol. Cell 40, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tavender T. J., Springate J. J., Bulleid N. J. (2010) Recycling of peroxiredoxin IV provides a novel pathway for disulfide formation in the endoplasmic reticulum. EMBO J. 29, 4185–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zito E., Chin K. T., Blais J., Harding H. P., Ron D. (2010) ERO1-β, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J. Cell Biol. 188, 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. (2002) A thiol peroxidase is an H2O2 receptor and redox transducer in gene activation. Cell 111, 471–481 [DOI] [PubMed] [Google Scholar]
- 70. Okazaki S., Tachibana T., Naganuma A., Mano N., Kuge S. (2007) Multistep disulfide bond formation in Yap1 is required for sensing and transduction of H2O2 stress signal. Mol. Cell 27, 675–688 [DOI] [PubMed] [Google Scholar]
- 71. Bozonet S. M., Findlay V. J., Day A. M., Cameron J., Veal E. A., Morgan B. A. (2005) Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J. Biol. Chem. 280, 23319–23327 [DOI] [PubMed] [Google Scholar]
- 72. Vivancos A. P., Castillo E. A., Biteau B., Nicot C., Ayté J., Toledano M. B., Hidalgo E. (2005) A cysteine sulfinic acid in peroxiredoxin regulates H2O2 sensing by the antioxidant Pap1 pathway. Proc. Natl. Acad. Sci. U.S.A. 102, 8875–8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shaikhali J., Heiber I., Seidel T., Ströher E., Hiltscher H., Birkmann S., Dietz K. J., Baier M. (2008) The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol. 8, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yan Y., Sabharwal P., Rao M., Sockanathan S. (2009) The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol redox-dependent activation of GDE2. Cell 138, 1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shichi H., Demar J. C. (1990) Non-selenium glutathione peroxidase without glutathione S-transferase activity from bovine ciliary body. Exp. Eye Res. 50, 513–520 [DOI] [PubMed] [Google Scholar]
- 76. Kim T. S., Sundaresh C. S., Feinstein S. I., Dodia C., Skach W. R., Jain M. K., Nagase T., Seki N., Ishikawa K., Nomura N., Fisher A. B. (1997) Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J. Biol. Chem. 272, 2542–2550 [DOI] [PubMed] [Google Scholar]
- 77. Chen J. W., Dodia C., Feinstein S. I., Jain M. K., Fisher A. B. (2000) 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J. Biol. Chem. 275, 28421–28427 [DOI] [PubMed] [Google Scholar]
- 78. Manevich Y., Fisher A. B. (2005) Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic. Biol. Med. 38, 1422–1432 [DOI] [PubMed] [Google Scholar]
- 79. Fisher A. B., Dodia C., Feinstein S. I., Ho Y. S. (2005) Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal type phospholipase A2. J. Lipid Res. 46, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 80. Kim T. S., Dodia C., Chen X., Hennigan B. B., Jain M., Feinstein S. I., Fisher A. B. (1998) Cloning and expression of rat lung acidic Ca2+-independent PLA2 and its organ distribution. Am. J. Physiol. 274, L750–L761 [DOI] [PubMed] [Google Scholar]
- 81. Sorokina E. M., Feinstein S. I., Zhou S., Fisher A. B. (2011) Intracellular targeting of peroxiredoxin 6 to lysosomal organelles requires MAPK activity and binding to 14-3-3ϵ. Am. J. Physiol. Cell Physiol. 300, C1430–CC1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chang T. S., Jeong W., Choi S. Y., Yu S., Kang S. W., Rhee S. G. (2002) Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J. Biol. Chem. 277, 25370–25376 [DOI] [PubMed] [Google Scholar]
- 83. Zykova T. A., Zhu F., Vakorina T. I., Zhang J., Higgins L. A., Urusova D. V., Bode A. M., Dong Z. (2010) T-LAK cell-originated protein kinase (TOPK) phosphorylation of Prx1 at Ser-32 prevents UVB-induced apoptosis in RPMI7951 melanoma cells through the regulation of Prx1 peroxidase activity. J. Biol. Chem. 285, 29138–29146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Parmigiani R. B., Xu W. S., Venta-Perez G., Erdjument-Bromage H., Yaneva M., Tempst P., Marks P. A. (2008) HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc. Natl. Acad. Sci. U.S.A. 105, 9633–9638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Park J. W., Mieyal J. J., Rhee S. G., Chock P. B. (2009) Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J. Biol. Chem. 284, 23364–23374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Park J. W., Piszczek G., Rhee S. G., Chock P. B. (2011) Glutathionylation of peroxiredoxin I induces decamer to dimer dissociation with concomitant loss of chaperone activity. Biochemistry 50, 3204–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fang J., Nakamura T., Cho D. H., Gu Z., Lipton S. A. (2007) S-Nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 104, 18742–18747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Koo K. H., Lee S., Jeong S. Y., Kim E. T., Kim H. J., Kim K., Song K., Chae H. Z. (2002) Regulation of thioredoxin peroxidase activity by C-terminal truncation. Arch. Biochem. Biophys. 397, 312–318 [DOI] [PubMed] [Google Scholar]